Abstract
Objectives
Calpain inhibition has an enhancing effect on myocardial perfusion and improves myocardial density by up regulating by inhibition of GSK-3β and up regulating downstream signaling pathways including the insulin/PI3K and WNT/β-catenin pathways in a pig model of chronic myocardial ischemia in the setting of metabolic syndrome.
Methods
Pigs were fed a high fat diet for 4 weeks, then underwent placement of an ameroid constrictor to their left circumflex artery. Three weeks later animals received either: no drug (high cholesterol control: HCC), a high (HCI) or low dose (LCI) calpain inhibitor, or a GSK-3β inhibitor (GSK-3βI). The diets and CI/GSK-3βI were continued for 5 weeks and the myocardial tissue was harvested.
Results
Calpain and GSK-3β inhibition caused an increase in myocardial perfusion ratios at rest and during pacing compared to the control. Pigs in the LCI and HCI groups had increased vessel densities in the ischemic and pigs in the GSK-3βI group had increased vessel densities in the ischemic and non-ischemic myocardium compared to the control. Calpain inhibition modulates proteins involved in the insulin/PI3K and WNT/βcatenin pathways. Quantitative proteomics revealed that CI and GSK-3βI significantly modulated expression of proteins enriched in cytoskeletal regulation, metabolism and respiration, and calcium binding pathways.
Conclusions
In the setting of Metabolic Syndrome, calpain or GSK-3β inhibition increase vessel density in both the ischemic and non-ischemic myocardial tissue. Calpain inhibition may exert these effects by inhibition of GSK-3β and up regulating downstream signaling pathways including the insulin/PI3K and WNT/β-catenin pathways.
INTRODUCTION
Metabolic Syndrome is associated with induction of apoptosis, increased myocardial oxidative stress and attenuation of cell survival pathways in ischemic myocardium. 1–6 Calpains are calcium dependent thiol-proteases. While some calpains are ubiquitously expressed, others are thought to be localized to specific tissues. For example, calpain 1 and 2 are thought to be localized to the endothelial cells 7 In situations of stress, there is an influx of extracellular calcium into cells. This influx of calcium may lead to calpain activation and has been associated with cellular apoptosis, increased leukocyte trafficking and cytoskeletal degradation. This pathway has been associated with renal tubule, endothelial and myocardial cell injury in situations of stress including ischemia and glucose intolerance. 8–10 When calpains become activated they cleave a broad spectrum of intracellular proteins involved in cellular apoptosis, inflammation, platelet function, cytoskeletal structure, cell adhesion, angiogenesis and cell migration. 7,11 Hyper-activation of calpain disrupts the endothelial cell cytoskeleton, causes defective capillary morphogenesis and promotes tissue hypoxia.7 In metabolic syndrome, hyper-activation of calpain has been linked to myocardial and vascular inflammation and impaired collateral vessel formation.11,12 Modest suppression of calpain activity restores cytoskeletal structure and promotes a functional neovasculature which results in reduced tissue hypoxia 7,11,12
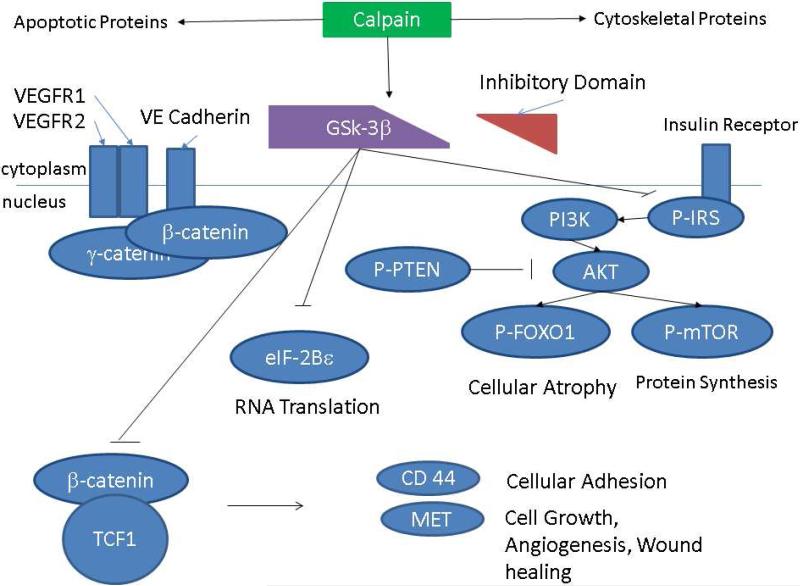
Calpain is also known to cleave the inhibitory domain of glycogen synthase kinase- 3β (GSK-3β) keeping it in an active state. 13,14 GSK-3β is a serine/threonine kinase that plays a role in a broad range of activities in the cell. 15 GSK-3β is an essential regulator in the WNT/ β-catenin pathway where it phosphorylates β-catenin committing it to degradation. 16 GSK-3β also regulates the insulin/PI3K pathway. [Figure 1] In fact, inhibition of GSK-3β in an animal model of diabetes has been found to reduce hyperglycemia and insulin resistance. 17 GSK-3β activity, like calpain, has been found to be increased in endothelial cells in the presence of hypoxia. 18,19 Moderate inhibition of GSK-3β improves the architecture and function of pathologic blood vessels in the setting of ischemia and is cardio protective against ischemia/reperfusion and pressure overload. 18–20
Figure 1.
Schematic Presentation of the Proposed Mechanisms by which Calpain Inhibition may Modulate Expression of WNT/ GSK-3β β Catenin and GSK-3β /Insulin/AKT Pathways
We recently found that calpain activity is significantly increased in the ischemic myocardium of pigs with diet-induced metabolic syndrome and that calpain inhibition increases coronary perfusion, improves endothelium-dependent microvessel relaxation, increases vessel density in ischemic myocardial tissue and decreases myocardial apoptosis. 1,2 Inhibition of GSK-3β also increases blood flow, vessel density and expression of proteins involved in angiogenesis and cell survival. 21
We hypothesized that calpain inhibition has an enhancing effect on myocardial perfusion and improves myocardial density by up regulating angiogenic and cell survival pathways, such as WNT/ β- catenin and Insulin/PI3K in a pig model of chronic myocardial ischemia in the setting of metabolic syndrome.
METHODS
Animal Model
The animal model used in this study has been previously described. 1,21 To induce metabolic syndrome, 33 (7 week old) Yorkshire swine (E.M. Parsons and Sons, Hadley MA) were fed a high fat/ high cholesterol diet. To induce chronic myocardial ischemia, four weeks later, the pigs underwent placement of an ameroid constrictor on the left circumflex artery. Three weeks later, animals were split into four groups and received either: no drug, (high cholesterol control group, HCC; n= 8); a low dose of the of Calpain inhibitor MDL28170 (CI) drug (0.12 mg/kg daily; LCI, n= 9); a high dose of Calpain inhibitor MDL28170 (CI) drug (0.25 mg/kg daily; HCI, n= 8) or a dose of GSK-3β inhibitor IM 12 (GSK-3βI) drug (1.5mg/kg, GSK-3βI, n=8). MDL28170 is a cell permeable selective peptidomimetic inhibitor of calpain 1 and 2 that binds to the protease enzymatic site and inhibits its action. 22,23IM-12 is a novel cell permeable indolyl maleimide which inhibits GSK-3β by competing for ATP binding. 24 The MDL28170 and IM-12 powder was dissolved in dimethyl sulfoxide (DMSO) and administered to the pigs orally. Pigs in the HCC received a placebo of DMSO without any drug dissolved. The high fat/ high cholesterol diet and drug were continued for five weeks. The pigs were then euthanized and the left ventricular myocardium was harvested. Preliminary experiments using these animals were previously reported. 1,2,21 The Institutional Animal Care and Use Committee of the Rhode Island Hospital approved all experiments.1
Immunohistochemical Staining
Immunohistochemistry staining procedures have been previously described. 2 Tissue slides were incubated with antibodies against p- vascular endothelial-cadherin (p-VE-cadherin) (Cell Signaling, Danvers, MA), endothelial marker CD-31 (R&D Systems, Minneapolis, MN) and smooth muscle actin (SMA) (Sigma-aldrich, St Louis, MO). Slides were then incubated with the appropriate alexa fluorconjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA). A Nikon E800 Eclipse microscope (Nikon, Tokyo, Japan) was used to capture images at x20 magnification in five random fields from each animal (4 animals per group). Image J (Image Processing and Analysis in Jave) was used to analyze vascular density. Capillaries were defined as CD31 positive structures between 10-800 pixels and arteries were defined as smooth muscle actin positive structures with lumens greater than 800-infinity pixels.
Myocardial Blood Flow
Myocardial perfusion methods described below have been previously described. 2 Briefly, during the ameroid placement procedure the left circumflex artery was constricted for two minute while gold microspheres (Biopal, Inc Worcester, MA) were simultaneously injected into the left atrium. During the harvest procedure, europium and lutetium microspheres were injected into the left atrium (both at rest and during pacing of 150 beats per minute) while simultaneously withdrawing blood from a femoral arterial line. Ventricular tissue samples obtained after the final harvest procedure were divided into 10 sections and sent to BioPal, Inc (Worcester, MA) along with the femoral arterial line blood where they were exposed to a neutron beam and microspheres measured with a gamma counter. 2
Protein Expression
Protein expression was performed as previously described. 1 Tissue was lysed (Boston BioProducts, Ashland, MA), fractionated (NuPage Novex Gel), transferred to polyvinylidene diflouride membrane (Millipore, Billerica, MA) and incubated with primary antibodies against GSK-3β, β Catenin, p- β Catenin (ser 675), eIF-2Bε, p-eIF-2Bε (ser539), phosphorylated phosphatase and tensin homologue deleted on chromosome ten protein (p-PTEN (ser380)), CD 44, Met, matrix metallopeptidase-7 (MMP-7), c-Myc, c-Jun, CyclinD1, Lymphoid Enhancer Factor-1 (LEF-1), Transcription Factor-1 (TCF-1), p-FOXO1 (ser256), FOXO1, phosphorylated-Insulin Receptor Substrate (p-IRS (ser 612)), Vascular Endothelial cadherin (VE-Cadherin), Phosphoinositide 3-Kinase (PI3Kp85), phospho-Phosphoinositide-dependent protein kinase 1 ser241 (p-PDK-1), mTOR, p-mTOR, AKT, γ-catenin, VEGF Receptor-1, and VEGF Receptor-2 (Cell Signaling, Danvers, MA). Membranes were incubated with the appropriate secondary antibody at room temperature (Jackson ImmunoResearch, West Grove, PA). Enhanced chemi-luminescence was used to visualize images with a digital camera system (G-Box, Syngene, Cambridge, England). Image J software was used to quantify band densitometry as arbitrary light.1
Data Analysis
Immunohistochemistry data is reported as mean of specimens from each group +/− SEM. Protein density data for each pig was normalized to GAPDH or α-tubulin for each pig. An average for each group is reported as fold change values compared to the HCC group +/− SEM. Kruskal- Wallis One-way Analysis of Variance (ANOVA) followed by a Dunn's post-hoc test was used to determine differences between the three or more groups (Graph Pad Prism 5.0 m San Diego, Ca). A Mann- Whitney U test was used to determine differences between two groups (Graph Pad Prism 5.0 m San Diego, Ca).
Proteomics
Ventricular tissue from the ischemic territory of each pig was homogenized in a dounce homogenizer and trypsin digested. Spectra was then collected with a Q Exactive mass spectrometer. Peptide and protein identifications were obtained by matching metabolic syndrome spectra to the pig genomic sequences using Mascot software. Biological replicates were aggregated using either Scaffold or ProteoIQ. Results were analyzed using ingenuity pathway analysis (Silicon Valley, CA) and DDAVID Bioinformatics Database (Frederick, MD). Gene sequences were compared to human sequence when pig sequences were unavailable. Gene lists were submitted to Panther Classification System Database and sorted by protein class.
RESULTS
Arteriolar and Capillary Density
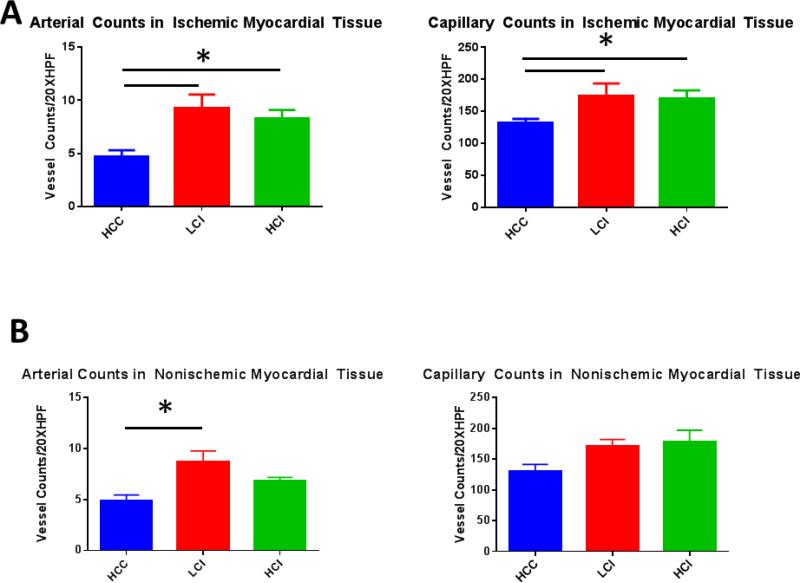
Pigs in the low dose calpain inhibitor (LCI) and high dose calpain inhibitor (HCI) groups had greater arteriolar and capillary densities in the chronically ischemic myocardium compared to the control (HCC) group. [Figure 2A and C]. This reanalysis confirmed previous findings of increased vessel density in chronically ischemic myocardium. 2 In this study, we also determined that calpain inhibition had global effects on myocardial vasculogenesis as the non-ischemic myocardium had increased arteriolar density in the LCI group compared to the control group. [Figure 2B and D]. There were also increases in capillary density in the non-ischemic myocardium of both the LCI and HCI groups however this did not reach statistical significance. [Figure B and 2D].
Figure 2. Calpain Inhibition Increases Vessel Density in Ischemic and Non Ischemic Myocardial Tissue.
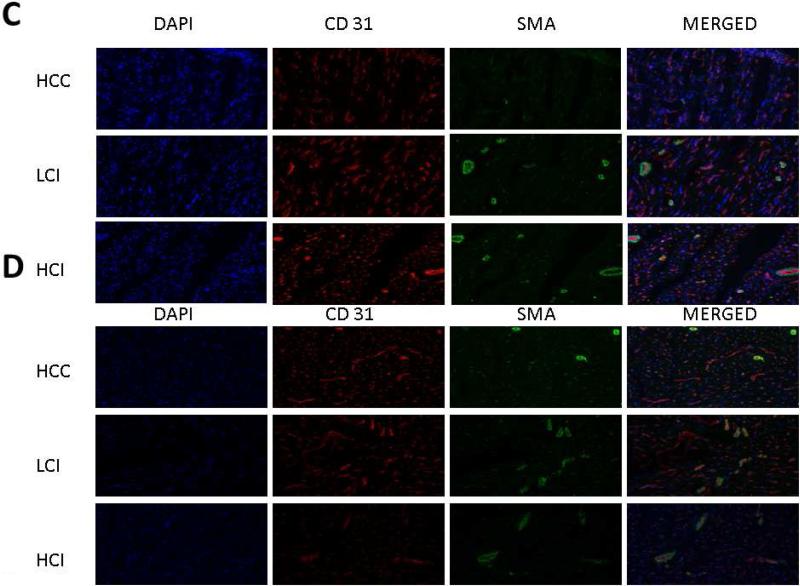
A. Ischemic Myocardium: Arteriolar cell density staining for smooth muscle actin (SMA) with SMA specific antibody in tissue sections. Capillary cell density staining for endothelial marker CD31 with CD31 specific antibody in tissue sections. The bar diagrams show significant increases in LCI and HCI groups compared to control. B. Non-Ischemic Myocardium: Arteriolar cell density staining for smooth muscle actin (SMA) with SMA specific antibody in tissue sections. Capillary cell density staining for endothelial marker CD31 with CD31 specific antibody in tissue sections. The bar diagrams show an increase in arteriolar density in the LCI compared to control C. Ischemic Myocardium: Representative images in 20XHPF: CD31 is red. SMA is green. D: Non-Ischemic Myocardium: Representative images in 20XHPF: CD31 is red. SMA is green. HCC- High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor; *= p<0.05 by Kruskal-Wallis and Dunn's Post Hoc Comparisons
Myocardial Blood Flow
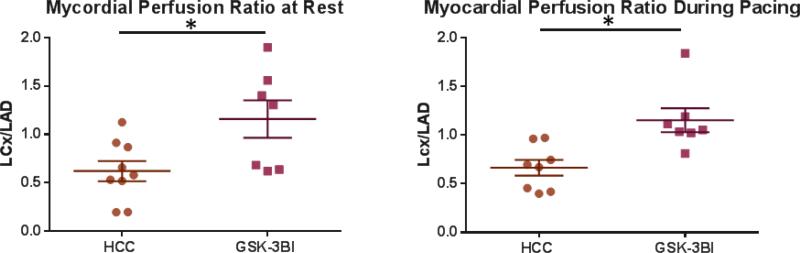
Inhibition of GSK-3β recapitulated many of the effects of calpain inhibition. Pigs in the GSK-3β inhibited (GSK-3βI) group (n=8) had a significant increase in ischemic to non-ischemic myocardial perfusion ratios (LCx/LAD) both at rest and during pacing compared to the HCC group. [Figure 3] These results are consistent with previously reported preliminary results. 21
Figure 3. GSK-3β Inhibition Increased Myocardial Blood Flow at Rest and During Pacing.
HCC- High Cholesterol Control, GSK-3βI- GSK-3βI Inhibition, *= p<0.05 by Mann-Whitney U-test
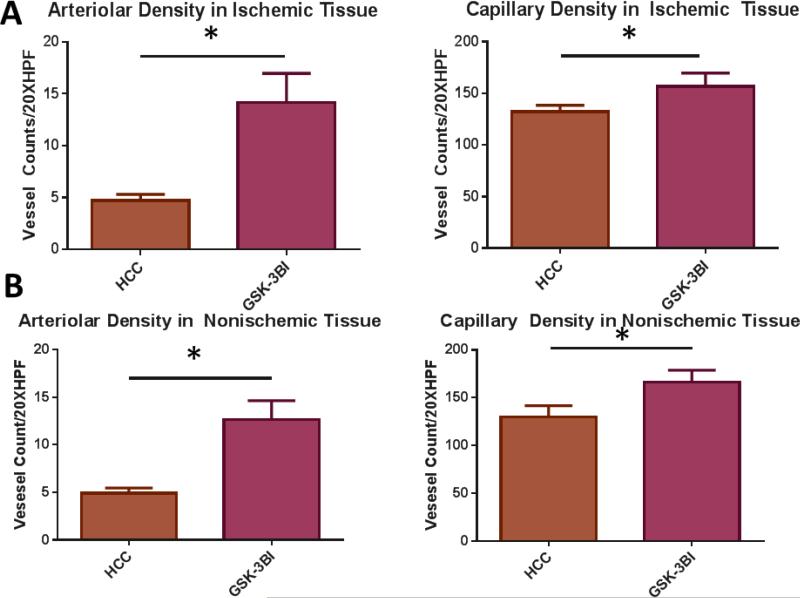
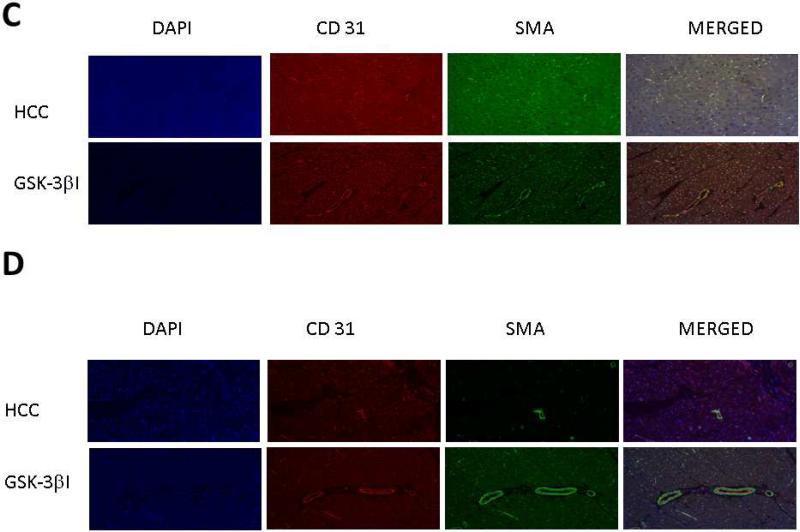
Similar to calpain inhibition groups, pigs in the GSK-3βI group had increased arteriolar and capillary densities in the ischemic and non-ischemic myocardial tissue compared to the HCC group. [Figure 4A-D]
Figure 4. GSK-3 β inhibition Increases Vessel Density in Ischemic and Non-Ischemic Myocardial Tissue.
A. Ischemic Myocardium: Arteriolar cell density staining for smooth muscle actin (SMA) with SMA specific antibody in tissue sections. Capillary cell density staining for endothelial marker CD31 with CD31 specific antibody in tissue sections. The bar diagrams show significant increases arteriolar and capillary density in GSK-3 β I group compared to control. B. Non-Ischemic Myocardium: Arteriolar cell density staining for smooth muscle actin (SMA) with SMA specific antibody in tissue sections. Capillary cell density staining for endothelial marker CD31 with CD31 specific antibody in tissue sections. The bar diagrams show significant increases arteriolar and capillary density in GSK-3 β I group compared to control. C. Ischemic Myocardium: Representative images in 20XHPF: CD31 is red. SMA is green. D: Non-Ischemic Myocardium: Representative images in 20XHPF: CD31 is red. SMA is green HCC- High Cholesterol Control, GSK-3βI- GSK-3βI Inhibition, *= p<0.05 by Mann-Whitney U-test
Pro- Angiogenic Protein Expression
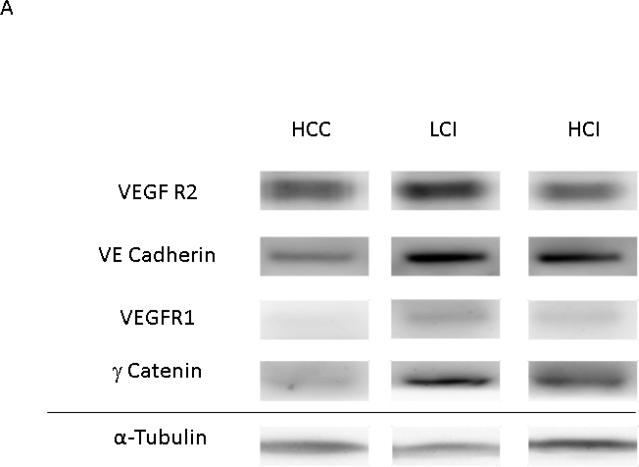
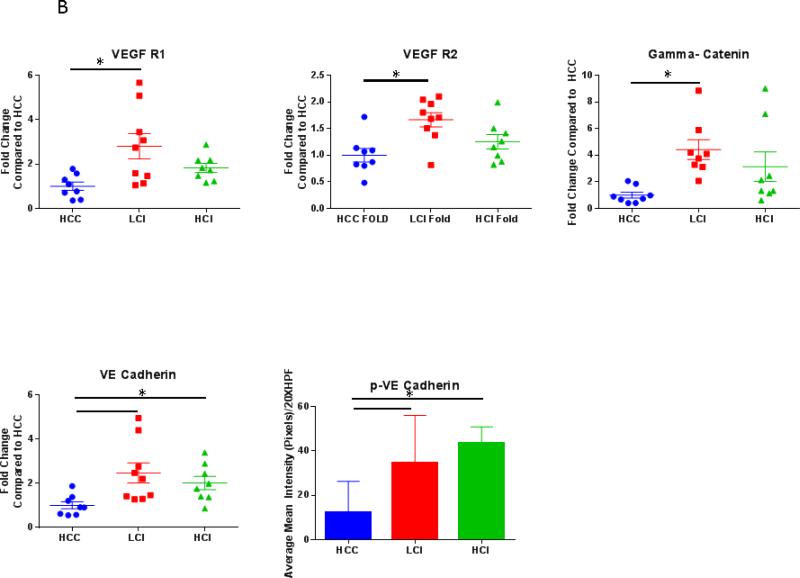
VEGF Receptor 1 and p-VE Cadherin expression levels were greater in the LCI and HCI groups compared to the HCC group. There was also greater expression of VEGF Receptor- 2, VE Cadherin and γ-catenin in the LCI group compared to the HCC group, however there appeared to be a biphasic dose effect as increased expression of these proteins was non-significant in the HCI group compared to the control. [Figure 5]
Figure 5. Calpain Inhibition Increases Expression of Angiogenic Proteins.
A: Representative Images from Western Blot with protein specific antibodies as shown at the left. All images shown in for each protein are from the same Western blot membrane using different antibodies as indicated. Alpha Tubulin shows representative images for loading control. B: The bar diagrams show significant changes in angiogenic proteins. HCC-_ High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor; *= p<0.05 by ANOVA with Dunn's Post Hoc Comparisons
Cell Growth and Proliferation Signaling
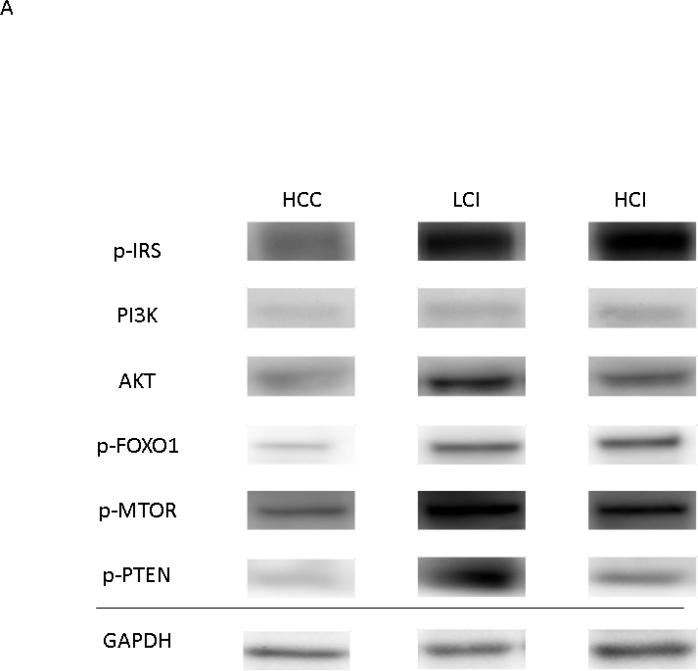
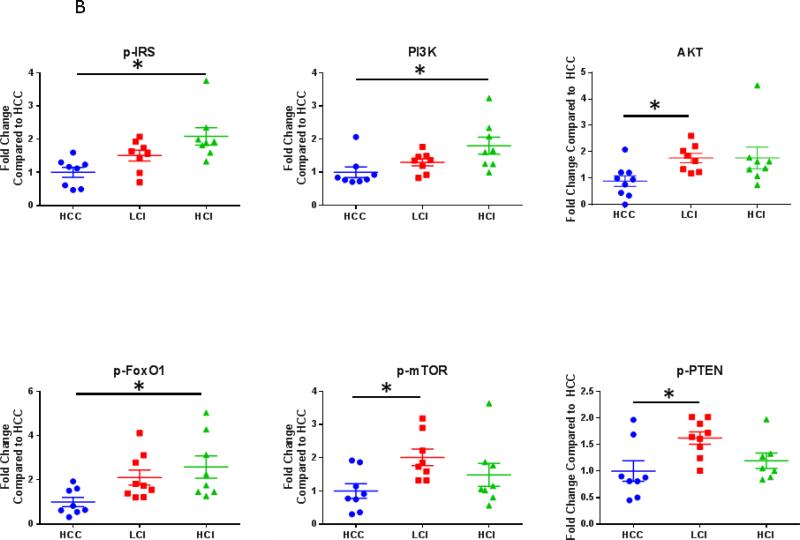
Calpain inhibition was correlated with increased expression of the growth and proliferation proteins akt, p-PTEN and p-mTOR in the LCI group compared to the HCC group. There was greater expression of phosphorylated-FOXO1 in both the LCI and HCI group compared to the HCC group. There was greater expression of p-IRS and PI3-K in the HCI group compared to the HCC group. [Figure 6] There were no significant differences in levels of total FOXO1 or mTOR. [Table 1]
Figure 6. Calpain Inhibition Increases Expression of Proteins involved in Cell Growth and Proliferation.
A: Representative Images from Western Blot with protein specific antibodies as shown at the left. All images shown in for each protein are from the same Western blot membrane using different antibodies as indicated. GAPDH shows representative images for loading control. B: The bar diagrams show significant changes in pro survival signaling proteins. HCC-_ High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor; *= p<0.05 by Kruskal-Wallis and Dunn's Post Hoc Comparisons
Table 1. Proteins that were Non Affected by Calpain Inhibition.
HCC- High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor; Analyzed by Kruskal- Wallis and Dunn's Post Hoc Comparisons.
| Proteins | HCC | LCI | HCI | P Value |
|---|---|---|---|---|
| FOXO1 | 1.00+/−0.28 | 1.24+/−0.18 | 1.59+/−0.23 | 0.31 |
| MTOR | 1.00+/−0.14 | 0.56+/−0.10 | 0.63+/−0.10 | 0.5 |
| c-Myc | 1.00+/−0.08 | 1.0+/−0.07 | 1.04+/−0.08 | 0.84 |
| c-Jun | 1.00+/− 0.1 | 1.19+/−0.05 | .24+/−0.10 | 0.26 |
| Cyclin D1 | 1.00+/−0.19 | 0.78+/−0.10 | 1.05 +/− 0.10 | 0.29 |
| Lymphoid Enhancer Factor-1 | 1.00 +/− 0.18 | 0.72+/−0.07 | 1.07+/−0.12 | 0.10 |
| MMP-7 | 1.00+/− 0.14 | 0.85+/−0.10 | 1.39+/−0.18 | 0.04* |
indicates significant different between LCI and HCI groups only
Wnt/ GSK-3β / β-Catenin Pathway
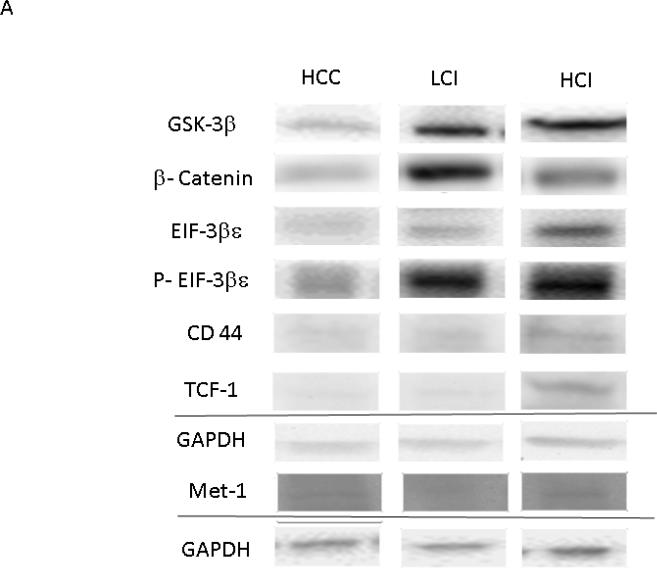
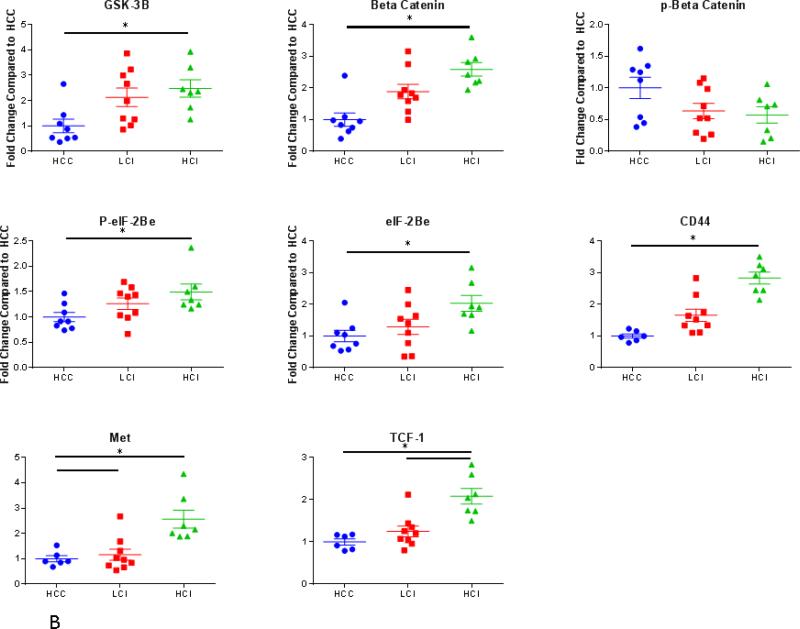
The Wnt/ GSK-3β / β-Catenin pathway regulates cell development and differentiation. There was greater expression of total GSK-3β in the HCI group compared to the control (with non-significant increase in the LCI group compared to the control). Consistent with GSK-3β inhibition, there the expression level of β-catenin was greater in both calpain inhibited groups compared to the control. Similarly, there was decreased phosphorylation of β-catenin in both HCI and LCI groups compared to the control however this did not reach statistical significance (P=0.09). The expression of EIF-2Bε and p- EIF-2Bε (ser 539) were increased in the LCI and HCI groups, however these differences were only significant in the HCI group.
Calpain inhibition was also correlated with increased the expression of downstream targets of GSK-3β involved in cellular adhesion, growth and angiogenesis including CD44, TCF-1, and Met. There was greater expression of CD 44 in the HCI and LCI group compared to the control. There was greater expression of TCF-1 and Met in the HCI group compared to the control group. [Figure 7] Calpain inhibition did not affect the expression of downstream targets of both β catenin and GSK-3β involved in proliferation including c-Myc, c-Jun, CyclinD1, and LEF-1. There was increased expression in MMP-7 in the HCI group compared to the LCI group. [Table 1]
Figure 7. Calpain Inhibition Increases Expression of Proteins in WNT/β Catenin Pathway.
A: Representative Images from Western Blot with protein specific antibodies as shown at the left. All images shown in for each protein are from the same Western blot membrane using different antibodies as indicated. GAPDH shows representative images for loading control. B: The bar diagrams show significant changes in pro survival signaling proteins. TCF-1- transcription factor-1, HCC-_ High Cholesterol Control, LCI- Low Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor; *= p<0.05 by Kruskal-Wallis and Dunn's Post Hoc Comparisons
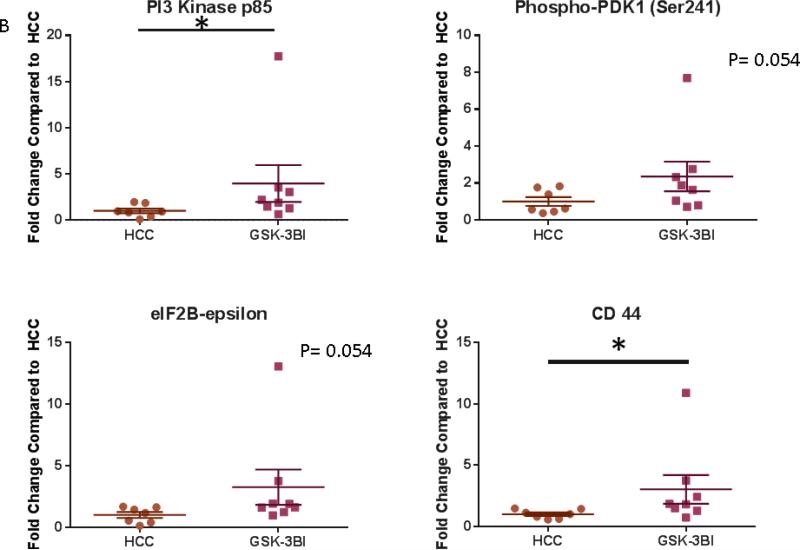
GSK-3β inhibition was associated with increased expression of PI3-K and CD44 compared to the control pigs. There was a trend toward increase in expression of P-PDK1 and eIF2Bε in the GSK-3β inhibited tissue compared to the control. [Figure 8]
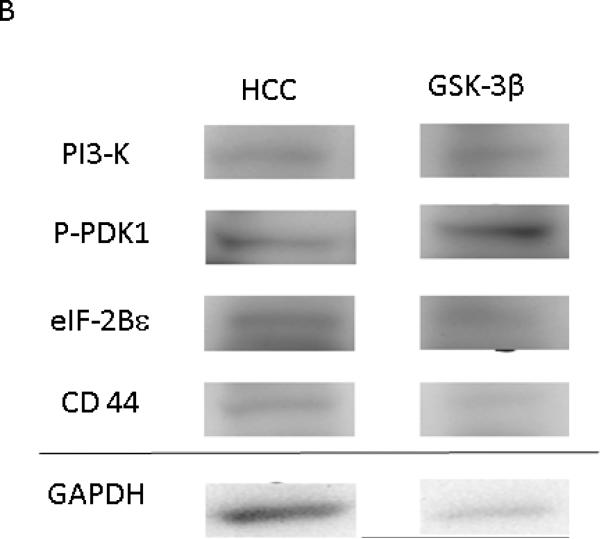
Figure 8. GSK-3β Increases Expression of Proteins involved Cell Growth and the WNT/ β Catenin Pathway.
A: Representative Images from Western Blot with protein specific antibodies as shown at the left. All images shown in for each protein are from the same Western blot membrane using different antibodies as indicated. GAPDH shows representative images for loading control. B: The bar diagrams show significant changes in pro survival signaling proteins. PI3Kp85- Phosphoinositide 3-Kinase, p-PDK-1 – Phospho-Phosphoinositide-dependent protein kinase 1 (ser 241) HCC- High Cholesterol Control, GSK-3βI- GSK-3β inhibited group *= p<0.05 by Mann-Whitney U-test
Proteomics
Quantitative proteomics and systems analysis revealed that CI and GSK-3βI significantly modulated expression of proteins enriched in cytoskeletal regulation, metabolism and respiration, and calcium binding pathways compared to the HCC group including up-regulation of β1-catenin in the GSK-3βI group compared to the control.
Thirty-five proteins were increased in the GSK-3βI group compared to the control group (P<0.05). Twenty-Eight proteins were increased in the HCI group compared to the control group (P<0.05). Twenty-Five proteins were increased in the LCI group compared to the control group (P<0.05). Two proteins related to sarcomere structure and function were up-regulated in all three experimental groups (GSK-3βI, HCI and LCI) compared to the control group. Five proteins related to inflammation, rRNA translation, ATP breakdown, triglyceride catabolism and ATP dependent motor protein movement were increased in the GSK-3βI and LCI groups compared to the control group. Five proteins involved in inflammation, signal transduction, ATPase activity and cytoskeletal structure were increased in the GSK-3βI and HCI groups compared to the control group. Three proteins involved in insulin resistance, redox reactions and cellular homeostasis (including calpain -1 catalytic subunit) were increased in the HCI and LCI groups compared to the control group. [Table 2]
Table 2. Proteomic Analysis: Protein Expression that Increased in GSK-3βI, HCI and LCI Groups Compared to HCC Group.
Proteins in pink were increased in the GSK-3βI and LCI group compared to the control. Proteins in yellow were increased in the GSK-3βI and HCI group compared to the control. Proteins in green were increased in the HCI and LCI group compared to the control. Proteins listed in blue were increased in all three experimental groups compared to the control. All proteins listed showed statistically significant differences in expression between appropriate experimental group (GSK-3βI, HCI and LCI) and the HCC group by Scaffold or ProteoIQ analysis. Pink indicates proteins that were increased in GSK-3βI and LCI group compared to the control. Orange indicates proteins that were increased in the GSK-3βI and HCI group compared to the control. Green indicated proteins that were increased in the HCI and LCI group compared to the control. Blue indicates proteins that were increased in all three experimental groups (GSK-3βI, LCI and HCI) compared to the control. HCC High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI- High Dose Calpain Inhibitor, GSK-3βI- GSK-3β Inhibitor
| GSK-3p Protein Expression> HCC | LCI Protein Expression>HCC | HCI Protein Expression>HCC |
|---|---|---|
| 60S ribosomal protein L32 | 26S proteasome non-ATPase regulatory subunit 5 | Alpha-soluble NDK attachment protein |
| AHNAK nucleoprotein | 60S ribosomal protein L32 | Annexin A6 |
| Alpha-actinin-1 | Apolipoprotein A-1 | Armadillo repeat containing protein 10 |
| Alpha-actinin-4 | Calpain-1 catalytic subunit | Beta-2-syntrophin |
| Alpha-soluble NSF attachment protein | Glucosamine-6-phosphate isomerase | Calpain-1 catalytic subunit |
| Annexin A6 | Homer protein homolog 1 (Homer-1) | Calreticulin |
| Apolipoprotein E | Integrin-linked protein kinase | Coiled-coil domain-containing protein 141 |
| Beta-catenin | MHC class II antigen | Fatty-acid amide hydrolase 1 |
| Calreticulin | Mitochondrial import inner membrane translocase subunit TIM50 | Fumarate hydrolase, mitochondrial |
| Cat eye syndrome critical region protein | MYL2 | Glucosamine-6-phosphate isomerase |
| Chaperorin containing, TCP | Myomesin-3 (Myomesin family member 3) | GTP-AMP phosphotransferase AK3 |
| Complexin-1 | Myosin-9 | GTP-binding nuclear protein Ran |
| Cysteine-rich motor neuron 1 protein | Myotilin | Homer IF (Homer protein homolog 1) |
| Cytoplasmic aconitate hydratase | NADH-ubiquinone oxidoreductase chain 4 | Myomesin-3 |
| Domain-containing protein 4 | Polypyrimidine tract binding protein 1 | Myotilin |
| Integrin alpha-V | Probably C->U editing enzyme APOBEC-2 (EC3.4.5) | NADH-ubiquinone oxidoreductase chain 4 |
| LIM domain only protein 7 | Protein CDV3 homolog | NAP1L1, NRP |
| Myomesin-3 | Pyridoxal kinase (EC 2.7.1.35) Pyridoxine kinase | Phosphoacetylglucosamine mutase |
| Myotilin | Ribosomal Protein | Proteasome subunit alpha type 6 |
| NAD(P) transhydrogenase, mitochondrial | Ryanodine receptor 2 | Protein NipSnap homolog 3A |
| NADH dehydrogenase (ubiquinone)1 | Tropomodulin-1 (Erythrocyte tropomodulin) | RuvB-like 1 |
| Obscurin | Voltage-dependent anion- selective channel protein 2 (VDAC-2) | Sorting nexin 18 |
| Peptidyl-Prolylcis-trans isomerase | Spectrin alpha chain, non-erythrocytic 1 | |
| Protein CDV3 homolog | Synemin (Desmuslin) | |
| Pyridoxal kinase | Talin-1 | |
| RuvB like 1 | Uncharacterized Protein TMEM70 | |
| Synemin | ||
| Talin-1 | ||
| Tubulin beta chain | ||
| Uncharacterized protein (ILK) | ||
| Uncharacterized protein (FN1) | ||
| Unconventional myosin-X | ||
| Vitamin D binding protein |
Ten proteins were decreased in the GSK-3βI group compared to the control group (P<0.05). Eighteen proteins were decreased in the HCI group compared to the control group (P<0.05). Eleven proteins were decreased in the LCI group compared to the control group (P<0.05). Two proteins related to carbohydrate metabolism and coagulation were decreased in the GSK-3βI and LCI groups compared to the control group. One protein involved in the tricarboxylic acid cycle was decreased in the GSK-3βI and HCI groups compared to the control group. Six proteins involved in serine and threonine metabolism, ATP synthesis, the electron transport chain, hemoglobin binding, and cellular hemostasis were decreased in the HCI and LCI groups compared to the control group. [Table 3]
Table 3. Proteomic Analysis: Expression of Proteins that was Decreased in GSK-3βI, HCI and LCI Groups Compared to HCC Group.
Proteins in pink were decreased in the GSK-3βI and LCI group compared to the control. Proteins in yellow were decreased in the GSK-3βI and HCI group compared to the control. Proteins in green were decreased in the HCI and LCI group compared to the control. Proteins listed in blue were decreased in all three experimental groups compared to the control. All proteins listed showed statistically significant differences in expression between appropriate experimental group (GSK-3βI, HCI and LCI) and the HCC group by Scaffold or ProteoIQ analysis. Pink indicates proteins that were decreased in GSK-3βI and LCI group compared to the control. Orange indicates proteins that were decreased in the GSK-3βI and HCI group compared to the control. Green indicated proteins that were decreased in the HCI and LCI group compared to the control. Blue indicates proteins that were decreased in all three experimental groups (GSK-3βI, LCI and HCI) compared to the control. HCC- High Cholesterol Control, LCI- Low Dose Calpain Inhibitor, HCI-High Dose Calpain Inhibitor, GSK-3βI- GSK-3β Inhibitor
| GSK-3P Protein Expression< HCC | LCI Protein Expression<HCC | HCI Protein Expression<HCC |
|---|---|---|
| Alpha-1,4, glucan phosphorylase | Alanine aminotransferase 1 (ALT1) | 14-3-3 protein theta |
| Fibrinogen beta chain | Alpha-1,4 glucan phosphorylase | 40S ribosomal protein SA |
| Ribonuclease inhibitor | ATP synthase F(0) complex subunit B1, mitochondrial | Alanine-glyoxylate aminotransferase |
| Serum deprivation-response protein | ATP Synthase protein 8 | Antithrombin protein |
| Succinyl-CoA ligase subunit beta | Carbonic Anhydrase 1 | ATP synthase protein 8 |
| Uncharacterized Protein (CHCHD3) | Cytochrome c oxidase subunit 5A | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial |
| Uncharacterized Protein (COX6B) | Fibrinogen beta chain | Cytochrome c oxidase subunit 5A |
| Uncharacterized Protein (PURA) | Hemoglobin subunit alpha (Alpha globin) ( Hemoglobin alpha chain) | Glyceraldehyde-3-phosphate dehydrogenase |
| Uncharacterized Protein (COX5A) | LETM1 and EF-hand domain containing protein 1, mitochondrial | Glycogen phosphorylase |
| Voltage-dependent anion selective channel protein 1 (VDAC 1) | Hemoglobin subunit alpha | |
| V type proton ATPase catalytic subunit A | LETM1 and EF-hand domain containing protein 1, mitochondrial | |
| Malate dehydrogenase, mitochondrial | ||
| NADH ubiquinone oxidoreductase chain 4 | ||
| Peptidyl-prolyl cis-trans isomerase | ||
| Succinate dehydrogenase | ||
| Succinyl-CoA ligase subunit beta | ||
| Voltage dependent anion-selective channel protein 1 (VDAC-1) |
DISCUSSION
In this study, we demonstrate the following in a pig model of metabolic syndrome and chronic myocardial ischemia: 1) both calpain and GSK-3β inhibition improve vessel density in the ischemic and non-ischemic myocardial tissue 2) GSK-3β inhibition improves myocardial blood flow both at rest and with pacing 3) calpain inhibition correlates with increased expression of proteins that serve as angiogenic markers and 4) that calpain inhibition likely has beneficial effects by modulating activity of GSK-3β and proteins involved in the Wnt/ β Catenin and insulin/PI3K pathways. [Figure 9]
Central Picture: Figure 1A.
Schematic Presentation of the Proposed Mechanisms by which Calpain Inhibition may Modulate Expression of WNT/ GSK-3β β Catenin and GSK-3β /Insulin/AKT
We recently found that calpain activity is significantly increased in the ischemic myocardium of pigs with metabolic syndrome and that calpain inhibition improves myocardial blood flow, increases endothelial-dependent microvascular relaxation, increases vessel density and decreases proteins involved in apoptosis. 1,2 Calpain is a ubiquitously expressed protein with a broad range of function. Calpain activity is overexpressed during times of stress and moderate inhibition of this overexpression has been found to be beneficial. GSK-3β is a downstream target of calpain. By looking at GSK-3β we hope to localize the beneficial of effect of calpain and narrow the range of activity of our medication. In related preliminary studies, we found that GSK-3β inhibition improves myocardial blood flow, increases microvascular density and modulates proteins involved in the ischemic myocardium of pigs with metabolic syndrome. 21 The current study validated some of our previous findings and expanded upon this previous work both in extent of physiologic findings including vessel density and myocardial perfusion and increased scope of the investigated signaling pathways and potential mechanisms.
We found that calpain inhibition increased vessel densities (arteriolar and capillary) in both the high and low dose calpain inhibited groups in the ischemic myocardial tissue compared to control pigs. In addition, we found that GSK-3β inhibition increased the ischemic to non-ischemic myocardial perfusion ratios (LCx/LAD) both at rest and during pacing compared to the control group. Importantly, our data is consistent with some of our preliminary studies which showed an increase in arteriolar and capillary density in the low dose calpain inhibited group and an increase in the high dose group compared to the control group which did not reach statistical significance. 2 In the current study, vessel density was evaluated in five 20XHPF instead of three 20XHPF which explains the difference in significance.
In this study, we also performed vessel density analysis in the non-ischemic myocardial tissue. While the only significant difference from the control group was found in arteriolar density in the low dose calpain inhibited group we did observe an overall trend of calpain inhibition to increase both arteriolar and capillary densities compared to the control. This finding suggests that calpain inhibition not only promotes angiogenesis in ischemic myocardium, but also has a global effect on the myocardium to induce angiogenesis.
Next we evaluated the effect of GSK-3β inhibition on vessel density in both the ischemic and non-ischemic myocardial tissue, as GSK-3β may be activated and is a direct downstream target of calpain. We found that there was a significant increase in capillary and arteriolar counts in both the ischemic and non-ischemic tissue in the GSK-3β inhibited group compared to the control. This is consistent with previous preliminary results. 21 Our findings demonstrate that both calpain and GSK-3β inhibition induce new vessel growth in the ischemic myocardial tissue of pigs in the setting of metabolic syndrome. In addition, GSK-3β inhibition may be a more potent stimulus to promote vessel growth in the non-ischemic myocardial tissue. Therefore, the beneficial effect of calpain inhibition and GSK-3β inhibition is likely related to their ability to promote new vessel growth both in the ischemic and non-ischemic myocardial tissue. 1,2,11,12
Interestingly, the beneficial effect of calpain inhibition on angiogenesis seems to be more significant in the ischemic myocardium while the beneficial effect of GSK-3β is present in both the ischemic and nonischemic myocardium. This is thought to occur because calpain activity is required for normal physiological functioning while calpain over activation in situations of stress, including ischemia, is detrimental. Therefore, moderate inhibition of calpain in the ischemic myocardial tissue only, where calpain activity is over activated, would bring calpain activity back to physiologic parameters. GSK-3β is not regulated in a similar fashion and therefore we do not see a difference of effect in the ischemic and nonischemic myocardium.
We then evaluated the signaling pathways that calpain and GSK-3β inhibition may be affecting to exert the changes in vessel density. 2 VEGF receptors activate several intracellular signaling processes that promote angiogenesis. In this study, we verified that calpain inhibition up-regulates VEGF receptors 1 and 2 similar to our previous work that demonstrated up regulation of VEGF and its associated receptors. 2 We then further extended our analysis of potential mechanistic signaling pathways and examined the Wnt/GSK-3β /β-Catenin signaling pathway. β-catenin is an integral VE-cadherin cell-cell adhesion adaptor protein. Up regulation of VE-cadherin in the cell membrane of vascular cells serves as an indicator of increased angiogenesis. VE-cadherin binds to γ-catenin and β-catenin on the cell membrane. Interestingly, we identified up-regulation of VE-Cadherin, p-VE-cadherin, γ-catenin and β-catenin in the calpain inhibited groups compared to the control group. We also previously showed that GSK-3β inhibition up regulates the expression of the similar proteins including (VEGF-R1+2, VE-Cadherin, γ-catenin and β-catenin) in the same pig model. 21 These findings corroborate the increase in vessel density seen in the calpain and GSK-3 β inhibited groups compared to the control group described above. Cytosolic β-catenin is known to function as a transcriptional co-regulator which promotes cell growth and differentiation.25 Therefore, these findings also suggest that calpain inhibition may be having an effect via up regulation of cytosolic β-catenin and its downstream signaling pathways.
The Wnt/ β Catenin pathway is essential for cell fate and angiogenesis. One of the most well described cellular functions of GSK-3β is its regulation of Wnt/ β-catenin signaling. 26 In this study, we found that calpain inhibition increases the expression of total GSK-3β suggesting that in the calpain inhibited group there is less cleavage of GSK-3β by calpain possibly leading to less GSK-3β activity. To test this hypothesis, we investigated proteins downstream of GSK-3β. GSK-3β works to phosphorylate β-catenin which marks it for degradation. Stabilization of β-catenin allows it to translocate to the nucleus where it interacts with transcription factor-1 (TCF-1), displaces co-repressors and recruits additional co-activators of Wnt target genes (such as CD 44 and Met). 27 CD 44 is a transmembrane glycoprotein that mediates cell-cell and cell-matrix interaction. Met is a tyrosine kinase receptor involved in cell growth. We found that calpain inhibition is associated with increased expression levels of not only β-catenin but also its co activator TCF-1 and its downstream targets CD 44 and Met. We also found that GSK-3β inhibition is associated with increased expression levels of CD 44. GSK-3β also works to directly phosphorylate eIF2Bε at ser535 (after it is primed by another kinase via phosphorylation at ser539) which inhibits its ability to initiate mRNA translation. 15 We found that up regulation of both eIF2Bε and p-eIF2Bε (ser539) in the calpain inhibited groups. We found a trend toward increase in eIF2Bε expression in the GSK-3β inhibited group compared to the control. The next step will be to evaluate the expression of the inhibited form, p-eIF2Bε (ser535) as this will be a more direct way to measure GSK-3β function. These data suggest that calpain inhibition modulates proteins involved in the Wnt/ β Catenin pathway via GSK-3β. Future work will need to evaluate GSK-3β activity in the calpain inhibited and GSK-3β inhibited groups.
The insulin/PI3K pathway is known to promote cell survival. Tyrosine phosphorylation of IRS allows association with phosphoinostitide-3-kinase (PI3K). PI3K activation leads to phosphorylation of phosphoinositide-dependent protein kinase-1 (PDK-1) which then leads to activation of akt, a serine kinase, which phosphorylates and modulates numerous effectors proteins (such as mTOR and FOXO1). MTOR is an intracellular protein involved in protein synthesis. FOXO1 is a member of the forkhead box transcription factor family and is important in cellular atrophy. FOXO1 is directly phosphorylated by AKT which inactivates it and excludes it from the nucleus. 28,29 PTEN is a tumor suppression protein that functions to dephosphorylate and turn off akt activity. Phosphorylation of PTEN suppresses its ability to turn off akt. 30 We identified increases in these proteins (akt, p-PTEN, PI3K, p-IRS, p-mTOR) in the ischemic myocardium of pigs with calpain inhibition compared to the control pigs. These data suggest that calpain inhibition may function by up regulation of the insulin/PI3K pathway and modulation of its downstream pathways. Importantly, GSK-3 β is known to degrade IRS-1 which is an upstream protein in the insulin/PI3K pathway. Interestingly, we found increased expression of activated PI3K (p85) and a trend towards increased expression of activated p-PDK1 (Ser241) in the GSK-3β inhibited group compared to the control. We previously found that GSK-3β inhibition also increases the expression of many of these proteins in ischemic myocardium in calpain inhibited animals (including akt, FOXO1, and p-FOXO1). 21 Therefore it is possible that calpain inhibition is modulating the insulin/PI3K pathways via GSK-3β. Future work will need to look into this mechanism further.
Finally, we used proteomic analysis to evaluate differences in protein expression between the groups. We found that calpain and GSK-3β inhibition significantly modulated expression of proteins enriched in cytoskeletal regulation, metabolism and respiration and calcium binding pathways compared to the control group. These pathways are critical for tissue survival under stress. These data support the overall idea that calpain and GSK-3β inhibition have a protective effect on the heart. Importantly, quantitative mass spectrometry studies confirmed western analysis demonstrating significant increase in β- catenin in the GSK-3β group compared to the control. 21 However, not all proteins investigated using western blots were found in our unbiased proteomic analysis as certain limitations of both methods likely play a role, specifically that the proteomics methods employed are most suitable to detect changes in very highly expressed proteins in the myocardium.
Calpain overexpression is induced by the increased calcium released by cells in situations of stress. Therefore, we would expect to see similar changes in the ischemic tissue of pigs on a normal diet. Several early large animal studies looking at chronic myocardial ischemia and angiogenesis showed promising results, however most of these studies failed to translate to successful phase I and II clinical trials. One likely explanation for these failures in human subjects is that patients with end-stage coronary arteries disease have multiple comorbid conditions including hypertension, hyperlipidemia, diabetes and endothelial dysfunction making them vastly different from healthy animals with induced coronary ischemia. 31 Our animal model has established a clinically relevant metabolic syndrome pig model to approximate the comorbidities seen in patients with chronic myocardial ischemia. Therefore, we chose to begin our study by looking at metabolic syndrome pigs. However, in the future it would be interesting to compare our results with those of normal diet controls.
Limitations
The data presented in this study did not determine the optimal dose of calpain inhibition required to have a beneficial effect. The optimal dose of calpain inhibition plausibly may lie in between the doses used in our LCI and HCI protocol. Future dose-response studies will be required to obtain precise optimal level of calpain inhibition necessary for positive cardiovascular effects. In addition, the optimal time for treatment will require further investigation. Our western blot data identify differences in protein expression in the pig myocardial tissue compared to loading controls. These differences could be due to differences in protein stability or in differences in the relative proportions of cell populations expressing different quantities of proteins. Our animal model is designed to emulate patients with coronary artery disease (ameroid constrictor model) and its associated comorbidities (diet induced metabolic syndrome). However, it is important to note humans develop these complications due to a variety of complex factors over many years. While our animal model is very instructive to elucidating early changes associated with chronic ischemia, numerous differences may exist and extrapolation of our findings to pathophysiologic processes in human coronary artery disease and ischemia should use considerable caution.
Conclusion
In summary, we found that in the setting of metabolic syndrome, calpain and GSK-3β inhibition both increase vessel density in the ischemic and non-ischemic myocardial tissue. Importantly, we also found that calpain inhibition may have exerted these effects by inhibition of GSK-3β and up regulation of its downstream effectors including the GSK-3β /insulin/PI3K pathway and WNT/GSK-3β /β-catenin pathways. This study confirmed that GSK-3β inhibition increases blood flow to ischemic myocardial tissue both at rest and during pacing. These findings have important implications for new vessel formation in the setting of myocardial ischemia under conditions known for reduced angiogenesis and collateral vessel formation.
Funding Statement: Acknowledgments
Funding for this research was provided by the National Heart, Lung, and Blood Institute (R01HL46716, RO1HL128831 Dr. Sellke); NIH/NIGMS Training Grant 2T32 GM065085 to Dr. Potz; NIH Training grant 5T32-HL094300-03, (Dr. Sabe and Dr. Elmadhun); AHA Grant-in Aid GRNT20460376 (Dr Clements); National Institute of General Medical Sciences (NIGMS) /National Institute of Health (NIH) grant 1P20GM103652 (Project# 3) (to MRA) and American Heart Association (AHA) Grant-in-Aid 14GRNT20460291 (to MRA).
Glossary of Abbreviations
- HCC
High cholesterol control group
- GSK-3βI
GSK-3βinhibited group
- HCI
High dose calpain inhibited group
- LCI
Low dose calpain inhibited group
- p-PTEN
phosphorylated phosphatase and tensin homologue deleted on chromosome ten
- MMP-7
matrix metallopeptidase-7
- LEF-1
Lymphoid Enhancer Factor-1
- TCF-1
Transcription Factor-1
- p-IRS
phosphorylated-Insulin Receptor Substrate
- Lcx
left circumflex artery (Lcx)
- VE-Cadherin
vascular endothelial cadherin
- PI3Kp85
Phosphoinositide 3-Kinase p65
- p-PDK-1
Phospho-Phosphoinositide-dependent protein kinase 1 (ser 241)
Biography



Perspective Statement: Metabolic syndrome is associated with endothelial dysfunction, accelerated atherosclerosis and a diminished angiogenic response to myocardial ischemia. Modification of major risk factors for cardiovascular disease (CVD) reduce the likelihood of developing CVD and its associated complications. Calpain may serve as a potential medical therapy for patients with chronic coronary artery disease and MS.
Central Message: Calpain inhibition may improve vessel density by inhibition of GSK-3β activity.
L7. CALPAIN INHIBITION MODULATES GSK-3? PATHWAYS IN A SWINE MODEL OF CHRONIC MYOCARDIAL ISCHEMIA IN THE SETTING OF METABOLIC SYNDROME: A PROTEOMIC AND MECHANISTIC ANALYSIS. Paper presented by Brittany A. Potz, M.D., Providence, Rhode Island. brittany_potz@brown.edu
Discussion by Juan A. Crestanello, M.D., Columbus, Ohio. juan.crestanello@osumc.edu Dr. J. Crestanello (Columbus, Ohio):
I would like to congratulate Dr. Potz and colleagues for a well-designed set of experiments and for an excellent presentation.
This study addresses an important topic given the increased incidence of morbid obesity, diabetes, hypercholesterolemia, and metabolic syndrome in patients with chronic ischemic heart disease.
The authors examined the effects of calpain inhibition and of a downstream intermediary, GSK-3ß, in a pig model of chronic myocardial ischemia in the setting of metabolic syndrome.
They found that calpain inhibition and GSK-3ß inhibition increases angiogenesis, capillary and arteriolar density is ischemic and nonischemic myocardium and myocardial perfusion. This effect appears to be mediated by the increased expression of angiogenic proteins, cytoskeletal regulation, increasing the proteins in both the regulation of metabolism and respiration and in calcium-binding pathways.
I have the following questions: What evidence do you have that calpain is overexpressed in your metabolic syndrome pig model? And if you do, is there a relationship between the severity of the metabolic syndrome and the level of expression of calpain?
L7. CALPAIN INHIBITION MODULATES GSK-3? PATHWAYS IN A SWINE MODEL OF CHRONIC MYOCARDIAL ISCHEMIA IN THE SETTING OF METABOLIC SYNDROME: A PROTEOMIC AND MECHANISTIC ANALYSIS. Response by Brittany A. Potz, M.D., Providence, Rhode Island.
DR. POTZ: Thank you for that review and for the question.
The evidence that we have for increased activity of calpain actually comes from research of a collaborator of ours (Dr Donald R. Senger). Using a small animal model, he has shown that ischemia increases calpain activity and that moderate calpain inhibition improves cytoskeletal structure and reduces underlying hypoxia. In our study, we used the same calpain inhibitor at similar dosages.
We have not yet looked at calpain activity officially in our pig model but instead we look at the expression levels of specific calpain substrates and inferred decreased activity in the calpain inhibited groups in that way.
DR. CRESTANELLO: So the following question is: Why did the effect of calpain inhibition on angiogenesis seem to be limited to or more significant in the ischemic myocardium while the effect of GSK-3ß is present in both the ischemic and nonischemic myocardium?
DR. POTZ: Thank you. That's a really good question. I think the reason we see a greater effect of calpain inhibition in the ischemic tissue is that calpain is activated by stress and the release of calcium (among other things). So in the ischemic tissue there is release of calcium and subsequent overactivation of calpain. When this overactivation gets inhibited, the calpain activity is brought to an appropriate level for cellular function. We do not see this same ischemic vs nonischemic effect with GSK-3B because, as far as we know, GSK-3B has a more broad spectrum of activation and activity.
DR. CRESTANELLO: And my final question is probably the most important. Did this improvement in myocardial angiogenesis and flow result in any improvement in ventricular function or any decrease in the size of the myocardial infarction?
DR. POTZ: That is a very good question. We were mostly looking at blood flow and angiogenesis. We did insert catheters into the left ventricle and the ascending aorta to measure pressure. We haven't analyzed that data, but we plan on doing that.
And in terms of determining the ischemic area of the heart, we injected gold microspheres into the heart after occluding the left circumflex artery. We later isolated those gold microspheres in the tissue and read their levels (BioPal, MA). This helped us to determine what area of the myocardium was ischemic versus non-ischemic. We did not measure the size of infarct in our pigs. However, to determine the effect of calpain inhibition on tissue ischemia, we measured vessel density, blood flow and oxidative stress in the ischemic myocardial tissue from the control and experimental pigs and found that calpain inhibition was beneficial. Thank you.
L7. CALPAIN INHIBITION MODULATES GSK-3? PATHWAYS IN A SWINE MODEL OF CHRONIC MYOCARDIAL ISCHEMIA IN THE SETTING OF METABOLIC SYNDROME: A PROTEOMIC AND MECHANISTIC ANALYSIS. Paper presented by Brittany A. Potz, M.D., Providence, Rhode Island. brittany_potz@brown.edu
Discussion by Marc Ruel, M.D., M.P.H., FRCSC, Ottawa, Ontario, Canada. mruel@ottawaheart.ca Dr. M. Ruel (Ottawa, Ontario): It looks like this is one of perhaps many master switches that occur in metabolic syndrome. Do we know how it interrelates with other ones such as methylglyoxal and eNOS uncoupling? Have you been able to kind of figure out the story together? And what does it do to insulin resistance and the overall effects of the metabolic syndrome?
L7. CALPAIN INHIBITION MODULATES GSK-3? PATHWAYS IN A SWINE MODEL OF CHRONIC MYOCARDIAL ISCHEMIA IN THE SETTING OF METABOLIC SYNDROME: A PROTEOMIC AND MECHANISTIC ANALYSIS. Response by Brittany A. Potz, M.D., Providence, Rhode Island.
DR. POTZ: That's a very good question. Our calpain inhibitor inhibits calpain 1 and 2, which is localized both in the heart and in the endothelium. We have thus far really only looked at the effect of calpain on the blood flow and have focused on changes in vessel density and the endothelium
However, we agree that calpain inhibition may be important in regulating metabolic syndrome and we're now looking to explore that further.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Potz BA, Sabe AA, Elmadhun NY, et al. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery. 2015;158(2):445–452. doi: 10.1016/j.surg.2015.03.034. doi:10.1016/j.surg.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabe AA, Potz BA, Elmadhun NY, et al. Calpain Inhibition Improves Collateral Dependent Perfusion in a Hypercholesterolemic Swine Model of Chronic Myocardial Ischemia. J Thorac Cardiovasc Surg. 2016;151(1):245–252. doi: 10.1016/j.jtcvs.2015.08.101. doi:10.1016/j.jtcvs.2015.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmadhun Nassrene Y., MD, Lassaletta Antonio D., MD, Chu Louis M., MD, YL, Feng Jun, MD, Sellke M Frank W. Atorvastatin incresaes oxidative stress and modulates angiogensis in Ossabaw Swine With Metabolic Syndrome. J Thorac Cardiovasc Surg. 2012;144(6):1486–1493. doi: 10.1016/j.jtcvs.2012.08.065. doi:10.1016/j.jtcvs.2012.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Damrauer SM, Lee M, Sellke FW, Ferran C, Abid R. Endothelium-Dependent Coronary Vasodilatation Requires NADPH Oxidase – Derived Reactive Oxygen Species. Arter Thromb Vasc Biol. 2010;30:1703–1710. doi: 10.1161/ATVBAHA.110.209726. doi:10.1161/ATVBAHA.110.209726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafique E, Choy WC, Liu Y, et al. Oxidative stress improves coronary endothelial function through activation of the pro survival kinase AMPK. Aging (Albany NY) 2013;5(7):515–530. doi: 10.18632/aging.100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boodhwani, Boodhwani MunirMunir, MD, MMSca, Sodha Neel R., MDb, Mieno Shigetoshi, MD, PhDb B, Ramlawi, Xu Shu-Hua, PhDb, Feng Jun, MD, PhDb, Clements P Richard T., Ruel Marc, MD, MPHa, Sellke Frank W., Md Insulin Treatment Enhances the Myocardial Angiogenic Response in Diabetes. J Thorac Cardiovasc Surg. 2007;134(6):1453–1460. doi: 10.1016/j.jtcvs.2007.08.025. doi:doi:10.1016/j.jtcvs.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potz BA, Sabe AA, Abid MR, Sellke FW. Calpains and Coronary Vascular Disease. Circ J. 2016;80(1):4–10. doi: 10.1253/circj.CJ-15-0997. doi:10.1253/circj.CJ-15-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters SL, Sarang SS, Wang KKW, Schnellmann RG. Calpains Mediate Calcium and Chloride Influx During the Late Phase of Cell Injury 2. J Pharmacol Exp Ther. 1997;283(3):1177–1184. [PubMed] [Google Scholar]
- 9.Pandurangan M, Hwang I, Orhirbat C, Jieun Y, Cho SH. The calpain system and diabetes. Pathophysiology. 2014;21(2):161–167. doi: 10.1016/j.pathophys.2014.01.003. doi:10.1016/j.pathophys.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad H a, Lu L, Ye S, Schwartz GG, Greyson CR. Calpain inhibition preserves talin and attenuates right heart failure in acute pulmonary hypertension. Am J Respir Cell Mol Biol. 2012;47(3):379–386. doi: 10.1165/rcmb.2011-0286OC. doi:10.1165/rcmb.2011-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang MV, Smith LESD. Calpain inhibitors reduce retinal hypoxia in ischemic retinopathy by improving neovascular architecture and functional perfusion. Biochim Biophys Acta. 2011;1812(64):997–1549 - 557003. doi: 10.1016/j.bbadis.2010.08.008. doi:10.1016/j.biotechadv.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoang MV, Nagy J a., Fox JEB, Senger DR. Moderation of calpain activity promotes neovascular integration and lumen formation during VEGF-induced pathological angiogenesis. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013612. doi:10.1371/journal.pone.0013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonĩ-Oliver Paloma, Lucas José J., Avila Jesús, FH N-terminal Cleavage of GSK-3 by Calpain. J Biol Chem. 2007;282(31):22406–22413. doi: 10.1074/jbc.M702793200. doi:10.1074/jbc.M702793200. [DOI] [PubMed] [Google Scholar]
- 14.Feng1 Ye, Xia1 Yiyuan, Yu1 Guang, Shu2 Xiji, Ge1 Haoliang, Kuan Zeng1 JW, Xiaochuan W. Cleavage of GSK-3β by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3β activity induced by H2O2. J Neurochem. 2013;126(2):234–242. doi: 10.1111/jnc.12285. doi:10.1111/jnc.12285. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland C. What Are the bona fide GSK3 Substrates ? Int J Alzheimers Dis. 2011;201 doi: 10.4061/2011/505607. (Article ID 505607):23 pages. doi:10.4061/2011/505607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Pan W. GSK3 : a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2009;35(3):161–168. doi: 10.1016/j.tibs.2009.10.002. doi:10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cline GW, Johnson K, Regittnig W, et al. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (falfa) rats. Diabetes. 2002;51(10):2903–2910. doi: 10.2337/diabetes.51.10.2903. doi:10.2337/diabetes.51.10.2903. [DOI] [PubMed] [Google Scholar]
- 18.Sambasivarao SV. Moderate GSK-3B Inhibition Improves Neovascular Architecture, Reduces Vascular Leakage and Reduces Retinal Hypoxia in A Model of Ischemic Retinopathy. Angiogenesis. 13(3):269–277. doi: 10.1007/s10456-010-9184-y. doi:10.1016/j.micinf.2011.07.011.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang Mien V., Nagy Janice A., Senger DR. Cbc42-mediated Inhibition of GSk-3B improves angio-architecture and lumen formation during VEGF-driven pathological angiogenesis. Microvasc Res. 81(1)(9):34–43. doi: 10.1016/j.mvr.2010.09.001. doi:10.1016/j.micinf.2011.07.011.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura T, Miki T. GSK-3 β , a Therapeutic Target for Cardiomyocyte Protection. Circ J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 21.Potz Brittany A., Sabe Ashraf A., MD, Elmadhun Nassrene Y., MD, Feng Jun, MD, PhD, Clements Richard T, PhD, Ruhul Abid M, MD, PhD, Sellke M. Frank W. Glycogen Synthase Kinase 3B Inhibition Improves Myocardial Angiogenesis and Collateral-dependent Perfusion in a Swine Model of Metabolic Syndrome. J Am Heart Assoc. 2016;5(7):e003694. doi: 10.1161/JAHA.116.003694. doi:doi: 101161/JAHA.116.003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian J, Cuerrier D, Davies PL, Li Z, Powers JC. Co-crystal structures of primed side-extending α-ketoamide inhibitors reveal novel calpain-inhibitor aromatic interactions. J Med Chem. 2008;51(17):5264–5270. doi: 10.1021/jm800045t. doi:10.1021/jm800045t.Co-crystal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Choonara YE, Pillay V. In Silico Affinity Profiling of Neuroactive Polyphenols for Post-Traumatic Calpain Inactivation: A Molecular Docking and Atomistic Simulation Sensitivity Analysis. Molecules. 2015;20:135–168. doi: 10.3390/molecules20010135. doi:10.3390/molecules20010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osakada F, Takahashi M. Forum Minireview Drug Development Targeting the Glycogen Synthase Kinase-3 β ( GSK-3 β ) - Mediated Signal Transduction Pathway : Targeting the Wnt Pathway and Transplantation Therapy as Strategies for Retinal Repair. J Pharmacol Sci. 2009;109:168–173. doi: 10.1254/jphs.08r19fm. [DOI] [PubMed] [Google Scholar]
- 25.Birdsey GM, Shah AV, Dufton N, et al. The Endothelial Transcription Factor ERG Promotes Vascular Stability and Growth through Wnt / b -Catenin Signaling. Dev Cell. 32:82–96. doi: 10.1016/j.devcel.2014.11.016. doi:10.1016/j.devcel.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Weijun, Ph.D, Dianqing Wu P. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2011;35(3):161–168. doi: 10.1016/j.tibs.2009.10.002. doi:10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadigan KM, Waterman ML. TCF / LEFs and Wnt Signaling in the Nucleus. Cold Spring Harb Symp Quant Biol. 2012;4(11):1–22. doi: 10.1101/cshperspect.a007906. doi:10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dharaneeswaran H, Abid MR, Yuan L, et al. FOXO1-mediated activation of akt plays a critical role in vascular homeostasis. Circ Res. 2014;115(2):238–251. doi: 10.1161/CIRCRESAHA.115.303227. doi:10.1161/CIRCRESAHA.115.303227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abid MR, Shih SC, Otu HH, et al. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J Biol Chem. 2006;281(46):35544–35553. doi: 10.1074/jbc.M608620200. doi:10.1074/jbc.M608620200. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN Tail Acts as an Inhibitory Switch by Preventing Its Recruitment into a Protein Complex. J Biol Chem. 2001;276(52):48627–48630. doi: 10.1074/jbc.C100556200. doi:10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- 31.Elmadhun NY, Sabe AA, Robich MP, Chu LM, Lassaletta AD, Sellke FW. The pig as a valuable model for testing the effect of resveratrol to prevent cardiovascular disease. Ann N Y Acad Sci. 2013;1290(Jul):130–135. doi: 10.1111/nyas.12216. doi:10.1111/nyas.12216. [DOI] [PubMed] [Google Scholar]