Abstract
Acute hepatitis C virus (HCV) is typically defined as new viremia and antibody seroconversion. Rates and immunologic correlates of hepatitis C clearance have therefore been based on clearance of viremia only in individuals who initially had an antibody response. We sought to characterize the immunological correlates of clearance in patients with acute hepatitis C and their sexual contacts. We prospectively determined CD4+ and CD8+ cytotoxic T-lymphocyte responses in index patients with acute HCV and their sexual contacts who developed acute infection, either with or without spontaneous clearance, as well as those contacts who never developed viremia. Responses were measured using proliferation and ELISpot assays for CD4+ and CD8+ responses. We demonstrate in this prospective study that cellular immune responses can develop in exposed but persistently aviremic and antibody-negative individuals as well as those individuals with spontaneous clearance of acute HCV. These findings lend further credence to the importance of cellular immune responses in recovery from HCV and suggest that low exposure to HCV may lead to development of HCV-specific immune responses without ongoing HCV replication. This finding has important implications for HCV vaccine and therapeutic development.
Hepatitis C virus (HCV) is a frequent cause of liver disease, leading to chronic infection in as many as 170 million persons worldwide. Vaccine development for HCV, like that for human immunodeficiency virus type 1 (HIV-1), is limited by the quasispecies nature of the virus as well as a lack of clear evidence that humoral immune responses protect against infection. Unlike patients with HIV-1, some individuals infected with acute hepatitis C can recover, although the asymptomatic nature of acute infection in most infected persons and the relative difficulty in conducting prospective studies have limited our understanding of the correlates of recovery from infection. Therefore, most studies have relied upon retrospective identification of subjects who previously cleared HCV to ascertain the correlates of protective immunity. These studies have shown that vigorous polyclonal cellular immune responses are associated with spontaneous recovery, whereas chronic infection is associated with a less vigorous immune response in the peripheral blood and liver (2, 6, 9, 11, 19, 22, 31). The only animal model of HCV infection, the chimpanzee, has provided additional valuable insights into the importance of both CD4+ and CD8+ responses in controlling infection (4, 8, 30). However, the small numbers of individuals who can be identified as having spontaneous recovery and the retrospective nature of such studies may underestimate the true rates of clearance.
The major risk factor for transmission of HCV is percutaneous or parenteral exposure to infected blood or blood products. However, sexual transmission may play a role in some cases, although the exact extent to which this is true and the precise determinants of transmission are as yet unknown. In this study we took advantage of a prospective study of sexual transmission between heterosexual couples to determine whether immune responses developed in partners of patients with acute hepatitis C. We determined that a substantial number of individuals who are exposed but remain persistently aviremic and antibody negative develop CD4+ and CD8+ cellular immune responses.
MATERIALS AND METHODS
Patients.
This study is a part of a larger survey for detection of HCV among health care workers (HCWs) in several Egyptian centers. All HCWs were screened for HCV, and those who were negative were rescreened if a needle stick or related sharp injury was reported. When an HCW reported a needle stick injury he or she was tested for HCV by enzyme-linked immunosorbent assay and HCV PCR. The mean time between a needle stick and the first positive PCR was 4.2 ± 2.5 weeks (range, 3 to 7 weeks). Index patients were enrolled in the present study if they fulfilled the following criteria: seroconversion from negative anti-HCV antibody status at baseline to positive anti-HCV antibody status by third-generation enzyme-linked immunosorbent assay (Roche Diagnostics, Branchburg, N.J.), a positive PCR for HCV RNA (Cobas Amplicor HCV version 2.0, with a lower limit of detection of 100 copies/ml; Roche Diagnostics), and a serum alanine transferase (ALT) level elevated >10 times above the upper limit of normal with or without symptoms. Their spouses were also screened immediately after identification of the index case (range, 1 to 3 days postexposure).
If the index case was defined as acute HCV and the sexual contact was seronegative with the absence of HCV RNA confirmed by PCR (lower limit of detection of 100 copies/ml), the patients were enrolled and prospectively followed. Peripheral blood mononuclear cell (PBMC) and serum samples were collected at weeks 0, 2, 4, 8, 12, 18, 24, and 48. Fifty-two subjects (male-to-female ratio, 32:20 [Table 1 ]) fulfilled the inclusion criteria and served as index cases. All index patients had genotype 4 as determined by a second-generation reverse hybridization line-probe assay (Inno-LiPA HCV II; Innogenetics, Zwijndrecht, Belgium). Four index patients (7.7%) had symptoms and jaundice, while 48 patients had mild, nonspecific symptoms. Fifty-two spouses (male-to-female ratio, 20:32 [Table 1]) with documented negative HCV status were enrolled in the study. PBMCs from 20 healthy donors served as negative controls for HCV-specific proliferative responses and cytokine ELISpot assays.
TABLE 1.
Baseline demographic characteristics of index patients with acute HCV and their sexual contacts
| Parameter | Index patients | Contacts |
|---|---|---|
| No. of patients | 52 | 52 |
| Male/Female | 32/20 | 20/32 |
| Mean age ± SD (yr) | 31.4 ± 5.3 | 30.7 ± 4.6 |
| No. of patients with indicated risk factor for HCV transmission: | ||
| Occupational exposure (needle sticks, sharps) | 52 | 0 |
| Blood transfusion | 0 | 0 |
| Intravenous drug use | 0 | 0 |
| Sexual activity | 0 | 52 |
| Mean total bilirubin ± SD (mg/dl) | 1.9 ± 0.7 | 0.5 ± 0.15 |
| Mean ALT ± SD (U/liter) | 544.3 ± 162.7 | 18.4 ± 4.3 |
| Mean aspartate transaminase ± SD (U/liter) | 547.6 ± 165.3 | 12.5 ± 7.6 |
| No. of infected patients (%) | 52 (100) | 8 (15) |
| No. of patients with symptoms/jaundice (%) | 4 (7.7) | 0/8 (0) |
| No. of patients with spontaneous recovery/no. infected (%) | 9/52 (17) | 4/8 (50) |
Clinical and biochemical laboratory values (serum ALT, aspartate transaminase, albumin, bilirubin concentrations, and prothrombin time) as well as positive anti-HCV antibody status by third-generation enzyme-linked immunosorbent assay (Roche Diagnostics) were determined in index patients and their contacts at enrollment and then at weeks 2, 4, 8, 12, 18, and 24 during the study and monthly thereafter until the end of follow-up (48 weeks). HCV RNA (Cobas Amplicor HCV version 2.0, with a lower limit of detection of 100 copies/ml; Roche Diagnostics) was performed at the same time points.
HLA typing of index patients and contacts was performed by standard serological techniques according to the manufacturer's (Behringer Ingelheim, Manheim, Germany) instructions.
The study protocol was reviewed and approved by the Ethics Committee of each of the participating centers: Ain Shams University, Albert Ludwig University, and Beth Israel Deaconess Medical Center. All patients participating in the study presented a written informed consent before enrollment and before any study-related procedures. The protocol and all study procedures were conducted in conformity with the ethical guidelines of the Declaration of Helsinki.
Antigens.
Purified recombinant HCV proteins (HCV core, amino acids [aa] 2 to 120; NS3, aa 1192 to 1457; NS4, aa 1569 to 1931; and NS5, aa 2054 to 2995) derived from the HCV type 1 prototype sequence were purchased from Chiron (Emeryville, Calif.). All antigens were expressed as COOH-terminal fusion proteins with human superoxide dismutase (SOD) in yeast. Yeast and SOD were used as controls for nonspecific stimulation in proliferation assays and ELISpot assays. As positive controls, 4 μg of phytohemagglutinin (PHA)/ml at a 1:200 dilution (Murex Diagnostics, Chatillon, France) and 1 μg of tetanus toxoid (TT)/ml (Wyeth Laboratories, St. Davids, Pa.) were used.
Synthetic peptides.
A panel of 18 peptides, each with a length of 9 or 10 amino acids corresponding to the amino acid sequences of the HCV type 1 strain core, envelope (E1), and nonstructural (NS4) regions of the HCV genotype la, grouped in five peptide pools was used to study recognition of the peptides by specific cytotoxic T lymphocytes (CTL). Peptides were synthesized as free acids by using the Mimotopes procedure (Chiron, Victoria, Australia) and the 9-fluorenylmethoxy carbonyl method (14). Peptides were selected according to known HLA-A2 binding motifs (14, 15, 21). The amino acid sequences of the peptides, their positions within the HCV amino acid sequence, and their degrees of relative conservation with regard to genotype 4a are presented in Table 2. All peptides were reconstituted in sterile distilled water containing 10% dimethyl sulfoxide (Sigma Chemical Co.) and 1 mM dithiothreitol (Sigma Chemical Co.).
TABLE 2.
Sequences of HCV peptides used in ELISpot assays
| No. | Peptide pool | HCV region | Position | Length (aa) | Amino acid sequence | Conservation of genotype 4a (%) |
|---|---|---|---|---|---|---|
| 1 | P1 | Core | 36-44 | 9 | LLPRRGPRL | 100 |
| 2 | P1 | Core | 132-140 | 9 | DLMGYIPLV | 100 |
| 3 | P1 | Core | 150-158 | 9 | ALAHGVRVL | 92 |
| 4 | P1 | NS4 | 1610-1618 | 9 | CLIRLKPTL | 100 |
| 5 | P1 | NS4 | 1623-1631 | 9 | PLLYRLGAV | 100 |
| 6 | P2 | Core | 136-144 | 9 | YIPLVGAPL | 100 |
| 7 | P2 | Core | 125-133 | 9 | TLTCGFADL | 100 |
| 8 | P2 | Core | 118-126 | 9 | NLGKVIDTL | 100 |
| 9 | P2 | Core | 161-169 | 9 | GVNYATGNL | 100 |
| 10 | P2 | E1 | 245-253 | 9 | TVATRDGKL | 89 |
| 11 | P3 | E1 | 212-220 | 9 | IVYEAADAI | 90 |
| 12 | P3 | Core | 168-176 | 9 | NLPGCSFSI | 100 |
| 13 | P3 | E1 | 332-340 | 9 | LVMAQLLRI | 78 |
| 14 | P4 | Core | 154-162 | 9 | GVRVLEDGV | 100 |
| 15 | P4 | E1 | 276-284 | 9 | YVGDLCGCV | 78 |
| 16 | P4 | E1 | 219-227 | 9 | AILHTPGCV | 90 |
| 17 | P5 | E1 | 337-346 | 10 | DLIAQPIRLL | 50 |
| 18 | P5 | E1 | 363-372 | 10 | SMVGNWAKVL | 100 |
Cell preparation.
PBMCs from study subjects and controls were isolated immediately from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation using standard techniques and immediately cryopreserved. Cryopreserved PBMCs were tested in a proliferation assay and ELISpot assays.
The CD4+ or CD8+ T-cell populations were isolated from the PBMC by positive selection using paramagnetic microbeads conjugated to a monoclonal mouse anti-human anti-CD8+ or anti-CD4+ antibody (Micro Beads; Miltenyl Biotec, Bergisch Gladbach, Germany) as previously described (29, 30). The viability and purity of isolated CD4+ or CD8+ T-cell populations were checked by flow cytometry, which revealed more than 95% purity.
The human HLA-A2.1-positive mutant cell line 0.174×CEM.T2 (T2) is defective for peptide transporter molecules and constitutively expresses empty HLA-A2.1 molecules on its cell membranes due to its inability to present peptides that had undergone intracellular processing (15, 21). The T2 cells were loaded efficiently with synthetic peptides and were used as target cells in ELISpot assays.
Proliferation assays.
Proliferation assays were performed for all study subjects and controls at weeks 0, 4, 8, 12, 24, and 48 using cryopreserved PBMCs (2 × 105) as previously described (11, 13) with the HCV proteins described above at concentrations of 2 μg/ml and control antigens. All proliferation assays were performed after 5 days of culture with HCV antigens or control antigens. All values were obtained in triplicate. A stimulation index (SI) of 3 or more, which represents 3 standard deviations (SD) above the mean SI of normal control subjects, was considered significant. No healthy controls (0 of 20) mounted a significant response to any HCV-specific antigen (mean SIcore, 1.0; SINS3, 0.8; SINS4, 0.9; SINS5, 0.8 [data not shown]). CD4+ and CD8+ depletion assays performed as previously described (29, 30), using paramagnetic microbeads conjugated to a monoclonal mouse anti-human anti-CD8+ or anti-CD4+ antibody (Micro Beads), showed that proliferative responses are mediated by CD4+ cells (data not shown).
ELISpot assay.
ELISpot assays were performed as previously described (9, 12). Cryopreserved PBMCs (200,000 cells/well) were thawed and incubated at 37°C overnight in R-10 medium. Ninety-six-well nitrocellulose plates were coated with 2.5 μg of recombinant human anti-gamma interferon (IFN-γ) or interleukin 4 (IL-4) antibody (Endogen, Woburn, Mass.)/ml in a carbonate and bicarbonate buffer (pH 9.6) overnight at 4°C. For T-helper-cell assays, PBMCs were incubated with soluble protein antigens, HCV proteins (2 μg/ml), positive control proteins (PHA at 5 μg/ml and TT at 1 μg/ml), SOD, yeast stimulation, and medium as a negative control in 96-well U-bottomed plates overnight and then transferred directly into the ELISpot plate. For the cytotoxicity assays, 105 purified CD8+ T cells were added per well. Control wells contained unstimulated CD8+ T cells alone or CD8+ T cells with either T2 cells or peptide alone. Then, mitomycin C-treated T2 cells (7.5 × 104/well) and the peptides at a final concentration of 100 μg/ml were added and incubated. After incubation at 37°C for 24 h for IFN-γ assays and 40 h for IL-4 assays, the plates were washed, labeled with 0.25 mg of biotin-labeled anti-human IFN-γ (Endogen)/ml, and developed by incubating with streptavidin-alkaline phosphatase (Bio-Rad) followed by incubating with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium (Bio-Rad) in Tris buffer (pH 9.5). The reaction was stopped by washing with tap water, and the mixture was allowed to dry before the spots were counted on an automated ELISpot reader (AID, Strassberg, Germany). To exclude the possibility of nonspecific stimulation by the peptide mix, patient PBMCs were tested with a control mix of overlapping peptides. In addition, PBMCs of 20 healthy, anti-HCV-antibody-negative controls were tested with HCV proteins and HCV peptide mixes. No significant responses were observed. A response was scored as positive if it was greater than the mean response in healthy, anti-HCV-antibody-negative control subjects plus 3 SD and more than threefold greater than the background response (buffer control for proteins or dimethyl sulfoxide control for peptides) in HCV-infected patients.
To elucidate the cell fraction producing the HCV-specific response, unfractionated PBMCs and CD4+- and CD8+-depleted cells were also tested in the ELISpot assay.
Statistical analysis.
Results were expressed as means ± SD and analyzed using paired and unpaired Student's t, χ2, nonparametric Mann-Whitney U, Wilcoxon rank sum, or Fisher exact testing where appropriate. Correlation between different parameters was performed using Pearson's or Spearman's rank test. All hypothesis tests were two tailed, and statistical significance was assessed at the 0.05 levels. All statistical procedures were performed using an SPSS package version 11 for Windows (SPSS Inc., Chicago, Ill.).
RESULTS
Outcome of acute hepatitis C.
In this cohort, 9 (17%) of the 52 index patients had spontaneous recovery, and 43 (83%) became chronically infected. Of the sexual contacts, 8 (15%) out of 52 developed HCV viremia. Four of these eight individuals subsequently resolved viremia spontaneously, for a spontaneous recovery rate of 50%. In all eight cases, genotypic and detailed virologic analysis of the E2 hypervariable and NS5b regions revealed transmission of concordant virus (data not shown).
HCV-specific CD4+ cells are detected in subjects with spontaneous recovery and persistent aviremia.
Index cases and contacts were tested prospectively for the development of immune responses against hepatitis C. At baseline none of the index patients or contacts mounted any significant HCV T-cell responses to the antigens tested (Fig. 1). The HCV-specific T-cell-proliferative responses were due to CD4+ T cells as determined by CD4+ and CD8+ T-cell depletion assays (data not shown). As expected, in this cohort the magnitude and breadth of CD4+ T-cell responses as measured by lymphoproliferative assays were significantly higher in patients and contacts who had spontaneous recovery from acute infection than in those who had evolution to chronic infection (Fig. 1 and 2). In individuals with spontaneous recovery, responses were detected early and were maintained throughout the study period; in contrast, those individuals who went on to chronic evolution never mounted lymphoproliferative responses. Fourteen contacts were negative for HCV by antibody testing and PCR at baseline, and some had previous documentation of negative HCV status. Interestingly, these 14 contacts were never found to have developed viremia by conventional testing (enzyme-linked immunosorbent assay and PCR) or antibody response detection and yet mounted detectable proliferative responses against HCV proteins at different time points through the study. The proliferative responses were significantly lesser in magnitude and breadth than those detected in individuals who had spontaneous recovery, yet they were maintained throughout the study period (Fig. 1 and 2).
FIG. 1.
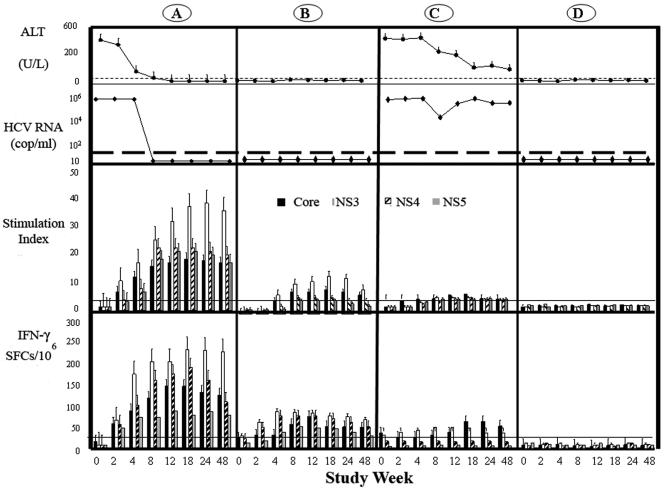
Time course of ALT, HCV RNA, and immune responses. Serum ALT, HCV RNA,CD4+ proliferative response, and IFN-γ in subjects with spontaneous recovery (A), contacts with persistent aviremia but detectable HCV-specific immune responses (B), contacts with chronic evolution (C), and noninfected contacts with no HCV (D). The upper two rows show the ALT (40 U/liter; filled circles) and mean serum HCV RNA levels (filled diamonds). The third row shows CD4+ proliferative T-cell responses to four HCV proteins (core, NS3, NS4, and NS5) expressed as SI (y axis). The cutoff for a positive response is an SI of 3. Bars represent mean responses to a given antigen, and lines represent SDs. The lower row shows numbers of SFCs expressing IFN-γ in the ELISpot assay after stimulation with core protein, NS3, NS4, and NS5. Bars represent mean responses, and lines represent SDs. Horizontal lines represent cutoff values.
FIG. 2.
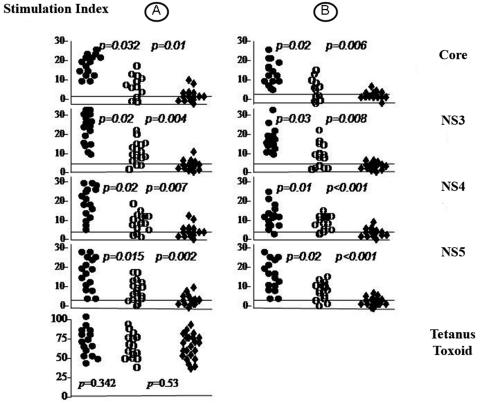
HCV-specific CD4+ proliferative responses in patients and contacts. HCV-specific CD4+ T-cell proliferative response in subjects with spontaneous recovery (filled circles), persistent aviremia (open circles), and chronic evolution (filled diamonds) are shown. Responses at week 12 (A) and the end of follow-up (B) are shown. Each point represents a subject. Each horizontal line represents the cutoff for a given response. The lower left panel represents the response to tetanus toxoid at baseline.
We confirmed these findings using the ELISpot assay, which also provided measurement of the qualitative nature of the immune response. As expected, subjects with spontaneous recovery mounted a more vigorous CD4+ response characterized by production of IFN-γ than those with chronic infection (Fig. 1). These responses were broader and of significantly greater magnitude than those in subjects with chronic infection (Tables 3 and 4). HCV-specific responses were determined to be strongly biased to production of IFN-γ in the acute infection setting, since we could not detect IL-4 against any HCV antigen at any time point (data not shown). As with the lymphoproliferative response, we found that a substantial proportion of the contacts developed production of IFN-γ against HCV antigens in the absence of viremia and seroconversion, which again was statistically greater than that of subjects who went on to chronic infection although less than that of subjects who had spontaneous recovery after viremia. Thirty spouses were noninfected and did not display any significant IFN-γ response at any time point (Fig. 1).
TABLE 3.
IFN-γ production in response to HCV proteins (ELISpot) at week 12a
| HCV patient profile (no. of patients) | Mean no. of cells producing IFN-γ per million PBMCs ± SD
|
||||
|---|---|---|---|---|---|
| Core HCV protein | NS3 | NS4 | NS5 | Tetanus toxoid | |
| Spontaneous recovery (13)b | 483 ± 120.6 | 645 ± 123 | 593 ± 215 | 470 ± 154 | 813 ± 346 |
| Persistent aviremia (14)c | 158 ± 73 | 112 ± 56 | 132 ± 19 | 179 ± 47 | 765 ± 213 |
| Chronic evolution (47)d | 56 ± 34 | 21 ± 3 | 13 ± 7 | 20 ± 2 | 793 ± 243 |
P values for patients with the indicated conditions in response to core HCV protein, NS3, NS4, NS5, and tetanus toxoid were as follows: spontaneous recovery versus persistent aviremia, 0.004, 0.004, 0.02, 0.09, and 0.95, respectively; spontaneous recovery versus chronic evolution, 0.001, 0.006, 0.004, 0.009, and 0.657, respectively; persistent aviremia versus chronic evolution, 0.01, 0.020, 0.005, 0.002, and 0.300, respectively.
Nine index patients and four contacts.
Aviremic contacts who had HCV responses.
Forty-three index patients and four infected contacts.
TABLE 4.
Cytotoxic T-lymphocyte responses: IFN-γ production in response to HCV peptide pools at different time pointsa
| HCV patient profile | No. of patients | No. of patients with positive ELISpot (%)
|
No. of pools
|
||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Week 48 | Baseline | Week 12 | Week 48 | ||
| Spontaneous recovery | 9 index patients | 0 | 9 (100) | 8 (100) | 0 | 4 | 3 |
| 4 contacts | 0 | 4 (100) | 4 (100) | 0 | 5 | 3 | |
| Persistent aviremia with detectable responses | 8 contacts | 0 | 5 (63) | 5 (63) | 0 | 3 | 2 |
| Persistent aviremia with no detectable responses | 7 contacts | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic evolution | 9 index patients | 0 | 2 (22) | 2 (22) | 0 | 2 | 1 |
| 4 infected contacts | 2 (50) | 2 (50) | 0 | 2 | 1 | ||
CTL responses were tested in a subset of subjects who were HLA A2 positive (18 of 52 index patients and 23 of 52 contacts, including eight infected contacts [four with spontaneous recovery and four with chronic evolution], eight contacts with persistent aviremia, and seven noninfected contacts) by using a panel of peptides representing CTL epitopes restricted by HLA.
In all experiments, stimulation with PHA and TT induced similar numbers of IFN-γ and IL-4 spot-forming cells (SFCs) in all subject groups, thus indicating the capacity of T cells to produce these cytokines (data not shown). To elucidate the cell fraction producing the HCV-specific response, unfractionated PBMCs and CD4+- and CD8+-depleted cells were also tested in the ELISpot assay. Depletion experiments confirmed that IFN-γ secretion was limited to CD4+ T cells (data not shown).
Cytotoxic T-lymphocyte responses are present in exposed seronegative contacts as well as subjects with spontaneous recovery.
In a subset of subjects who were HLA A2 positive (18 of 52 index patients [nine with spontaneous recovery and nine with chronic evolution] and 23 of 52 contacts, including 8 infected contacts [4 with spontaneous recovery and 4 with chronic evolution], 8 contacts with persistent aviremia, and 7 noninfected contacts) we tested CTL responses using a panel of peptides representing CTL epitopes restricted by HLA A201 (Table 2). Using this technique, we also found that CTL responses against this defined panel of epitopes were strongest in subjects with spontaneous recovery. At baseline no CTL responses could be detected in any of the index cases or their sexual contacts (Fig. 3A, Table 4). At week 12 after the first positive HCV RNA test, CTL responses could be detected in 5 of 8 tested contacts with persistent aviremia, while weak CTL responses were detected in only 4 of 13 subjects who evolved to chronic infection (Fig. 3B). Twelve subjects with resolved acute infection tested positive with 90% of the tested peptides compared to four (31%) subjects with chronic hepatitis C who responded to only 40% of relevant peptides (P = 0.001). However, five (63%) of eight tested contacts with persistent aviremia responded to 60% of tested peptides (P = 0.02 for subjects with spontaneous resolution versus aviremic exposed sexual contacts). At the end of follow-up, we could detect HCV-specific IFN-γ production in 12 (92%) of 13 subjects with resolved infection in response to 60% of relevant peptides tested in comparison to 4 subjects (30%) with chronic evolution (P = 0.008). HCV-specific IFN-γ production was demonstrated in six (75%) of eight contacts with persistent aviremia. CD8+ T-cell responses were maintained in all subjects with resolved acute HCV infection and in six of eight tested seronegative contacts at the end of follow-up (Fig. 3C). In contrast, only two of four subjects with chronic evolution had detectable CD8+ T-cell responses at the end of follow-up (Table 4).
FIG. 3.
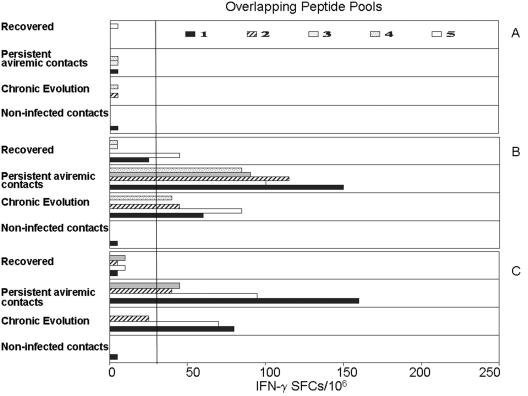
HCV-specific CTL activity from HLA-A2-positive HCV patients stimulated with peptide pools, including the following: subjects with spontaneous recovery, contacts with persistent aviremia but detectable immune responses, subjects with chronic evolution, and noninfected subjects with no detectable HCV-specific immune responses. At the baseline (A) no CTL responses were detected in any index patients or contacts. At week 12 (B) and at the end of follow-up (C) HCV-specific CTL responses are more vigorous in subjects with spontaneous recovery and persistent aviremia than in those with evolution to chronic disease. Each bar represents the mean response to a given peptide pool. The details of the peptide pools are given in Materials and Methods. The vertical line represents the cutoff for each response.
DISCUSSION
In this study, we demonstrated that (i) individuals with acute HCV and spontaneous recovery mount polyclonal CD4+ and CD8+ T-cell responses that are greater in magnitude and breadth than those of individuals who fail to recover and (ii) a substantial proportion of exposed but persistently aviremic and antibody-seronegative individuals mount a CD4+ and CD8+ response that is comparable in vigor and breadth to that of individuals with acute HCV infection and spontaneous recovery.
This is a prospective demonstration in humans that cellular immune responses against HCV may be mounted following exposure even in the absence of antibody seroconversion. Previous retrospective studies have suggested that some individuals with acute HCV may lose antibody over time. Takaki et al. examined a large cohort of individuals with known acute HCV many years after resolution of the disease and demonstrated that cellular immune responses were maintained without corresponding humoral immune responses, suggesting that antibodies could be lost over time (31, 32). We have previously shown that several health care workers with needle stick injury had detectable CD4+ and CD8+ responses, although definitive exposure to HCV and a lack of antibody seroconversion could not be proven (17). Similar findings were noted in long-term partners of HCV-positive persons, although again it could not be proven that the partners had not lost antibody (1, 28). However, in this study we demonstrate that polyclonal immune responses are present following known exposure even in individuals who fail to develop antibody responses and viremia. Given the polyclonal nature of the immune response, this suggests that viral replication at very low levels, below those measured by presently available assays, occurred and primed the immune response. This is consistent with a recent report that inoculation of chimpanzees with very low levels of HCV (1 to 10 copies) might induce cellular immune responses without corresponding viremia (29), but the relevance to human disease, where there are extremely high levels of circulating virus in the blood, was unclear until this report. We cannot completely exclude a transient but detectable viremia that might have resolved in between our frequent measurements of HCV RNA, but most prospective studies of acute infection have been able to detect viremia for weeks when it occured.
Similar findings have been noted in persons repetitively exposed to HIV-1 without seroconversion. HIV-specific CD4+ T-helper cells and CTL have been detected in persons exposed through sexual contact (18, 25) or contact with contaminated blood (3, 23) as well as in seronegative infants of infected women (5, 26). These studies have shown recognition of diverse epitopes in these highly exposed but seronegative individuals, although the repertoire of epitopes recognized by HIV-infected and uninfected persons may be different (21, 24).
Our results provide additional support to previous studies demonstrating the role of cellular immunity in the resolution of acute HCV, even as measured solely by antibody seroconversion. Clearance of HCV is associated with the development of a polyclonal CD8+ response in humans (20, 27, 32) and chimpanzees (4). In contrast, individuals who go on to chronic infection fail to mount such a response or may have inadequate production of the cytokine essential to control of viral replication (19). CTL may be present in the liver tissue of chronically infected patients (15, 16) but are ineffective at completely controlling viral replication. Resolution of acute HCV is also marked by the development of polyclonal CD4+ T-helper-cell responses in blood (6, 7, 10, 13, 22) and liver (9) that in some cases can be maintained for years after recovery from acute disease (31). Given the high seroprevalence rate of HCV in the region, we cannot completely exclude that the contacts may have had prior, resolved HCV infection and thus that the responses here represent memory responses, but we believe this to be unlikely. Even if this were the case, it would reinforce the notion that cryptic infection can occur and elicit relatively broad immune responses. Failure of the memory CD4+ and CD8+ responses was recently shown to be essential to long-term control of HCV, with incomplete control of viral replication by CD8+ T cells in the absence of sufficient memory CD4+ cells leading to viral persistence and emergence of CTL escape mutants (8, 30). Given the limited number of patients with acute HCV viremia who go on to resolve infection, determining the epitopes recognized by individuals who remain uninfected despite repetitive exposure may be another method of defining the nature of the protective immune response.
One implication of these findings is that definition of infection solely on the basis of the presence of antibody at a single point in time may significantly underestimate the true rate of infection, and future prospective studies assessing factors associated with risk of infection or clearance may have to include measurement of cellular immune responses. It has been shown that some individuals at high risk for HCV, such as intravenous drug users, remain seronegative despite ongoing risk. Measurement of cellular immune responses in these populations might provide additional insight into the nature of protective immunity in HCV and further aid in vaccine development.
REFERENCES
- 1.Bronowicki, J. P., D. Vetter, G. Uhl, H. Hudziak, A. Uhrlacher, J. M. Vetter, and M. Doffoel. 1997. Lymphocyte reactivity to hepatitis C virus (HCV) antigens shows evidence for exposure to HCV in HCV-seronegative spouses of HCV-infected patients. J. Infect. Dis. 176:518-522. [DOI] [PubMed] [Google Scholar]
- 2.Chang, K. M., R. Thimme, J. J. Melpolder, D. Oldach, J. Pemberton, J. Moorhead-Loudis, J. G. McHutchison, H. J. Alter, and F. V. Chisari. 2001. Differential CD4 and CD8 T-cell responsiveness in hepatitis C virus infection. Hepatology 33:267-276. [DOI] [PubMed] [Google Scholar]
- 3.Clerici, M., J. M. Levin, H. A. Kessler, A. Harris, J. A. Berzofsky, A. Landay, and G. M. Sherer. 1994. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA 271:42-46. [PubMed] [Google Scholar]
- 4.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 5.De Maria, A., C. Cirillo, and L. Moretta. 1994. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected children born to HIV-1-infected mothers. J. Infect. Dis. 170:1296-1299. [DOI] [PubMed] [Google Scholar]
- 6.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, M. C. Jung, T. Gerlach, and G. R. Pape. 1996. The role of hepatitis C virus specific CD4+ T lymphocytes in acute and chronic hepatitis C. J. Mol. Med. 74:583-588. [DOI] [PubMed] [Google Scholar]
- 7.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 117:933-941. [DOI] [PubMed] [Google Scholar]
- 8.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302:659-662. [DOI] [PubMed] [Google Scholar]
- 9.Kamal, S., C. Graham, Q. He, L. Bianchi, J. Rasenack, A. Tawil, K. Khalifa, M. Massoud, and M. Koziel. 2004. Progression of fibrosis in chronic hepatitis C correlates with the kinetics of peripheral and intrahepatic hepatitis C-specific CD4+ T cell responses. J. Infect. Dis. 189:1140-1150. [DOI] [PubMed] [Google Scholar]
- 10.Kamal, S., A. Ismail, C. Graham, Q. He, J. Rasenack, T. Peters, A. Tawil, J. Fehr, E. Khalifa, M. Madwar, and M. Koziel. 2004. Pegylated interferon therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology 39:1721-1731. [DOI] [PubMed] [Google Scholar]
- 11.Kamal, S. M., L. Bianchi, A. Al Tawil, M. Koziel, K. El Sayed Khalifa, T. Peter, and J. W. Rasenack. 2001. Specific cellular immune response and cytokine patterns in patients coinfected with hepatitis C virus and Schistosoma mansoni. J. Infect. Dis. 184:972-982. [DOI] [PubMed] [Google Scholar]
- 12.Kamal, S. M., J. Fehr, B. Roesler, T. Peters, and J. W. Rasenack. 2002. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology 123:1070-1083. [DOI] [PubMed] [Google Scholar]
- 13.Kamal, S. M., J. W. Rasenack, L. Bianchi, A. Al Tawil, K. El Sayed Khalifa, T. Peter, H. Mansour, W. Ezzat, and M. Koziel. 2001. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4(+) T-cell and cytokine response. Gastroenterology 121:646-656. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, T., I. Nakamura, H. Kita, K. Hiroishi, T. Moriyama, and M. Imawari. 1996. Three new cytotoxic T cell epitopes identified within the hepatitis C virus nucleoprotein. J. Gen. Virol. 77:1305-1309. [DOI] [PubMed] [Google Scholar]
- 15.Koziel, M. J., D. Dudley, N. Afdhal, A. Grakoui, C. M. Rice, Q. L. Choo, M. Houghton, and B. D. Walker. 1995. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J. Clin. Investig. 96:2311-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koziel, M. J., D. Dudley, J. T. Wong, J. Dienstag, M. Houghton, R. Ralston, and B. D. Walker. 1992. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J. Immunol. 149:3339-3344. [PubMed] [Google Scholar]
- 17.Koziel, M. J., D. K. Wong, D. Dudley, M. Houghton, and B. D. Walker. 1997. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J. Infect. Dis. 176:859-866. [DOI] [PubMed] [Google Scholar]
- 18.Langlade-Demoyen, P., N. Ngo-Giang-Huong, F. Ferchal, and E. Oksenhendler. 1994. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J. Clin. Investig. 93:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 20.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubaki, M. N., M. A. Egan, R. F. Siliciano, K. J. Weinhold, and R. C. Bollinger. 1994. A novel method for detection and ex vivo expansion of HIV type 1-specific cytolytic T lymphocytes. AIDS Res. Hum. Retrovir. 10:1427-1431. [DOI] [PubMed] [Google Scholar]
- 22.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto, L. A., J. Sullivan, J. A. Berzofsky, M. Clerici, H. A. Kessler, A. L. Landay, and G. M. Shearer. 1995. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J. Clin. Investig. 96:867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Promadej, N., C. Costello, M. M. Wernett, P. S. Kulkarni, V. A. Robison, K. E. Nelson, T. W. Hodge, V. Suriyanon, A. Duerr, and J. M. McNicholl. 2003. Broad human immunodeficiency virus (HIV)-specific T cell responses to conserved HIV proteins in HIV-seronegative women highly exposed to a single HIV-infected partner. J. Infect. Dis. 187:1053-1063. [DOI] [PubMed] [Google Scholar]
- 25.Rowland-Jones, S., J. Sutton, K. Ariyoshi, T. Dong, F. Gotch, S. McAdam, D. Whitby, S. Sabally, A. Gallimore, T. Corrah, et al. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 26.Rowland-Jones, S. L., D. F. Nixon, M. C. Aldhous, F. Gotch, K. Ariyoshi, N. Hallam, J. S. Kroll, K. Froebel, and A. McMichael. 1993. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet 341:860-861. [DOI] [PubMed] [Google Scholar]
- 27.Sarobe, P., C. D. Pendleton, T. Akatsuka, D. Lau, V. H. Engelhard, S. M. Feinstone, and J. A. Berzofsky. 1998. Enhanced in vitro potency and in vivo immunogenicity of a CTL epitope from hepatitis C virus core protein following amino acid replacement at secondary HLA-A2.1 binding positions. J. Clin. Investig. 102:1239-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scognamiglio, P., D. Accapezzato, M. A. Casciaro, A. Cacciani, M. Artini, G. Bruno, M. L. Chircu, J. Sidney, S. Southwood, S. Abrignani, A. Sette, and V. Barnaba. 1999. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J. Immunol. 162:6681-6689. [PubMed] [Google Scholar]
- 29.Shata, M. T., N. Tricoche, M. Perkus, D. Tom, B. Brotman, P. McCormack, W. Pfahler, D. H. Lee, L. H. Tobler, M. Busch, and A. M. Prince. 2003. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology 314:601-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chien, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6:578-582. [DOI] [PubMed] [Google Scholar]
- 32.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]


