Abstract
The unique biologic characteristics of naked mole-rats (NMR, Heterocephalus glaber) include longevity, cancer resistance, hypoxia tolerance, and pain insensitivity, making NMR an attractive model for biomedical research on aging, cancer, and neurobiology. However, breeding and rearing NMR in captivity is challenging. Here, we report a method for breeding NMR by using a closed-colony mating system. We selected sexually mature male and female NMR from different natal colonies and mated them 1:1. The 5 original colonies had an annual parity of 3.20 ± 0.84 (mean ± 1 SD), with 38.80 ± 9.50 pups born, 33.80 ± 8.32 pups weaned, and a survival rate of 87.19% ± 6.09% after weaning. The average annual parity of 22 N1 pairs (established from the progeny of the 5 original pairs) was 3.09 ± 0.81, with 34.86 ± 10.66 total pups born during the year, 30.14 ± 10.23 pups weaned, and a survival rate after weaning of 85.51% ± 6.60%. The average annual parity of 29 N2 pairs (that is, offspring of N1 pairs) was 3.04 ± 0.87, with 33.69 ± 11.42 pups born, 28.17 ± 10.43 pups weaned, and a survival rate after weaning of 83.66% ± 10.75%. None of these measures differed among the 3 generations, with average reproductive success exceeding 70% for each. In addition, the reproduction and growth of the N1 and N2 generations was similar to the original colonies. Our breeding method remarkably increases the production of NMR, thus representing a great potential to promote experimental NMR research and its applications.
Abbreviations: NMR, naked mole-rat; N1 generation, progeny from the original colonies; N2 generation, progeny from the N1 generation
Naked mole-rats (NMR, Heterocephalus glaber) are a unique species of rodents that demonstrates high longevity,1,2 tumor resistance,22 hypoxia tolerance,21,23 and pain insensitivity.24 These features make NMR widely used mammalian models for biomedical research in aging,6,13,14 cancer,20,28 neurobiology,25,26 and other fields. However, breeding and rearing NMR in captivity is challenging.
NMR are a true eusocial species:16,18,19 only a single female (the queen) and 1 to 3 breeding males reproduce;16,17 the remaining colony members of both sexes are reproductively suppressed, although not sterile, and function as workers.7-10 The gestation of wild NMR is about 70 d. The average litter is 11 pups and typically ranges from 3 to 12 pups in size but may be as large as 28. In the wild, NMR usually breed only once each year, if the litter survives. In captivity, NMR give birth 2 to 4 times annually, and the average litter size is 7 to 13.27 This low reproductive efficiency of NMR limits their use in research applications. Although both nonbreeding female and male NMR in captive and wild colonies are reported to rapidly become reproductively active when socially suppressive cues are removed,5,7 previous studies assessed parameters such as changes in pituitary gonadotrophin secretion, luteinizing hormone concentrations, and physical contact.4,7,9,11,12 Whether these ‘reversed’ animals can successfully mate, breed, and give birth to offspring remains unknown.
The aim of the current study was to assess the reproductive performance of NMR in a novel closed-colony mating system under laboratory conditions.
Case Report
Animals and housing.
This study was performed in the animal research facility of the Second Military Medical University (Shanghai, China). The original NMR colonies were purchased from the Department of Zoology of the University of Cape Town (Cape Town, South Africa). All animal handling and study procedures complied with the current Chinese regulation,GB14925-2010: Laboratory animal requirements of environment and housing facilities (Chinese version). All NMR were maintained in accordance with the guidelines established by the IACUC of the Second Military Medical University (protocol no. 20120016). Animals were housed in clear plastic cages containing wood-shaving bedding. The housing unit (Chinese patent CN 201320608562) consisted of activity chambers (32 cm × 50 cm × 20 cm; divided into 4 interconnecting runways), chambers for feeding and resting (30 cm × 50 cm × 20 cm), and small chambers (18 cm × 50 cm × 20 cm) for defecation; various configurations of these chambers were assembled according to the number of animals. Cages were cleaned and sterilized once every 1 to 2 wk. Housing room conditions were maintained at 29 ± 2 °C, with a relative humidity of 40% to 70% and a photoperiod comprising 2 to 4 h of light and 18 to 22 h of dark. Animals received apples, potatoes, sweet potatoes, pumpkins, or cucumbers in the morning and afternoon; additional drinking water was not provided.
Breeding.
The average size (± 1 SD) of the 5 original colonies was 11.0 ± 1.6 NMR. Each colony was housed in separate containers and allowed to breed naturally (gestation period, 68 to 74 d) to produce offspring (N1 generation). At this point, we introduced the closed-colony mating system. Once they were fully weaned (age, 1 to 2 mo), all N1 NMR (females and males) were removed from their natal colony and placed into a new cage; at the age of 5 mo, female and male juveniles were housed as single-sex groups by natal colony. When sexually mature (12 mo for males and 10 mo for females), male and female N1 NMR from different natal colonies were mated at a 1:1 ratio, and each pair was placed in a new cage. Pairs were established according to the animal's body weight (that is, NMR of similar size were paired) and reproductive history of its parents. When male and female NMR were paired initially, they were observed continuously for at least 30 min until it was clear that both animals had accepted each other. When aggressive behaviors were observed, such as shoving, biting, and tugging,15 the NMR pair was separated and the 2 animals placed with other potential mates until compatible pairs were formed. The offspring of the N1 pairs (that is, the N2 generation) were themselves paired and bred in the same way as the N1 animals.
For all 3 populations (original, N1, and N2 colonies), parity, litter size, number of pups weaned, and survival rate after weaning were evaluated. Data are presented as mean ± 1 SD. One-way repeated-measure ANOVA (Excel, Microsoft, Redmond, WA) was used to determine differences between values; differences were considered statistically significant at a P value of 0.05 or less.
Reproductive performance of the original NMR colonies.
Of the 7 NMR colonies purchased from the University of Cape Town, 5 succeeded in breeding, for a reproductive success rate of 71.43%. The reproductive performance of the 5 original NMR colonies is summarized in Table 1. The average parity of the 5 original colonies (mean ± 1 SD) was 3.20 ± 0.84 litters per year, with 38.80 ± 9.50 total pups born and 33.80 ± 8.32 pups weaned annually. The average survival rate after weaning was 87.19% ± 6.09%.
Table 1.
Annual reproductive performance of the original NMR colonies
| No. of |
||||
| Colony no. | Litters | Pups born | Pups weaned | Survival rate after weaning (%) |
| 1 | 2 | 30 | 24 | 80.00 |
| 2 | 3 | 35 | 30 | 85.71 |
| 3 | 3 | 31 | 30 | 96.77 |
| 4 | 4 | 49 | 42 | 85.71 |
| 5 | 4 | 49 | 43 | 87.76 |
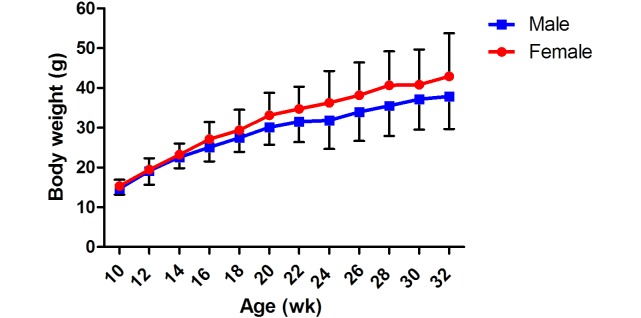
We recorded the weights of 60 of these offspring (30 females, 30 males; N1 generation) biweekly (Figure 1). The average body weight of 10-wk-old male N1 NMR was 14.68 ± 1.51 g, whereas their female counterparts weighed 15.33 ± 1.58 g. Both male and female pups increased steadily in weight, and beginning at 20 wk, female NMR weighed more (P <0.05) than did males of the same age.
Figure 1.
Body weight (mean ± 1 SD [error bars]) for the N1 generation of NMR.
Reproductive performance of N1 generation.
Sexually mature male and female NMR of the N1 generation were selected from different colonies and mated 1:1. Of the 30 pairs established, 22 pairs successfully produced offspring, for a reproductive success rate of 73.33%. The reproductive performance of the N1 pairs is summarized in Table 2. The average annual parity of 22 N1 pairs was 3.09 ± 0.81 litters, with 34.86 ± 10.66 total pups born and 30.14 ± 10.23 pups weaned per year. The survival rate after weaning was 85.51% ± 6.60%. None of these measures differed significantly from those of the original colonies, suggesting that the reproductive ability of the N1 generation was similar to that of the original colonies.
Table 2.
Annual reproductive performance of N1 pairs
| No. of |
||||
| Pair no. | Litters | Pups born | Pups weaned | Survival rate after weaning (%) |
| 1 | 3 | 36 | 31 | 86.11 |
| 2 | 2 | 18 | 15 | 83.33 |
| 3 | 4 | 37 | 32 | 86.49 |
| 4 | 3 | 30 | 25 | 83.33 |
| 5 | 4 | 40 | 36 | 90.00 |
| 6 | 2 | 26 | 23 | 88.46 |
| 7 | 3 | 38 | 35 | 92.11 |
| 8 | 4 | 33 | 28 | 84.85 |
| 9 | 4 | 42 | 39 | 92.86 |
| 10 | 2 | 22 | 17 | 77.27 |
| 11 | 4 | 49 | 47 | 95.92 |
| 12 | 3 | 35 | 30 | 85.71 |
| 13 | 2 | 20 | 17 | 85.00 |
| 14 | 2 | 24 | 21 | 87.50 |
| 15 | 4 | 57 | 48 | 84.21 |
| 16 | 3 | 31 | 27 | 87.10 |
| 17 | 4 | 46 | 38 | 82.61 |
| 18 | 4 | 45 | 39 | 86.67 |
| 19 | 2 | 21 | 13 | 61.91 |
| 20 | 3 | 31 | 26 | 83.87 |
| 21 | 4 | 51 | 46 | 90.20 |
| 22 | 3 | 35 | 30 | 85.71 |
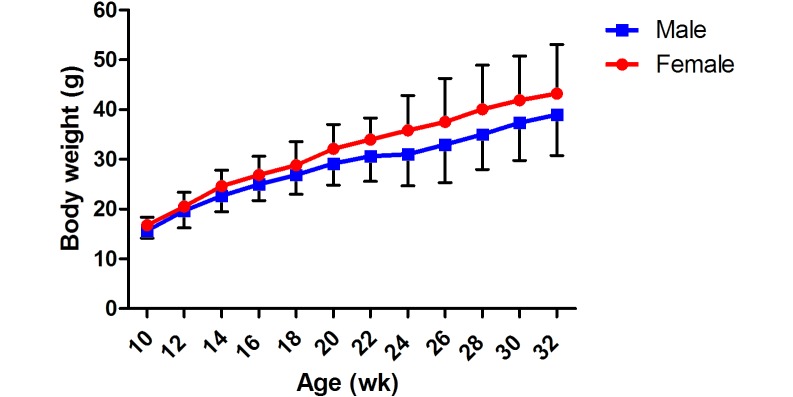
We weighed 60 of the offspring from these N1 pairs (30 females, 30 males; N2 generation) biweekly. The average weight of 10-wk-old male N2 NMR was 15.68 ± 1.53 g, and that of their female counterparts was 16.78 ± 1.60 g. N2 animals gained weight over time similarly to the N1 generation (Figure 2), and body weight did not differ between generations for NMR of the same age and sex (data not shown).
Figure 2.
Body weight (mean ± 1 SD [error bars]) for the N2 generation of NMR.
Reproductive performance of N2 animals.
We assessed the reproductive performance of the N2 pairs by using the same method as for the original and N1 animals. Of the 40 pairs established, 29 (72.50%) reproduced successfully (Table 3). The average annual parity of the N2 pairs was 3.04 ± 0.87 litters, comprising 33.69 ± 11.42 total pups and 28.17 ± 10.43 pups weaned over 1 y. The survival rate after weaning was 83.66% ± 10.75%. The N2 generation did not differ from the previous generations in terms of reproductive ability.
Table 3.
Annual reproductive performance of the N2 generation
| No. of |
||||
| Pair no. | Litters | Pups born | Pups weaned | Survival rate after weaning (%) |
| 1 | 3 | 30 | 26 | 86.67 |
| 2 | 4 | 39 | 35 | 89.74 |
| 3 | 3 | 32 | 28 | 87.50 |
| 4 | 2 | 19 | 15 | 78.95 |
| 5 | 3 | 35 | 28 | 80.00 |
| 6 | 2 | 30 | 23 | 76.67 |
| 7 | 3 | 26 | 25 | 96.15 |
| 8 | 4 | 56 | 48 | 85.71 |
| 9 | 4 | 46 | 41 | 89.13 |
| 10 | 3 | 39 | 31 | 79.49 |
| 11 | 1 | 5 | 5 | 100.00 |
| 12 | 3 | 37 | 30 | 81.08 |
| 13 | 4 | 39 | 34 | 87.18 |
| 14 | 2 | 31 | 16 | 51.61 |
| 15 | 4 | 42 | 40 | 95.24 |
| 16 | 3 | 31 | 28 | 90.32 |
| 17 | 4 | 42 | 32 | 76.19 |
| 18 | 2 | 13 | 10 | 76.92 |
| 19 | 3 | 30 | 25 | 83.33 |
| 20 | 4 | 56 | 40 | 71.43 |
| 21 | 3 | 29 | 25 | 86.21 |
| 22 | 4 | 42 | 39 | 92.86 |
| 23 | 2 | 22 | 17 | 77.27 |
| 24 | 4 | 49 | 47 | 95.92 |
| 25 | 3 | 34 | 29 | 85.29 |
| 26 | 2 | 21 | 20 | 95.24 |
| 27 | 4 | 39 | 34 | 87.18 |
| 28 | 3 | 35 | 30 | 85.71 |
| 29 | 2 | 28 | 16 | 57.14 |
Discussion
The purpose of our research has been to domesticate NMR and to establish a closed-colony system for their breeding by monitoring parameters indicative of their husbandry, social behavior, and reproduction under laboratory conditions.
During the first several months after their arrival at our facility, the NMR in the original colonies were timid and startled easily. However, they became acclimated and less frightened after 3 to 6 mo of fixed feeding and cleaning times. In addition, the adult NMR in all 3 generations ate, rested, and defecated in appropriate chambers of the housing unit, and the pups mimicked their parents in these behaviors; the estimated daily food consumption was 5 to 10 g for an adult NMR and 20 to 28 g for a pair with pups. Therefore the NMR adjusted well to our rearing scheme.
The observation that both nonbreeding female and male NMRs in captive and wild colonies rapidly become reproductively active when socially suppressive cues are removed5,7 was the theoretical principle underlying our breeding scheme using breeding pairs instead of the eusocial colonies that occur in the wild. According to our data, the reproductive success rate was approximately 73% for both the N1 and N2 pairs; these paired NMR copulated and reproduced year-round. The remaining compatible but nonproductive pairs remained together for more than 6 mo without producing a litter. In addition, the reproductive indexes and pup growth curves were similar between the original colonies and the N1 and N2 pairs. Thus, our breeding method was successful and efficient.
Under our closed-colony strategy, an N1 or N2 colony comprised a pair of breeders and a single litter of pups. In the present study, the gestational period of our NMR was 68 to 74 d, and newly paired NMR typically needed 3 to 4 mo to start breeding. We removed fully weaned pups (age, 1 to 2 mo) when the breeders gave birth to another litter; no subordinate NMR were present in the cage to help nurse and care for the pups. At the age of 5 mo, the juvenile female and male NMR were separated into same-sex groups.
In the wild, NMR colonies typically contain 75 to 80 members but can comprise 295 animals,3 complicating experimental observations and recordkeeping, particularly of genetic background. In addition to producing NMR more quickly than in the wild, our closed-colony breeding scheme facilitates tracking individual animals to collect detailed data. In addition, we successfully imported NMR from Africa for breeding and rearing research in China. Our studies provide additional experimental data and methodology to support the use of NMR in biomedical research.
Acknowledgments
We thank Pan Liu, Qiaoyun Xiao, Jishuai Cheng, and Bang Xiao for their assistance in maintaining the NMR research facility (Second Military Medical University). This work was supported by grants from the National Key Technology R&D Program (no. 2015BAI09B02), the Shanghai Committee of Science and Technology (nos. 14140900201 and 15140900200), the National Natural Science Foundation of China (No. 31402028), and PLA Special Issue of Animal Projects (nos. SYDW2014-005 and SYDW2014-007).
References
- 1.Buffenstein R, Jarvis JU. 2002. The naked mole-rat—a new record for the oldest living rodent. Sci SAGE KE 2002:pe7. [DOI] [PubMed] [Google Scholar]
- 2.Buffenstein R. 2005. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 60:1369–1377. [DOI] [PubMed] [Google Scholar]
- 3.Brett RA. 1991. The population structure of naked mole-rat colonies, p 97–136. In: Sherman PW, Jarvis JUM, Alexander RD. The biology of the naked mole-rat, Princeton (NJ): Princeton University Press. [Google Scholar]
- 4.Clarke FM, Faulkes CG. 1997. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc Biol Sci 264:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke FM, Faulkes CG. 1998. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc Biol Sci 265:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. 2007. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol 293:H919–H927. [DOI] [PubMed] [Google Scholar]
- 7.Faulkes CG, Abbott DH. 1991. Social control of reproduction in both breeding and nonbreeding male naked mole-rats, Heterocephalus glaber. J Reprod Fertil 93:427–435. [DOI] [PubMed] [Google Scholar]
- 8.Faulkes CG, Abbott DH. 1996. The physiology of a reproductive dictatorship: regulation of male and female reproduction by a single breeding female in colonies of naked mole-rats, p 302–334. In: Solomon NG, French JA. Cooperative breeding in mammals, Cambridge and New York (NY): Cambridge University Press. [Google Scholar]
- 9.Faulkes CG, Abbott DH, Jarvis JU. 1990. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fertil 88:559–568. [DOI] [PubMed] [Google Scholar]
- 10.Faulkes CG, Abbott DH, Jarvis JU. 1991. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J Reprod Fertil 91:593–604. [DOI] [PubMed] [Google Scholar]
- 11.Faulkes CG, Bennett NC. 2001. Family values: group dynamics and social control of reproduction in African mole-rats. Trends Ecol Evol 16:184–190. [DOI] [PubMed] [Google Scholar]
- 12.Faulkes CG, Trowell SN, Jarvis JU, Bennett NC. 1994. Investigation of numbers and motility of spermatozoa in reproductively active and socially suppressed males of 2 eusocial African mole-rats, the naked mole-rat (Heterocephalus glaber) and the Damaraland mole-rat (Cryptomys damarensis). J Reprod Fertil 100:411–416. [DOI] [PubMed] [Google Scholar]
- 13.Gladyshev VN, Zhang G, Wang J. 2011. The naked mole-rat genome: understanding aging through genome analysis. Aging (Albany NY) 3:1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimes KM, Lindsey ML, Gelfond JA, Buffenstein R. 2012. Getting to the heart of the matter: age-related changes in diastolic heart function in the longest-lived rodent, the naked mole-rat. J Gerontol A Biol Sci Med Sci 67A:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart AG, Ratnieks FL. 2004. Crossing the taxonomic divide: conflict and its resolution in societies of reproductively totipotent individuals. J Evol Biol 18:383–395. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis JU. 1981. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 212:571–573. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis JU. 1991. Reproduction of naked mole-rats, p 384–425. In: Sherman PW, Jarvis JUM, Alexander RD. The biology of the naked mole-rat, Princeton (NJ): Princeton University Press. [Google Scholar]
- 18.Jarvis JU, Bennett NC. 1991. Ecology and behaviour of the family Bathyergidae, p 66–97. In: Sherman PW, Jarvis JUM, Alexander RD. The biology of the naked mole-rat, Princeton (NJ): Princeton University Press. [Google Scholar]
- 19.Ke Z, Vaidya A, Ascher J, Seluanov A, Gorbunova V. 2014. Novel husbandry techniques support survival of naked mole-rat (Heterocephalus glaber) pups. J Am Assoc Lab Anim Sci 53:89–91. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, Marino SM, Sun X, Turanov AA, Yang P, Yim SH, Zhao X, Kasaikina MV, Stoletzki N, Peng C, Polak P, Xiong Z, Kiezun A, Zhu Y, Chen Y, Kryukov GV, Zhang Q, Peshkin L, Yang L, Bronson RT, Buffenstein R, Wang B, Han C, Li Q, Chen L, Zhao W, Sunyaev SR, Park TJ, Zhang G, Wang J, Gladyshev VN. 2011. Genome sequencing reveals insights into physiology and longevity of the naked mole-rat. Nature 479:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson J, Park TJ. 2009. Extreme hypoxia tolerance of naked mole-rat brain. Neuroreport 18:l634–l637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ. 2010. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber). Aging Cell 9:626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathaniel TI, Saras A, Umesiri FE, Olajuyigbe F. 2009. Tolerance to oxygen nutrient deprivation in the hippocampal slices of the naked mole-rats. J Integr Neurosci 08:123–136. [DOI] [PubMed] [Google Scholar]
- 24.Park TJ, Lu Y, Jüttner R, Smith ES, Hu J, Brand A, Wetzel C, Milenkovic N, Erdmann B, Heppenstall PA, Laurito CE, Wilson SP, Lewin GR. 2008. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber). PLoS Biol 6:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson BL, Larson J, Buffenstein R, Park TJ, Fall CP. 2012. Blunted neuronal calcium response to hypoxia in naked mole-rat hippocampus. PLoS One 7:e31568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson BL, Park TJ, Larson J. 2012. Adult naked mote-rat brain retains the NMDA receptor subunit GluN2D associated with hypoxia tolerance in neonatal mammals. Neurosci Lett 506:342–345. [DOI] [PubMed] [Google Scholar]
- 27.Sherman PW, Braude S, Jarvis JUM. 1999. Litter sizes and mammary numbers of naked mole-rats: breaking the one-half rule. J Mammal 80:720–733. [Google Scholar]
- 28.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A. 2013. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499:346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]