Abstract
There is no consensus regarding the best practice for detecting murine pinworm infections. Initially, we evaluated 7 fecal concentration methods by using feces containing Aspiculuris tetraptera (AT) eggs (n = 20 samples per method). Sodium nitrate flotation, sodium nitrate centrifugation, Sheather sugar centrifugation, and zinc sulfate centrifugation detected eggs in 100% of samples; zinc sulfate flotation and water sedimentation detected eggs in 90%. All had better detection rates than Sheather sugar flotation (50%). To determine optimal detection methods, Swiss Webster mice were exposed to Syphacia obvelata (SO; n = 60) or AT (n = 60). We compared the following methods at days 0, 30, and 90, beginning 21 or 28 d after SO and AT exposure, respectively: fecal concentration (AT only), anal tape test (SO only), direct examination of intestinal contents (cecum and colon), Swiss roll histology (cecum and colon), and PCR analysis (pooled fur swab and feces). Detection rates for SO-exposed mice were: PCR analysis, 45%; Swiss roll histology, 30%; intestinal content exam, 27%; and tape test, 27%. The SO detection rate for PCR analysis was significantly greater than that for the tape test. Detection rates for AT-exposed mice were: intestinal content exam, 53%; PCR analysis, 33%; fecal flotation, 22%; and Swiss roll histology, 17%. The AT detection rate of PCR analysis combined with intestinal content examination was greater than for PCR analysis only and the AT detection rate of intestinal content examination was greater than for Swiss roll histology. Combining PCR analysis with intestinal content examination detected 100% of infected animals. No single test detected all positive animals. We recommend combining PCR analysis with intestinal content examination for optimal pinworm detection.
Abbreviations: AT, Aspiculuris tetraptera; SO, Syphacia obvelata
Murine pinworm infections continue to be a problem for contemporary research colonies, as highlighted by recent vendor-associated outbreaks at many institutions. Syphacia obvelata (SO) and Aspiculuris tetraptera (AT) are the ‘cosmopolitan’ murine pinworms59 that should be excluded from and monitored for in mouse colonies, due to their effects on animal health and research, including behavior, gastrointestinal physiology, immunology, growth, and hematopoiesis.9,37,40,44,61 Furthermore, pinworm infections may limit the transfer of mice between institutions.16 A 2006 survey of 35 of the top 112 NIH-funded institutions spanning 25 states reported that approximately 75% of those institutions had detected SO and approximately 60% had detected AT in their mouse colonies.11
First described in the early 1800s,51,53 these endoparasitic oxyurid nematodes have direct life cycles and transmit infections through the ingestion of embryonated (infective) eggs.3,51 SO and AT can be distinguished by means of morphologic differences in the worms and eggs.3,51,59 SO primarily inhabits the cecum and has a prepatent period of 11 to 15 d; eggs are laid in the perianal region, where they embryonate in 5 to 20 h.12,59 Retroinfection has been postulated but remains unproven.12 AT primarily resides in the proximal colon and has a prepatent period of approximately 24 d.2,4,14 In contrast to SO eggs, AT eggs are passed in feces51 and embryonate in 5 to 8 d.1,13,30
The reported prevalence of murine pinworm infections varies markedly. Many factors affect prevalence, including age, sex, strain, host immune function, and environmental burden.5,7,12,18,22,27,33,38,39,47,51,55,58 Even though pinworm eggs have not been documented in the environment in modern vivaria, they have been shown to be resistant to disinfectants.8,19,36,43 Although Syphacia spp. have been eradicated successfully from rodent colonies by using oral anthelmintic treatment alone,31,36 no reports of eradication of AT without environmental decontamination have been published.62 The prevalence and potential for environmental persistence underlie the importance of instituting a biosecurity program that incorporates optimal detection methods.
Traditionally, pinworms are diagnosed in live mice by detecting eggs by means of the anal tape test (SO)3,24,29,51,54,59 and fecal concentration methods (AT).3,50,51,59 Antemortem pinworm detection can be challenging, given that testing can produce false-negative results due to testing during the prepatent period or intermittent egg shedding.10,15,16,50 In addition, pinworm detection can be affected by extrinsic factors including worm burden and the ability to transmit infections to soiled-bedding sentinels, which can be influenced further by the parasite's life cycle, including time to egg embryonation.16,23 Immune resistance can reduce both worm and egg burdens and has been reported regarding both pinworm species.6,7,15. Traditional antemortem testing methods generally are considered to be less sensitive than are postmortem testing methods.16 Fecal concentration techniques, including flotation and sedimentation methods, improve the recovery and identification of parasites.57 In the flotation method, a solution whose specific gravity is greater than that of the egg is used, allowing it to float to the top of the solution.57 Passive flotation generally is reported to be less sensitive than centrifugation flotation.57 However, this difference has not been evaluated with murine pinworm eggs. Flotation solutions include sodium chloride, sodium nitrate, sugar, and zinc sulfate and, for this purpose, should have a specific gravity of 1.20 to 1.30.49,57 Centrifugation improves the sensitivity of the test by pelleting the dense debris at the bottom of the tube, allowing the lighter parasite eggs to rise,57 increasing recovery.21 In the sedimentation method, a solution with a lower specific gravity than the egg is used allowing them to sink.57
The direct evaluation of cecal and colonic contents at necropsy is generally considered to be the ‘gold standard’ for detecting pinworms.20,25,26,46 The ‘Swiss roll’ technique, in which the intestine is rolled into a spiral prior to processing for histology, is another method for direct examination of the entire intestinal wall and luminal contents.45 Even though SO infections have been identified by ELISA,42,55 commercial serologic pinworm assays are not currently available. Recently, real-time PCR detection has been introduced as a sensitive and specific diagnostic method, with tests reported to detect fewer than 10 copies of DNA.20,26,28,35,48 However, because PCR analysis can detect nucleic acid from shed pinworm cells26 and dead nematodes, positive PCR results may not reflect an active infection.20
Our interest in this topic was stimulated by inconsistent test results during a recent vendor-associated outbreak. Even though recent studies have compared select pinworm detection methods, there is no consensus regarding the best practice for testing,17,20,23,26,28,52 and a comprehensive comparative study of methods has never been reported. Flotation solutions and methods have been described inconsistently in published manuscripts, thus preventing method replication or diagnostic use, and study findings are inconsistent, likely due to inadequate subject numbers.20,23,26,28,52 In addition, some studies used heavily infected pet-store mice, which may not accurately reflect testing on laboratory mice in which pinworms may be present at low levels. Surprisingly, no studies have been published regarding the sensitivity of PCR testing using commercial assays to detect SO. Authors of recent studies have suggested that using PCR analysis only is effective for antemortem screening. However, they concluded that pinworm PCR assays may simply augment current testing methods and may not eliminate the possibility of false-positive or -negative results.20,28
Although current antemortem detection methods appear to be insensitive, prior studies have not evaluated the optimal fecal concentration solution and method for murine pinworm detection. Furthermore, direct examination of intestinal contents has not been compared with Swiss roll histology as a postmortem detection method.
It is paramount that biosecurity programs use the most sensitive tests possible, to minimize false-negative results. Therefore, the goal of the current study was to determine the best method, or combination of methods, for detecting pinworm infections as a component of quarantine and colony health-monitoring programs, while considering detection rates and practicality. The hypothesis was that detection rates would be higher for PCR analysis, direct examination of intestinal contents, and Swiss roll histology than for other methods.
Materials and Methods
Animals.
Female Swiss Webster (n = 120; age, 3 to 5 wk; Tac:SW; Taconic Biosciences, Germantown, NY) mice were obtained. We selected this sex and strain of mice because they are commonly used as sentinels for colony health monitoring programs. Mice were specific pathogen-free for mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, murine norovirus, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyoma virus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, and Mycoplasma pulmonis. Mice also were free of Helicobacter spp., Salmonella spp., Clostridium piliforme, Corynebacterium kutscheri, Citrobacter rodentium, endoparasites, and ectoparasites on arrival. All mice were housed in a quarantine facility under Animal Biosafety Level 2 conditions in solid-bottom, polysulfone, IVC (Thoren Caging Systems, Hazelton, PA) on autoclaved aspen-chip bedding (PWI Industries Canada, Quebec, Canada); γ-irradiated feed (LabDiet 5058, PMI, St Louis, MO) and acidified water (pH, 2.5 to 2.8) were provided ad libitum. Cages were changed weekly in a class II type 2A biologic safety cabinet (NU S602-500, Series SP, Nuaire, Plymouth, MN). The holding room was ventilated with 95% filtered outside air at 15 air changes hourly. Other room conditions were 72 ± 2 °F (21.5 ± 1 °C), relative humidity at 30% and 70%, and a 12:12-h light:dark photoperiod.
Animal use was approved by Memorial Sloan Kettering Cancer Center's IACUC. The center's animal care and use program is AAALAC-accredited and operates in accordance with the recommendations provided in the Guide.32
Establishment of infected colonies.
Infected colonies were established from pinworm-infected mice maintained at our institution. We previously established colonies of Swiss Webster and NSG (NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ; The Jackson Laboratory, Bar Harbor, ME) mice infected with SO. NSG mice were used due to their increased susceptibility to parasitic infections. To establish an AT-infected colony, adult AT worms were collected from the cecum and colon of infected mice, both from dual-infected (SO and AT) mice maintained at MSKCC and from a mouse purchased from a pet store. The worms were macerated, and the eggs were collected and cultured in approximately 10 mL of sterile physiologic saline at 23 °C until embryonated (approximately 2 wk). Each dish contained approximately 500 to 2000 embryonated eggs. Embryonated eggs, suspended in saline, were administered by oral gavage of 0.1 to 0.15 mL into each naïve NSG mouse. In addition, embryonated eggs, suspended in saline, were added to powdered chow (LabDiet 5058) provided in a medicine cup within the cage. To further increase the likelihood of infection, infected dirty bedding from confirmed positive cages was transferred into the cages during weekly cage changes. Partial bedding changes were performed on confirmed infected cages to facilitate continual exposure to embryonated eggs.
Optimal fecal concentration method.
The optimal fecal concentration method was determined by using pooled fecal samples from the cages containing AT-infected mice. We tested 7 fecal concentration methods: sodium nitrate flotation (Fecasol, Vetoquinol USA, Fort Worth, TX; specific gravity, 1.2); sodium nitrate with centrifugation; zinc sulfate flotation (zinc sulfate heptahydrate, Sigma–Aldrich, St Louis, MO; specific gravity, 1.35); zinc sulfate with centrifugation; Sheather sugar flotation (Sheather Sugar Flotation Solution, Jorgensen Labs, Loveland, CO; specific gravity, 1.27); Sheather sugar with centrifugation; and sedimentation and centrifugation with water (tap; specific gravity, 1.0). Each fecal concentration detection method was repeated 20 times, each using a different fecal aliquot. A hydrometer (Specific Gravity Plain Form Hydrometer, VWR, Radnor, PA) was used to measure the specific gravity of each solution prior to use.
For each fecal concentration solution and method, a volume equal to approximately 10 fecal pellets was used. Feces evaluated by flotation were mixed with flotation solution in a fecal flotation device (Fecatector, Henry Schein, Dublin, OH), and flotation solution was added until a meniscus formed above the rim. A coverslip was placed on top of the meniscus. After 15 min, the coverslip was transferred to a microscope slide for examination. Feces evaluated by centrifugation were mixed with flotation solution in a 15-mL conical tube (Polystyrene Centrifuge Tubes, Jorgensen Labs) and centrifuged at 400 × g for 5 min, after which the flotation solution was added until a meniscus formed above the rim. A coverslip was placed on top of the meniscus for 10 min and then transferred to a microscope slide for examination. For sedimentation with water, feces was mixed with 10 mL water in a 15-mL conical tube and centrifuged at 400 × g for 5 min. The supernatant was decanted, and the remaining pellet was resuspended in a small amount of water. A few drops of this suspension were placed on a microscope slide, covered with a coverslip, and examined. For all methods, the entire coverslip was examined systematically under 100× magnification. A positive result was reported when at least one egg was detected. The number of eggs was not quantified.
Exposure of naïve subjects.
Naïve Swiss Webster mice (20 per time point; n = 60 total) were exposed to SO on day –21, and naïve Swiss Webster mice (20 per time point; n = 60 total) were exposed to AT on day –28, based on each species’ prepatent period.2,4,12,14,59 For each SO exposure, 5 cages of 4 Swiss Webster mice were housed with an SO-infected NSG mouse for 1 wk. On day 0 (21 d after exposure), 6 or 7 SO-exposed mice were selected randomly by using a randomization program (www.randomizer.org) and tested for SO infection. The remaining mice were recombined randomly into groups of 4 or 5 mice per cage. A similar procedure was undertaken on day 30, and the remaining subjects were collected on day 90. For each AT exposure, 4 cages of 5 Swiss Webster mice were exposed to AT as described for establishing AT infection in NSG mice. However, infected dirty bedding from confirmed-positive cages was transferred into the cages only once during the initial exposure. Collection of subjects began at day 0 (28 d after exposure) and continued on days 30 and 90 as described for SO. Selection of mice for testing at each time point and of the postmortem test group (examination of intestinal contents or Swiss roll histology) was performed by using the aforementioned randomization program.
Comparison of pinworm detection methods.
SO- and AT-exposed mice (n = 20 for each parasite) were randomly selected for testing and euthanized by CO2 asphyxiation on days 0, 30, and 90. Samples were blinded and all, except for Swiss rolls, were read by 2 examiners. The detection methods included sodium nitrate flotation (AT only; each mouse), tape test (SO only; each mouse), PCR analysis (each mouse), direct examination of cecal and colonic contents (n = 10 for each parasite per time point), and Swiss roll histology (n = 10 for each parasite per time point).
Sodium nitrate flotation.
Between 3 and 11 (mean, 7) fecal pellets were collected directly from each mouse prior to euthanasia. Fecal flotation was performed by using sodium nitrate flotation solution as previously described.
Anal tape test.
Approximately 2.5 cm of clear cellophane tape (Scotch Transparent Tape 600, 3M, St Paul, MN) was applied to the perianal region of each mouse for approximately 1 to 2 s prior to being adhered onto a microscope slide and systematically examined under 100× magnification.
PCR analysis.
Testing was conducted by using validated and established PCR assays (Charles River Research Animal Diagnostic Services, Wilmington, MA). Testing was performed on lysate obtained from a combination of a sticky swab (Puritan Medical Products, Guilford, ME) and a fecal pellet collected from each mouse. The swab was wiped systematically over the fur of the dorsum and ventrum prior to swabbing the perianal region. PCR testing was performed as previously described by using proprietary real-time fluorogenic 5′ nuclease PCR assays.28 All PCR primers and probes targeted 28S ribosomal RNA sequences. The testing and interpretation algorithm used by the testing laboratory was as follows. Isolated DNA was first screened with 2 primer and probe sets that target sequences common to a subset of nematodes within the superfamily Oxuroidea. When either of the screening assays was positive, DNA was reisolated from retained sample lysate and retested by the screening assays as well as 3 species-specific assays that target unique sequences for AT, SO, and Syphacia muris. A positive result was reported when the repeated screening assay or species-specific assay was positive (real-time PCR cycle threshold values equivalent to or greater than approximately 1 template copy per PCR reaction). To monitor for successful DNA recovery after extraction and for the presence of PCR inhibitors, a nucleic acid recovery control assay was performed. Exogenous algae DNA was added to the sample lysis prior to extraction to result in approximately 200 copies of isolated nucleic acid per PCR well (approximately 40 copies per µL; 5 µL nucleic acid total added to the PCR reaction tube), which subsequently was monitored by using a real-time PCR assay targeting the algae sequence. Nucleic acid recovery control assays for samples that demonstrated greater than a log10 loss of template copies compared with control wells were diluted 1:4 and retested or reextracted.
Examination of cecal and colonic (intestinal) contents.
The cecum and colon were harvested from each mouse after euthanasia, separately macerated for approximately 15 min in a culture dish containing warm tap water, and examined under 4× magnification.
Swiss roll histology.
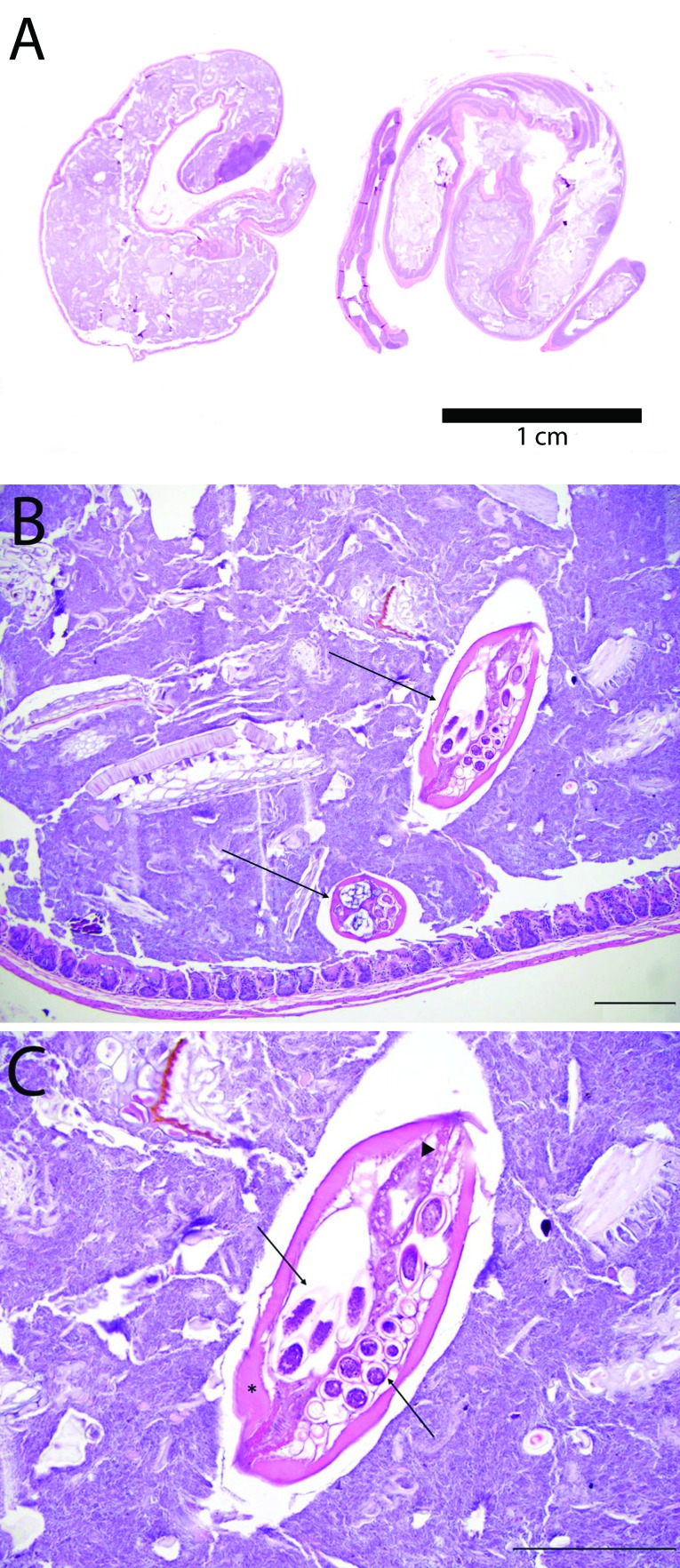
The large intestine (cecum and colon) was collected, and the cecum was separated from the colon. The cecum and colon were separately rolled longitudinally to form 2 Swiss roll samples. Both the cecum and rolled colon were placed into the same cassette in 10% buffered formalin for overnight fixation, followed by routine processing, paraffin embedding, sectioning at 4 µm, and staining with hematoxylin and eosin. The Swiss roll samples (Figure 1) were examined by a board-certified veterinary pathologist at 100× to 400× magnification.
Figure 1.
Swiss roll histology. (A) Subgross photomicrograph demonstrating a Swiss roll. (B) Transverse and oblique sections of a gravid female S. obvelata (arrows) within the lumen of the cecum. The bar = 200 µm. (C) Gravid female S. obvelata with characteristic platymyarian musculature (asterisk), gastrointestinal tract lined by uninucleate cuboidal epithelial cells (arrowhead), and thick-shelled, asymmetrically ellipsoidal eggs measuring approximately 30 × 80 μm (arrows). Hematoxylin and eosin stain. The bar = 200 µm.
Statistics.
The detection rate was calculated as the number of mice with positive test results divided by the number of animals exposed. The Fisher exact test was used for unpaired comparisons, including comparing examination of intestinal contents with Swiss roll histology and comparing PCR analysis combined with examination of intestinal contents with PCR analysis combined with Swiss roll histology. The McNemar test was used for paired comparisons, including comparing PCR analysis with tape tests, PCR analysis with fecal flotation, PCR analysis with examination of intestinal contents, PCR analysis with Swiss roll histology, PCR analysis only with PCR analysis combined with examination of intestinal contents, tape test combined with PCR analysis with examination of intestinal contents, fecal flotation combined with PCR analysis with examination of intestinal contents only, tape test combined with PCR analysis with Swiss roll histology only, fecal flotation combined with PCR analysis with Swiss roll histology only, tape test combined with PCR analysis with PCR analysis combined with examination of intestinal contents, and fecal flotation combined with PCR analysis with PCR analysis combined with examination of intestinal contents. Differences were considered to be significant when the P value was less than 0.05. All computations were performed by using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Optimal fecal concentration method.
Sodium nitrate flotation, sodium nitrate centrifugation, Sheather's sugar centrifugation, and zinc sulfate flotation detected AT eggs in 100% (20 of 20) of the samples evaluated by each method. Zinc sulfate flotation and water sedimentation each detected AT eggs in 90% (18 of 20) of the samples evaluated. All fecal concentration methods evaluated had significantly better detection rates than did Sheather's sugar flotation, which detected AT eggs in only 50% (10 of 20) of the samples (P < 0.0001).
Infection of naïve subjects.
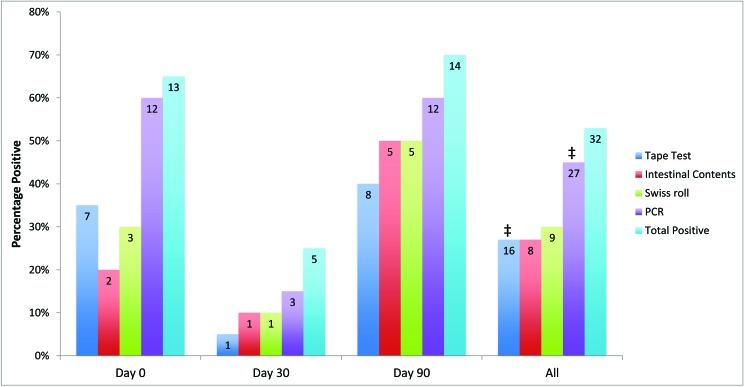
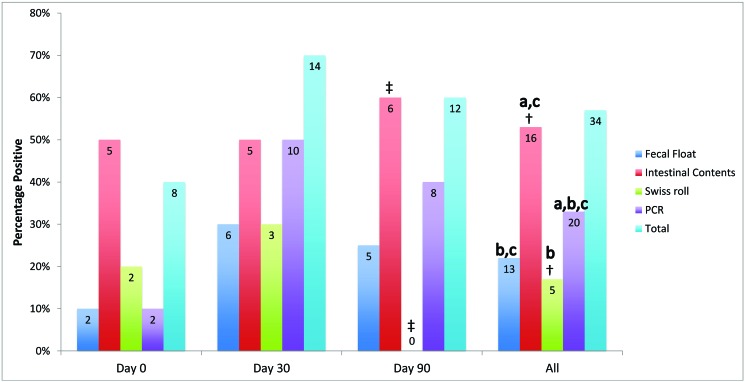
Thirteen (65%) of the 20 SO-exposed mice tested positive by at least one test method on day 0, 5 (25%) on day 30, 14 (70%) on day 90, and 32 (53%) overall (Figure 2). Eight (40%) of the 20 AT-exposed mice tested positive by at least one test method on day 0, 14 (70%) on day 30, 12 (60%) on day 90, and 34 (57%) overall (Figure 3).
Figure 2.
Detection rates (percentage positive) for S. obvelata-exposed mice, comparing the tape test (20 mice per time point; n = 60 total), examination of intestinal contents (10 mice per time point; n = 30 total), Swiss roll histology (10 mice per time point; n = 30 total), and PCR analysis (20 per time point; n = 60 total) on days 0, 30, 90, and for all time points combined. The ‘total positive’ data represent the number of mice positive by at least one test method (20 mice per time point; n = 60 total). The number within the bar represents the number of positive mice. ‡, P = 0.001 (McNemar test) for PCR analysis compared with the tape test for all time points combined.
Figure 3.
Detection rates (percentage positive) for A. tetraptera-exposed mice, comparing fecal flotation (20 mice per time point; n = 60 total), examination of intestinal contents (10 mice per time point; n = 30 total), Swiss roll histology (10 mice per time point; n = 30 total), and PCR analysis (20 mice per time point; n = 60 total) on days 0, 30, 90, and for all time points combined. The ‘total positive’ data represent the number of mice positive by at least one test method (20 mice per time point; n = 60 total). The number within the bar represents the number of positive mice. Detection rate differed significantly between examination of intestinal contents compared with Swiss roll histology on day 90 (‡, P = 0.001, Fisher exact test) and for all time points combined (†, P = 0.01, Fisher exact test); a, P = 0.002 (McNemar test) for PCR analysis combined with examination of intestinal contents compared with PCR analysis only for all time points combined; b, P = 0.02 (McNemar test) for PCR analysis combined with fecal flotation compared with Swiss roll histology for all time points combined; c, P = 0.004 (McNemar test) for PCR analysis combined with examination of intestinal contents compared with PCR analysis combined with fecal flotation for all time points combined.
Comparison of pinworm detection methods.
For SO, PCR had the highest overall detection rate, detecting SO nucleic acid in 45% (27 of 60) of the mice evaluated (Figure 2). The overall detection rates were lower and similar for Swiss roll, intestinal contents, and the tape test, with detection rates of 30% (9 of 30), 27% (8 of 30), and 27% (16 of 60), respectively (Figure 2).
When comparing antemortem tests, PCR was better at detecting SO than was the tape test for all time points combined (P = 0.001), and the tape test detected no additional positive animals than had been detected by PCR analysis. The total number of positive animals (that is, positive on at least one test) was lower on day 30 compared with earlier and later time points. When PCR analysis was combined with each of the 3 other tests, PCR analysis combined with Swiss roll histology yielded the highest detection rate at 57% (17 of 30), followed by PCR analysis combined with examination of intestinal contents at 50% (15 of 30), and, lastly, PCR analysis combined with the tape test at 45% (27 of 60). In addition, the sensitivity of the tape test and PCR analysis were each 75% (6 of 8) when compared with examination of intestinal contents as the historical gold standard.
When SO detection rates were adjusted to consider only animals that were positive on at least one test, the rates were higher with overall adjusted detection rates of 84% (27 of 32) for PCR analysis, 53% (8 of 15) for examination of intestinal contents, 53% (9 of 17) for Swiss roll histology, and 50% (16 of 32) for the tape test. The adjusted detection rate for combinations of tests was 100% (15 of 15) for PCR analysis combined with examination of intestinal contents, 100% (17 of 17) for PCR analysis combined with Swiss roll histology, and 84% (27 of 32) for PCR analysis combined with the tape test.
For AT, examination of intestinal contents yielded the highest overall detection rate at 53% (Figure 3; 16 of 30). The overall detection rates were lower for PCR analysis, fecal flotation, and Swiss roll histology, with detection rates of 33% (20 of 60), 22% (13 of 60), and 17% (5 of 30), respectively (Figure 3). Examination of intestinal contents had the highest or equivalent AT-detection rate for all time points evaluated (equivalent to PCR on day 30) and was better than Swiss roll histology at detecting AT on day 90 and overall (P = 0.01 and 0.001, respectively). The antemortem detection rate for PCR analysis was higher, albeit nonsignificantly, than for fecal flotation. Importantly, fecal flotation detected 4 AT-positive mice that were not detected by PCR analysis. Combining PCR analysis with fecal flotation was better at detecting AT than was Swiss roll histology alone (P = 0.02). PCR analysis combined with examination of intestinal contents was better at detecting AT than was either PCR analysis alone (P = 0.002) or PCR analysis combined with fecal flotation (P = 0.004). In addition, detection rates were lower on day 0. When PCR analysis was combined with each of the 3 other tests, PCR analysis combined with examination of intestinal contents yielded the highest detection rate (67%, 20 of 30), followed by PCR analysis combined with Swiss Roll histology (40%, 12 of 30) and PCR analysis combined with fecal flotation (40%, 24 of 60). When compared with examination of intestinal contents as the gold standard, PCR analysis was more sensitive than was fecal flotation, 38% (6 of 16) compared with 31% (5 of 16), respectively.
When AT detection rates were adjusted to consider only mice that were positive on at least a one test, overall adjusted detection rates were 80% (16 of 20) for examination of intestinal contents, 59% (20 of 34) for PCR analysis, 38% (13 of 34) for fecal flotation, and 36% (5 of 14) for Swiss roll histology. The adjusted detection rate for combinations of tests was 100% (20 of 20) for PCR analysis combined with examination of intestinal contents, 86% (12 of 14) for PCR analysis combined with Swiss roll histology, and 71% (24 of 34) for PCR analysis combined with fecal flotation.
Individual animal results.
We considered results for mice positive on at least one test. For SO, PCR analysis yielded the least false negative results with 5, followed by examination of intestinal contents with 7, Swiss roll histology with 8, and the tape test with 16 (Table 1). For AT, examination of intestinal contents produced the least false-negative results with 4, followed by Swiss roll histology with 9, PCR analysis with 14, and fecal flotation with 21 (Table 2). Sixteen mice were PCR-positive only—7 for SO and 9 for AT. Nineteen mice were PCR-negative but positive on other tests. For SO, 3 mice were positive by Swiss roll only and 2 mice were positive by examination of intestinal contents only. For AT, 9 mice were positive by examination of intestinal contents only, 2 by fecal flotation only, 1 by Swiss roll histology only, 1 by both fecal flotation and examination of intestinal contents, and 1 by both fecal flotation and Swiss roll histology.
Table 1.
Individual results for S. obvelata-exposed mice
| Day | Mouse | Tape test | Intestinal contents | Swiss roll | PCR |
| 0 | 1 | + | – | NA | + |
| 2 | + | + | NA | + | |
| 3 | + | + | NA | + | |
| 4 | + | – | NA | + | |
| 5 | + | – | NA | + | |
| 6 | – | – | NA | + | |
| 7 | – | – | NA | – | |
| 8 | – | – | NA | – | |
| 9 | – | – | NA | – | |
| 10 | – | – | NA | – | |
| 11 | – | NA | + | + | |
| 12 | – | NA | + | + | |
| 13 | – | NA | – | + | |
| 14 | – | NA | – | – | |
| 15 | – | NA | – | + | |
| 16 | + | NA | – | + | |
| 17 | – | NA | + | – | |
| 18 | + | NA | – | + | |
| 19 | – | NA | – | – | |
| 20 | – | NA | – | – | |
| 30 | 21 | – | – | NA | – |
| 22 | – | – | NA | – | |
| 23 | – | – | NA | – | |
| 24 | – | – | NA | – | |
| 25 | – | – | NA | – | |
| 26 | – | – | NA | – | |
| 27 | – | – | NA | – | |
| 28 | – | + | NA | – | |
| 29 | – | – | NA | – | |
| 30 | – | – | NA | + | |
| 31 | – | NA | + | – | |
| 32 | – | NA | – | – | |
| 33 | – | NA | – | – | |
| 34 | – | NA | – | + | |
| 35 | – | NA | – | – | |
| 36 | – | NA | – | – | |
| 37 | – | NA | – | – | |
| 38 | – | NA | – | – | |
| 39 | + | NA | – | + | |
| 40 | – | NA | – | – | |
| 90 | 41 | + | – | NA | + |
| 42 | + | + | NA | + | |
| 43 | + | – | NA | + | |
| 44 | – | – | NA | – | |
| 45 | – | – | NA | – | |
| 46 | + | + | NA | + | |
| 47 | – | + | NA | – | |
| 48 | + | + | NA | + | |
| 49 | + | + | NA | + | |
| 50 | – | – | NA | – | |
| 51 | – | NA | – | + | |
| 52 | – | NA | + | + | |
| 53 | + | NA | + | + | |
| 54 | – | NA | – | + | |
| 55 | + | NA | + | + | |
| 56 | – | NA | – | – | |
| 57 | – | NA | – | – | |
| 58 | – | NA | + | – | |
| 59 | – | NA | – | – | |
| 60 | – | NA | + | + |
NA, not applicable.
Table 2.
Individual results for A. tetraptera-exposed mice
| Day | Mouse | Fecal float | Intestinal contents | Swiss roll | PCR |
| 0 | 61 | – | + | NA | – |
| 62 | – | + | NA | – | |
| 63 | – | – | NA | – | |
| 64 | + | + | NA | – | |
| 65 | – | + | NA | – | |
| 66 | – | – | NA | – | |
| 67 | – | + | NA | – | |
| 68 | – | – | NA | – | |
| 69 | – | – | NA | – | |
| 70 | – | – | NA | – | |
| 71 | – | NA | – | –a | |
| 72 | + | NA | + | + | |
| 73 | – | NA | – | + | |
| 74 | – | NA | – | – | |
| 75 | – | NA | – | – | |
| 76 | – | NA | + | – | |
| 77 | – | NA | – | – | |
| 78 | – | NA | – | – | |
| 79 | – | NA | – | – | |
| 80 | – | NA | – | – | |
| 30 | 81 | – | – | NA | – |
| 82 | – | – | NA | – | |
| 83 | – | + | NA | + | |
| 84 | + | + | NA | + | |
| 85 | – | + | NA | – | |
| 86 | – | – | NA | + | |
| 87 | + | + | NA | + | |
| 88 | – | – | NA | + | |
| 89 | – | + | NA | – | |
| 90 | – | – | NA | – | |
| 91 | + | NA | + | + | |
| 92 | – | NA | – | + | |
| 93 | – | NA | – | + | |
| 94 | – | NA | – | – | |
| 95 | + | NA | + | – | |
| 96 | – | NA | – | – | |
| 97 | + | NA | + | + | |
| 98 | – | NA | – | – | |
| 99 | + | NA | – | – | |
| 100 | – | NA | – | + | |
| 90 | 101 | – | – | NA | – |
| 102 | – | + | NA | – | |
| 103 | – | + | NA | – | |
| 104 | – | – | NA | – | |
| 105 | – | – | NA | + | |
| 106 | – | – | NA | + | |
| 107 | – | + | NA | – | |
| 108 | + | + | NA | + | |
| 109 | + | + | NA | + | |
| 110 | – | + | NA | + | |
| 111 | – | NA | – | – | |
| 112 | – | NA | – | – | |
| 113 | – | NA | – | – | |
| 114 | – | NA | – | – | |
| 115 | + | NA | – | – | |
| 116 | + | NA | – | + | |
| 117 | + | NA | – | + | |
| 118 | – | NA | – | – | |
| 119 | – | NA | – | + | |
| 120 | – | NA | – | – |
NA, not applicable
PCR negative for A. tetraptera and PCR positive for S. obvelata.
Discussion
In spring 2014, we experienced a limited outbreak of SO when we received infected mice from a commercial vendor after feral mice had breached their production barrier. Inconsistent test results during the investigation of the outbreak prompted us to evaluate traditional and contemporary pinworm detection methods. Surprisingly, despite the continued prevalence of pinworms in contemporary laboratory mouse colonies, there is a dearth of publications evaluating available testing methods, particularly current molecular methods. In this study, we determined the optimal fecal concentration method for detection of AT prior to comparing pinworm detection methods in populations of Swiss Webster mice infected with either SO or AT.
Sodium nitrate flotation, sodium nitrate centrifugation, Sheather sugar centrifugation, water centrifugation, zinc sulfate flotation, and water sedimentation had similarly high detection rates, although zinc sulfate flotation and water sedimentation did not detect AT eggs in all of the positive samples. All methods had significantly better detection rates compared with Sheather sugar flotation, which only detected half of the positive samples. Although centrifugation typically improves the efficiency of egg recovery,57 detection rates were no better with centrifugation, with the exception of Sheather sugar centrifugation. However, we did not evaluate the efficiency of egg recovery (that is, comparing the number of eggs detected by each method) in this study. We selected sodium nitrate flotation for comparing pinworm detection methods because it is commonly used as a flotation solution and requires less preparation than centrifugation.
Even though the specific gravities of SO and AT eggs have not been published, they likely are similar to that of Enterobius vermicularis eggs, the human pinworm representative of the family Oxyuridae, which reportedly has a specific gravity of approximately 1.115.56 We recommend using sodium nitrate (specific gravity, 1.20) or a solution of a similar specific gravity, such as zinc sulfate, when using fecal flotation to detect eggs from murine oxyurid nematodes, as this technique will allow eggs and a limited amount of debris to float to the top of the solution.
To evaluate a range of flotation solution specific gravities, we opted to use zinc sulfate at a higher specific gravity of 1.35, even though it is usually used at the same specific gravity as sodium nitrate. We speculate that Sheather sugar flotation (specific gravity, 1.27) had a lower AT detection rate because it was more viscous than were the other solutions, resulting in inadequate softening of the fecal pellets with fewer eggs floating to the top of the solution.57 In addition, flotation solutions with a specific gravity greater than 1.20 (for example, Sheather sugar and zinc sulfate) resulted in markedly more debris on the cover slip, making it more difficult to identify AT eggs. The water sedimentation method also resulted in increased debris. Even though the sedimentation method may be cumbersome and impractical, additional washing or use of ethyl acetate, which aids in lipid removal,21 might reduce the amount of debris present, thus improving the visualization of eggs.
PCR analysis had the highest detection rate (45%) for SO-exposed mice. The overall detection rates were lower for Swiss roll histology (30%), examination of intestinal contents (27%), and the tape test (27%). When PCR analysis was combined with each of the 3 other tests, PCR analysis combined with Swiss roll histology detected the most infected mice (57%), followed by PCR analysis combined with examination of intestinal contents (50%), and PCR analysis combined with the tape test (45%). PCR analysis detected more infected mice than did the tape test, which detected no additional positive animals. This result is not surprising as it is consistent with a previous studies, including one in which an early PCR assay was superior to the tape test at detecting SO26 and one in which the combination of PCR with direct examination of cecal contents was better at detecting pinworm infections than was noninvasive testing, including tape tests.17 Although we did not prescribe the specific time of day for sample collection, most samples were collected mid- to late morning. Periodicity of egg shedding has been reported with S. muris, with the greatest number of eggs shed in the early afternoon.41,60 A similar shedding periodicity may have contributed to fewer tape-test–positive results in our current study.
The number of SO-exposed mice that tested positive decreased on day 30 (51 d after initial exposure to an SO-infected mouse). Whether this effect, and an apparent 57% (34 of 60) infection rate after exposure, was the result of inadequate exposure, the life cycle of this oxyurid nematode, or immunologic resistance or clearance is unknown. In rats infected with S. muris, egg shedding decreased to 0 at 2- to 3-wk intervals;41 the authors suggested that this effect was due to the life cycle of Syphacia spp., in which gravid female worms migrate to the anus, lay eggs, and die. Theoretically, rodents could therefore be free of worms and eggs until they are reinfected from the environment or from grooming by cage mates or by the host itself. Later time points had increased numbers of mice positive for both SO and AT, perhaps reflecting infections acquired throughout the study due to random rehousing of surviving mice at each time point. However, multiple studies have demonstrated age-associated resistance to SO as well as a robust humoral immune response. For example, most Swiss Webster mice stopped shedding SO eggs 14 wk after being infected at 6 to 8 wk of age.15 In contrast, another study demonstrated no difference in susceptibility to pinworms after 4 wk of age,27 whereas a separate study found that mice infected with SO at 8 wk of age had lower worm burdens than mice infected at 3 wk of age.47 In addition, a thymus-dependent, Th2 immune response resulting in specific IgG antibody production has been documented.33,42,55
We were surprised by the low detection rates for the methods evaluated in this study, especially for PCR analysis. These low detection rates likely reflected animals that were not infected at the time of testing, given that they were negative on all tests. Therefore, we recalculated the detection rates by including only mice that were positive on at least one test. Although there is inherent bias in considering only positive results, these adjusted detection rates may more closely represent the true sensitivity of these tests by eliminating truly negative mice from the calculations. When SO detection rates were adjusted to consider only animals that were positive on at least one test, the rates increased resulting in overall adjusted detection rates of 84% for PCR analysis, 53% for examination of intestinal contents, 53% for Swiss roll histology, and 50% for the tape test. However, even the PCR analysis produced 5 false-negative results. The adjusted detection rate for combinations of tests was 100% for PCR analysis combined with either examination of intestinal contents or Swiss roll histology. Unfortunately, even the optimal combination of antemortem tests, PCR analysis combined with the tape test, failed to detect all infected mice (84%), because the tape test resulted in no additional positive results.
Examination of intestinal contents had the highest detection rate (53%) for AT-exposed mice; overall detection rates were lower for PCR analysis (33%), fecal flotation (22%), and Swiss roll histology (17%). Even though the antemortem detection rate for PCR analysis was higher than for fecal flotation, the 2 detection rates were not significantly different. However, 4 additional positive mice were detected by fecal flotation that were not identified by PCR analysis. In addition, one AT-exposed mouse likely had a false-positive PCR result, because the mouse was PCR-positive for SO but PCR-negative for AT. Although we identified and macerated only AT worms for culture and gavage in the AT-exposed mice, it is possible (although unlikely) that SO worms were included inadvertently. However, SO worms and eggs are morphologically distinct and were not noted during culturing. In addition, if an inadvertent contamination of the AT egg culture had occurred, we would have expected the AT-exposed mice to have included other SO-positive mice.
When PCR analysis was combined with each of the 3 other tests, the PCR assay combined with examination of intestinal contents yielded the highest detection rate (67%), followed by PCR analysis combined with Swiss roll histology (40%) and PCR analysis combined with fecal flotation (40%). Notably, fecal flotation identified no additional infected animals compared with PCR analysis combined with examination of intestinal contents, whereas fecal flotation did lead to the identification of 2 additional infected mice when compared with PCR analysis combined with Swiss roll histology (47%; 14 of 30). Examination of intestinal contents had the highest or an equivalent AT-detection rate at all time points. Day 0 detection rates were lower than those for days 30 and 90, likely due to prepatent infections or a low pinworm burden.
When AT detection rates were adjusted to include only mice that were positive on at least one test, rates increased with overall adjusted detection rates of 80% for examination of intestinal contents, 59% for PCR analysis, 38% for fecal flotation, and 36% for Swiss roll histology. The PCR results are consistent with those published previously, which demonstrated that PCR detected 60% (3 of 5) of the mice that had AT in their intestinal contents and 61.5% (16 of 26) of mice from positive cages.20 When evaluating AT detection rates using a combination of tests, PCR analysis combined with examination of intestinal contents detected all infected mice, whereas PCR analysis combined with either Swiss roll histology or fecal flotation detected 86% and 71% of the AT-positive mice, respectively.
The superiority of detection achieved by PCR analysis combined with examination of intestinal contents across both SO and AT infected study groups is consistent with findings from previously published studies. One study suggested that PCR analysis combined with direct examination of intestinal contents is the most effective screening method, whereas another found that PCR and direct examination of cecal contents were better at detecting pinworm infections than testing of noninvasive samples, including PCR evaluation of cage swabs or feces (individual and pooled) and tape tests.17,20 However, the authors of one study17 acknowledged the need to improve their PCR assay, because inhibitors may have limited efficient DNA extraction.
Interestingly, Swiss roll histology was similar to examination of intestinal contents in detecting SO-infected mice but significantly less effective in detecting AT-infected mice. The reason for this difference remains unclear. A single longitudinal section was collected from each tissue block and examined. Therefore, oxyurid nematodes might have been present in the block but not in the section viewed by the pathologist. Collecting and examining additional sections to avoid these false negative results would be impractical. Therefore, we do not recommend Swiss roll histology as a pinworm detection method.
The finding that some animals tested negative on all tests suggests that they were not infected at the time of testing, either due to inadequate exposure or from immunologic resistance or clearance. Infection rates, which were calculated by using mice that were positive on at least one test, were never higher than 70% at any time point and overall were 53% and 57%, respectively for SO- and AT-exposed mice. Although a 100% infection rate would have been ideal and allowed for a less complicated analysis of the data, this goal would have been very difficult to achieve given the unpredictable nature of pinworm infections, as previously discussed in regard to immunologic resistance and clearance. Infection rates might have been improved by exposing naïve mice to SO-infected dirty bedding and infected colony mice for longer than 1 wk. Likewise, AT infection rates might have been improved by exposing naïve mice to more embryonated eggs. Eggs were not concentrated after collection from culture dishes for fear of losing embryonated eggs during the concentration process.
The results of the current study bring into question the use of direct examination of intestinal contents as the gold standard for diagnosing pinworm infections in mice. Compared with data for mice positive on at least one test, examination of intestinal contents yielded 11 false negative results, 7 for SO and 4 for AT. Nonetheless, when the sensitivity was calculated by using examination of intestinal contents as the gold standard, the tape test and PCR analysis each had a sensitivity of 75% for SO-infected mice. Similarly, the sensitivity was 38% for PCR analysis and 31% for fecal flotation for the detection of for AT-infected mice. Compared with those for SO-infected mice, the sensitivities for AT-infected mice were based on higher numbers (n = 16) of intestinal-content–positive mice. The differing sensitivities of PCR analysis may be the result of differences in life cycles and potentially false-positive SO PCR results.
This study revealed important differences between test methods. Sixteen mice were positive by PCR analysis only, 7 for SO and 9 for AT. Whether these results represent actual false positives, when considering individual animals, or true positives due to the increased sensitivity of PCR analysis is unclear. PCR analysis might have detected parasites from oxyurid nucleic acid shed by cage mates, given that 15 of the 16 PCR-positive-only mice (6 for SO and 9 for AT) were housed with at least a one cage mate that was positive at that time point by at least one other test method. The other test methods likely also yielded false-negative results. Assessing mice that were positive for SO on any test revealed 7 false-negative results for examination of intestinal contents, 8 for Swiss roll histology, and 16 for the tape test; for AT, there were 4 false-negative results for examination of intestinal contents, 9 for Swiss roll histology, and 21 for fecal flotation. False-negative results might have resulted from a failure to notice worms or eggs due to a low burden, recent clearance of worms, or passage of unembryonated eggs,20 even though we attempted to minimize these effects by using 2 examiners for each test. The possibility of false-negative results warrants consideration of prophylactic treatment of all mice received from noncommercial sources. In addition, the possibility of false-positive PCR results due to sample contamination cannot be excluded. In fact, false-positive PCR results for AT have been reported due to corncob bedding contaminated with rhabditid nematodes.34
Nineteen mice were PCR-negative but positive on other tests performed at the same time point, 5 for SO and 14 for AT. False negative PCR results were likely due to an absence of oxyurid nucleic acid, although fecal PCR inhibitors might contribute to this outcome.26,28 However, the inclusion of nucleic acid recovery control assays greatly reduces the likelihood of this possibility. Other potential explanations include prepatent infection, low-level infection, and intermittent egg shedding, as previously discussed in regard to S. muris.
In conclusion, when considered as single tests, PCR analysis and direct examination of intestinal contents detected the greatest number of SO- and AT-infected mice, respectively. However, no single method detected all infected mice. Therefore, we recommend using PCR analysis combined with direct examination of intestinal contents to optimize pinworm detection and reduce the likelihood of false negative results in quarantine and health monitoring programs. In addition, given the overall superiority of direct examination of intestinal contents and the additional time and effort required to process samples for Swiss roll histology, we do not recommend using Swiss roll histology for diagnosis of pinworm infections. Even though traditional antemortem test methods, fecal flotation and the tape test, are relatively inexpensive and easy to perform, the low detection rates we observed suggest that these methods are likely to produce false negative results. Furthermore, their use may be unnecessary when animals can be euthanized for direct examination of intestinal contents, because these antemortem tests identified no additional infected animals to those identified by using PCR analysis combined with direct examination of intestinal contents. However, in regard to survival detection methods only, we recommend using PCR analysis for SO detection and PCR analysis combined with fecal flotation using sodium nitrate solution for AT detection. In light of these results, we have modified our own quarantine and sentinel programs to include biannual examination of intestinal contents in addition to bimonthly PCR testing.
Acknowledgments
We thank the staff of the Laboratory of Comparative Pathology—especially Jacqueline Candelier, Desiree Powell, Julie White, and Sebastien Monette, and Andrew Gorman, Melissa Nashat, Nicholas Tataryn, Samantha Peneyra, Miranda Gallo, and Christopher Chellieutte-Nieves—for their diagnostic support. We also thank Charles River Research Animal Diagnostic Services for their generous technical and financial support of PCR testing.
This study was funded in part through the NIH/NCI Cancer Center Support Grant P30-CA008748 and the NIH Research Education Grant R25OD010447-02.
References
- 1.Anya AO. 1966. Studies on the biology of some oxyurid nematodes. I. Factors in the development of eggs of Aspiculuris tetraptera Schulz. J Helminthol 40:253–260. [DOI] [PubMed] [Google Scholar]
- 2.Anya AO. 1966. Studies on the biology of some oxyurid nematodes. II. The hatching of eggs and development of Aspiculuris tetraptera Schulz within the host. J Helminthol 40:261–268. [DOI] [PubMed] [Google Scholar]
- 3.Baker DG. 2007. Parasites of rats and mice, p 303–397, Chapter 11. In: Baker DG. Flynn's parasites of laboratory animals. 2nd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 4.Behnke JM. 1974. The distribution of larval Aspiculuris tetraptera Schulz during a primary infection in Mus musculus, Rattus norvegicus, and Apodemus sylvaticus. Parasitology 69:391–402. [DOI] [PubMed] [Google Scholar]
- 5.Behnke JM. 1975. Aspiculuris tetraptera in wild Mus musculus. The prevalence of infection in male and female mice. J Helminthol 49:85–90. [DOI] [PubMed] [Google Scholar]
- 6.Behnke JM. 1975. Immune expulsion of the nematode Aspiculuris tetraptera from mice given primary and challenge infections. Int J Parasitol 5:511–515. [DOI] [PubMed] [Google Scholar]
- 7.Behnke JM. 1976. Aspiculuris tetraptera in wild Mus musculus. Age resistance and acquired immunity. J Helminthol 50:197–202. [PubMed] [Google Scholar]
- 8.Boivin GP, Ormsby I, Hall JE. 1996. Eradication of Aspiculuris tetraptera by using fenbendazole-medicated food. Contemp Top Lab Anim Sci 35:69–70. [PubMed] [Google Scholar]
- 9.Bugarski D, Jovcic G, Katic-Radivojevic S, Petakov M, Krstic A, Stojanovic N, Milenkovic P. 2006. Hematopoietic changes and altered reactivity to IL17 in Syphacia obvelata-infected mice. Parasitol Int 55:91–97. [DOI] [PubMed] [Google Scholar]
- 10.Bunte RM, Nolan TJ. 2006. Searching for Aspiculuris tetraptera: lessons learned. J Am Assoc Lab Anim Sci 45:86. [Google Scholar]
- 11.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276. [DOI] [PubMed] [Google Scholar]
- 12.Chan KF. 1952. Life-cycle studies on the nematode Syphacia obvelata. Am J Hyg 56:14–21. [DOI] [PubMed] [Google Scholar]
- 13.Chan KF. 1953. The effect of storage at low temperatures on the infectivity of Aspiculuris tetraptera eggs. J Parasitol 39:42. [Google Scholar]
- 14.Chan KF. 1955. The distribution of larval stages of Aspiculuris tetraptera in the intestine of mice. J Parasitol 41:529–532. [PubMed] [Google Scholar]
- 15.Clarke CL, Perdue KA. 2004. Detection and clearance of Syphacia obvelata infection in Swiss Webster and athymic nude mice. Contemp Top Lab Anim Sci 43:9–13. [PubMed] [Google Scholar]
- 16.Clifford CB, Watson J. 2008. Old enemies still with us after all these years. ILAR J 49:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compton SR, Macy JD. 2015. Effect of cage-wash temperature on the removal of infectious agents from caging and the detection of infectious agents on the filters of animal bedding-disposal cabinets by PCR analysis. J Am Assoc Lab Anim Sci 54:745–755. [PMC free article] [PubMed] [Google Scholar]
- 18.Derothe JM, Loubes C, Orth A, Renaud F, Moulia C. 1997. Comparison between patterns of pinworm infection (Aspiculuris tetraptera) in wild and laboratory strains of mice, Mus musculus. Int J Parasitol 27:645–651. [DOI] [PubMed] [Google Scholar]
- 19.Dix J, Astill J, Whelan G. 2004. Assessment of methods of destruction of Syphacia muris eggs. Lab Anim 38:11–16. [DOI] [PubMed] [Google Scholar]
- 20.Dole VS, Zaias J, Kyricopoulos-Cleasby DM, Banu LA, Waterman LL, Sanders K, Henderson KS. 2011. Comparison of traditional and PCR methods during screening for and confirmation of Aspiculuris tetraptera in a mouse facility. J Am Assoc Lab Anim Sci 50:904–909. [PMC free article] [PubMed] [Google Scholar]
- 21.Dryden MW, Payne PA, Ridley R, Smith V. 2005. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther 6:15–28. [PubMed] [Google Scholar]
- 22.Eaton GJ. 1972. Intestinal helminths in inbred strains of mice. Lab Anim Sci 22:850–853. [PubMed] [Google Scholar]
- 23.Effler JC, Hickman-Davis JM, Erwin JG, Cartner SC, Schoeb TR. 2008. Comparison of methods for detection of pinworms in mice and rats. Lab Anim(NY) 37:210–215. [DOI] [PubMed] [Google Scholar]
- 24.Eguíluz C, Viguera E, Perez J. 2001. Modification of the anal tape method for detection of pinworms in rodents. Lab Anim(NY) 30:54–55. [DOI] [PubMed] [Google Scholar]
- 25.Farrar PL, Wagner JE, Kagiyama N. 1994. Syphacia spp., p 219–224. In: Waggie K Kagiyam N Allen A Nomura T. Manual of microbiological monitoring of laboratory animals. 2nd ed. Bethesda (MD): US Department of Health and Human Services, National Institutes of Health (publication no. 94-2498). [Google Scholar]
- 26.Feldman SH, Bowman SG. 2007. Molecular phylogeny of the pinworms of mice, rats, and rabbits, and its use to develop molecular beacon assays for the detection of pinworms in mice. Lab Anim(NY) 36:43–50. [DOI] [PubMed] [Google Scholar]
- 27.Grove KA, Smith PC, Booth CJ, Compton SR. 2012. Age-associated variability in susceptibility of Swiss Webster mice to MPV and other excluded murine pathogens. J Am Assoc Lab Anim Sci 51:789–796. [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson KS, Perkins CL, Havens RB, Kelly MJ, Francis BC, Dole VS, Shek WR. 2013. Efficacy of direct detection of pathogens in naturally infected mice by using a high-density PCR array. J Am Assoc Lab Anim Sci 52:763–772. [PMC free article] [PubMed] [Google Scholar]
- 29.Hill WA, Randolph MM, Mandrell TD. 2009. Sensitivity of perianal tape impressions to diagnose pinworm (Syphacia spp.) infections in rats (Rattus norvegicus) and mice (Mus musculus). J Am Assoc Lab Anim Sci 48:378–380. [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh KY. 1952. The effect of the standard pinworm chemotherapeutic agents on the mouse pinworm Aspiculuris tetraptera. Am J Hyg 56:287–293. [PubMed] [Google Scholar]
- 31.Huerkamp MJ, Benjamin KA, Zitzow LA, Pullium JK, Lloyd JA, Thompson WD, Webb SK, Lehner ND. 2000. Fenbendazole treatment without environmental decontamination eradicates Syphacia muris from all rats in a large, complex research institution. Contemp Top Lab Anim Sci 39:9–12. [PubMed] [Google Scholar]
- 32.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): The National Academic Press. [Google Scholar]
- 33.Jacobson RH, Reed ND. 1974. The thymus dependency of resistance to pinworm infection in mice. J Parasitol 60:976–979. [PubMed] [Google Scholar]
- 34.Leblanc M, Berry K, Graciano S, Becker B, Reuter JD. 2014. False-positive results after environmental pinworm PCR testing due to rhabditid nematodes in corncob bedding. J Am Assoc Lab Anim Sci 53:717–724. [PMC free article] [PubMed] [Google Scholar]
- 35.Leutenneger CM. 2001. The real-time TaqMan PCR and applications in veterinary medicine. Veterinary sciences tomorrow 1:1–15. [Google Scholar]
- 36.Lipman NS, Dalton SD, Stuart AR, Arruda K. 1994. Eradication of pinworms (Syphacia obvelata) from a large mouse breeding colony by combination oral anthelmintic therapy. Lab Anim Sci 44:517–520. [PubMed] [Google Scholar]
- 37.Lübcke R, Hutcheson FA, Barbezat GO. 1992. Impaired intestinal electrolyte transport in rats infested with the common parasite Syphacia muris. Dig Dis Sci 37:60–64. [DOI] [PubMed] [Google Scholar]
- 38.Mathies AW., Jr 1959. Certain aspects of the host–parasite relationship of Aspiculuris tetraptera, a mouse pinworm. I. Host specificity and age resistance. Exp Parasitol 8:31–38. [DOI] [PubMed] [Google Scholar]
- 39.Mathies AW., Jr 1959. Certain aspects of the host–parasite relationship of Aspiculuris tetraptera, a mouse pinworm. II. Sex resistance. Exp Parasitol 8:39–45. [DOI] [PubMed] [Google Scholar]
- 40.McNair DM, Timmons EH. 1977. Effects of Aspiculuris tetraptera and Syphacia obvelata on exploratory behavior of an inbred mouse strain. Lab Anim Sci 27:38–42. [PubMed] [Google Scholar]
- 41.Meade TM, Watson J. 2014. Characterization of rat pinworm (Syphacia muris) epidemiology as a means to increase detection and elimination. J Am Assoc Lab Anim Sci 53:661–667. [PMC free article] [PubMed] [Google Scholar]
- 42.Michels C, Goyal P, Nieuwenhuizen N, Brombacher F. 2006. Infection with Syphacia obvelata (pinworm) induces protective Th2 immune responses and influences ovalbumin-induced allergic reactions. Infect Immun 74:5926–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyaji S, Kamiya M, Shikata J. 1988. [Ovicidal effects of heat and disinfectants on Syphacia muris estimated by in vitro hatching.] Jikken Dobutsu 37:399–404.[Article in Japanese]. [PubMed] [Google Scholar]
- 44.Mohn G, Philipp EM. 1981. Effects of Syphacia muris and the anthelmintic fenbendazole on the microsomal monooxygenase system in mouse liver. Lab Anim 15:89–95. [DOI] [PubMed] [Google Scholar]
- 45.Moolenbeek C, Ruitenberg EJ. 1981. The ‘Swiss roll’: a simple technique for histologic studies of the rodent intestine. Lab Anim 15:57–59. [DOI] [PubMed] [Google Scholar]
- 46.Ooi HK, Oku Y, Kamiya M. 1994. Aspiculuris tetraptera, p 173–175. In: Waggie K Kagiyam N Allen A Nomura T. Manual of microbiological monitoring of laboratory animals. 2nd ed. Bethesda (MD): National Institutes of Health (publication no. 94-2498). [Google Scholar]
- 47.Panter HC. 1969. Studies on host–parasite relationships. Syphacia obvelata in the mouse. J Parasitol 55:74–78. [PubMed] [Google Scholar]
- 48.Parel JD, Galula JU, Ooi HK. 2008. Characterization of rDNA sequences from Syphacia obvelata, Syphacia muris, and Aspiculuris tetraptera and development of a PCR-based method for identification. Vet Parasitol 153:379–383. [DOI] [PubMed] [Google Scholar]
- 49.Parkinson CM, O'Brien A, Albers TM, Simon MA, Clifford CB, Pritchett-Corning KR. 2011. Diagnosis of ecto- and endoparasites in laboratory rats and mice. J Vis Exp 55:e2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillipson RF. 1974. Intermittent egg release by Aspiculuris tetraptera in mice. Parasitology 69:207–213. [DOI] [PubMed] [Google Scholar]
- 51.Pritchett KR.2007. Helminth parasites of laboratory mice, p 551–564. Chapter 22. In: Fox JG Davisson MT Quimby FW Barthold SW Newcomer CE Smith AL. The mouse in biomedical research, vol 2. 2nd ed. New York (NY): Academic Press. [Google Scholar]
- 52.Roble GS, Gillespie V, Lipman NS. 2012. Infectious disease survey of Mus musculus from pet stores in New York City. J Am Assoc Lab Anim Sci 51:37–41. [PMC free article] [PubMed] [Google Scholar]
- 53.Rudolphi CA. 1801. [Beobachtungen über die Eingeweidewurmer.] Arch f Zool u Zoot 2:1–65.[Article in German]. [Google Scholar]
- 54.Sasa M, Tanaka H, Fukui M, Takata A.1962. Internal parasites of laboratory animals, p 195–214. In: Harris RJC. The problems of laboratory animal disease. London ; New York: Academic Press. [Google Scholar]
- 55.Sato Y, Ooi HK, Nonaka N, Oku Y, Kamiya M. 1995. Antibody production in Syphacia obvelata-infected mice. J Parasitol 81:559–562. [PubMed] [Google Scholar]
- 56.Sawitz W. 1942. The buoyancy of certain nematode eggs. J Parasitol 28:95–102. [Google Scholar]
- 57.Smith PH, Wiles SE, Malone JB, Monahan CM. 2007. Collection, preservation, and diagnostic methods, p 1–13. In: Baker DG. Flynn's parasites of laboratory animals. 2nd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 58.Stahl W. 1961. Influences of age and sex on the susceptibility of albino mice to infection with Aspiculuris tetraptera. J Parasitol 47:939–941. [PubMed] [Google Scholar]
- 59.Taffs LF. 1976. Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13. [DOI] [PubMed] [Google Scholar]
- 60.van der Gulden WJ. 1967. Diurnal rhythm in egg production by Syphacia muris. Exp Parasitol 21:344–347. [DOI] [PubMed] [Google Scholar]
- 61.Wagner M. 1988. The effect of infection with the pinworm (Syphacia muris) on rat growth. Lab Anim Sci 38:476–478. [PubMed] [Google Scholar]
- 62.Zenner L. 1998. Effective eradication of pinworms (Syphacia muris, Syphacia obvelata, and Aspiculuris tetraptera) from a rodent breeding colony by oral anthelmintic therapy. Lab Anim 32:337–342. [DOI] [PubMed] [Google Scholar]