Abstract
Rodent euthanasia using exposure to increasing concentrations of CO2 has come under scrutiny due to concerns of potential pain during the euthanasia process. Alternatives to CO2, such as isoflurane and barbiturates, have been proposed as more humane methods of euthanasia. In this study, we examined 3 commonly used euthanasia methods in mice: intraperitoneal injection of pentobarbital–phenytoin solution, CO2 inhalation, and isoflurane anesthesia followed by CO2 inhalation. We hypothesized that pentobarbital–phenytoin euthanasia would cause fewer alterations in cardiovascular response, result in less behavioral evidence of pain or stress, and produce lower elevations in ACTH than would the isoflurane and CO2 methods, which we hypothesized would not differ in regard to these parameters. ACTH data suggested that pentobarbital–phenytoin euthanasia may be less stressful to mice than are isoflurane and CO2 euthanasia. Cardiovascular, behavioral, and activity data did not consistently or significantly support isoflurane or pentobarbital–phenytoin euthanasia as less stressful methods than CO2. Euthanasia with CO2 was the fastest method of the 3 techniques. Therefore, we conclude that using CO2 with or without isoflurane is an acceptable euthanasia method. Pathologic alterations in the lungs were most severe with CO2 euthanasia, and alternative euthanasia techniques likely are better suited for studies that rely on analysis of the lungs.
Abbreviations: CRR, chamber replacement rate; DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure
Several available euthanasia techniques for mice are either acceptable or acceptable with conditions as described in the AVMA guidelines.13 These methods are often categorized according to methodology: injectable, inhalant, or physical methods. Pentobarbital is an injectable agent that is considered the ‘gold standard’ for euthanasia of most species and is acceptable for rodent euthanasia.13 Pentobarbital is both rapid and induces a painless death, the goal for all euthanasia techniques. However, due to the frequent need to euthanize several mice at the same time, an injectable agent is often considered to be too labor-intensive for general use. Therefore, alternative euthanasia techniques are desirable at research institutions where a large number of animals must be euthanized or when pentobarbital might alter experimental outcomes.
Inhalant agents are practical because multiple animals can be euthanized simultaneously, and the use of controlled substances can be avoided. However, rodent euthanasia by using exposure to increasing concentrations of CO2 has come under scrutiny due to concerns of potential pain during the euthanasia process. When euthanizing with any agents including inhalants, the potential for pain or suffering exists only from the time of exposure to the inhalant until the time that the animal becomes unconscious. The guidelines from the Canadian Council on Animal Care regarding the euthanasia of animals used in science recommend the use of anesthetics prior to CO2 euthanasia.4 This recommendation is based on studies documenting that the time period between the development of aversive behavior and of unconsciousness is shorter when rats are exposed to isoflurane compared with CO2.12,15 The Canadian Council states that “because animals are exposed to aversive concentrations of gas for a shorter duration, initial induction with inhalant anesthetics appears to be more humane than euthanasia with CO2 alone.”4 The AVMA guidelines on euthanasia do not require the use of anesthetics before CO2 euthanasia in rodents, but they do include a provision that parallels some of the concern for the use of CO2 by the Canadian Council. The AVMA accepts CO2 inhalation as a method of euthanizing rodents provided that a controlled chamber-replacement rate (CRR) of 10% to 30% is used. This recommendation is based on both aversion of rodents to the gas6,11,12 and the potential pain of CO2 at higher CRR.13
Choosing a euthanasia agent should be based on sound scientific data. Surrogate studies, such as the use of aversion or avoidance, are beneficial for defining aspects of the response to inhalant agents, but they do not adequately define whether pain or distress is experienced, nor do they replicate the euthanasia experience in its entirety. In fact, independent of CRR using CO2, the CO2 concentration at which rats and mice leave a chamber is similar (about 12% to 15%).18,20,21 This finding does not mean that pain is present in the rodents, given that pain is not believed to occur until 40% CO2 concentration.18 Therefore, other methods to evaluate the euthanasia experience are needed. When selecting a euthanasia agent, choosing a humane method that induces a rapid, painless, and distress-free death is imperative.13 To assess whether pain or distress is present, prior investigations into euthanasia have examined behavioral, stress hormone, and neurologic responses to the procedure.1-3,17-21,31 Behavioral assessments include changes in activity (escape behaviors, rearing, sniffing, grooming, vocalizations) or rely on reaction to stimuli.2,19-21 A rat study examining escape, the righting reflex, and the pedal withdrawal reflex to toe pinch during euthanasia with CO2 or isoflurane demonstrated that mice euthanized with CO2 but not isoflurane were insensible when initially recumbent.19 However, this technique may not be an adequate method of assessing consciousness, because movement and response to stimuli occur without supraspinal structures.1 A second method to evaluate euthanasia is measuring the stress response of the animal. Stress hormones such as ACTH have previously been shown to be elevated during euthanasia procedures.2 The third method of assessment examines neural responses during euthanasia. This technique has commonly been used to assess euthanasia during decapitation, cervical dislocation, isoflurane, potassium chloride, and CO2.3,17,31
In the current study, we asked whether inducing general anesthesia with the inhalant, volatile anesthetic isoflurane prior to euthanasia with CO2 is an improvement over using CO2 only, and we compared both isoflurane and CO2 with sodium pentobarbital–phenytoin (for example, Euthasol [Virbac Animal Health, Fort Worth, TX]) administered by intraperitoneal injection. We hypothesized that pentobarbital–phenytoin euthanasia would cause fewer alterations in cardiovascular response, have less behavioral evidence of pain or stress, and have lower elevations in ACTH than would the isoflurane and CO2 methods. In addition, we hypothesized that the isoflurane and CO2 euthanasia methods would not differ from each other in regard to the evaluated parameters.
Materials and Methods
Mice.
Male C57BL/6NTac mice (n = 57; age, 16 wk; Taconic, Hudson, NY) were used for all procedures. Mice were individually housed in open rodent ‘shoebox’ cages (Allentown Caging, Allentown, NJ) on a 12:12-h light:dark cycle (lights on, 0700 to 1900). Male mice were used because of their increased size, which facilitates placement of the carotid catheter. Because male mice were used in this study, they were housed individually to avoid fighting and to best acclimate them to the study housing scenario. The room temperature was maintained at 23.3° C with a mean humidity of 52.6% ± 6.0%. Mice were fed a commercial rodent diet (Teklad 8640, Harlan, Indianapolis, IN), received tap water in bottles without restriction, and were housed on Sani-Chip bedding (Harlan) with cotton squares (Ancare, Bellmore, NY) provided. Results of vendor surveillance and colony sentinel monitoring showed that the mice were free from pathogenic agents including ectromelia virus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, mouse adenovirus strains 1 and 2, mouse hepatitis virus, mouse parvovirus, minute virus of mice, polyoma virus, pneumonia virus of mice, reovirus type 3, Theiler murine encephalomyelitis virus, Sendai virus, endoparasites, and ectoparasites. All experimental procedures were approved by the Wright State University IACUC.
Surgery.
At 7 to 9 d after arrival of the mice at the housing facility, all surgeries were performed by using aseptic techniques in a dedicated rodent surgery suite. Mice were anesthetized with isoflurane (1% to 4%) in oxygen (induced in a chamber and maintained by mask). The ventral neck was shaved and prepped 3 times with alternating povidone–iodine and alcohol scrubs followed by a final swab of povidone–iodine solution. The mice were monitored continuously for depth of anesthesia according to their responses to nociceptive stimulation, movement, and respiratory rate and were kept on a heating pad to prevent hypothermia during the procedure. A 1-cm incision was made in the ventral neck and the muscle bluntly dissected to expose the carotid artery. A telemetry pressure-transmitter probe (TA11PA-C10, Data Sciences International, St Paul, MN) was inserted into the carotid artery and ligated in place. The body of the transmitter was inserted subcutaneously on the left flank, and the incision was closed by using 5-0 nonabsorbable black nylon monofilament sutures (Arosurgical, Newport Beach, CA). Pain and discomfort were alleviated by an initial subcutaneous dose of carprofen (5 mg/kg; Penn Vet, Lancaster, PA) at the time of surgery and an additional dose of carprofen at 24 h postoperatively. Selection of carprofen over an opioid is best practice for this type of dissection-associated pain, where tissue trauma is the primary factor for analgesia.24 After surgery, mice were housed singly to prevent a cage mate from disturbing the wound site and to accustom mice to single housing for individual blood pressure measurements.
Telemetry Measurement.
Mice were allowed to recover for 7 to 10 d after surgery. They were individually housed on data-acquisition receiver boards (RPC1, Data Sciences International) to ensure signal integrity and were randomized for euthanasia method. Radiotelemetry measurements were collected by using Ponemah software (Data Sciences International). Heart rate (HR), blood pressure (BP), and activity data were collected continuously every second (sample rate 1000 Hz) during both baseline and testing measurements. For data collection, the transmitters were turned on and all personnel left the room. After we allowed at least 30 min for the readings to stabilize, baseline data were collected for at least 1 h (starting at approximately 1000). On subsequent days, mice were euthanized after a similar 30-min stabilization period. The stabilization period was used to minimize the effect of cage movement on the cardiovascular parameters and mouse activity. Only one person entered the room during testing; that person remained silent during the procedure.
Isoflurane euthanasia.
To minimize handling-associated stress, all mice were euthanized in their home cage in the housing room. Euthanasia was performed between 1000 and 1300. To set up the euthanasia chamber, the mouse home cage (5.8 L, Allentown Caging) was placed in a 22-L transparent polycarbonate euthanasia chamber (44 cm × 23.5 cm × 21 cm) in the same location in which the home cage was positioned on the telemetry pad. The euthanasia chamber was covered with an acrylic lid that included ports for the gas inlet and outlet. Isoflurane was provided from a vaporizer at 5% with oxygen flow rate at 1 L/min. Mice were monitored continuously during the procedure, and once the mouse was immobile (except for breathing) for 1 min, compressed CO2 gas was provided at 100% chamber volume per minute. A total of 11 mice were euthanized by isoflurane followed by CO2; cardiovascular recordings were obtained successfully from 10 of the 11 mice, and ACTH, behavioral response, and lungs for histology were collected from all 11 mice. Mice were monitored until 30 s after complete cessation of heart beat and blood pressure.
CO2 euthanasia.
Mice were euthanized as previously reported at CRR of 15%, 30%, 50%, or 100% (volume per minute).2 The same chamber setup, parameters, and procedures as described for isoflurane euthanasia were used.
Pentobarbital–phenytoin euthanasia.
Mice were euthanized with an intraperitoneal injection of saline-diluted Euthasol (150 mg/kg [0.08 mL]; Virbac Animal Health) containing pentobarbital sodium (390 mg/mL) and phenytoin sodium (50 mg/mL) as the active ingredients. Briefly, the mice were picked up by hand, scruffed, and inverted; the mouse's head was down at a slight (approximate 20°) angle, and the injection was given in the lower left abdominal quadrant. Mice were returned to their home cages immediately after injection. A total of 14 mice were euthanized with pentobarbital–phenytoin solution; 12 cardiovascular recordings were obtained, and ACTH, behavioral responses, and lungs for histology were collected from all 14 mice. The same person performed all euthanasia procedures.
Behavior.
Mice were video-recorded (model C920 camera, Logitech, Newark, CA) during all euthanasia procedures. Videos were analyzed for activity (hopping, walking/running, sedentary, standing/rearing), breathing pattern (normal, cessation of breathing), ataxia, face wiping, grooming, recumbency or cessation of walking, and loss of muscle tone or nose resting on the bedding. Ataxia was defined as the first point at which an uncoordinated movement was made, including stumbling, mis-stepping, and wobbling. Nose down (full recumbency) was the time that the mouse no longer raised its head off of the bedding and no movements other than breathing were made. The time until the HR reached 0 bpm was established as the point of death. The HR had to be 0 bpm for 2 consecutive seconds for definition of death, and spontaneous electrical activity after this time did not produce heart beats that were measurable by the telemetry probe. Time to the initiation of the activity and number of occurrences were recorded. The same person, who was blinded regarding animal group, conducted all video analyses.
Histology.
Lungs were inflation-fixed with 10% neutral buffered formalin and harvested from each mouse after euthanasia. They were then processed through a gradient of alcohols and xylene, embedded in paraffin, cut at 5 micron thickness, and stained with hematoxylin and eosin. Lungs were examined for acute hemorrhagic lesions, congestion and perivascular and peribronchiolar edema. Changes were scored on a 4-point scale: 0, normal; 1, mild change (involvement of 1% to 10% of the tissue); 2, moderate change (involvement of 11% to 50% of the tissue); and 3, severe change (involvement of 51% to 100% of the tissue). Scoring was done by a single person, who had more than 25 y of experience in murine pathology and was blind to the method of euthanasia.
Stress hormone.
Blood (0.3 to 1.0 mL) was collected by cardiocentesis in EDTA collection tubes immediately after euthanasia. Blood samples were centrifuged at 385 × g for 15 min at room temperature, and the plasma was removed. Plasma samples for ACTH analysis were stored at –80 °C until assay. ACTH samples were analyzed by using a commercially available kit (ImmunChem Double Antibody ACTH Radioimmunoassay, MP Biomedicals, Santa Ana, CA). Plasma samples were diluted 1:7 with assay diluent before being processed according to the manufacturer's instructions (with standards ranging from 5 to 707 pg/mL). None of the samples were below the manufacturer's reported minimal detectable dose of 5.7 pg/mL. The intraassay coefficient of variation was 12.7%.
Statistics.
AUC.
The AUC above baseline data captures the change in the sum of the values of a defined time period. The baseline value was defined as the average value of the parameter for the mice during the baseline data collection period, as described in the preceding telemetry section. Values below baseline were treated as 0. The rate of AUC change (AUC/s) represents the average increase over baseline and thus corrects for the variable length of time between endpoints for the different euthanasia methods. Isoflurane and pentobarbital–phenytoin euthanasia processes were compared with each other and with 4 CO2 flow rates (15%, 30%, 50%, and 100%) from a prior report.2 All analyses were done by using SAS version 9.4 (SAS Institute Inc., Cary, NC). Analysis of covariance was used for all analyses where model assumptions were met to control for the baseline measurements. The data analysis was done for total HR, HR/s, total systolic BP (SBP), SBP/s, total diastolic BP (DBP), DBP/s, total mean BP (MBP), and MBP/s. The analysis was performed for time until the mouse was ataxic, time until the mouse did not lift its head off the bedding (nose down or full recumbency), and time until death. When required, natural logarithm transformations were performed on response variables to meet model assumptions. For time until mouse was ataxic, natural logarithm transformations were performed on total HR, total DBP, and total MBP. For time until nose down, natural logarithm transformations were performed on total HR, total SBP, total DBP, DBP/s, total MBP, and MBP/s. For time until death, natural logarithm transformations were performed on all 8 response variables.
An α level of 0.0125 was used to define significance for all inferences to control for type 1 error, given that 4 different outcomes were analyzed simultaneously. Tukey multiple-comparison testing was performed for all post hoc pairwise comparisons except for HR/s until ataxic, which violated the ANOVA assumption of constant variance and had to be analyzed with the nonparametric Kruskal–Wallis test. Individual Wilcoxon rank-sum tests were performed for all HR/sec until ataxic post hoc pairwise comparisons, with another Bonferroni correction being made and α being adjusted to 0.0011.
Activity.
Activity levels including the amount of activity (that is, distance traveled) and the time spent moving across the 6 methods of euthanasia were compared. Because 2 outcomes were analyzed here, a Bonferroni correction was applied, yielding a level of significance α = 0.025. This analysis was done for the total amount of activity until ataxia (ataxia activity level), average activity until ataxia (ataxia activity level per second), total amount of activity until nose down (nose down activity level), and average activity until nose down (nose down activity level per second). Because the outcome is ordinal and there were 6 levels of the treatment, the nonparametric Kruskal–Wallis test was used for all comparisons. Individual Wilcoxon rank-sum tests were performed for all post hoc pairwise comparisons, with another Bonferroni correction and adjustment to α = 0.0028.
The time spent moving during each of the euthanasia methods was analyzed. The total time (in seconds) spent moving until ataxia (activity time until ataxia), average time spent moving until ataxia (activity time until ataxia per second), total time spent moving until nose down (activity time until nose down), and average time spent moving until nose down (activity time until nose down per second) were analyzed by using a one-way ANOVA with method of euthanasia as the factor. Natural logarithm transformations were necessary for activity time until ataxia, activity time until nose down, and activity time until death to meet model assumptions. Tukey multiple-comparison testing was performed for all posthoc pairwise comparisons.
Behavior.
The frequency with which the mice in the 6 groups wiped their faces, stood, groomed themselves, or walked during the course of the experiment was recorded. Because of the few data points in some cells, Fisher exact tests were performed. Because 4 separate tests were performed, an overall level of significance α = 0.0125 was used to control for inflated type I error.
Peak values.
Peak values were analyzed for SBP, DBP, MBP, HR, and activity. One-way ANOVA was used for SBP, DBP, MBP, and HR, whereas the Kruskal–Wallis test was used for activity. A Bonferroni correction was applied to the 4 cardiovascular outcomes, resulting in a level of significance of α = 0.0125 for that portion of the analysis. Individual Wilcoxon rank-sum tests were performed on activity to reveal where those differences might lie. Because 9 comparisons were made, another Bonferroni correction was performed, yielding a level of significance α = 0.0011 for this portion of the analysis.
Time until peak value.
Time until peak value was assessed for SBP, DBP, MBP, HR, and activity to reveal any relationship between when peak values occurred, on average. Because time until an event is of interest, log rank tests were conducted for each outcome, with a P value of 0.0125 considered significant.
Histology.
The nonparametric Kruskal–Wallace tests was used for the histology analysis. A level of significance of α = 0.017 was used to control for potentially inflated type I error, given that 3 tests were performed. The Dwass–Steel–Critchlow–Flinger method was performed for all post hoc pairwise comparisons.
Stress hormone.
The ACTH analysis used one-way ANOVA with method of euthanasia as the factor. A natural logarithm transformation was performed on the response variable to meet the model assumption of constant variance. Tukey multiple comparison was performed on the log-transformed data to identify potential differences. A P value of 0.05 was considered significant.
Results
The results of 3 different euthanasia methods on cardiovascular parameters, activity, behavior, lung histology, and plasma ACTH values in mice are presented. Our previous study examined differences between CO2 CRR.2 The current study was designed to examine the physiologic and behavioral differences between pentobarbital–phenytoin, isoflurane, and the CO2 euthanasia data previously collected; not reported here are the differences previously seen between the different CO2 CRR.2 The time until death was examined by using 2 prior time points used that potentially represent changes in consciousness. The first time point represents the time when the mice became ataxic. The mean time until ataxia was significantly shorter for pentobarbital–phenytoin than for either 15% or 30% CO2 CRR (P < 0.0001 and P = 0.0053, respectively) and significantly shorter for isoflurane than for 15% and 30% CO2 CRR (P < 0.0001 and P = 0.0004, respectively, Table 1). The second time point of nose down is the time at which the mouse no longer raised its head off of the bedding. The mean time until nose down was significantly longer for pentobarbital–phenytoin than isoflurane, 50% CO2 CRR, and 100% CO2 CRR (P = 0.0008, P < 0.0001, and P < 0.0001, respectively), shorter for isoflurane than 15% CO2 CRR (P < 0.0001), but longer for isoflurane than 100% CO2 CRR (P < 0.0001, Table 1). Finally, the time until the HR reached 0 bpm was established as the point of death. The mean time until death was significantly longer for pentobarbital–phenytoin than for all other methods of euthanasia (P < 0.0001 for all except isoflurane, where P = 0.0014) and longer for isoflurane than for 30%, 50%, or 100% CO2 CRR (P < 0.0001 for all comparisons, Table 1).
Table 1.
Time (s; mean ± 1 SD) until ataxia, full recumbency, or death due to various euthanasia methods
| 15% CO2 | 30% CO2 | 50% CO2 | 100% CO2 | Isoflurane | Pentobarbital– phenytoin | |
| Ataxia | 79.4 ± 13.4a,b | 49.6 ± 8.8a,b | 31.7 ± 5.3 | 24.7 ± 4.8 | 32.6 ± 13.0 | 35.6 ± 11.0 |
| Nose down | 106.3 ± 17.6a | 70.2 ± 6.0 | 45.5 ± 13.6b | 32.6 ± 5.5a,b | 61.9 ± 13.6 | 105.8 ± 51.3 |
| Death | 204.1 ± 29.0b | 160.3 ± 25.2a,b | 100.9 ± 17.1a,b | 70.6 ± 7.2a,b | 236.6 ± 35.0b | 343.3 ± 110.3a |
CO2 data have been published previously.2
Value significantly (P < 0.0001) different from that for isoflurane
Value significantly (P < 0.0001) different from that for pentobarbital–phenytoin
Cardiovascular effects.
Differences in the telemetry data on time until death are represented in Tables 1 through 4; Differences between time until death and nose down are represented by the inclusion of telemetry data collected after the mice were presumed to be unconscious. Using recumbency as a proxy for loss of consciousness for CO2 euthanasia is justified because during recumbency induced by CO2 euthanasia rapid disruption of cortical function and alterations in brain waves occur,3 and mice are insensitive, having lost the righting reflex and toe pinch reaction.19 However, this assumption may not be applicable for mice euthanized by isoflurane given that sensitivity to handling remains for a short time after recumbency.19 The same behavioral time points were used to maintain comparability.
Table 4.
Total AUC (mean ± 1 SD) above baseline until death for various cardiovascular parameters (heart rate [HR], bpm; blood pressure [BP], mm Hg)
| 15% CO2 | 30% CO2 | 50% CO2 | 100% CO2 | Isoflurane | Pentobarbital–phenytoin | P | |
| Total HRa | 10,549 ± 5883 | 8569 ± 4060 | 4159 ± 1888b,c | 3354 ± 1892b,c | 8578 ± 3020 | 16,349 ± 5829 | <0.0001 |
| HR/sa | 51.7 ± 7.9 | 54.9 ± 26.0 | 41.8 ± 20.0 | 47.8 ± 27.0 | 35.6 ± 13.6 | 49.4 ± 15.6 | 0.0395 |
| Total SBPa | 1862 ± 1280c | 1816 ± 706c | 1315 ± 702c | 845 ± 319 | 771 ± 665 | 459 ± 395 | 0.0014 |
| SBP/sa | 9.0 ± 6.0c | 11.7 ± 5.2b,c | 12.7 ± 5.4b,c | 11.9 ± 4.4b,c | 3.1 ± 2.5 | 1.6 ± 1.7 | <0.0001 |
| Total DBPa | 2082 ± 1527c | 2091 ± 1023c | 1481 ± 859c | 931 ± 374 | 639 ± 447 | 556 ± 586 | <0.0001 |
| DBP/sa | 10.0 ± 6.9c | 13.5 ± 7.2b,c | 14.2 ± 7.1b,c | 13.1 ± 5.1b,c | 2.5 ± 1.7 | 2.0 ± 2.9 | <0.0001 |
| Total MBPa | 1891 ± 1339c | 1874 ± 831c | 1316 ± 741 | 851 ± 339 | 722 ± 544 | 516 ± 491 | 0.0034 |
| MBP/sa | 9.1 ± 6.1c | 12.1 ± 5.9b,c | 12.7 ± 5.9b,c | 12.0 ± 4.6b,c | 2.9 ± 2.0 | 1.8 ± 2.3 | <0.0001 |
DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure
CO2 data have been published previously.2
Data were transformed for analysis.
Value significantly different from that for isoflurane.
Value significantly different from that for pentobarbital–phenytoin.
There was a significant increase above baseline in all cardiovascular parameters during isoflurane or pentobarbital–phenytoin euthanasia (Figures 1 and 2). HR was significantly increased until ataxia for pentobarbital–phenytoin compared with all other methods (P < 0.0001 for all except 15% CO2 CRR [P = 0.0035] and 30% CO2 CRR [P = 0.0001]) and until nose down for pentobarbital–phenytoin compared with all other euthanasia methods (P < 0.0001, Tables 2 and 3). In addition, HR was increased until nose down for isoflurane compared with 50% and 100% CO2 CRR (P < 0.0001, Table 3). Furthermore, HR/s was increased for pentobarbital–phenytoin compared with all other methods until ataxia (P < 0.0001 for all except 100% CO2 CRR [P = 0.0004]) and nose down (P < 0.0001 for all) and for isoflurane compared with 15% CO2 CRR (P < 0.0001), 30% CO2 CRR (P < 0.0014), 50% CO2 CRR (P < 0.0001), and 100% CO2 CRR (P = 0.0074) until nose down (Table 3).
Figure 1.
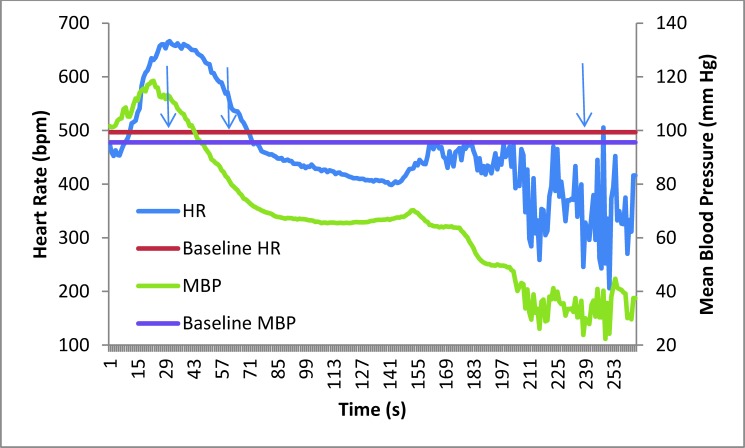
Mean heart rate (HR) and mean blood pressure (MBP) of mice (n = 12) at baseline and during pentobarbital–phenytoin euthanasia. Graphed are the baseline and average data for mice euthanized with pentobarbital–phenytoin. The arrows indicate the average times until ataxia, full recumbency, and death, respectively.
Figure 2.
Mean heart rate (HR) and mean blood pressure data (MBP) of mice (n = 10) at baseline and during isoflurane euthanasia. The arrows indicate the average times until ataxia, full recumbency, and death, respectively.
Table 2.
Total AUC (mean ± 1 SD) above baseline until ataxia for various cardiovascular parameters (heart rate [HR], bpm; blood pressure [BP], mm Hg)
| 15% CO2 | 30% CO2 | 50% CO2 | 100% CO2 | Isoflurane | Pentobarbital–phenytoin | P | |
| Total HRa | 4632 ± 3195 | 3104 ± 1606c | 1476 ± 566c | 2156 ± 783c | 2851 ± 2107c | 7656 ± 2654 | <0.0001 |
| HR/s | 58.4 ± 38.4c | 65.2 ± 39.6c | 46.1 ± 14.0c | 86.6 ± 23.5c | 83.3 ± 33.3c | 219.9 ± 62.2b | <0.0001 |
| Total SBP | 1262 ± 621b,c | 1131 ± 381b,c | 853 ± 308 | 728 ± 167 | 476 ± 334 | 322 ± 325 | <0.0001 |
| SBP/s | 16.0 ± 7.7 | 23.2 ± 8.5b | 27.8 ± 10.9c | 30.0 ± 7.2c | 16.2 ± 10.9 | 9.3 ± 8.4 | <0.0001 |
| Total DBPa | 1049 ± 517 | 879 ± 423 | 69 ± 302 | 54 ± 192 | 402 ± 255 | 391 ± 442 | 0.0133 |
| DBP/s | 13.3 ± 6.3 | 17.8 ± 8.8 | 22.7 ± 10.2 | 22.0 ± 7.9 | 13.9 ± 8.3 | 11.3 ± 11.3 | 0.0931 |
| Total MBPa | 1131 ± 559c | 986 ± 389c | 746 ± 297 | 636 ± 172 | 449 ± 308 | 362 ± 387 | 0.0057 |
| MBP/s | 14.4 ± 6.8 | 20.1 ± 8.4 | 24.4 ± 10.2c | 26.1 ± 6.7c | 15.5 ± 10.3 | 10.5 ± 10.0 | 0.0067 |
DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure
CO2 data have been published previously.2
Data were transformed for analysis.
Value significantly different from that for isoflurane.
Value significantly different from that for pentobarbital–phenytoin.
Table 3.
Total AUC (mean ± 1 SD) above baseline until nose down for various cardiovascular parameters (heart rate [HR], bpm; blood pressure [BP], mm Hg)
| 15% CO2 | 30% CO2 | 50% CO2 | 100% CO2 | Isoflurane | Pentobarbital–phenytoin | P | |
| Total HRa | 6079 ± 3746c | 4075 ± 2342c | 1835 ± 843b,c | 2078 ± 964b,c | 6365 ± 1834c | 16,787 ± 6607b | <0.0001 |
| HR/s | 57.7 ± 34.5b,c | 57.5 ± 32.4b,c | 40.0 ± 15.5b,c | 63.6 ± 25.4c | 104.1 ± 27.2c | 168.1 ± 51.5b | <0.0001 |
| Total SBPa | 1520 ± 955 | 1377 ± 716 | 1003 ± 438 | 744 ± 216 | 744 ± 596 | 423 ± 396 | 0.0148 |
| SBP/s | 13.4 ± 9.7 | 20.6 ± 10.1c | 25.3 ± 12.0b,c | 23.7 ± 7.2c | 11.8 ± 8.8 | 4.5 ± 4.3 | <0.0001 |
| Total DBPa | 1437 ± 907 | 1320 ± 732 | 1008 ± 559 | 717 ± 268 | 636 ± 448 | 519 ± 573 | 0.0316 |
| DBP/sa | 13.6 ± 8.3 | 18.4 ± 11.0c | 23.0 ± 11.0c | 21.8 ± 7.5c | 10.2 ± 6.5 | 5.7 ± 6.3 | 0.0021 |
| Total MBPa | 1466 ± 921 | 1350 ± 689 | 1007 ± 522 | 719 ± 235 | 712 ± 525 | 480 ± 485 | 0.0375 |
| MBP/sa | 13.9 ± 8.5 | 19.3 ± 10.2 | 23.2 ± 11.1c | 22.2 ± 7.0c | 11.4 ± 7.8 | 5.2 ± 5.3 | <0.001 |
DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure
CO2 data have been published previously.2
Data were transformed for analysis.
Value significantly different from that for isoflurane.
Value significantly different from that for pentobarbital–phenytoin.
In contrast to the HR changes, many of the DBP, MBP, and SBP measurements were significantly lower for pentobarbital–phenytoin and isoflurane euthanasia compared with CO2 euthanasia (Tables 2 through 4). For example, DBP/s until nose down was significantly lower for pentobarbital–phenytoin compared with 50% and 100% CO2 CRR (P = 0.0076 and P = 0.0028, respectively, Table 3). Significant decreases in MBP until ataxia were present between pentobarbital–phenytoin and 15% and 30% CO2 CRR (P = 0.0078 and P = 0.0113, respectively, Table 2). Further significant decreases in MBP/s until nose down were present between pentobarbital–phenytoin and 30%, 50%, and 100% CO2 CRR (P = 0.0049, P = 0.0020, and P = 0.0008, respectively, Tables 2 and 3). Significant decreases in SBP until ataxia were found for both pentobarbital–phenytoin and isoflurane compared with 15% and 30% CO2 CRR (P < 0.0001 and P = 0.0002, respectively for pentobarbital–phenytoin; P = 0.0004 and P = 0.0048, respectively for isoflurane, Table 2). SBP/s was significantly lower for pentobarbital–phenytoin than 50% and 100% CO2 CRR at ataxia (P = 0.0005) and 30%, 50%, and 100% at nose down (P = 0.0018, P < 0.0001, and P = 0.0003, respectively). In addition, SBP/s was significantly lower for isoflurane compared with 50% CO2 CRR at nose down (P = 0.0116, Tables 2 and 3).
Analysis of the peak values for HR and BP showed that the mean peak HR for pentobarbital–phenytoin was significantly higher than that for 15%, 30%, and 50% CO2 CRR (P = 0.0081, P = 0.0010, and P < 0.0001, respectively, Table 5). Analysis of the HR data showed that isoflurane had a median time to peak HR that was longer than the median time to peak for 50% CO2 CRR (P = 0.0059, Table 6). Peak BP did not differ between euthanasia methods (Table 5). Pentobarbital–phenytoin had a significantly shorter median time to peak DBP than did 15% and 30% CO2 CRR (P < 0.0001 andP < 0.0006, respectively) and a shorter median time to peak MBP than did 15% CO2 CRR (P = 0.0033). Isoflurane had a significantly shorter median time to peak SBP than did 15% CO2 CRR (P = 0.0059), a shorter median time to peak DBP than did 15% and 30% CO2 CRR (P = 0.0002 and P = 0.0069, respectively), and a shorter median time to peak MBP than did 15% CO2 CRR (P = 0.0016, Table 6).
Table 5.
Peak (mean ± 1 SD) cardiovascular (heart rate [HR], bpm; blood pressure [BP], mm Hg) and activity values
| 15% | 30% | 50% | 100% | Isoflurane | Pentobarbital–phenytoin | P | |
| HR | 678 ± 71.5b | 661 ± 43.2b | 640 ± 39.5b | 709 ± 93.7 | 708 ± 75.8 | 772 ± 45.6 | <0.0001 |
| SBP | 140.3 ± 14.2 | 150.6 ± 15.3 | 145.5 ± 12.8 | 151.7 ± 6.3 | 141.9 ± 11.9 | 134.4 ± 20.5 | 0.0360 |
| DBP | 116.1 ± 10.5 | 117.5 ± 10.0 | 115.4 ± 7.8 | 115.6 ± 4.0 | 109.4 ± 8.4 | 108.6 ± 22.2 | 0.3462 |
| MBP | 125.7 ± 11.0 | 130.9 ± 12.2 | 127.5 ± 11.0 | 130.6 ± 4.3 | 125.1 ± 9.6 | 121.4 ± 21.1 | 0.4391 |
| Activity | 129.6 ± 80.0 | 117.7 ± 51.1b | 68.8 ± 32.7a,b | 55.3 ± 23.1a,b | 142.0 ± 32.3 | 187.2 ± 39.6 | <0.0001 |
DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure
CO2 data have been published previously.2
Value significantly different from that for isoflurane.
Value significantly different from that for pentobarbital–phenytoin.
Table 6.
Time (s; mean ± 1 SD) until peak cardiovascular values
| 15% CO2 | 30% CO2 | 50% CO2 | 100% CO2 | Isoflurane | Pentobarbital– phenytoin | P | |
| HR | 52.0 ± 48.1b | 25.1 ± 20.3a,b | 7.4 ± 6.8a | 22.7 ± 25.8a,b | 42.7 ± 43.0 | 21.2 ± 16.8 | <0.0001 |
| SBP | 51.5 ± 21.4a,b | 37.4 ± 22.5a,b | 17.6 ± 6.7 | 17.5 ± 4.3 | 16.9 ± 7.2 | 19.8 ± 19.7 | <0.0001 |
| DBP | 57.2 ± 27.5a,b | 52.5 ± 20.6a,b | 31.2 ± 13.8 | 23.1 ± 6.6 | 17.9 ± 6.0 | 11.8 ± 12.1 | <0.0001 |
| MBP | 53.0 ± 24.1a,b | 37.0 ± 23.3a,b | 20.7 ± 9.4 | 17.2 ± 5.6 | 17.4 ± 6.5 | 17.7 ± 19.2 | <0.0001 |
DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure
CO2 data have been published previously.2
Significantly different from isoflurane.
Significantly different from pentobarbital–phenytoin.
Activity.
Activity was measured as the amount of distance traveled, and the amount of time that the mice were moving. The total distance traveled (activity) until ataxia was significantly greater for pentobarbital–phenytoin than for 30%, 50%, and 100% CO2 CRR (P = 0.0021, P < 0.0001, and P = 0.0004, respectively) and for isoflurane compared with 50% and 100% CO2 CRR (P = 0.0004 and P = 0.0006, respectively, Table 7, Figures 3 and 4). The median activity level per second until ataxia was significantly higher for pentobarbital–phenytoin than for all CO2 levels (P = 0.0011 for 15%, P = 0.0006 for 30%, P = 0.0002 for 50%, and P = 0.001 for 100% CO2 CRR) and higher for isoflurane compared with 30%, 50%, and 100% CO2 CRR (P = 0.0012, P = 0.0002, and P = 0.0013, respectively, Table 7).
Table 7.
Distance traveled (activity [arbitrary units]; mean ± 1 SD) until ataxia and nose down
| 15% CO2 | 30% CO2 | 50% CO2 | 100% CO2 | Isoflurane | Pentobarbital– phenytoin | P | |
| Ataxia activity | 458 ± 606 | 334 ± 96.7b | 168.5 ± 157.4a,b | 148.4 ± 81.3a,b | 723 ± 734 | 766 ± 411 | <0.0001 |
| Ataxia activity/s | 6.5 ± 9.8b | 7.0 ± 5.7ab | 5.3 ± 4.4a,b | 6.2 ± 3.5a,b | 24.3 ± 27.8 | 22.7 ± 10.8 | <0.0001 |
| Nose down activity | 627 ± 673b | 574 ± 382b | 273 ± 185.2a,b | 181.4 ± 109.8a,b | 835 ± 267 | 2043 ± 674 | <0.0001 |
| Nose down activity/s | 6.1 ± 6.6b | 8.4 ± 5.9b | 5.7 ± 3.4a,b | 5.7 ± 3.4a,b | 14.0 ± 4.8 | 22.0 ± 9.9 | <0.0001 |
CO2 data have been published previously.2
Significantly different from isoflurane.
Significantly different from pentobarbital–phenytoin.
Figure 3.
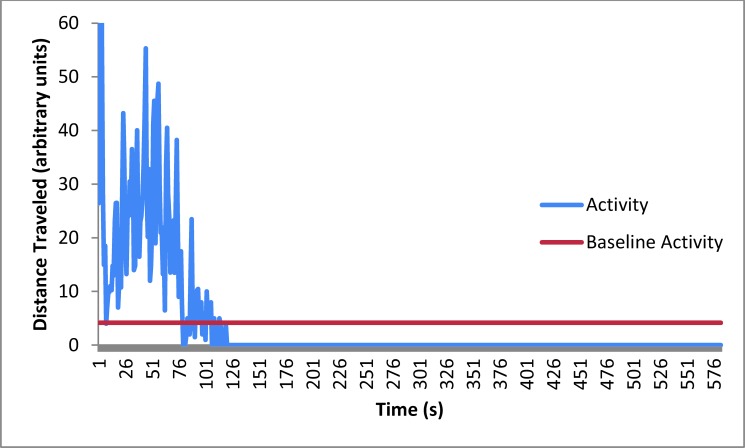
Mean activity of mice (n = 12) at baseline and during pentobarbital–phenytoin euthanasia. The amount of time the mice were active, the distance traveled (how high the peaks are), and percentage of each compared with the total time the mice were active are presented in the Results section.
Figure 4.
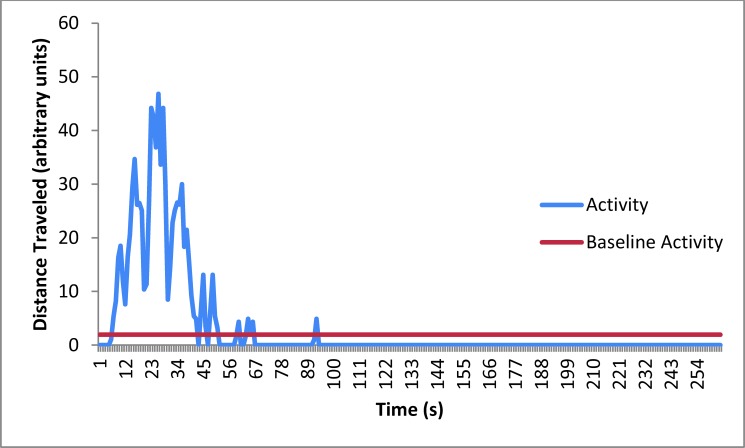
Mean activity of mice (n = 10) at baseline and during isoflurane euthanasia. The amount of time during which the mice were active, the distance they traveled (that is, peak height), and the percentage of each compared with the total time that the mice were active are presented in the Results section.
The amount of time the mouse moved until ataxia for pentobarbital–phenytoin was significantly higher compared with the 50% and 100% CO2 CRR (P = 0.0050 and P = 0.0059, respectively, Table 8). The activity per second until ataxia for pentobarbital–phenytoin was significantly higher than all 4 CO2 CRR (P = 0.0144 for 15%, P = 0.0151 for 30%, P = 0.0005 for 50%, and P = 0.0194 for 100% CO2 CRR) and for isoflurane compared with the 15%, 30%, and 50% CO2 CRR (P = 0.0144, P = 0.0151, and P = 0.0077, respectively, Table 8).
Table 8.
Amount of time (s; mean ± 1 SD) that mice were active during euthanasia
| 15% CO2 | 30% CO2 | 50% CO2 | 100% CO2 | Isoflurane | Pentobarbital–phenytoin | P | |
| Activity time until ataxiaa | 10.4 ± 10.2 | 6.8 ± 6.1 | 4.0 ± 3.6c | 4.0 ± 2.2c | 10.2 ± 2.1 | 13.0 ± 7.8 | 0.0006 |
| Activity time until ataxia/s | 0.14 ± 0.16b,c | 0.14 ± 0.11b,c | 0.13 ± 0.11b,c | 0.17 ± 0.10c | 0.34 ± 0.08 | 0.38 ± 0.18 | <0.0001 |
| Activity time until nose downa | 12.7 ± 11.4c | 10.9 ± 7.0c | 6.9 ± 4.4b,c | 4.7 ± 3.1b,c | 15.7 ± 5.1 | 35.8 ± 10.6 | <0.0001 |
| Activity time until nose down/s | 0.12 ± 0.11c | 0.16 ± 0.11c | 0.15 ± 0.09b,c | 0.15 ± 0.09b,c | 0.27 ± 0.09 | 0.37 ± 0.13 | <0.0001 |
CO2 data have been published previously.2
Data were transformed for analysis.
Value significantly different from that for isoflurane.
Value significantly different from that for pentobarbital–phenytoin.
The median activity level (distance moved) and amount of activity time until nose down was significantly higher for pentobarbital–phenytoin than for any other method of euthanasia (for distance moved: P = 0.0003 compared with 15% CO2 CRR, P = 0.0002 compared with isoflurane, and P < 0.0001 compared with 30%, 50%, and 100% CO2 CRR; for activity time: P = 0.0247 compared with isoflurane and P < 0.0001 compared with 15%, 30%, 50%, and 100% CO2 CRR) and for isoflurane compared with 50% and 100% CO2 CRR (for distance moved: P = 0.0002 for each; for activity time: P = 0.0130 and P = 0.0011, respectively, Table 8). The activity per second and amount of time moving per second until nose down were significantly higher for pentobarbital–phenytoin than for 15%, 30%, 50%, and 100% CO2 CRR (for distance moved: P = 0.0003, P = 0.0008, P < 0.001, and P = 0.0001, respectively; for activity time: P < 0.0001 for all). For isoflurane, the median activity level (distance moved) until nose down was significantly higher compared with that for 50% and 100% CO2 CRR (P = 0.0006 and P = 0.0012, respectively), and the mean activity time for isoflurane was higher than that for 15% CO2 CRR (P = 0.0181, Table 8).
The median peak activity for pentobarbital–phenytoin was significantly higher than the median peak activity for 30%, 50%, or 100% CO2 CRR (P = 0.0015, P < 0.0001, and P < 0.0001, respectively, Table 5). The median peak activity for isoflurane was significantly higher than the median peak activity for 50% or 100% CO2 CRR (P = 0.0003 and P < 0.0001, respectively).
Behavior.
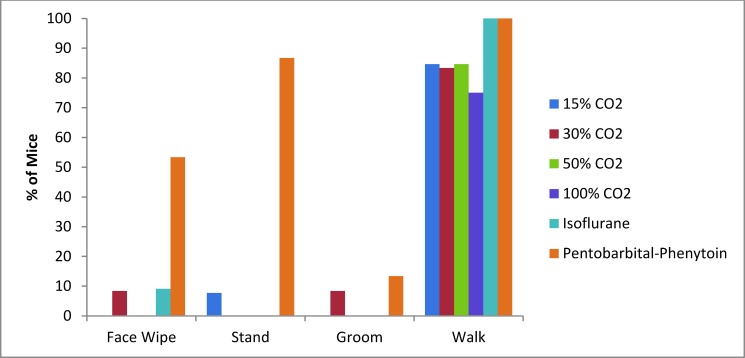
Mice euthanized with pentobarbital–phenytoin were more likely to wipe their face and to stand compared with all other euthanasia techniques (P < 0.0001, Figure 5). In addition, mice euthanized with pentobarbital–phenytoin displayed what has been described as an escape response,19 which was characterized as a paddling motion of the hindlegs that often pushed the mouse forward. This behavior occurred after the mice were recumbent, but nose-down data were recorded only after this movement stopped. There was no external motivation to stimulate this movement. There were no significant differences in incidence of grooming or walking between euthanasia methods (P = 0.386 and P = 0.241 respectively).
Figure 5.
Percentages of mice exhibiting behavioral responses to various euthanasia methods.
Histology.
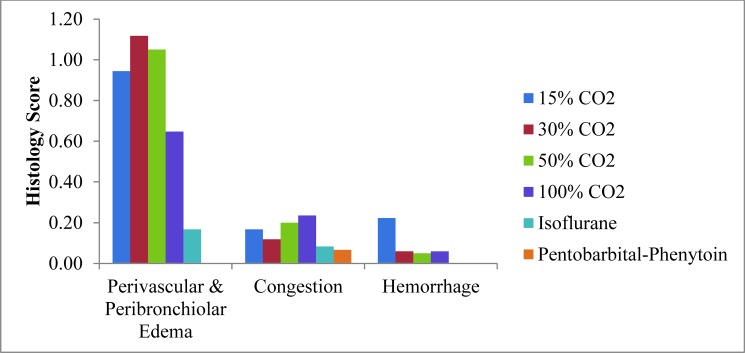
Histologic damage characterized by mild to moderate perivascular and peribronchiolar edema has been reported in CO2-euthanized mice.2 In contrast, mice euthanized with isoflurane followed by CO2 or euthanized with pentobarbital–phenytoin had no to mild lesions (Figure 6). The incidence of perivascular and peribronchiolar edema was significantly lower after pentobarbital–phenytoin euthanasia compared with all CO2 euthanasia techniques (P ≤ 0.0061) and after isoflurane compared with 50% CO2 CRR (P = 0.0073).
Figure 6.
Mean scores of lung histologic lesions of mice in response to various euthanasia methods.
Stress hormone.
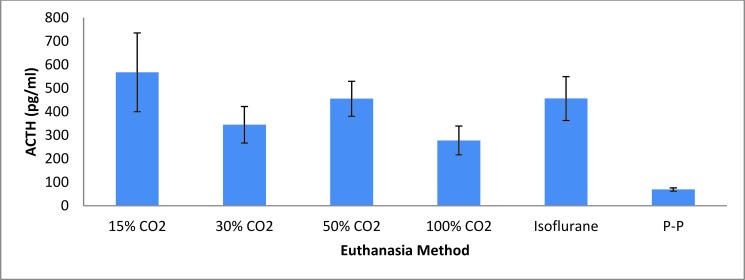
The plasma ACTH level of mice euthanized by pentobarbital–phenytoin was significantly lower than that for all other euthanasia methods (P = 0.0006, Figure 7).
Figure 7.
Stress hormone (ACTH) response (mean ± SEM) of mice to various euthanasia methods. P-P, pentobarbital-phenytoin.
Discussion
The AVMA Panel on Euthanasia provides guidelines for appropriate euthanasia techniques in all species.13 These methods are categorized into chemical (subdivided into inhalant and injectable) and physical techniques. The most common chemical methods used to euthanize mice are CO2 exposure, inhalant anesthetic overdose such as isoflurane, and pentobarbital injection using a euthanasia solution such as pentobarbital–phenytoin. To address whether the use of isoflurane prior to CO2 is an improvement over CO2 alone for euthanasia, we examined the physiologic, stress hormone, lung histology, and behavioral responses of mice to these euthanasia techniques. In addition, the gold standard of euthanasia methods, pentobarbital injection, was included to allow comparison with both inhalant procedures. Note that a comparison between CO2 CRR was the focus of a previous study2 and that the intent of the current study was to compare the CO2 response with those to isoflurane and pentobarbital–phenytoin. Significant differences occurred between euthanasia techniques in all areas studied.
One of the major focuses of the current study was to analyze cardiovascular parameters to determine differences in the stress response associated with the euthanasia technique. All 3 euthanasia methods lead to increases in HR and BP. Whereas peak BP did not differ between euthanasia methods, the HR peak was higher for pentobarbital–phenytoin compared with several of the CO2 CRR, and there was no difference between isoflurane and any of the CO2 CRR. The link of HR values to pain intensity is tenuous, and interpretation of these results might be difficult.2,8,14,28 Our interpretation of the peak value data suggests that the stress or pain associated with the inhalant methods studied is not higher than that with the injectable method, but further analysis is required to make a conclusive determination. In addition, the difference in time to peak values correlates well with the alterations in behavior that we observed visually. The earlier peak in values for the pentobarbital–phenytoin group is consistent with a response to stress or pain associated with restraint and intraperitoneal injection, whereas the stress or pain associated with isoflurane and CO2 came later, as the gases started to reach effective levels.
Interestingly, the AUC for HR and the average HR for pentobarbital–phenytoin- and isoflurane-euthanized mice were increased compared with those for some of the CO2 CRR. In contrast, the BP AUC for both pentobarbital–phenytoin and isoflurane were decreased compared with those for the CO2 euthanasia techniques. It is difficult to interpret the implications of these alterations in the cardiovascular data given the possible effects of the anesthetic agent on the cardiovascular system. Intraperitoneal injection of pentobarbital reduces the HR and BP of mice during anesthesia.10,34 Likewise, isoflurane inhalation depresses HR and BP.16,23 In contrast, hypercapnia causes increases in both HR and BP.22 As a result, a second peak in HR occurred in mice euthanized with CO2,2 whereas HR showed a steady decline in association with pentobarbital–phenytoin euthanasia and, to a lesser extent, isoflurane euthanasia. The increase in HR response and erratic recordings in the isoflurane-euthanized mice after 150 s of exposure to isoflurane (Figure 2) corresponded to when the CO2 was turned on and likely reflects the stimulation of the HR associated with hypercapnia. Despite these possible effects of the agents on the cardiovascular system, the cardiovascular AUC results do not reveal any significant differences between euthanasia procedures and highlight that all of the procedures cause cardiovascular changes consistent with a stress-like response in adult male C57BL/6NTac mice.
The activity levels of mice euthanized by isoflurane or pentobarbital–phenytoin were higher than those of mice euthanized by many of the CO2 CRR. Although mice are more likely to avoid CO2 than isoflurane by moving to a different chamber with normal air,12,15 there was no apparent relationship between avoidance behavior and activity.12 Interestingly, the mice euthanized with pentobarbital–phenytoin had the most behavioral alterations, although whether the observed behaviors (face wiping and standing) were related to stress, distress, or pain is unclear. Therefore, our data provide no evidence that mouse activity or behaviors were indicative of more distress due to CO2 euthanasia compared with isoflurane or pentobarbital–phenytoin euthanasia.
In contrast to the activity data, lung histology revealed a clear benefit to using pentobarbital–phenytoin or isoflurane compared with CO2. The lungs of CO2-euthanized mice had increased perivascular and peribronchiolar edema, perhaps due to the severe gasping that occurs during CO2 euthanasia. Whether lung changes are painful is unknown, because it is unclear whether the changes occur prior to loss of consciousness, when pain perception is possible. The use of isoflurane prior to CO2 during euthanasia is a valid refinement for preventing this pathologic change in the lungs.
One of our most interesting findings is the observation that mice euthanized with pentobarbital–phenytoin had significantly lower plasma ACTH levels than did those euthanized with isoflurane or CO2; in addition, ACTH levels did not differ between mice euthanized with isoflurane and CO2. These findings indicate that both isoflurane and CO2 are stressors that strongly activate the hypothalamic–pituitary–adrenal axis, whereas pentobarbital–phenytoin does not, consistent with the AVMA's acceptance of pentobarbital–phenytoin as an acceptable euthanasia agent.13 In addition, our current results are consistent with a study in ponies that showed a marked stress response (ACTH and cortisol) in response to halothane anesthesia but not pentobarbitone anesthesia.27 A few studies include an analysis of isoflurane, CO2, or pentobarbital on ACTH or corticosterone levels in mice.5,29,30,33 Corticosterone results are often limited in practical interpretation because rodent studies indicate that approximately 4 min are required for corticosterone levels to increase in response to a stressful event.7,9,25 Therefore, studies examining corticosterone levels as a marker of stress prior to that time point may not be valid. In a previous study, we found most of the CO2 euthanasia events were less than 4 min in duration.2 However, both the current and previous studies indicate that both isoflurane and pentobarbital euthanasia methods require more than 4 min.5,29,30,33 Understanding this limitation, we reviewed a study analyzing the effect of pentobarbital on acute stress in Sprague–Dawley rats,33 in which corticosterone in the pentobarbital-treated rats was significantly increased at 5 min after treatment, but this effect was attributed to the pentobarbital-induced response to the injection, given that a control injection of saline led to a similar increase in corticosterone.33 Clearly the handling procedure and injection process themselves are able to induce a stress response by activating the hypothalamic–pituitary–adrenal axis.
Another study that examined plasma ACTH and corticosterone in rats euthanized by decapitation after preanesthesia for 2 min with CO2, or preanesthesia for 5 min after injection of 150 mg/kg pentobarbital sodium, or 10 s after handling without anesthesia found significant increases in ACTH in CO2- (13-fold increase) and pentobarbital- (2-fold increase) anesthetized rats over decapitation alone.30 Elevations in corticosterone were seen only in the pentobarbital group, consistent with the claim that 4 min are needed before elevations can be seen.30 We observed a similar, pronounced increase in the ACTH level of CO2-euthanized mice, with only mild increases in the pentobarbital–phenytoin-euthanized mice. In another publication ACTH levels were examined in 2 arms of a study,26 in which handling had little effect on ACTH in one arm, whereas the other showed a marked handling-associated response.26 We did not see a strong ACTH response due to the handling and injection events during pentobarbital–phenytoin injection, but HR and BP increased due to these procedures.
There were several limitations to the study. We used only male mice of a single strain. Although we do not expect sex- or strain-associated differences in the stress response, additional studies should be done in female mice and in additional strains for confirmation. In addition, all mice were individually housed; different results might occur due to cohousing of mice or coeuthanizing of mice. As noted earlier, the interpretation of the cardiovascular data is complicated by the pharmacologic effect of each of the agents selected for euthanasia. Pentobarbital–phenytoin injection led to high variability in the time until death; this result perhaps was due to inadvertent inaccurate injection into the abdominal cavity or to different absorption rates of the 2 drugs, leading to high biologic variability. Two complications were potential confounders for the isoflurane euthanasia. Rats have been shown to be more averse to a second exposure to isoflurane than to a first exposure.32 If mice (like rats) are more reactive to a second exposure to isoflurane, the surgical use of isoflurane during insertion of the telemetry units may have presensitized the mice to isoflurane euthanasia. Using an alternative anesthesia method during telemetry implantation surgery may help to elucidate this possibility. Furthermore, we examined only a single flow rate of oxygen as the carrier for isoflurane. Higher CRR might yield different results, likely leading to faster anesthesia of the mice; this hypothesis should be explored in future studies.
In conclusion, we obtained sporadic evidence that pentobarbital–phenytoin euthanasia may be less stressful than isoflurane and CO2 euthanasia in mice. However this evidence is based on the ACTH results, because none of the cardiovascular, behavioral, or activity data revealed significant improvements when pentobarbital–phenytoin euthanasia was used. In addition, we did not obtain any consistent differences between the isoflurane and CO2 euthanasia methods to substantiate the claim that, compared with CO2, isoflurane reduces euthanasia-associated stress. Therefore, we conclude that use of CO2 with or without isoflurane anesthesia is an acceptable euthanasia method for use in mice. Pathologic alterations in the lungs were most severe with CO2 euthanasia, suggesting that alternative euthanasia techniques may be better suited when studies rely on analysis of the lungs.
Acknowledgments
The project described was supported by the Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
References
- 1.Antognini JF, Barter L, Carstens E. 2005. Movement as an index of anesthetic depth in humans and experimental animals. Comp Med 55:413–418. [PubMed] [Google Scholar]
- 2.Boivin GP, Bottomley MA, Dudley ES, Schiml PA, Wyatt CN, Grobe N. 2016. Physiologic, behavioral, and histologic response to different CO2 chamber replacement rates in C57BL/6N male mice. J Am Assoc Lab Anim Sci 55:451–461. [PMC free article] [PubMed] [Google Scholar]
- 3.Cartner SC, Barlow SC, Ness TJ. 2007. Loss of cortical function in mice after decapitation, cervical dislocation, potassium chloride injection, and CO2 inhalation. Comp Med 57:570–573. [PubMed] [Google Scholar]
- 4.Canadian Council on Animal Care. [Internet] 2010. Canadian Council on: euthanasia of animals used in science. [Cited 05 December 2016]. Available at: http://www.ccac.ca/Documents/Standards/Guidelines/Euthanasia.pdf
- 5.Chesnokova V, Auernhammer CJ, Melmed S. 1998. Murine leukemia inhibitory factor gene disruption attenuates the hypothalamo-pituitary-adrenal axis stress response. Endocrinology 139:2209–2216. [DOI] [PubMed] [Google Scholar]
- 6.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161. [DOI] [PubMed] [Google Scholar]
- 7.Coover GD, Heybach JP, Lenz J, Miller JF. 1979. Corticosterone “basal levels” and response to ether anesthesia in rats on a water deprivation regimen. Physiol Behav 22:653–656. [DOI] [PubMed] [Google Scholar]
- 8.Cowen R, Stasiowska MK, Laycock H, Bantel C. 2015. Assessing pain objectively: the use of physiologic markers. Anaesthesia. 70:828–847. [DOI] [PubMed] [Google Scholar]
- 9.Davidson JM, Jones LE, Levine S. 1968. Feedback regulation of adrenocorticotropin secretion in “basal” and “stress” conditions: acute and chronic effects of intrahypothalamic corticoid implantation. Endocrinology 82:655–663. [DOI] [PubMed] [Google Scholar]
- 10.Erhardt W, Hebstedt A, Aschenbrenner G, Pichotka B, Blumel G. 1984. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res Exp Med (Berl) 184:159–169. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins P, Playle L, Golledge H, Leach M, Banzett R, Coenen A, Cooper J, Danneman P, Flecknell P, Kirkden R, Niel L, Raj M. [Internet]. 2006. Newcastle Consensus Meeting on Carbon Dioxide Euthanasia of Laboratory Animals. London (UK): National Centre for the Replacement, Refinement and Reduction of Animals in Science.[Cited 05 December 2016]. Available at: https://www.nc3rs.org.uk/sites/default/files/documents/Events/First%20Newcastle%20consensus%20meeting%20report.pdf.
- 12.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Aversion to gaseous euthanasia agents in rats and mice. Comp Med 52:249–257. [PubMed] [Google Scholar]
- 13.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. 2013AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 05 December 2016]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
- 14.Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF. 2014. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur J Pain 18:301–314. [DOI] [PubMed] [Google Scholar]
- 15.Makowska IJ, Weary DM. 2009. Rat aversion to induction with inhalant anaesthetics. Appl Anim Behav Sci 119:229–235. [Google Scholar]
- 16.Merin RG. 1993. Are differences in cardiopulmonary effects among the inhalation agents clinically important? Proc ASA Refresher Course Lectures, Washington DC: Lecture; 226:1-6. [Google Scholar]
- 17.Mikeska JA, Klemm WR. 1975. EEG evaluation of humaneness of asphyxia and decapitation euthanasia of the laboratory rat. Lab Anim Sci 25:175–179. [PubMed] [Google Scholar]
- 18.Moody CM, Chua B, Weary DM. 2014. The effect of carbon dioxide flow rate on the euthanasia of laboratory mice. Lab Anim 48:298–304. [DOI] [PubMed] [Google Scholar]
- 19.Moody CM, Maakowska IJ, Weary DM. 2015. Testing 3 measures of mouse insensibility following induction with isoflurane or carbon dioxide gas for a more humane euthanasia. Appl Anim Behav Sci 163:183–187. [Google Scholar]
- 20.Niel L, Stewart SA, Weary DM. 2008. Effect of flow rate on aversion to gradual-fill carbon dioxide exposure in rats. Appl Anim Behav Sci 109:77–84. [Google Scholar]
- 21.Niel L, Weary DM. 2006. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl Anim Behav Sci 100:295–308. [Google Scholar]
- 22.O'Regan RG, Majcherczyk S. 1982. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol 100:23–40. [DOI] [PubMed] [Google Scholar]
- 23.Pachon RE, Scharf BA, Vatner DE, Vatner SF. 2015. Best anesthetics for assessing left ventricular systolic function by echocardiography in mice. Am J Physiol Heart Circ Physiol 308:H1525–H1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rätsep MT, Barrette VF, Winterborn A, Adams MA, Croy BA. 2013. Hemodynamic and behavioral differences after administration of meloxicam, buprenorphine, or tramadol as analgesics for telemeter implantation in mice. J Am Assoc Lab Anim Sci 52:560–566. [PMC free article] [PubMed] [Google Scholar]
- 25.Riley V, Fitzmaurice MA, Spackman DH. 1981. Psychoneuroimmunologic factors in neoplasia: Studies in animals.p 31–102. In: Ader R. ed. Psychoneuroimmunology. New York (NY): Academic Press. [Google Scholar]
- 26.Roper JA, Craighead M, O'Carroll A-M, Lolait SJ. 2010. Attenuated stress response to acute restraint and forced swimming stress in arginine vasopressin 1b receptor subtype (Avpr1b)receptor knockout mice and wild-type mice treated with a novel Avpr1b receptor antagonist. J Neuroendocrinol 22:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor PM. 1990. The stress response to anaesthesia in ponies: barbiturate anaesthesia. Equine Vet J 22:307–312. [DOI] [PubMed] [Google Scholar]
- 28.Tousignant-Laflamme Y, Rainville P, Marchand S. 2005. Establishing a link between heart rate and pain in healthy subjects: a gender effect. J Pain 6:341–347. [DOI] [PubMed] [Google Scholar]
- 29.Vachon P, Moreau JP. 2001. Serum corticosterone and blood glucose in rats after 2 jugular vein blood sampling methods: comparison of the stress response. Contemp Top Lab Anim Sci 40:22–24. [PubMed] [Google Scholar]
- 30.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. 2005. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–E828. [DOI] [PubMed] [Google Scholar]
- 31.Vanderwolf CH, Buzsaki G, Cain DP, Cooley RK, Roberson B. 1988. Neocortical and hippocampal electrical activity following decapitation in the rat. Brain Res 451:340–344. [DOI] [PubMed] [Google Scholar]
- 32.Wong D, Makowska IJ, Weary DM. 2012. Rat aversion to isoflurane versus carbon dioxide. Biol Lett 9:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu XY, Hu YT, Guo L, Lu J, Zhu QB, Yu E, Wu JL, Shi LG, Hang ML, Bao AM. 2015. Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol Behav 145:118–121. [DOI] [PubMed] [Google Scholar]
- 34.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. 1999. Echocariographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol 277:H1967–H1974. [DOI] [PubMed] [Google Scholar]