Abstract
The local anesthetic bupivacaine is valuable for perioperative analgesia, but its use in the postoperative period is limited by its short duration of action. Here, we evaluated the application of a slow-release liposomal formulation of bupivacaine for postoperative analgesia. The aim was to assess whether liposomal bupivacaine effectively attenuates postoperative mechanical and thermal hypersensitivity in a rat model of incisional pain. Rats (n = 36) were randomly assigned to 1 of 5 treatment groups: saline, 1 mL/kg SC every 12 h for 2 d; buprenorphine HCl, 0.05 mg/kg SC every 12 h for 2 d (Bup HCl); 0.5% bupivacaine, 2 mg/kg SC local infiltration once (Bupi); liposomal bupivacaine, 1 mg/kg SC local infiltration once (Exp1); and liposomal bupivacaine, 6 mg/kg SC local infiltration once (Exp6). Mechanical and thermal hypersensitivity were evaluated daily on days –1, 0, 1, 2, 3, and 4. The saline group exhibited both hypersensitivities through all 4 evaluated postoperative days. Bup HCl attenuated mechanical hypersensitivity for 2 d and thermal hypersensitivity for 1 d. Bupi attenuated only thermal hypersensitivity for 4 d. Rats in the Exp1 group showed attenuation of both mechanical and thermal hypersensitivity for 4 d, and those in the Exp6 group had attenuation of mechanical hypersensitivity on day 0 and thermal hypersensitivity for 4 d. These data suggest that a single local infiltration of liposomal bupivacaine at a dose of 1 mg/kg SC effectively attenuates postoperative mechanical and thermal hypersensitivity for 4 d in a rat model of incisional pain.
Abbreviations: Bup HCl, buprenorphine HCl; Bupi, 05% bupivacaine; Exp1, liposomal bupivacaine at 1 mg/kg; Exp6, liposomal bupivacaine at 6 mg/kg
Postoperative analgesia is an important component of laboratory animal wellbeing. It is important not only from an ethical and regulatory standpoint,24 but also for high-quality research data.8 Opioids, although considered a ‘gold standard’ for postoperative analgesia, may be contraindicated in some research projects due to their systemic adverse effects, including nausea, vomiting, constipation, sedation, bradycardia, respiratory depression, pica, and immunomodulation.3,39,46 Local anesthetics (for example, bupivacaine, lidocaine), which are commonly used in multimodal analgesic techniques, provide analgesia through blockade of voltage-gated Na channels, thereby preventing activation of sensory pain pathways and central sensitization.34 As noncontrolled substances with few side effects, local anesthetics are widely used for surgical procedures, but their contribution toward postoperative analgesia is limited by a relatively short duration of action.28
Extended-release liposomal bupivacaine is a slow-release bupivacaine formulation intended to provide extended postoperative analgesia after single-dose administration.20 It is now commercially available for humans and has been a beneficial component of multimodal analgesic techniques, increasing the median time until opioid rescue and decreasing overall opioid usage.16,19,41 The liposomal formulation contains bupivacaine encapsulated in multivesicular liposomes.9 The honeycomb-appearing, nonconcentric lipid bilayers allow gradual drug release over 96 h as the lipid membranes erode and reorganize.15 Liposomal bupivacaine reportedly attenuates hyperalgesia for 48 to 72 h in humans.16,21 Due to its long-lasting analgesia, liposomal bupivacaine has promise for use as either a sole analgesic or as part of multimodal analgesic techniques in research facilities. Therefore, the aim of the current study was to investigate whether liposomal bupivacaine effectively attenuated hypersensitivity in rats during the postoperative period.
Materials and Methods
Animals.
Adult male Sprague–Dawley rats (Rattus norvegicus; n = 36; weight, 387.6 ± 2.1 g; Charles River, Wilmington, MA) were used. Sentinel animals were free of Kilham rat virus, rat Theiler virus, rat coronavirus, rat minute virus, Toolan H1 virus, rat parvovirus, reovirus type 3, lymphocytic choriomeningitis virus, murine adenovirus types 1 and 2, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, and endo- and ectoparasites. Rats were singly housed in static microisolation cages (Allentown, Allentown, NJ) on ALPHA-dri paper bedding (Shepherd Specialty Papers, Milford, NJ). All rats were fed a commercial diet (Teklad Global 18% Protein Rodent Diet 2018, Harlan Laboratories, Madison, WI), provided bottles with reverse-osmosis–purified water, and offered Rat Retreats (Bio-Serv, Flemington, NJ) for enrichment. Rooms were maintained on a 12:12-h dark:light cycle at 70 to 74 °F (21 to 23 °C) and 30% to 70% relative humidity. Experiments were approved by Stanford University's Administrative Panel for Laboratory Animal Care, and all rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals.24 Rats were weighed daily from the day prior to surgery until euthanasia. Upon study conclusion, rats were euthanized by carbon dioxide asphyxiation followed by a secondary physical method.
Surgery.
General anesthesia was induced in rats by using 1% to 5% isoflurane with 100% O2 in an induction chamber, followed by maintenance using 0.8% to 2.5% isoflurane in 100% O2 by mask. Sterile ophthalmic ointment was applied, and rats were supported on a circulating warm-water blanket. Cefazolin (20 mg/kg SC; GlaxoSmithKline, Research Triangle Park, NC) and warm 0.9% NaCl (10 mL/kg SC) were administered prior to incision.
Each rat was placed in sternal recumbency, and the plantar surface of the left (ipsilateral) hindpaw was surgically prepared and draped. The surgery was performed as previously described for the incisional pain model.7 At 0.5 cm from the tibiotarsal joint, a 1-cm longitudinal incision through skin and fascia was made in the left hindpaw, extending toward the digits. The plantaris muscle was identified, elevated, and incised longitudinally without interrupting the muscle attachments. The incision was closed with 2 interrupted horizontal mattress sutures (5-0 polyglactin 910, Ethicon, Somerville, NJ) and triple antibiotic ointment applied (Taro Pharmaceuticals, Hawthorne, NY). Rats were monitored continuously in a heated recovery cage and returned to their home cages after full recovery from anesthesia.
Study design.
Rats were randomly assigned to 1 of 5 treatment groups: saline (n = 6; 1 mL/kg SC; 0.9% NaCl, Hospira, Lake Forest, IL) at surgery and then every 12 h for 2 d; Bup HCl (n = 6; 0.05 mg/kg SC; buprenorphine hydrochloride, 0.3 mg/mL, Hospira) at surgery and then every 12 h for 2 d; Bupi (n = 8; 2 mg/kg SC local infiltration once, 0.15 mL; 0.5% Bupivacaine HCl, 5 mg/mL, Hospira); Exp1 (n = 8; 1 mg/kg SC local infiltration once, 0.03 mL; Exparel, 13.3 mg/mL, Pacira Pharmaceuticals, Parsippany, NJ); or Exp6 (n = 8; 6 mg/kg SC local infiltration once, 0.17 mL; Exparel, 13.3 mg/mL, Pacira Pharmaceuticals). All experimental compounds were administered 5 min prior to skin incision. For the Bupi, Exp1, and Exp6 groups, local anesthetics were injected into the plantar surface of rats’ hindpaws along the surgical site by using a 25-gauge needle. All volumes were sufficient to cover the length of the incision. After completion of each injection, digital pressure was applied to the injection site for 5 s to prevent leakage.
Behavioral testing.
Rats were acclimated to the testing environment daily for 3 d prior to surgery, from 0900 to 1200. Mechanical and thermal hypersensitivity behavioral testing was conducted daily for 3 d prior to surgery and averaged to acquire baseline data and then at 2 h after surgery (day 0), followed by daily for 4 consecutive days (days 1 through 4) between 0900 and 1200 each day. Prior to each behavioral testing session, rats were transported to the behavioral testing room and allowed a minimum of 15 min to acclimate.
Mechanical hypersensitivity testing.
To assess responses to mechanical stimuli, each rat was placed in a clear acrylic enclosure (20 × 9.5 × 12.5 cm) on an elevated mesh stand (Electronic von Frey Mesh Stand, IITC Life Science, Woodland Hills, CA) with 0.64-cm ‘waffle’ holes. Rats were given 15 min to acclimate within the testing enclosure before von Frey monofilaments with calibrated bending forces (10 g, Aesthesio, DanMic Global, San Jose, CA) were applied to the plantar surface of each hindpaw for 10 trials. Each mechanical stimulus was administered for 1 s on various locations of the plantar surface, with care taken to avoid the pads, toes, and heels. Withdrawal responses were defined as the number of times a rat lifted his paw off the mesh after von Frey stimulation during 10 applications of monofilaments. Mechanical hypersensitivity was defined as a significant increase (P < 0.05) in paw withdrawal frequency as a result of punctate mechanical stimuli. The right (contralateral) hindpaw of each rat served as a control.
Thermal hypersensitivity testing.
To assess responses to thermal stimuli, each rat was placed in a clear acrylic enclosure (20 × 9.5 × 12.5 cm) on a tempered-glass surface preheated to 30 °C (Plantar Analgesia Meter, IITC Life Science). Rats were given 15 min to acclimate within the testing enclosure before focal (4 × 6 mm) radiant heat from a 50-W light bulb was directed to the plantar surface of each hindpaw for 4 trials. A 20-s cutoff was set to prevent tissue injury. Each hindpaw was tested 4 times, with a minimum of 1 min between trials. The mean of the last 3 trials was set as the withdrawal latency. Thermal hypersensitivity was defined as a significant (P < 0.05) increase in paw withdrawal frequency after focal thermal stimuli. The right (contralateral) hindpaw of each rat served as a control.
Statistical analyses.
To evaluate differences in withdrawal responses by group and over time, repeated-measures ANOVA with Bonferroni correction for multiple comparisons (R Development Core Team, 2015) was performed. Data were expressed as mean ± SEM. A P value of less than 0.05 was considered significant.
Results
Body weight.
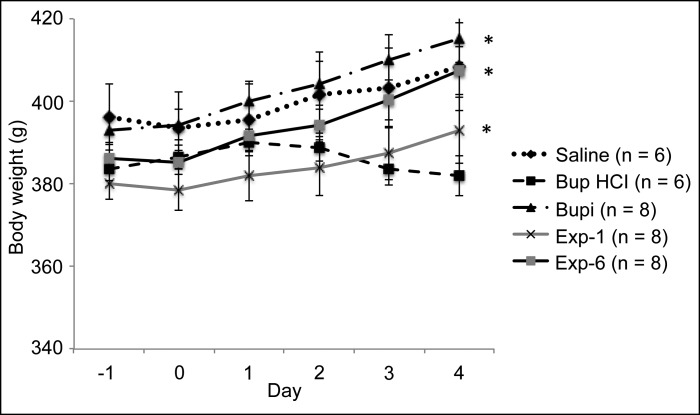
Except for the Bup HCl group, the weights of rats in all treatment groups gradually increased from the first day (day –1) through the last day (day 4) of the experiment (Figure 1). The increase in weight was statistically significant (P < 0.05) only in the Bupi, Exp1, and Exp6 treatment groups. The weights of the Bup HCl rats did not differ between day –1 (383 ± 2.7 g) and day 4 (382 ± 5.0 g), nor did body weight differ between groups prior to the experiment.
Figure 1.
Body weights of rats throughout the study. *, Day 4 value is significantly (P < 0.05) different from the day –1 (baseline) value for the same treatment group.
Mechanical hypersensitivity.
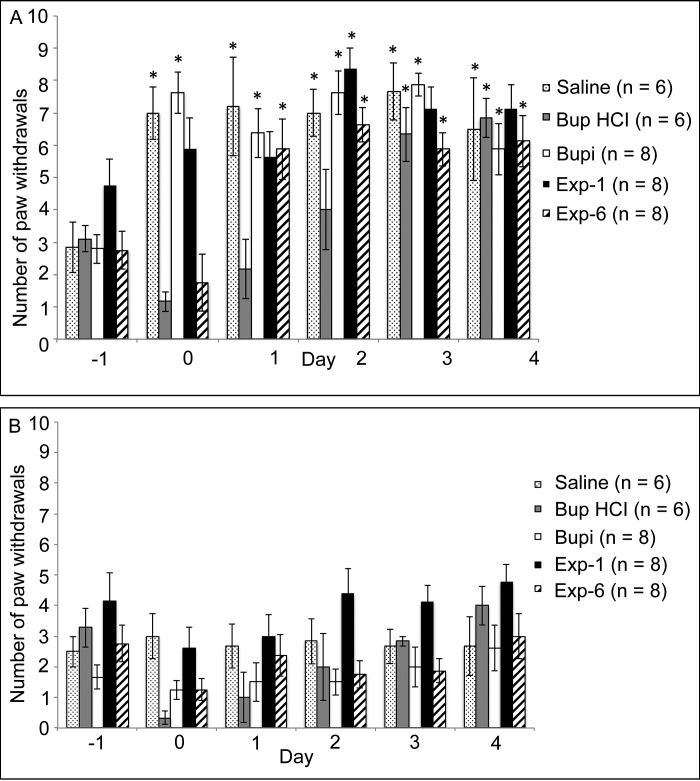
Mechanical hypersensitivity did not differ between groups prior to treatment. In the ipsilateral hindpaw (Table 1, Figure 2 A), the mechanical hypersensitivity of rats in the saline group was increased significantly (P < 0.05) on days 0 through 4 compared with day –1 (baseline). The mechanical hypersensitivity of rats in the Bup HCl group was not increased on days 0, 1, or 2 compared with day –1. Similar to that of the saline group, the mechanical hypersensitivity of rats in the Bupi group was increased on days 0, 1, 2, 3, and 4 compared with day –1. The mechanical hypersensitivity of rats in the Exp1 group was increased relative to baseline only on day 2. In the Exp6 group, mechanical hypersensitivity was increased on days 1 through 4 compared with day –1. In the contralateral hindpaws, mechanical hypersensitivity did not differ between time points in all treatment groups (Figure 2 B).
Table 1.
Mechanical hypersensitivity of the ipsilateral hindpaw
| Day |
||||||
| –1 | 0 | 1 | 2 | 3 | 4 | |
| Saline | 2.8 ± 0.8 | 7.0 ± 0.8a | 7.2 ± 1.5a | 7.0 ± 0.7a | 7.7 ± 0.9a | 6.5 ± 1.6a |
| Bup HCl | 3.1 ± 0.4 | 1.2 ± 0.3 | 2.2 ± 0.9 | 4.0 ± 1.2 | 6.3 ± 0.8a | 6.8 ± 0.6a |
| Bupi | 2.8 ± 0.4 | 7.6 ± 0.7a | 6.4 ± 0.8a | 7.6 ± 0.7a | 7.9 ± 0.4a | 5.9 ± 0.8a |
| Exp1 | 4.8 ± 0.8 | 5.9 ± 0.9 | 5.6 ± 0.8 | 8.4 ± 0.6a | 7.1 ± 0.7 | 7.1 ± 0.8 |
| Exp6 | 2.7 ± 0.6 | 1.8 ± 0.9 | 5.9 ± 0.9a | 6.6 ± 0.5a | 5.9 ± 0.5a | 6.1 ± 0.8a |
Data are shown as no. of paw withdrawals (mean ± SEM).
Significantly (P < 0.05) different from the day –1 (baseline) value for the same treatment group
Figure 2.
Mechanical hypersensitivity (no. of paw withdrawals; mean ± SEM) of the (A) ipsilateral and (B) contralateral hindpaws. Day 0 testing occurred at 2 h after surgery. *, Value is significantly (P < 0.05) different from the day –1 (baseline) value for the same treatment group.
Thermal hypersensitivity.
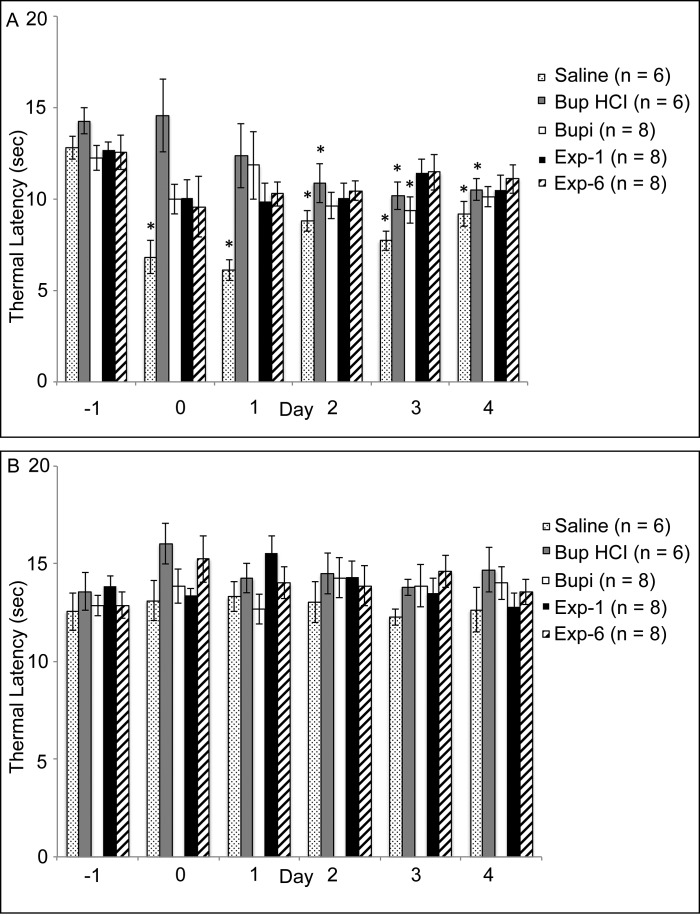
Thermal hypersensitivity did not differ between groups before treatment. In the ipsilateral hindpaw (Table 2, Figure 3 A), the thermal hypersensitivity of rats in the saline group increased significantly (P < 0.05) on days 0 through 4 compared with day –1 (baseline). Thermal hypersensitivity of rats in the Bup HCl group was increased on days 2 through 4 compared with day –1, whereas that in the Bupi group differed relative to baseline only on day 3. The thermal hypersensitivity of rats in the Exp1 and Exp6 groups did not differ on days 0 through 4 compared with day –1. Thermal hypersensitivity in the contralateral hindpaw did not differ between any time points or groups (Figure 3 B).
Table 2.
Thermal hypersensitivity of the ipsilateral hindpaw
| Day |
||||||
| –1 | 0 | 1 | 2 | 3 | 4 | |
| Saline | 12.8 ± 0.6 | 6.8 ± 0.9a | 6.1 ± 0.6a | 8.8 ± 0.6a | 7.7 ± 0.5a | 9.2 ± 0.7a |
| Bup HCl | 14.3 ± 0.7 | 14.6 ± 2.0 | 12.4 ± 1.8 | 10.9 ± 1.1a | 10.2 ± 0.8a | 10.5 ± 0.6a |
| Bupi | 12.3 ± 0.7 | 10.0 ± 0.8 | 11.8 ± 1.9 | 9.6 ± 0.7 | 9.4 ± 0.7a | 10.1 ± 0.6 |
| Exp1 | 12.7 ± 0.4 | 10.1 ± 1.0 | 9.9 ± 1.0 | 10.0 ± 0.9 | 11.4 ± 0.7 | 10.5 ± 0.8 |
| Exp6 | 12.6 ± 0.9 | 9.6 ± 1.7 | 10.3 ± 0.7 | 10.5 ± 0.5 | 11.5 ± 1.0 | 11.1 ± 0.8 |
Data are shown as thermal latency (s; mean ± SEM).
Significantly (P < 0.05) different from the day –1 (baseline) value for the same treatment group
Figure 3.
Thermal hypersensitivity (measured as latency [s] to withdrawal; mean ± SEM) of the (A) ipsilateral and (B) contralateral hindpaws. Day 0 testing occurred at 2 h after surgery. *, Value is significantly (P < 0.05) different from the day –1 (baseline) value for the same treatment group.
Discussion
This study demonstrates that infiltration of a single dose of liposomal bupivacaine (1 mg/kg SC) effectively attenuates both mechanical and thermal hypersensitivity for 4 d in a rat incisional pain model. At the higher dose (6 mg/kg SC), liposomal bupivacaine attenuated mechanical hypersensitivity for 1 d and thermal hypersensitivity for 4 d. Bupivacaine (2 mg/kg SC once) attenuated thermal hypersensitivity for 4 d, but had no effect on mechanical hypersensitivity. Rats of all treatment groups maintained their body weights throughout the duration of the study. Daily measurement of body weight is a simple adjunctive objective measure of postoperative wellbeing and can reflect various side effects of medications.6 The current data support our hypothesis that liposomal bupivacaine attenuates postoperative hypersensitivity in a rat incisional pain model.
The aim of this study was to refine postoperative analgesia in rodents by examining the ability of liposomal bupivacaine to attenuate hypersensitivity. We chose an incisional pain model because our lab has extensive experience with this model as a means to study potential analgesics for postoperative pain in rats. In this model, mechanical and thermal hypersensitivity lasted 4 d, consistent with results from the originally described incisional pain model7 and our previous studies,30,40 in which both hypersensitivities variably continued for as long as 6 d after surgery.
In this study, Bup HCl attenuated mechanical hypersensitivity for 2 d, with a return to hypersensitivity on cessation of therapy. These results are consistent with previous studies10,30,37 and align with data showing that although opioids effectively reduce postoperative pain, they can enhance central sensitization and lead to rebound hypersensitivity.10 Bup HCl attenuated thermal hypersensitivity for 1 d in the current study, in contrast to 2 d in our previous study,30 suggesting that Bup HCl more effectively attenuated mechanical compared with thermal hypersensitivity. Similarly, in a previous plantar incision model, the median effective dose of another opioid, morphine, for attenuating mechanical hypersensitivity (1.5 mg/kg SC) was lower than that for thermal hypersensitivity (1.8 mg/kg SC).48 Therefore, the doses required to attenuate thermal hypersensitivity may be higher than those needed to ameliorate mechanical hypersensitivity. In addition, opioid-induced hypersensitivity has been reported in literature,27 but has been more often linked with chronic administration of full μ-opioid agonists13,29 or ultralow doses of buprenorphine than with clinically relevant doses.14,47 We included Bup HCl in the current study because it remains a cornerstone of effective rodent postoperative analgesia. Its advantages include its control of mild to severe pain, multiple routes of delivery, relatively long duration of action (6 to 12 h in most species), and minimal cardiorespiratory depression.33,38,45 Disadvantages of Bup HCl include its controlled drug status, ceiling effect, difficulty to antagonize, and potential for immunomodulation.12
In the present study, at 2 h after surgery (day 0 testing), whereas buprenorphine attenuated mechanical hypersensitivity, 0.5% bupivacaine did not. Although bupivacaine has local anesthetic properties, its reported duration of mechanical analgesia has been variable. For example, von Frey monofilament testing after local infiltration of 0.5% bupivacaine into rat hindpaws suggested reduced mechanical hypersensitivity of only approximately 23 min18 or, when infiltrated subcutaneously along a back incision, approximately 2 h.32 In humans, the median analgesia duration according to pinprick testing after intradermal injection of 0.5% bupivacaine into the lower back area was 1 h.17 If we had performed behavioral tests sooner than 2 h after surgery, we likely would have detected this attenuation of mechanical hypersensitivity, but we decided not to perform immediate postoperative testing to avoid confounding anesthetic effects within 2 h after surgery. However, bupivacaine did attenuate thermal hypersensitivity for 4 d, consistent with another study reporting that bupivacaine prevented the onset of thermal hypersensitivity for at least 3 d.44 The activation of C-fibers, afferent nociceptive nerve fibers, is well known to induce thermal hypersensitivity, and this activation can be blocked by bupivacaine.1,42,44 The blockade of afferent peripheral fibers is thought to decrease central sensitization (or ‘wind-up’), such that the attenuation of thermal hypersensitivity is sustained even when bupivacaine is no longer present at pharmacologic levels.44 We also investigated 2 different doses of liposomal bupivacaine; the 1-mg/kg dose attenuated both mechanical and thermal hypersensitivity on all 4 d of testing. Other studies similarly reported that liposomal bupivacaine effectively attenuated both mechanical (from 2 to 4 d)22 and thermal hypersensitivity (up to 3 d).23 The 6-mg/kg dose of liposomal bupivacaine attenuated mechanical hypersensitivity only on day 0 (2 h after surgery), but attenuated thermal hypersensitivity for 4 d. This result, although surprising, was similar to another report in which liposomal bupivacaine provided mechanical analgesia for approximately 3 h in a Sprague–Dawley rat paw wound model.18 Although liposomal bupivacaine appears to provide dose-dependent analgesia in humans,5 liposomal bupivacaine did not attenuate both hypersensitivities in a dose-dependent manner in our rat study. We suspect that the large volumes of bupivacaine and liposomal bupivacaine (at 6 mg/kg) relative to the size of the rat's hindpaw (0.15 and 0.17 mL, respectively, for a 380-g rat in contrast to 0.03 mL for the 1-mg/kg dose) caused tissue distension and irritation, which may have intensified hypersensitivity. In humans, the recommended dose of liposomal bupivacaine is based on the volume required to cover the surgical site, and a ceiling effect may occur20 once all local nociceptors have been exposed to the minimal amount of local anesthetic necessary for analgesia. Therefore, the surgical-site volume of a rat's hindpaw likely would favor a smaller volume and dose of local anesthetic. We believe that the 6-mg/kg dose of liposomal bupivacaine would be effective if infiltrated in a larger surgical site. Other possible factors contributing to the variability of our findings include injection pressure, speed of injection, and site or tissue density of the local infiltration.43 The transiently significant differences in bupivacaine's effects on thermal hypersensitivity (day 3) and mechanical hypersensitivity (1-mg/kg dose on day 2) were not clinically significant, given the absence of clinical signs and the normal weight gain of the study animals.
From a practical standpoint, assuming a 380-g rat, the current cost of Bup HCl (0.05 mg/kg every 12 h for 3 d) is US$5.78, bupivacaine (2 mg/kg once) is US$0.03, and liposomal bupivacaine is US$0.54 (1 mg/kg once) or US$3.24 (6 mg/kg once); this estimate does not include labor charges for repeated dose administration (Bup HCl). Bupivacaine, although inexpensive, would not itself provide sufficient postoperative analgesia for most surgeries. Nonetheless, the expense of liposomal bupivacaine remains a limiting consideration for veterinary patients because it is marketed as a human drug and sold only in 20-mL vials. Due to the small administration volumes needed for rodents and the manufacturer's recommendation that vials be used within 4 h of opening, its use in laboratories where few daily rodent surgeries are performed may not be financially feasible. However, liposomal bupivacaine is currently under FDA review for the veterinary market. If FDA approval is granted to a veterinary pharmaceutical company, the aforementioned limitations may no longer apply, as the diversity of species and patient sizes will necessitate the distribution of small-volume vials for single-dose use.
In human studies, local administration of liposomal bupivacaine lacked a clinical effect on wound and bone healing. Both the wound-healing profiles of liposomal bupivacaine and the occurrence of adverse effects,2 such as cardiotoxicity,4,9,26 were similar to those of bupivacaine. Other common side effects in humans are pruritus, constipation, tachycardia, nausea, vomiting, dizziness, headache, and fever.31 Studies in dogs, pigs, and rabbits have demonstrated a favorable safety profile for liposomal bupivacaine.11,25 A mild, self-limiting granulomatous inflammation was reported in some cases, which was deemed to be a normal, expected reaction to foreign matter (that is, degradation of multivesicular liposomes).26,35,36 No adverse cardiac, CNS, or wound-healing effects were observed clinically in the rats treated with liposomal bupivacaine compared with other treatment groups.
According to the results of our current study, a single dose of liposomal bupivacaine (1 mg/kg local infiltration) is recommended for providing 4 days of postoperative analgesia in a rat incisional pain model. Although the 1-mg/kg dosage effectively attenuated hypersensitivity in the current study, we still recommend multimodal postoperative analgesic techniques for most surgical procedures, due to the complexity of nociceptive pathways. We suggest additional research with liposomal bupivacaine to determine a range of appropriate infiltration volumes, to suggest dosages for larger surgical sites, to examine its application in different surgical pain models and with other laboratory animal species, to assess its use as a component of multimodal analgesia, and to perform sequential histologic examinations of surgical infiltration sites.
Acknowledgments
We thank Mikhail Klukinov for his assistance in the experimental set-up, Mike Alvarez for helping us secure a behavioral testing room, and Janis Atuk-Jones for her assistance in formatting and editing the manuscript. This work was partly supported by the NIH Research Education Program for Laboratory Animal Veterinarians Training Grant (5R25OD104524).
References
- 1.Banik RK, Brennan TJ. 2004. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain 112:204–213. [DOI] [PubMed] [Google Scholar]
- 2.Baxter R, Bramlett K, Onel E, Daniels S. 2013. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from 10 prospective, controlled clinical studies. Clin Ther 35:312–320.e5. [DOI] [PubMed] [Google Scholar]
- 3.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. 2008. Opioid complications and side effects. Pain Physician 11:S105–S120. [PubMed] [Google Scholar]
- 4.Bergese SD, Onel E, Morren M, Morganroth J. 2012. Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med 37:145–151. [DOI] [PubMed] [Google Scholar]
- 5.Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. 2012. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res 5:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan MP, Sinusas AJ, Horvath TL, Collins JG, Harding MJ. 2009. Correlation between body weight changes and postoperative pain in rats treated with meloxicam or buprenorphine. Lab Anim (NY) 38:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan TJ, Vandermeulen EP, Gebhart GF. 1996. Characterization of a rat model of incisional pain. Pain 64:493–501. [DOI] [PubMed] [Google Scholar]
- 8.Carbone L, Austin J. 2016. Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS One 11:e0155001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chahar P, Cummings KC., 3rd 2012. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res 5: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtin LI, Grakowsky JA, Suarez M, Thompson AC, DiPirro JM, Martin LB, Kristal MB. 2009. Evaluation of buprenorphine in a postoperative pain model in rats. Comp Med 59:60–71. [PMC free article] [PubMed] [Google Scholar]
- 11.Damjanovska M, Cvetko E, Hadzic A, Seliskar A, Plavec T, Mis K, Vuckovic Hasanbegovic I, Stopar Pintaric T. 2015. Neurotoxicity of perineural vs intraneural-extrafascicular injection of liposomal bupivacaine in the porcine model of sciatic nerve block. Anaesthesia 70:1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeClue AE, Yu DH, Prochnow S, Axiak-Bechtel S, Amorim J, Tsuruta K, Donaldson R, Lino G, Monibi F, Honaker A, Dodam J. 2014. Effects of opioids on phagocytic function, oxidative burst capacity, cytokine production and apoptosis in canine leukocytes. Vet J 200:270–275. [DOI] [PubMed] [Google Scholar]
- 13.Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. 2002. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci 22:6747–6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhold KJ, Drdla-Schutting R, Honsek SD, Forsthuber L, Sandkuhler J. 2015. Pronociceptive and antinociceptive effects of buprenorphine in the spinal cord dorsal horn cover a dose range of 4 orders of magnitude. J Neurosci 35:9580–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golembiewski J, Dasta J. 2015. Evolving role of local anesthetics in managing postsurgical analgesia. Clin Ther 37:1354–1371. [DOI] [PubMed] [Google Scholar]
- 16.Gorfine SR, Onel E, Patou G, Krivokapic ZV. 2011. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum 54:1552–1559. [DOI] [PubMed] [Google Scholar]
- 17.Grant GJ, Barenholz Y, Bolotin EM, Bansinath M, Turndorf H, Piskoun B, Davidson EM. 2004. A novel liposomal bupivacaine formulation to produce ultralong-acting analgesia. Anesthesiology 101:133–137. [DOI] [PubMed] [Google Scholar]
- 18.Grant GJ, Lax J, Susser L, Zakowski M, Weissman TE, Turndorf H. 1997. Wound infiltration with liposomal bupivacaine prolongs analgesia in rats. Acta Anaesthesiol Scand 41:204–207. [DOI] [PubMed] [Google Scholar]
- 19.Haas E, Onel E, Miller H, Ragupathi M, White PF. 2012. A double-blind, randomized, active-controlled study for posthemorrhoidectomy pain management with liposome bupivacaine, a novel local analgesic formulation. Am Surg 78:574–581. [DOI] [PubMed] [Google Scholar]
- 20.Hadzic A, Abikhaled JA, Harmon WJ. 2015. Impact of volume expansion on the efficacy and pharmacokinetics of liposome bupivacaine. Local Reg Anesth 8:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchins JL, Kesha R, Blanco F, Dunn T, Hochhalter R. 2016. Ultrasound-guided subcostal transversus abdominis plane blocks with liposomal bupivacaine compared with nonliposomal bupivacaine for postoperative pain control after laparoscopic hand-assisted donor nephrectomy: a prospective randomised observer-blinded study. Anaesthesia 71:930–937. [DOI] [PubMed] [Google Scholar]
- 22.Ickowicz DE, Golovanevski L, Domb AJ, Weiniger CF. 2014. Extended duration local anesthetic agent in a rat paw model. Int J Pharm 468:152–157. [DOI] [PubMed] [Google Scholar]
- 23.Ickowicz DE, Golovanevski L, Haze A, Domb AJ, Weiniger CF. 2014. Extended release local anesthetic agents in a postoperative arthritic pain model. J Pharm Sci 103:185–190. [DOI] [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 25.Joshi GP, Patou G, Kharitonov V. 2015. The safety of liposome bupivacaine following various routes of administration in animals. J Pain Res 8:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambrechts M, O'Brien MJ, Savoie FH, You Z. 2013. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence 7:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. 2011. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 14:145–161. [PubMed] [Google Scholar]
- 28.Lemke KA, Dawson SD. 2000. Local and regional anesthesia. Vet Clin North Am Small Anim Pract 30:839–857. [DOI] [PubMed] [Google Scholar]
- 29.Mao J, Sung B, Ji RR, Lim G. 2002. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 22:8312–8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeon GP, Pacharinsak C, Long CT, Howard AM, Jampachaisri K, Yeomans DC, Felt SA. 2011. Analgesic effects of tramadol, tramadol-gabapentin, and buprenorphine in an incisional model of pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 50:192–197. [PMC free article] [PubMed] [Google Scholar]
- 31.Noviasky J, Pierce DP, Whalen K, Guharoy R, Hildreth K. 2014. Bupivacaine liposomal compared with bupivacaine: comparative review. Hosp Pharm 49:539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohri R, Wang JC, Pham L, Blaskovich PD, Costa D, Nichols G, Hildebrand W, Scarborough N, Herman C, Strichartz GR. 2014. Prolonged amelioration of experimental postoperative pain by bupivacaine released from microsphere-coated hernia mesh. Reg Anesth Pain Med 39:97–107. [DOI] [PubMed] [Google Scholar]
- 33.Pascoe PJ. 2000. Opioid analgesics. Vet Clin North Am Small Anim Pract 30:757–772. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen JL, Crawford ME, Dahl JB, Brennum J, Kehlet H. 1996. Effect of preemptive nerve block on inflammation and hyperalgesia after human thermal injury. Anesthesiology 84:1020–1026. [DOI] [PubMed] [Google Scholar]
- 35.Richard BM, Ott LR, Haan D, Brubaker AN, Cole PI, Nelson KG, Ross PE, Rebelatto MC, Newton PE. 2011. The safety and tolerability evaluation of DepoFoam bupivacaine (bupivacaine extended-release liposome injection) administered by incision wound infiltration in rabbits and dogs. Expert Opin Investig Drugs 20:1327–1341. [DOI] [PubMed] [Google Scholar]
- 36.Richard BM, Rickert DE, Newton PE, Ott LR, Haan D, Brubaker AN, Cole PI, Ross PE, Rebelatto MC, Nelson KG. 2011. Safety evaluation of EXPAREL (DepoFoam bupivacaine) administered by repeated subcutaneous injection in rabbits and dogs: Species comparison. J Drug Deliv 2011:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richebé P, Rivat C, Laulin JP, Maurette P, Simonnet G. 2005. Ketamine improves the management of exaggerated postoperative pain observed in perioperative fentanyl-treated rats. Anesthesiology 102:421–428. [DOI] [PubMed] [Google Scholar]
- 38.Roughan JV, Flecknell PA. 2004. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol 15:461–472. [DOI] [PubMed] [Google Scholar]
- 39.Sacerdote P, Franchi S, Panerai AE. 2012. Nonanalgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 18:6034–6042. [DOI] [PubMed] [Google Scholar]
- 40.Seymour TL, Adams SC, Felt SA, Jampachaisri K, Yeomans DC, Pacharinsak C. 2016. Postoperative analgesia due to sustained-release buprenorphine, sustained-release meloxicam, and carprofen gel in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 55:300–305. [PMC free article] [PubMed] [Google Scholar]
- 41.Smoot JD, Bergese SD, Onel E, Williams HT, Hedden W. 2011. The efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammaplasty: a randomized, double-blind, active-control study. Aesthet Surg J 32:69–76. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki S, Gerner P, Colvin AC, Binshtok AM. 2009. C-fiber-selective peripheral nerve blockade. Open Pain J 2:24–29. [Google Scholar]
- 43.Tateno K, Inoue K, Sato T, Fukayama H. 2008. Differences in the degree of infiltration of local anesthesia according to the site of injection in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:e6–e10. [DOI] [PubMed] [Google Scholar]
- 44.Themistocleous A, Kamerman P, Mitchell D. 2007. Pre-emptive ring-block with bupivacaine prevents the development of thermal hyperalgesia, but not sustained mechanical hyperalgesia, in rat tails exposed to ultraviolet A light. J Pain 8:208–214. [DOI] [PubMed] [Google Scholar]
- 45.Tranquilli WJ, Thurmon JC, Grimm KA. 2007. Lumb and Jones’ veterinary anesthesia and analgesia, 4th ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 46.Vallejo R, de Leon-Casasola O, Benyamin R. 2004. Opioid therapy and immunosuppression: a review. Am J Ther 11:354–365. [DOI] [PubMed] [Google Scholar]
- 47.Wala EP, Holtman JR., Jr 2011. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol 651:89–95. [DOI] [PubMed] [Google Scholar]
- 48.Zhu CZ, Nikkel AL, Martino B, Bitner RS, Decker MW, Honore P. 2006. Dissociation between postsurgical pain behaviors and spinal fos-like immunoreactivity in the rat. Eur J Pharmacol 531:108–117. [DOI] [PubMed] [Google Scholar]