Abstract
Objectives
Earlier studies suggest the involvement of osteoprotegerin (OPG), RANK and RANK ligand (RANKL) in OA subchondral bone metabolism; however, few studies have looked at their functional consequences on chondrocytes. We compared the expression/production of OPG, RANK and RANKL on human normal and OA chondrocytes, and evaluated, on OA chondrocytes, their modulation by some catabolic factors. Furthermore, the role of OPG and RANKL on the production of catabolic/anabolic factors was assessed.
Methods
Expression was determined using real-time PCR, production of RANK and RANKL by flow cytometry and that of OPG by ELISA. Modulation of these factors was determined upon treatment with IL-1β, TNF-α and PGE2. The functional consequences were examined following treatment with soluble RANKL or OPG-Fc (OPG without the heparin-binding domain).
Results
OPG, RANK and RANKL were expressed and produced by human chondrocytes. Membranous RANK was produced only by an OA chondrocyte subpopulation (29%) localized throughout the cartilage. The OPG/RANKL ratio was significantly (P=0.05) reduced on the OA chondrocytes, whereas the RANK/RANKL ratio was significantly (P<0.03) increased. OPG and membranous RANKL levels were significantly enhanced by IL-1β, TNF-α and PGE2, whereas membranous RANK was significantly increased only with IL-1β. Administration of soluble RANKL had no effect on the OA chondrocytes. However, addition of OPG-Fc significantly stimulated MMP-13 (P=0.05) and protease-activated receptor-2 (PAR-2) (P<0.04) production.
Conclusions
Our findings showed that human chondrocytes express and produce OPG, RANK and RANKL. OA chondrocyte treatment with catabolic factors pointed towards an increased biological effect of OPG. Interestingly, OPG appears to be involved in OA progression by increasing two catabolic factors involved in cartilage pathophysiology.
Keywords: Osteoarthritis, Chondrocytes, OPG, RANK, RANKL
Introduction
OA is characterized by the degradation and loss of articular cartilage, inflammation of the SM, osteophyte formation and changes in the subchondral bone [1–7]. Although it is well-known that bone, and particularly the subchondral bone remodelling process, is tightly controlled by a molecular triad composed of osteoprotegerin (OPG), RANK and RANK ligand (RANKL) [8–10], very little data exists regarding the expression/production of these factors by human chondrocytes. Only one study [11] showed that human chondrocytes express and produce each member of this molecular triad; immunohistochemical analysis detected OPG, RANK and RANKL only in the superficial zone of normal cartilage; whereas in OA cartilage, their production was extended to the middle zone. Although the RANKL– RANK system appears to be non-functional in these cells [11], the functional consequences of OPG in human cartilage remain to be investigated.
RANKL is produced by the osteoblastic lineage cells and is essential for mediating bone resorption through the regulation of osteoclastogenesis and the activation of mature osteoclasts. RANKL stimulates osteoclastogenesis and osteoclast activity by binding to the cell surface receptor RANK, located on precursor and mature osteoclasts [8–10]. OPG is secreted by the stromal cells and other cell types including osteoblasts. By binding to RANKL, OPG inhibits the interaction of RANKL–RANK, thereby preventing RANK activation and subsequent osteoclastogenesis [8–10].
Apart from its role in the bone system, OPG has also been demonstrated to have other cellular functions. These include the survival of tumour cells by preventing TNF-related apoptosis inducing ligand (TRAIL) from exerting its apoptotic effect [12–14]. This molecular triad is also important in maintaining an immune response; RANKL expressed by activated T cells binds to RANK that is expressed on the dendritic cells, and such binding regulates the function and survival of these latter cells [15–17]. There is also accumulating evidence for the potential roles of OPG and RANKL in the vascular system, as they have been found to be associated with pathologies such as coronary artery disease, atherosclerosis and vascular calcification [18–20].
The broad spectrum of molecular and cellular mechanisms of action of OPG–RANK–RANKL in various systems led us to further complement the data on the presence of this triad in human chondrocytes, by comparing OA to normal and by the modulation of each member of this triad upon treatment with catabolic factors. The functional consequences of treating OA chondrocytes with either soluble human RANKL or OPG-Fc (OPG without the heparin-binding domain) were also evaluated. Our study is the first to report that OPG up-regulates two catabolic factors, MMP-13 and protease-activated receptor-2 (PAR-2), on human OA chondrocytes.
Materials and methods
Specimen selection
Human cartilage was obtained from normal individuals within 12 h of death [mean age (S.D.) 56 (16)]. The cartilage was examined macroscopically and microscopically to ensure that only normal tissue was used. Human OA specimens were obtained from patients undergoing total knee arthroplasty [mean age (S.D.) 67 (9)]. All patients were evaluated as having OA according to ACR criteria [21]. These specimens represented moderate to severe OA as defined according to macroscopic criteria. At the time of surgery, the patients had symptomatic disease requiring medical treatment in the form of acetaminophen, NSAIDs or selective cyclooxygenase (COX)-2 inhibitors. None had received IA steroid injections within 3 months prior to surgery. The institutional Ethics Committee Board of the University of Montreal Hospital Centre approved the use of the human articular tissues.
Chondrocyte culture
Chondrocytes were released from full-thickness strips of cartilage followed by sequential enzymatic digestion at 37°C as previously described [22]. Cells were seeded at high density (105 cells/cm2) in tissue culture flasks and cultured to confluence in DMEM (Wisent, Saint-Bruno, QC, Canada) supplemented with 10% heat-inactivated fetal bovine serum (FBS; PAA Laboratories, Etobicoke, ON, Canada) and an antibiotics mixture (100 U/ml of penicillin base and 100 μg/ml of streptomycin base; Wisent) at 37°C in a humidified atmosphere. To ensure the phenotype, only first-passage cultured chondrocytes were used.
To determine which cells within the cartilage produce RANK, OA chondrocytes of the superficial and upper intermediate layers (superficial zone) were discriminated from the lower intermediate and deep layers (deep zone). Hence, full thickness cartilage specimens were cut in two distinct zones and OA chondrocytes from these two distinct zones of the cartilage were isolated and cultured as above.
To separate the two OA chondrocyte subpopulations (RANK-positive and -negative), we used the magnetic activated cell sorting (MACS) high gradient magnetic separation column for selection of large cells from Miltenyi Biotec (Auburn, CA, USA). In brief, primary OA chondrocytes (1 × 106) were labelled with 2 μg of a mouse anti-human RANK antibody (20 μg/ml; Abcam, Cambridge, MA, USA) for 15 min at 4°C. Cells were washed to remove unbound primary antibodies, and then magnetically labelled with 20 μl of rat anti-mouse immunoglobulin 1 (IgG1) microbeads for 15 min at 4°C. The cell suspension was further loaded onto a MACS column, which was placed in the magnetic field on a MACS separator. The magnetically labelled cells were retained on the column. The column was then eluted twice with 1% BSA/PBS, pH 7.5, and unlabelled cells, RANK negative, were collected. After removal of the column from the magnetic field, the magnetically retained cells were eluted with the 1% BSA/phosphate buffered saline (PBS), pH 7.5, and constituted the RANK-positive cells. Each population of chondrocytes was then processed for RNA extraction, reverse transcription (RT) and real-time PCR. Quantitative PCR for RANK gene expression was performed first to ensure the purity of each subpopulation.
The effects of the factors were assessed on OA chondrocytes by incubating cells in DMEM/0.5% FBS with the factors under study for 20 h for mRNA determination and 72 h for protein determination. The factors tested were IL-1β (100 pg/ml; R&D Systems, Minneapolis, MN, USA), TNF-α (5 ng/ml; R&D Systems) and PGE2 (500 nM; Cayman Chemical, Ann Arbor, MI, USA). The effects of RANKL and OPG on OA chondrocytes were assessed by incubating the cells with either soluble human RANKL (50 and 100 ng/ml) or OPG-Fc (50 and 100 ng/ml) (both generously provided by Amgen, Thousand Oaks, CA, USA).
RNA extraction, RT and real-time PCR
Total cellular RNA from human chondrocytes was extracted with the TRIzol reagent (Invitrogen, Burlington, ON, Canada), the RNA was quantitated using the RiboGreen RNA quantitation kit, and the RT reactions were primed with random hexamers [23].
Real-time quantitation of mRNA was performed as previously described [23] in the Rotor-Gene 6 RG-3000A (Corbett Research, Mortlake, NSW, Australia) with the 2× Quantitect SYBR Green PCR Master Mix (Qiagen, Mississauga, ON, Canada). The primer sequences were as in Table 1. The data were given as a threshold cycle (CT). Data were calculated as the ratio of the number of molecules of the target gene/number of molecules of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The data were expressed in arbitrary units relative to control which was given the value of 1. The primer efficiencies for the test genes were the same as for the GAPDH gene. The standard curves were generated with the same plasmids as the target sequences.
Table 1.
Primer sequences
| Gene | Sense | Antisense |
|---|---|---|
| OPG | 5′-GCTTGAAACATAGGAGCTG | 5′-GTTTACTTTGGTGCCAGG |
| RANK | 5′-ATGCGGTTTGCAGTTCTTCTC | 5′-ACTCCTTATCTCCACTTAGG |
| RANKL | 5′-CACTATTAATGCCACCGAC | 5′-GGGTATGAGAACTTGGGATT |
| IL-1β | 5′-CCTGTACGATCACTGAACTG | 5′-TGGGCAGACTCAAATTCCAG |
| IL-6 | 5′-CACCTCTTCAGAACGAATTG | 5′-CTAGGTATACCTCAAACTCC |
| TNF-α | 5′-AGCCCATGTTGTAGCAAACC | 5′-GACTCGGCAAAGTCGAGATA |
| IL-17 | 5′-ACAATCCCACGAAATCCAG | 5′-TTGCCTCCCAGATCACAGAG |
| PAR-2 | 5′-GAAGCCTTATTGGTAAGGTTG | 5′-CAGAGAGGAGGTCAGCCAAG |
| MMP-1 | 5′-CTGAAAGTGACTGGGAAACC | 5′-AGAGTTGTCCCGATGATCTC |
| MMP-2 | 5′-CACTGTTGGTGGGAACTCAG | 5′-GTGTAAATGGGTGCCATCAG |
| MMP-9 | 5′-CCTTCACTTTCCTGGGTAAG | 5′-CCATTCACGTCGTCCTTATG |
| MMP-13 | 5′-CTTAGAGGTGACTGGCAAAC | 5′-GCCCATCAAATGGGTAGAAG |
| TIMP-1 | 5′-AGACCACCTTATACCAGCGT | 5′-AAGCAATGAGTGCCACTCTG |
| Collagen type II α1 | 5′-AGTTTCAGGTCTCTGCAGGT | 5′-AACTGGCAAGCAAGGAGACA |
| Aggrecan | 5′-GCCTATCAGGACAAGGTCTC | 5′-ATGATGGCACTGTTCTGCAG |
| GAPDH | 5′-CAGAACATCATCCCTGCCTCT | 5′-GCTTGACAAAGTGGTCGTTGAG |
Membranous RANK and RANKL determination: flow cytometry
Cells were washed once in 1% BSA/PBS, detached with the enzyme-free cell dissociation buffer (Invitrogen) at 37°C, and centrifuged at 500 g for 5 min at 4°C. The cells were re-suspended in 1% BSA/PBS and a 500 μl suspension prepared, with a concentration of 1 × 106 cells/ml. The suspension was incubated for 30 min at room temperature and divided into tubes. For the determination, one served as negative control to which a mouse IgG (15μg/ml; Chemicon International, Billerica, MA, USA) for RANKL and a mouse IgG coupled to phycoerythrin (IgG-PE: 20 μg/ml; R&D Systems) for RANK was added, and the other was labelled with either a mouse anti-human RANKL antibody (15μg/ml; R&D Systems) or a mouse anti-human RANK-PE (20μg/ml; Abcam) for 30 min at 4°C. For RANKL, after this period, cells were washed and a goat anti-mouse FITC-conjugated secondary antibody (7.5μg/ml; R&D Systems) was added for another 30 min at 4°C. Cells were then re-suspended in PBS and analysed by using a flow cytometry apparatus (Facscalibur; BD Biosciences, Mississauga, ON, Canada). The control sample was used to determine the background fluorescence and compared with that of the sample incubated with the specific antibody. The percentage of positive cells as well as the level of fluorescence was measured by a FACS using the CellQuest program (BD Biosciences). The level of fluorescence was calculated as the mean fluorescent intensity of positive cells and data expressed in fold of control. OA chondrocytes producing membranous RANK are represented in the gate M1.
PAR-2 determination: western blot
Total proteins were extracted with 0.5% SDS (Invitrogen) supplemented with protease inhibitors. The protein level was determined using the bicinchoninic acid protein assay, and 10μg of the protein electrophoresed on a discontinuous 4–12% SDS gel polyacrylamide. The proteins were transferred electrophoretically onto a nitrocellulose membrane (Bio-Rad Laboratories, Mississauga, ON, Canada) for 1 h at 4°C.
The membranes were incubated overnight at 4°C with 5% skimmed milk in SuperBlock in Tris–buffered saline (Pierce, Rockford, IL, USA). The membranes were then washed once with tween tris buffered saline (TTBS) 1× (Tris 20mM, NaCl 150mM, pH 7.5, and 0.1% Tween 20) for 10 min and incubated in SuperBlock 1:10 in TTBS 1×, supplemented with the mouse anti-human PAR-2 antibody (1:500; Santa Cruz Biotechnology, Santa-Cruz, CA, USA) overnight at 4°C. The membranes were washed with TTBS 1× and incubated for 1 h at room temperature with the second antibody (1:20 000; anti-mouse IgG horseradish peroxidase conjugated; Pierce) and washed again with TTBS 1×. Detection was performed by chemiluminescence using the Super Signal ULTRA chemiluminescent substrate (Pierce) and exposure to Kodak Biomax photographic film (GE Healthcare, Baie d’Urfé, QC, Canada). The band intensity was measured by densitometry using TotalLab TL 100 Software (Nonlinear Dynamics, Newcastle Upon Tyne, UK), and data were expressed as arbitrary units.
OPG and MMP-13 protein determination: ELISA
OPG (Medicorp, Montreal, QC, Canada) and MMP-13 (GE Healthcare) production were determined in the culture media by specific ELISA. All determinations were performed in duplicate.
Statistical analysis
Data are expressed as the mean (S.E.M.). Statistical significance was assessed by the two-tailed Student’s t-test, and P-values ≤0.05 were considered as statistically significant.
Results
OPG–RANK–RANKL expression by normal and OA chondrocytes
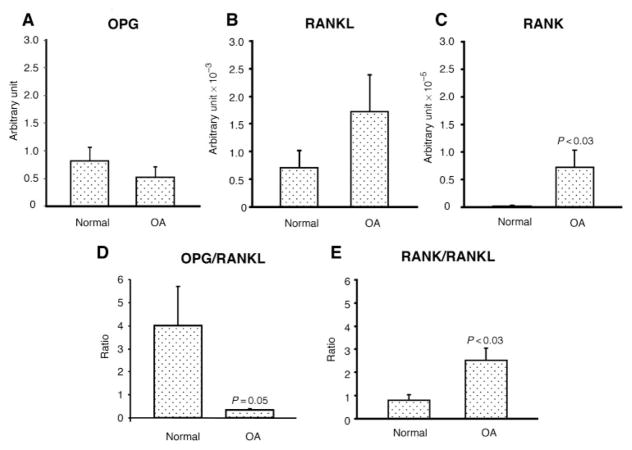
To investigate the presence of each member of the triad, OPG, RANK and RANKL, we first looked at their expression levels by real-time PCR, comparing normal to OA. Data showed that both normal (n=8) and OA (n=8) chondrocytes expressed these factors (Fig. 1). Of note, human chondrocytes had a much higher level of OPG expression compared with RANKL and RANK; the levels of RANKL and RANK gene expression were ~103 and 105 less elevated, respectively, than OPG. Although OPG showed a lower level in OA than normal and the opposite was found for RANKL, these did not reach statistical difference (Fig. 1A and B). The level of RANK was very low in normal and found significantly increased in OA (Fig. 1C).
Fig. 1.
Gene expression of (A) OPG, (B) RANKL, (C) RANK, and the ratio of (D) OPG/RANKL and (E) RANK/RANKL in human normal (n=8) and OA chondrocytes (n=8). Total RNA was extracted and processed for real-time PCR, and the data are expressed as the mean (S.E.M.) of arbitrary unit as described in ‘Materials and methods’ section. Of note, the RANKL arbitrary unit is represented as ×10−3 and RANK as ×10−5. Statistical significance was assessed by the Student’s t-test vs normal.
In bone biology, although the levels of these factors are of key importance, the ratio of OPG/RANKL or RANK/RANKL is considered to better reflect environmental signals than the level of each of these factors individually. Data showed that the OPG/RANKL ratio is significantly decreased (P=0.05) in OA chondrocytes in favour of RANKL, whereas the RANK/RANKL ratio is significantly increased (P<0.03) in the OA cells in favour of RANK (Fig. 1D and E).
OPG, RANKL and RANK production by OA chondrocytes
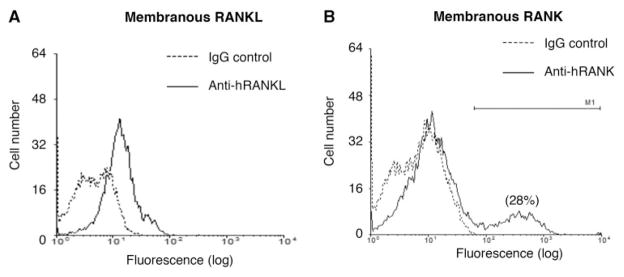
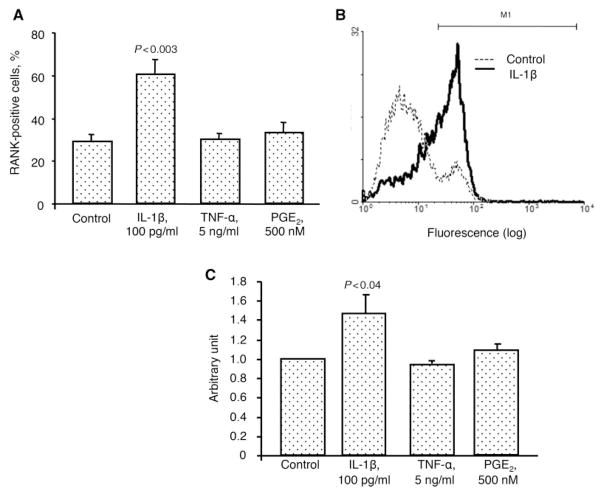
OPG is produced by OA chondrocytes and a level of 1993 ± 349 pmol/mg protein (n=9) was found. Similarly, both RANKL (n=5) and RANK (n=11) were detected on the membranous surface of the OA chondrocytes (Fig. 2). Interestingly, although membranous RANKL was present in all the chondrocytes, membranous RANK appeared to be produced only by an OA chondrocyte subpopulation, and 29 ± 4% (n=11) of the chondrocytes produced RANK. In an attempt to discriminate a localization pattern of membranous RANK in the OA cartilage (n=3), chondrocytes from the superficial (superficial and upper intermediate layers) and from the deep (deep and lower intermediate layers) zones of the OA cartilage were isolated separately and the RANK level was determined. Data revealed similar levels in the superficial (29 ± 5%) and deep (29 ± 1%) zones, suggesting that OA chondrocytes producing RANK are localized throughout the cartilage layers.
Fig. 2.
Representative histograms of the membranous (A) RANKL (n=5) and (B) RANK (n=11) flow cytometry on human OA chondrocytes. A mouse IgG served as control (dotted line) for background fluorescence, whereas specific fluorescence (solid line) was obtained with an anti-human RANKL (anti-hRANKL) or anti-human RANK (anti-hRANK) antibody as described in ‘Materials and methods’ section. Of note, the gate M1 represents the number of OA chondrocytes expressing membranous RANK, and in this sample 28% of the cells were RANK positive.
Moreover, in order to have a better understanding of these two subpopulations, OA chondrocytes (n=5) were separated into RANK-positive and -negative cells, and the expression levels of two chondrocyte markers, aggrecan and collagen type II, were evaluated. Data showed that these OA chondrocyte subpopulations expressed these genes differently, the RANK-positive chondrocytes showing a higher level than the RANK-negative ones. However, statistical significance was reached for aggrecan (1.4 ± 0.1 fold increase; P=0.003) but not for collagen type II (5.5 ± 2.3 fold increase; P=0.08).
Modulation of OPG, RANK and RANKL by catabolic factors
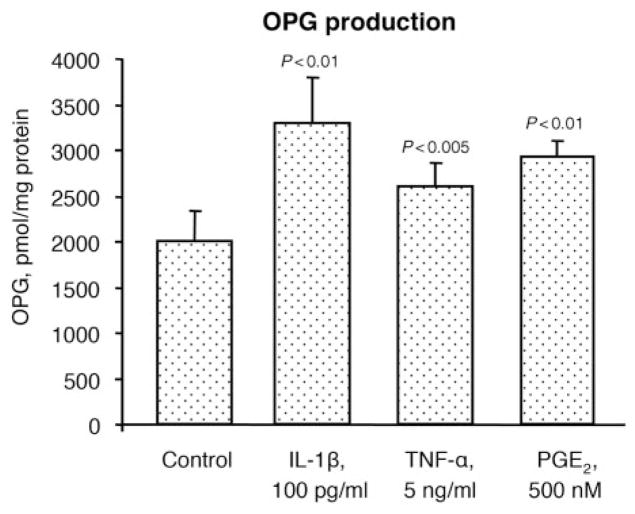
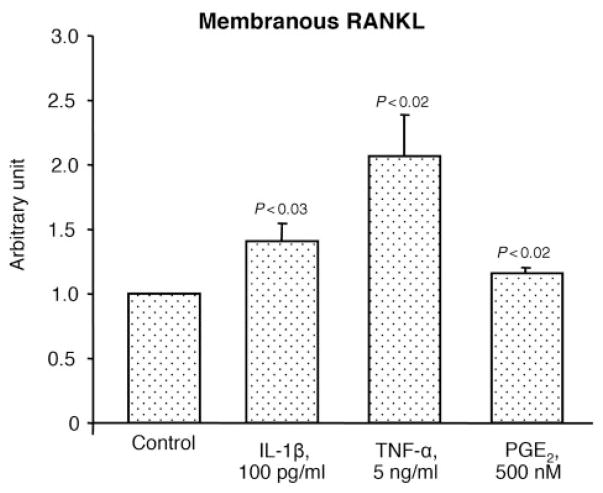
To better reflect the modulation of each member of this triad during the micro-environmental conditions that may occur in OA, some factors known to be involved in the pathophysiology of this disease, including IL-1β, TNF-α and PGE2, were tested. Data revealed (Fig. 3) that IL-1β, TNF-α and PGE2 treatment on OA chondrocytes (n=9) significantly up-regulated OPG production by 1.7-, 1.3- and 1.5-fold, respectively. Membranous RANKL (Fig. 4) was also up-regulated with a significantly increased level upon treatment with IL-1β (1.4-fold), TNF-α (2.1-fold) and PGE2 (1.2-fold) (n=8). The percentage of RANK-positive cells (n=6) was also significantly increased, but only with IL-1β (2.1-fold) (Fig. 5A). Representative histograms of the membranous RANK flow cytometry in the absence or in the presence of IL-1β clearly show the modulation of the RANK subpopulation in the gate M1 (Fig. 5B). Of note, the gate M1 represents the cell number of OA chondrocytes expressing membranous RANK. However, when data are calculated by the intensity of membranous RANK/cell (n=6) (Fig. 5C), the RANK intensity/cell is similarly increased upon IL-1β. The fact that the effect of OPG is mediated by its binding to RANKL at the cell membrane, together with the above-mentioned data, indicates that the fold increase of each member of this molecular triad favours an increased OPG biological effect.
Fig. 3.
OPG protein production in human OA chondrocytes (n=9) incubated in the absence (Control) or presence of the catabolic factors IL-1β, TNF-α and PGE2 at the indicated concentrations. Statistical analysis was assessed by the paired Student’s t-test vs control.
Fig. 4.
Membranous RANKL level on human OA chondrocytes (n=8) incubated in the absence (Control) or presence of IL-1β, TNF-α and PGE2. Data are expressed as arbitrary unit calculated from fluorescence intensity over control, which was attributed a value of 1. Statistical analysis was assessed by paired Student’s t-test vs control.
Fig. 5.
(A) Percentage of cells positively labelled for membranous RANK on human OA chondrocytes (n=6) incubated in the absence (Control) or presence of IL-1β, TNF-α and PGE2. (B) Representative histogram of the membranous RANK flow cytometry in the absence (Control, dotted line) or in the presence (solid line) of IL-1β. Of note, the gate M1 represents the number of OA chondrocytes expressing membranous RANK. (C) Membranous RANK level on OA chondrocytes (n=6) incubated in the absence (Control) or presence of the above factors. Data are expressed as arbitrary unit calculated from fluorescence intensity over control, which was attributed a value of 1. Statistical analysis was assessed by paired Student’s t-test vs control.
Functional consequence of RANKL and OPG on OA chondrocytes
The functional consequence of increasing RANKL and OPG on OA chondrocytes was investigated with human soluble RANKL and OPG-Fc. OPG contains a heparin-binding domain and glycosaminoglycans bind to this particular domain [24, 25]. In order to exclude such interaction, which prevents OPG from binding to RANKL, an OPG-Fc, i.e. without this heparin-binding domain, was used. The concentration of OPG-Fc was determined following dose curves from preliminary experiments showing that the maximum effect of OPG-Fc was obtained at 50 and 100 ng/ml. Gene expression levels of the catabolic factors including IL-1β, -6, -17, TNF-α, MMP-1, -2, -9, -13, tween tris buffered saline (TIMP)-1 and PAR-2 were determined as well as the collagen type II α1.
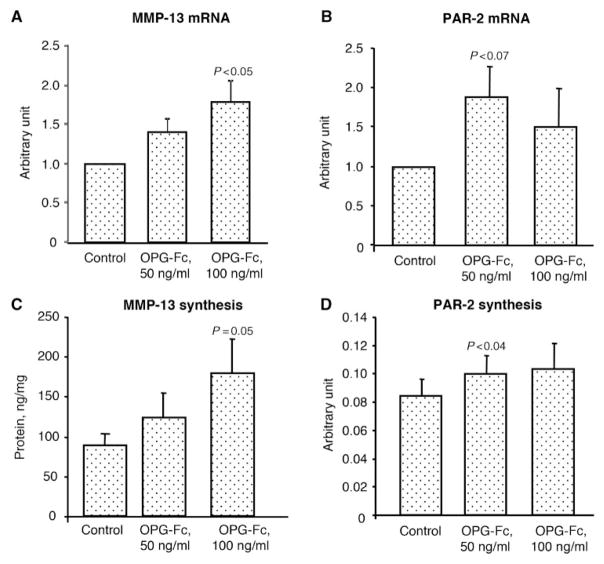
Treatment of OA chondrocytes with soluble human RANKL showed no effect for all tested factors at either concentration, 50 or 100 ng/ml (n=8) (data not shown). However, treatment with OPG-Fc (n=7) significantly up-regulated the gene expression level of MMP-13 (Fig. 6A). PAR-2 was also up-regulated with OPG-Fc; however, the increase did not quite reach statistical significance (Fig. 6B). None of the other factors tested was affected by OPG-Fc (data not shown). To further validate that the OPG effect was due to its binding to the RANKL receptor, experiments were carried out in which OA chondrocytes (n=7) were pre-incubated with a neutralizing anti-RANKL (40 ng/ml; R&D Systems #6262) for 24 h in DMEM/0.5% FBS, and incubated with the anti-RANKL in the absence or presence of OPG-Fc (50 ng/ml) for 20 h and PAR-2 expression level was determined. PAR-2 expression was 1.8-fold higher in OA chondrocytes treated with OPG-Fc than the control, and 1.1-fold higher in those treated with the OPG-Fc in conjunction with the anti-RANKL, indicating that the effect of OPG-Fc was mediated by RANKL.
Fig. 6.
Gene expression level (n=7) of (A) MMP-13 and (B) PAR-2, and protein production of (C) MMP-13 (n=5) and (D) PAR-2 (n=8) on human OA chondrocytes upon treatment in the absence (Control) or presence of OPG-Fc at concentrations of 50 and 100 ng/ml. The gene expression level is expressed as arbitrary units over control, which was attributed a value of 1. The protein production is expressed as nanograms per milligram of protein for MMP-13 and as arbitrary units for PAR-2. Statistical analysis was assessed by paired Student’s t-test vs control.
We then determined if the production of the two factors affected by OPG-Fc followed the expression levels. Following treatment with OPG-Fc, MMP-13 production level (n=5) (Fig. 6C) had the same profile as its expression; a dose-dependent increase with statistical significance reached at the higher concentration (100 ng/ml). Both concentrations of OPG-Fc elevated the level of PAR-2 production (Fig. 6D) (n=8), and statistical significance was reached at 50 ng/ml.
Discussion
Although significant progress has been made in the understanding of the OA pathophysiological pathways, much remains to be done to develop a specific therapy that could effectively slow down or prevent the progression of the disease. To this end, the literature suggests that in addition to cartilage, other articular tissues, including the subchondral bone, should be targeted. Hence, factors involved in the control of bone homoeostasis, such as OPG and RANKL, were investigated in OA subchondral bone osteoblasts, and data demonstrated their abnormal regulation in the remodelling process of this tissue [6, 7]. Moreover, recent studies performed on mice reported a beneficial effect of OPG against the progression of OA [26, 27]. We therefore expanded these data by looking at the implication of OPG, RANK and RANKL in human OA cartilage metabolism and, more specifically, by identifying the factors targeted by this system.
On the one hand, although statistical significance was not reached, our data first showed that the OPG mRNA level was lower and RANKL level was higher in OA compared with normal. On the other hand, the RANK expression level was significantly increased in OA compared with normal. These data contrast those of Komuro et al. [11], which showed that the OPG mRNA level was higher in OA cartilage and that RANKL and RANK did not differ. This difference could be explained by the methods utilized to determine the mRNA levels; a quantitative methodology (real-time PCR) was employed in our study, whereas the latter authors [11] used semi-quantitative PCR. However, in agreement with their data, our study also showed no effect of exogenous soluble RANKL on the OA chondrocytes on a variety of factors involved in this disease process. Such absence of effect could be related to the fact that only a small subpopulation of OA chondrocytes produces RANK. Moreover, this could also indicate that the membranous RANK on human OA chondrocytes is in an incomplete, truncated or mutated form, thus not able to respond to RANKL treatment. Surprisingly, our data also revealed that treatment with OPG-Fc significantly increased the levels of two catabolic mediators.
Although OPG, RANK and RANKL are gaining importance in the progression of inflammatory joint diseases, such as RA and PsA [28–32], their participation in OA has until now been under-evaluated. In this disease, recent efforts have been devoted to a better comprehension of this triad in the bone tissue, particularly in the cancellous [33] and subchondral bone [6, 7], and data show that OPG and RANKL are produced in abnormal levels in this tissue during OA. Komuro et al. [11] reported that in human cartilage, the factors of this triad are produced by chondrocytes. Our results confirmed that these factors are expressed and produced by human chondrocytes. Interestingly, further investigation revealed that only a subpopulation of OA chondrocytes (~29%) produced membranous RANK. This subpopulation was found to be distributed throughout the whole cartilage, as both the superficial and the deep zones of cartilage showed an equivalent percentage of RANK-positive cells. Interestingly, these two subpopulations could be discriminated according to some chondrocyte markers. Indeed, the gene expression levels of the RANK-positive OA chondrocytes were higher for the aggrecan and collagen type II than the RANK-negative cells, although statistically significant difference was reached only for the aggrecan. The higher expression level for the RANK-positive cells could reflect an attempt by these chondrocytes to produce more of these macromolecules. Further investigation into the properties and activities of these two subpopulations is currently ongoing.
Although OPG and RANKL mRNA levels were not quite significantly modulated in OA compared with normal, the ratio of OPG/RANKL was significantly decreased in OA, indicating increased RANKL, thus favouring an OPG–RANKL interaction. At the same time, the RANK/RANKL ratio was significantly increased in the OA chondrocytes compared with the normal; however, our study did not reveal a specific role for RANKL binding to RANK. This observation, as mentioned above, could reflect that only a small subpopulation of OA chondrocytes produces RANK, and therefore the biological consequence of RANKL might be too low to be detected.
OPG, through its different domains, has been suggested to have roles other than acting as a decoy receptor for soluble RANKL. On some tumour cells, OPG was reported to prevent TRAIL from exerting its apoptotic effect [12–14]. Such effect was also recently reported on a mouse model of OA when injected IA with human OPG [26]. Of note, the beneficial anti-apoptotic effect of OPG on chondrocytes can be somewhat detrimental when applied to other arthritic articular cells. For example, OA as well as RA SM demonstrate hyperplasia, and one of the therapeutic strategies involves increasing cell apoptosis in this tissue. Hence, the anti-apoptotic effect of OPG would further increase the number of cells in the SM, thereby exerting a damaging effect. In cartilage, another negative consequence of OPG could be through its heparin-binding domain that binds glycosaminoglycans such as CS, heparan sulphate and dermatan sulphate, and proteoglycans such as syndecan-1, interactions which could in turn modulate glycosaminoglycan bioavailability. In this context, Standal et al. [34] demonstrated that exogenous OPG interacts with syndecan-1 at the cellular membrane, forming a molecular complex that is internalized and degraded by the cells. Although speculative and not yet tested, a similar negative event might occur in chondrocytes; since syndecan-1 is suggested to be involved in an attempt to repair OA cartilage, degradation of this proteoglycan which is up-regulated by the diseased chondrocytes might be unfavourable [35–37]. In the same line of thought, another proteoglycan from this cell surface family susceptible to interact with OPG is the TGF-β receptor III or β-glycan [38], which is well-known for its role in orchestrating the TGF-β superfamily signalling. Thus, although speculative, interaction with the OPG heparin-binding domain could regulate this receptor’s availability. In the present study, these effects were not studied as such interaction was excluded by the use of a recombinant OPG in which the heparin-binding domain was removed (OPG-Fc).
Various in situ mediators are responsible for orienting the development of OA. Data showed that upon treatment with the catabolic factors IL-1β, PGE2 (the fold increase of OPG compared with RANKL was greater) and TNF-α, induced OPG and RANKL levels with an overall fold increase favouring RANKL. Yet, an elevation in either OPG or RANKL increases OPG–RANKL interaction thus favouring the biological activity of OPG. Similarly, IL-1β significantly increased the percentage of chondrocytes producing RANK as well as its number per cell. By augmenting membranous RANK levels, IL-1β favours an increased interaction of soluble RANKL with membranous RANK. Since OPG is known to bind to both soluble and membranous RANKL, the higher production of RANK would favour the binding of soluble RANKL to RANK, thereby promoting the potential binding of OPG to membranous RANKL, allowing OPG to exert its biological effect on these cells.
The alterations that occur in OA cartilage are numerous and involve morphological changes in chondrocyte metabolism resulting in biochemical and structural alterations in the extracellular macromolecules. During the disease process, higher production by the OA chondrocytes of enzymes responsible for the cartilage matrix breakdown is encountered. While various types of proteases participate in this matrix turnover, a family of key enzymes, the MMPs, has been specifically related to articular tissues. In addition to the MMPs, considerable evidence has been accumulated indicating that pro-inflammatory factors synthesized and released by the OA chondrocytes are crucial to the catabolic processes in this tissue. We thus further looked at the functional consequences of OPG and RANKL on the OA chondrocytes by investigating their effect on MMPs, TIMP-1 and inflammatory factors known to be involved in the disease process as well as on collagen type II. Data first showed that treatment of OA chondrocytes with soluble RANKL induced no effect on the tested factors. However, unexpectedly, OPG was found to significantly increase two catabolic factors involved in the degradation of OA cartilage, MMP-13 and PAR-2. Such finding is important, as OPG has been proposed as a therapeutic strategy against some arthritic diseases. No effect was seen on the other factors tested, including TIMP-1 which, if increased, could have negatively regulated MMP-13 activity, or on the collagen type II expression levels. The latter finding concurs with that of Kadri et al. [27] in which the effects of in vivo injection of OPG in mice had no effect on the release from the cartilage of another matrix macromolecule, the proteoglycan.
The data showing that OPG-induced MMP-13 and PAR-2 levels are of importance, as both factors are involved in the catabolic activity of OA cartilage. MMP-13 is responsible for the excessive degradation of the cartilage matrix by not only acting on the degradation of native collagen but also on a wide range of non-collagenous macromolecules [39–42]. PAR-2 is a factor linked to the catabolic and inflammatory cascades that occur in human joints [43–46]. Recent data demonstrated a significantly higher level of PAR-2 in human OA chondrocytes and that its activation up-regulates the production of MMP-13 and COX-2 [47]. Interestingly, it was also recently demonstrated on human subchondral bone osteoblasts that activation of PAR-2 up-regulates the level of membranous RANKL [48]. Thus, if this latter phenomenon also occurs in the chondrocytes, PAR-2 would induce a catabolic cascade of events in which the membranous RANKL level would be expected to increase and allow more OPG to bind to it, thereby affecting the cartilage metabolism. Indeed, data showed that incubation of cells in the presence of OPG and a RANKL antibody was effective at inhibiting the effect of OPG on the PAR-2 expression level.
This finding of OPG increasing the level of two catabolic factors appears to contradict the studies performed on a murine model of OA in which OPG was shown to be effective at reducing the progression of the disease [26, 27]. However, one of these studies suggested that the mechanism of action was that the injection of human recombinant OPG in mice with surgically induced OA prevented chondrocyte apoptosis [26], whereas the other using the same model concluded that the effect of OPG administration was indirect and occurred via its effect on the bone [27]. As no effect of RANKL was observed on OA chondrocytes, the well-known role of OPG as decoy receptor for RANKL may not be applied to cartilage. Moreover, data from studies in mice and humans, especially those reported for OA, are not always in agreement, which could be related to the fact that these small animals do not possess some proteases and cytokines involved in human OA. Moreover, in those studies [26, 27], human recombinant OPG was injected into the diseased articulation. Although OPG from the mice and humans showed 85% homology, the difference could affect its bioavailability by decreasing its binding to proteoglycan heparin domains, and/or cause a weaker interaction with the mouse RANKL at the cell membrane.
In conclusion, the findings of this study are of importance in providing a new basis for the rationalization of a therapeutic strategy targeting OA. Indeed, this study demonstrated that the ratios of OPG/RANKL and RANK/RANKL in human OA chondrocytes are significantly different from normal, favouring an increased membranous level of RANKL, hence increased interaction of OPG at the cell membrane. Moreover, some catabolic factors, especially IL-1β, induced an up-regulation of each factor of this triad, the offset being an increased biological effect of OPG. In turn, exogenous OPG enhanced two catabolic factors involved in the pathophysiology of the disease. Therefore, targeting the factor that mediates the effect of OPG, for example, by inhibiting RANKL, could lead to a new therapeutic approach against OA.
Such inhibition of RANKL has been proposed as a therapeutic in some osteolytic diseases [49, 50]. The ultimate goal in the treatment of OA is to improve or preserve the patient’s joint structure by preventing its destruction. As recent data indicate a key role for subchondral bone in OA, it is thus plausible that therapies that interfere with both cartilage and subchondral bone simultaneously could block or at least attenuate the progression of OA. The potential of RANKL inhibition as a disease-modifying osteoarthritis drug is thus very appealing. However, only appropriate clinical trials will be able to provide the information needed to answer this important question.
Rheumatology key messages.
OPG enhanced two catabolic factors involved in OA.
Inhibiting RANKL could lead to a new therapeutic approach against OA.
Acknowledgments
The authors are grateful to Dr Marika Sarfati, Dr Guy Delespesse and Manuel Rubio from the Immunoregulation and Allergy Research Laboratories at the University of Montreal Hospital Research Centre for their expertise in flow cytometry. The authors also thank François-Cyril Jolicoeur, François Mineau and Changshan Geng for their expert technical assistance, and Virginia Wallis for the manuscript preparation, all from the Osteoarthritis Research Unit of the University of Montreal Hospital Research Centre. Amgen Inc., Thousand Oaks, California generously provided the soluble human RANKL and OPG-Fc. Steeve Kwan Tat was the recipient of fellowship awards from ESCEO/Amgen and Mentor/Canadian Institutes of Health Research. Nathalie Amiable was the recipient of a graduate student award from Mentor/Canadian Institutes of Health Research.
Funding: This study was supported by internal funds of the Osteoarthritis Research Unit, University of Montreal Hospital Research Centre (CRCHUM).
Footnotes
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop. 1986;213:34–40. [PubMed] [Google Scholar]
- 2.Bailey AJ, Mansell JP. Do subchondral bone changes exacerbate or precede articular cartilage destruction in osteoarthritis of the elderly? Gerontology. 1997;43:296–304. doi: 10.1159/000213866. [DOI] [PubMed] [Google Scholar]
- 3.Mansell JP, Tarlton JF, Bailey AJ. Biochemical evidence for altered subchondral bone collagen metabolism in osteoarthritis of the hip. Br J Rheumatol. 1997;36:16–19. doi: 10.1093/rheumatology/36.1.16. [DOI] [PubMed] [Google Scholar]
- 4.Lajeunesse D, Massicotte F, Pelletier JP, Martel-Pelletier J. Subchondral bone sclerosis in osteoarthritis: not just an innocent bystander. Mod Rheumatol. 2003;13:7–14. doi: 10.3109/s101650300001. [DOI] [PubMed] [Google Scholar]
- 5.Martel-Pelletier J, Lajeunesse D, Pelletier JP. Etiopathogenesis of osteoarthritis. In: Koopman Moreland., editor. Arthritis & allied conditions. A textbook of rheumatology. 15. Baltimore: Lippincott, Williams & Wilkins; 2005. pp. 2199–226. [Google Scholar]
- 6.Kwan Tat S, Pelletier JP, Lajeunesse D, Fahmi H, Lavigne M, Martel-Pelletier J. The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells. Clin Exp Rheumatol. 2008;26:295–304. [PMC free article] [PubMed] [Google Scholar]
- 7.Tat SK, Pelletier JP, Lajeunesse D, Fahmi H, Duval N, Martel-Pelletier J. Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: influence of osteotropic factors. Bone. 2008;43:284–91. doi: 10.1016/j.bone.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 9.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komuro H, Olee T, Kuhn K, et al. The osteoprotegerin/receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand system in cartilage. Arthritis Rheum. 2001;44:2768–76. doi: 10.1002/1529-0131(200112)44:12<2768::aid-art464>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–16. [PubMed] [Google Scholar]
- 13.Nyambo R, Cross N, Lippitt J, et al. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J Bone Miner Res. 2004;19:1712–21. doi: 10.1359/JBMR.040703. [DOI] [PubMed] [Google Scholar]
- 14.De Toni EN, Thieme SE, Herbst A, et al. OPG is regulated by beta-catenin and mediates resistance to TRAIL-induced apoptosis in colon cancer. Clin Cancer Res. 2008;14:4713–8. doi: 10.1158/1078-0432.CCR-07-5019. [DOI] [PubMed] [Google Scholar]
- 15.Cremer I, Dieu-Nosjean MC, Marechal S, et al. Long-lived immature dendritic cells mediated by TRANCE-RANK interaction. Blood. 2002;100:3646–55. doi: 10.1182/blood-2002-01-0312. [DOI] [PubMed] [Google Scholar]
- 16.Wiethe C, Dittmar K, Doan T, Lindenmaier W, Tindle R. Enhanced effector and memory CTL responses generated by incorporation of receptor activator of NF-kappa B (RANK)/RANK ligand costimulatory molecules into dendritic cell immunogens expressing a human tumor-specific antigen. J Immunol. 2003;171:4121–30. doi: 10.4049/jimmunol.171.8.4121. [DOI] [PubMed] [Google Scholar]
- 17.Yu Q, Gu JX, Kovacs C, Freedman J, Thomas EK, Ostrowski MA. Cooperation of TNF family members CD40 ligand, receptor activator of NF-kappa B ligand, and TNF-alpha in the activation of dendritic cells and the expansion of viral specific CD8+ T cell memory responses in HIV-1-infected and HIV-1-uninfected individuals. J Immunol. 2003;170:1797–805. doi: 10.4049/jimmunol.170.4.1797. [DOI] [PubMed] [Google Scholar]
- 18.Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardus A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22:860–6. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- 20.Orita Y, Yamamoto H, Kohno N, et al. Role of osteoprotegerin in arterial calcification: development of new animal model. Arterioscler Thromb Vasc Biol. 2007;27:2058–64. doi: 10.1161/ATVBAHA.107.147868. [DOI] [PubMed] [Google Scholar]
- 21.Altman RD, Asch E, Bloch DA, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.Boileau C, Pelletier JP, Tardif G, et al. The regulation of human MMP-13 by licofelone, an inhibitor of cyclooxygenases and 5-lipoxygenase, in human osteoarthritic chondrocytes is mediated by the inhibition of the p38 MAP kinase signaling pathway. Ann Rheum Dis. 2005;64:891–8. doi: 10.1136/ard.2004.026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tardif G, Hum D, Pelletier JP, Boileau C, Ranger P, Martel-Pelletier J. Differential gene expression and regulation of the bone morphogenetic protein antagonists follistatin and gremlin in normal and osteoarthritic human chondrocytes and synovial fibroblasts. Arthritis Rheum. 2004;50:2521–30. doi: 10.1002/art.20441. [DOI] [PubMed] [Google Scholar]
- 24.Theoleyre S, Kwan Tat S, Vusio P, et al. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: role in the interactions with receptor activator of nuclear factor kappaB ligand (RANKL) and RANK. Biochem Biophys Res Commun. 2006;347:460–7. doi: 10.1016/j.bbrc.2006.06.120. [DOI] [PubMed] [Google Scholar]
- 25.Irie A, Takami M, Kubo H, Sekino-Suzuki N, Kasahara K, Sanai Y. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone. 2007;41:165–74. doi: 10.1016/j.bone.2007.04.190. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu S, Asou Y, Itoh S, et al. Prevention of cartilage destruction with intraarticular osteoclastogenesis inhibitory factor/osteoprotegerin in a murine model of osteoarthritis. Arthritis Rheum. 2007;56:3358–65. doi: 10.1002/art.22941. [DOI] [PubMed] [Google Scholar]
- 27.Kadri A, Ea HK, Bazille C, Hannouche D, Liote F, Cohen-Solal ME. Osteoprotegerin inhibits cartilage degradation through an effect on trabecular bone in murine experimental osteoarthritis. Arthritis Rheum. 2008;58:2379–86. doi: 10.1002/art.23638. [DOI] [PubMed] [Google Scholar]
- 28.Gravallese EM, Manning C, Tsay A, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–8. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Takayanagi H, Iizuka H, Juji T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–69. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–31. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritchlin CT, Schwarz EM, O’Keefe RJ, Looney RJ. RANK, RANKL and OPG in inflammatory arthritis and periprosthetic osteolysis. J Musculoskelet Neuronal Interact. 2004;4:276–84. [PubMed] [Google Scholar]
- 32.Saidenberg-Kermanac’h N, Cohen-Solal M, Bessis N, De Vernejoul MC, Boissier MC. Role for osteoprotegerin in rheumatoid inflammation. Joint Bone Spine. 2004;71:9–13. doi: 10.1016/S1297-319X(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 33.Fazzalari NL, Kuliwaba JS, Atkins GJ, Forwood MR, Findlay DM. The ratio of messenger RNA levels of receptor activator of nuclear factor kappaB ligand to osteoprotegerin correlates with bone remodeling indices in normal human cancellous bone but not in osteoarthritis. J Bone Miner Res. 2001;16:1015–27. doi: 10.1359/jbmr.2001.16.6.1015. [DOI] [PubMed] [Google Scholar]
- 34.Standal T, Seidel C, Hjertner O, et al. Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood. 2002;100:3002–7. doi: 10.1182/blood-2002-04-1190. [DOI] [PubMed] [Google Scholar]
- 35.Barre PE, Redini F, Boumediene K, Vielpeau C, Pujol JP. Semiquantitative reverse transcription-polymerase chain reaction analysis of syndecan-1 and -4 messages in cartilage and cultured chondrocytes from osteoarthritic joints. Osteoarthritis Cartilage. 2000;8:34–43. doi: 10.1053/joca.1999.0286. [DOI] [PubMed] [Google Scholar]
- 36.Redini F. Structure and regulation of articular cartilage proteoglycan expression. Pathol Biol (Paris) 2001;49:364–75. doi: 10.1016/s0369-8114(01)00145-6. [DOI] [PubMed] [Google Scholar]
- 37.Salminen-Mankonen H, Saamanen AM, Jalkanen M, Vuorio E, Pirila L. Syndecan-1 expression is upregulated in degenerating articular cartilage in a transgenic mouse model for osteoarthritis. Scand J Rheumatol. 2005;34:469–74. doi: 10.1080/03009740500304338. [DOI] [PubMed] [Google Scholar]
- 38.Cheifetz S, Massague J. Transforming growth factor-beta (TGF-beta) receptor proteoglycan. Cell surface expression and ligand binding in the absence of glycos-aminoglycan chains. J Biol Chem. 1989;264:12025–8. [PubMed] [Google Scholar]
- 39.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 40.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes: a role in osteoarthritis. J Clin Invest. 1996;97:2011–19. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiller O, Lichte A, Oberpichler A, Kocourek A, Tschesche H. Matrix metalloproteinases collagenase-2, macrophage elastase, collagenase-3, and membrane type 1-matrix metalloproteinase impair clotting by degradation of fibrinogen and factor XII. J Biol Chem. 2000;275:33008–13. doi: 10.1074/jbc.M001836200. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–95. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 43.Ferrell WR, Lockhart JC, Kelso EB, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abe K, Aslam A, Walls AF, Sato T, Inoue H. Up-regulation of protease-activated receptor-2 by bFGF in cultured human synovial fibroblasts. Life Sci. 2006;79:898–904. doi: 10.1016/j.lfs.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 45.Xiang Y, Masuko-Hongo K, Sekine T, et al. Expression of proteinase-activated receptors (PAR)-2 in articular chondrocytes is modulated by IL-1beta, TNF-alpha and TGF-beta. Osteoarthritis Cartilage. 2006;14:1163–73. doi: 10.1016/j.joca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Busso N, Frasnelli M, Feifel R, et al. Evaluation of protease-activated receptor 2 in murine models of arthritis. Arthritis Rheum. 2007;56:101–7. doi: 10.1002/art.22312. [DOI] [PubMed] [Google Scholar]
- 47.Boileau C, Amiable N, Martel-Pelletier J, Fahmi H, Duval N, Pelletier JP. Activation of proteinase-activated receptor 2 in human osteoarthritic cartilage upregulates catabolic and proinflammatory pathways capable of inducing cartilage degradation: a basic science study. Arthritis Res Ther. 2007;9:R121. doi: 10.1186/ar2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amiable N, Kwan Tat S, Lajeunesse D, et al. Proteinase-activated receptor (PAR)-2 activation impacts bone resorptive properties of human osteoarthritic subchondral bone osteoblasts. Bone. 2009;44:1143–50. doi: 10.1016/j.bone.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakashima T, Wada T, Penninger JM. RANKL and RANK as novel therapeutic targets for arthritis. Curr Opin Rheumatol. 2003;15:280–7. doi: 10.1097/00002281-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–66. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]