Abstract
Does eating good-tasting food influence body weight? To investigate, we first established some concentrations of sucralose and mineral oil in chow that mice strongly preferred. Then, in Experiment 1, we compared groups of 16 mice fed plain chow (i.e., chow with no additives) to groups fed chow with added (a) sucralose, (b) mineral oil, (c) sucralose and mineral oil, or (d) sucralose on odd days and mineral oil on even days. During a 6-week test, the body weights and body compositions of the five groups never differed. In Experiment 2, we compared groups of 18 mice fed plain chow or plain high-fat diet to groups fed these diets with added sucralose. During a 9-week test, the high-fat diet caused weight gain, but the body weights of mice fed the sucralose-sweetened diets did not differ from those fed the corresponding plain versions. Two-cup choice tests conducted at the end of each experiment showed persisting strong preferences for the diets with added sucralose and/or mineral oil. In concert with earlier work, our results challenge the hypothesis that the orosensory properties of a food influence body weight gain. A good taste can stimulate food intake acutely, and guide selection toward nutrient-dense foods that cause weight gain, but it does not determine how much is eaten chronically.
Keywords: Taste, obesity, food choice, nonnutritive sweeteners, mineral oil
There is a common adage that “if it tastes good, it must be bad for you,” implying that good-tasting foods cause obesity and related health problems. However, empirical support for this is weak, at best. Work often cited as evidence that body weight is influenced by “good taste” (used here in the general sense, to include flavor and texture) involves feeding rats combinations of tasty foods, sometimes for several weeks, but these studies confound food taste with variety and/or macronutrient content [e.g., (19,31,43); reviewed in (22)]. In fact, we know of only three investigations where food taste was independently manipulated and body weight monitored.
First, Wene et al. (41) reported that baboons gained weight when they were fed chow with a preferred flavor (first punch flavor, then orange flavor) for 9 weeks. However, this study involved only three subjects and had no control group, so the effects attributed to good taste may have been due to experience, including age. Moreover, the increase in body weight reported as significant—“t(2) = 2.76, p = 0.055”—is actually nonsignificant using the standard criterion, even with a one-tailed t-test.
Second, Ramirez (29) found that rats gained significantly more weight if fed a saccharin-sweetened wet diet than an unsweetened wet diet. However, contrary to an explanation based on the saccharin taste causing hyperphagia and weight gain, (a) there was a latent period of several days between first giving the saccharin-containing food and the onset of hyperphagia, (b) the effect was present only with a diet containing 80% water and not one containing 60% water, (c) the effect was absent in rats given previous experience consuming saccharin, and (d) more generally, nonnutritive sweeteners do not increase food intake when mixed into food [e.g., (15,24,37)], or when provided as a separate taste solution [e.g., (28)]. Swithers and colleagues have argued that saccharin can cause weight gain by disrupting the normal relationship between sweet taste and its anticipated physiological outcome (38,39), but recent work undermines the evidence for this (7). Certainly, interpreting any effects of nonnutritive sweetener ingestion as due to “good taste” is complicated by the potential contribution of sweet receptors in the gut and pancreas [e.g., (18,20); review (13)], and by the action of nonnutritive sweeteners on gut bacteria that influence metabolism [reviews (27,34)].
Third, Naim et al. (25,26) compared groups of 12–15 rats fed plain diet for 23 days with others exposed to a “cafeteria-variety” condition, involving concurrent access to three foods, each with a different preferred flavor (25) presented in three different food textures, and with the three foods switched twice daily for another three preferred-flavor foods. This experiment was conducted first with the flavors embedded in AIN-76A diet (a nutritionally complete moderate-energy density diet) and then in a high-fat, high-carbohydrate diet. In each case, there were no differences in food intake or body weight between rats in the plain diet and cafeteria-variety conditions.
The work by Naim et al. (26) is meticulous but not without problems. One is that the treatment condition confounded good taste with variety, so even if the results had been positive it would not be possible to attribute any effects to “good taste” alone. Another is that rats fed plain AIN-76A diet and those fed plain high-fat, high-carbohydrate diet had similar body weights. This seems awry because there are many demonstrations that feeding high-fat, high-carbohydrate diet promotes obesity. It raises the specter of a technical error, perhaps with diet formulation, or perhaps the twice-daily commotion associated with weighing and changing dozens of food cups disturbed the rats' normal feeding patterns in a manner that compromised body weight gains.
Given these limitations, the tremendous importance of taste for the enjoyment of food, and the substantial funds spent by the food industry to improve the chemosensory attributes of their products, we thought it worthwhile to revisit the issue. One problem with the work of Naim et al. is that proprietary flavors were used, making a direct replication difficult. It has also been suggested that the flavors may not have been as strongly liked as are the flavors of typical high-macronutrient density foods. To avoid these potential problems, here we manipulated diet acceptability with sucralose, a nonnutritive sweetener, and mineral oil, a nonnutritive oily “taste.” First, in an initial pretest, we established concentrations of sucralose and mineral oil in chow that C57BL/6J mice strongly preferred in two-cup choice tests. Then, in Experiment 1, we compared the body weights and compositions of mice fed plain chow with those fed chow containing various combinations of added sucralose and/or mineral oil. Finally, in Experiment 2, we compared the body weights and compositions of mice fed chow or a high-fat diet with those fed the same diets containing added sucralose.
METHODS AND RESULTS
General methods
Subjects, maintenance, and diets
Subjects were naïve male C57BL/6J mice, purchased from The Jackson Laboratory (Bar Harbor, Maine). The mice were 10 – 12 weeks old when they arrived in our facility. They were individually housed in plastic “tub” cages (26.5 cm × 17 cm × 12 cm) with stainless steel grid lids, and wood shavings scattered on the floor. The vivarium was maintained at 23°C on a 12:12 h light/dark cycle, with lights off at 1800 h. The mice had deionized water to drink from a glass bottle with a stainless steel sipper tube, and they were fed Teklad 8604 chow. This is a cereal-based diet manufactured by Harlan (Madison, WI), with an energy density of 13 kJ/g (3.1 kcal/g), derived from 30% protein, 58% carbohydrate, and 12% fat. The diet was provided as pellets while the mice acclimated to our vivarium, and as a powder during experiments. The powder was presented in a glass jar (1-oz capacity; U-line, no. S-17073P). To reduce food spillage, this was held upright in the center of a 3” (7.6-cm) diameter acrylic disk (U.S. Plastics Corp., no. 44185) by three clear 8–32” × 7/8” screw fasteners (U.S. Plastics Corp., no. 32016).
Sucralose (Sigma-Aldrich., no. 69293) and mineral oil (CVS Health, Woonsocket, RI) were mixed into the chow using a commercial-grade food mixer. For Experiment 2, some mice were fed a “Corbit and Stellar” high-fat diet [(9) ~21 kJ/g (5.1 kcal/g)]. This was made by heating on a hotplate a glass beaker containing a weighed quantity of vegetable shortening (Crisco brand; J.M. Smucker Co.) until liquid, then removing it from the heat and stirring in 2 grams of Teklad 8604 chow (with sucralose, if needed) for each gram of vegetable shortening. Spoonfuls of the mixture were then scooped into glass feeding jars, and allowed to cool and solidify.
Measurement of body weight and composition
At the beginning and end of each experiment, and weekly in Experiments 1 and 2, mice were weighed by placing them on a top-loading balance with 0.1-g precision. In Experiments 1 and 2, body composition was assessed every 3 weeks. Each mouse was carried to an adjacent room housing a Bruker Minispec LF110; the mouse was placed into a plastic restraining tube, and then inserted into the core of the machine for ~90 sec while its body composition was assessed by magnetic resonance technology. The mouse was then returned to its home cage. However, a technical fault with the Minispec invalidated several body composition values, so here we provide only those measurements collected at the end of the experiments.
Two-cup choice tests
In each experiment, 48- or 96-h two-cup choice tests were used to assess preferences for diets containing sucralose and/or mineral oil. At the beginning of each two-cup choice test and then at 48 h and 96 h, the food jars were weighed with 0.1-g precision. Spillage was collected and accounted for. The positions of the two jars were switched every 24 h to control for side preferences (2).
The food eaten from each cup was calculated by subtracting the weight of the food remaining at the end of each 48-h period from the weight of food given (corrected for spillage). Preference scores for the sucralose or mineral oil were calculated based on the formula: preference score = intake of food with additive/total intake of food from both cups × 100.
Pretest
Methods
The pretest to establish some highly preferred concentrations of sucralose and mineral oil was conducted with two groups of 12 mice weighing (mean ± SE) 23.3 ± 0.3 g (range; 21.7 – 25.4 g). The mice received six 48-h two-cup choice tests. For both groups, the first test involved a choice between two cups of plain diet. Then, one group received a choice between chow and ascending concentrations of sucralose [0.25, 0.5, 1, 2, and 4% wt/wt (i.e., g sucralose/100 g diet)]; the other received a choice between chow and ascending concentrations of mineral oil (1, 2, 4, 8, and 16% wt/wt). Fresh cups of plain chow were introduced every 48 h, at the same time that fresh chow containing a new concentration of sucralose or mineral oil was introduced.
One mouse in two 48-h tests involving sucralose, and one mouse in one 48-h test involving sucralose, contaminated its plain food with feces to an extent that we could not make accurate weight measurements. Values for these three tests were interpolated, so as to simplify statistical analyses.
Results
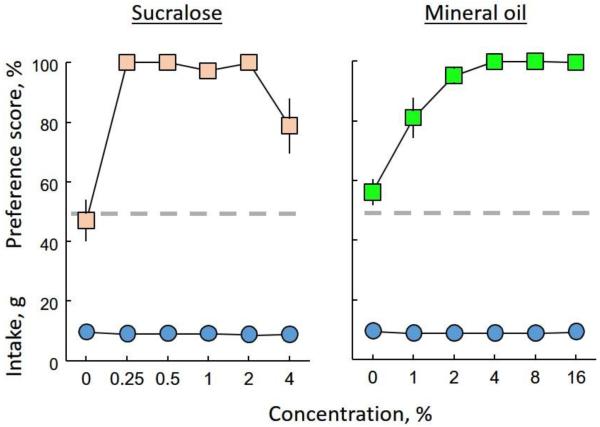
Preference scores for chow containing 0.25, 0.5, 1 or 2% sucralose were 100%, or very close to it; that is, the mice ate almost all their food from the cup containing the sweetened chow. Preference scores for the highest (4%) concentration of sucralose were slightly lower than for the lower concentrations (i.e., 78%; Fig. 1). Preference scores for chow containing 2 – 16% mineral oil were also ~100%; the preference scores for chow containing 1% mineral oil were slightly lower (i.e., 81%; Fig. 1). Based on these findings, in the following experiments, we used 0.25% sucralose and 2% mineral oil—the lowest concentrations producing 100% preference scores.
Figure 1.
Results of 48-h two-cup choice tests between plain chow and chow with ascending concentrations of sucralose (left; n = 12) or mineral oil (right; n = 12). Circles show total daily food intake (i.e., intake of plain food + intake of food with palatability agent); square symbols show preference scores (i.e., intake of food with palatability agent/total food intake × 100). Horizontal dashed lines show 50% preference score (indifference). Vertical bars on symbols are SEMs; where not shown, the SEM was smaller than the symbol).
There was no effect of either palatable agent on total food intakes (Fig. 1). A germane casual observation was that mice often defecated in the cup containing the plain diet. Mineral oil is a laxative but we did not observe any soft fecal boli from the mice eating it, even at high concentrations.
Experiment 1: Effect of sucralose and/or mineral oil added to chow on body weight and composition
Methods
This experiment investigated the effect on body weight and composition of various combinations of sucralose and/or mineral oil added to chow. Eighty mice were given a week to acclimate to our vivarium, and then were weighed weekly. After a baseline week, the mice weighed 24.1 ± 0.2 g (range 20.8 – 27.4 g). They were assigned to five groups of 16, matched for body weight and body composition. The groups were fed a single cup of (1) plain chow, (2) chow + 0.25% sucralose, (3) chow + 2% mineral oil, (4) chow + 0.25% sucralose + 2% mineral oil, and (5) switching: chow + 0.25% sucralose on odd days and chow + 2% mineral oil on even days. The mice remained on the diets for 6 weeks. During the 7th week, the first four groups listed above received 96-h two-cup choice tests. We did not assess preferences of the group that switched daily between sucralose and mineral oil.
Weekly changes in body weights were analyzed using a two-way mixed-design analysis of variance (ANOVA), with factors of group and time (week 0 – 6). Body fat content in week 6 was analyzed using a one-way ANOVA.
Results
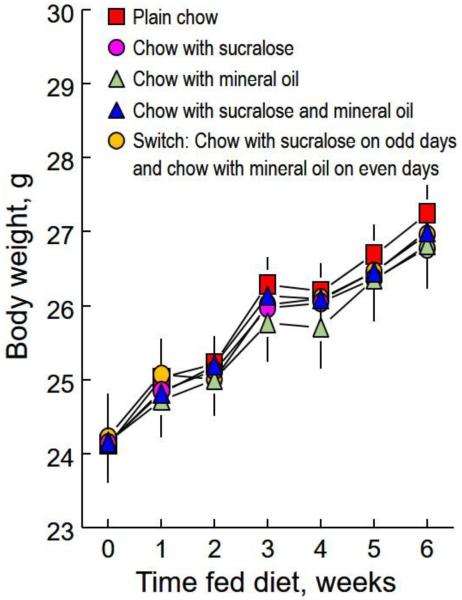
Over the six-week experiment, all five groups of mice gained body weight progressively [Fig. 2; main effect of time, F(5,375) = 37.3, p < 0.0001]. There were no differences among the groups in body weight either throughout the experiment [main effect of group, F(4,75) = 0.80, p = 0.5284] or at any time [group × time interaction, F(20,375) = 1.06, p = 0.3864]. Consistent with this, there were no group differences in body fat content or the percentage of body weight that was fat [F(4,75) = 0.43, p = 0.7807; means ± SE, percent fat; plain chow = 3.7 ± 0.4, chow + sucralose = 3.1 ± 0.4, chow + mineral oil = 4.0 ± 0.5, chow + sucralose + mineral oil = 3.4 ± 0.2, switching group = 3.4 ± 0.4].
Figure 2.
Mean (± SE) body weights of groups of 16 male C57BL/6J mice fed for 6 weeks plain chow or chow with 0.25% sucralose and/or 2% mineral oil in various combinations. There were no significant differences among the five groups.
Two-cup choice tests at the end of the experiment confirmed that the foods with sucralose and mineral oil were strongly preferred, despite 6 weeks of exposure to them. The group fed chow received two cups of chow and ate from them equally (preference score 47 ± 6%). Preference scores of the other groups were: chow + sucralose = 77 ± 5%, chow + mineral oil = 88 ± 3%, chow + sucralose + mineral oil = 97 ± 1%).
Experiment 2: Effect of sucralose added to chow or high-fat diet on body weight and composition
Methods
This experiment investigated the effect on body weight and composition of sucralose added to chow or high-fat diet. It involved 72 mice weighing 25.6 ± 0.2 g (range, 20.8 – 29.1 g). The mice were assigned to one of four groups, matched for body weight and composition. According to a 2 × 2 design, two groups of 18 mice were fed chow, the other two were fed high-fat diet; one version of each diet contained 0.25% sucralose. Body weights were monitored for 9 weeks. During the 10th week, 48-h two-cup choice tests were conducted.
Weekly changes in body weights were analyzed using a three-way mixed-design ANOVA, with factors of diet type (chow or high-fat), diet sweetness (plain or sweetened) and time (week 0 – 9). Carcass fat at week 9 was analyzed using a two-way ANOVA.
Results
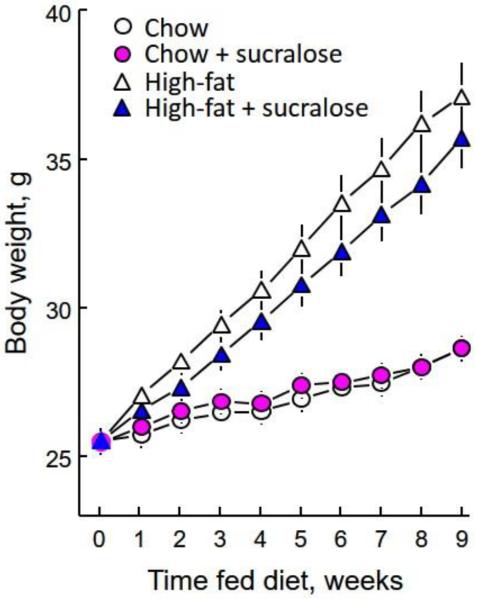
As expected, there was a strong effect of dietary fat to increase body weight [diet × time interaction, F(8,544) = 7.59. p < 0.0001]. Sucralose had no significant effect on body weight gain [Fig. 3; main effect of sucralose, F(1,68) = 2.78, p = 0.0998; sucralose × time interaction, F(8,544) = 1.62, p = 0.1158; sucralose × diet × time interaction, F(8,544) = 1.15, p = 0.3267].
Figure 3.
Mean (± SE) body weights of groups of 18 male C57BL/6J mice fed for 9 weeks plain chow or chow with 0.25% sucralose, or plain high-fat diet, or high-fat diet with 0.25% sucralose. Both groups fed high-fat diet weighed significantly more than did those fed chow; the mice fed sucralose in high-fat diet weighed significantly less than those fed the plain high-fat diet.
At the end of the experiment (week 9), the two groups fed chow had identical mean body weights (28.7 ± 0.4 g vs. 28.7 ± 0.4 g); the mice fed plain high-fat diet weighed slightly but not significantly more than did those fed the high-fat diet + sucralose (37.2 ± 1.1 g vs. 34.8 ± 1.1 g). Body fat content of the mice fed plain chow did not differ from those fed chow + sucralose (5.8 ± 0.6% versus 4.7 ± 0.4%). However, mice fed the plain high-fat diet had a significantly higher percentage body fat than did those fed the high-fat diet + sucralose diet (23.9 ± 1.6% versus 17.2 ± 1.6%), leading to a significant interaction between diet type and sweetness, F(1,68) = 5.59, p<0.0208.
Two-cup choice tests conducted at the end of the experiment confirmed that the mice had strong preferences for the diets with added sucralose. Preference scores of all 72 mice combined for the sweetened versions of their diets were 91 ± 2%, and for each group separately they were: plain chow group = 81 ± 3%, chow + sucralose group = 91 ± 5%, plain high-fat group = 94 ± 2%, high-fat + sucralose group = 97 ± 1%.
DISCUSSION
C57BL/6J mice had very strong and persistent preferences for food containing sucralose and mineral oil. Yet mice fed chow containing these ingredients for 6 weeks did not gain any more body weight or fat than did controls fed plain diet (Experiment 1). Similarly, mice fed high-fat diet for 9 weeks became obese, but the obesity was not exacerbated by adding sucralose—if anything, sucralose tended to reduce body weight gain and it significantly reduced fat stores (Experiment 2). These results add strength to the argument that “good taste” does not cause or contribute to obesity.
The most thorough previous attempt to investigate whether eating good-tasting food influences body weight was conducted by Naim et al. [(26); see introduction]. Our work differs from, and thus extends, this earlier work in several ways. First, Naim et al. (26) used rats whereas we used mice. Second, Naim et al. (26) gave their subjects food flavors with strong odor components whereas we used sucralose and mineral oil. The attraction of sucralose is its sweet taste; preferences for sucralose solutions are absent in mice with genetic ablation of molecular components of the sweet taste transduction cascade [e.g., (11,40)]. It is less clear why mice are attracted to mineral oil; its primary orosensory property, at least to humans, is oiliness (a texture); it may also have a greasy (16) or fatty taste [reviews (6,17,21) but see (33,42)], and it has an odor to rats (14). Our work with sucralose and mineral oil suggests that Naim et al.'s (26) findings cannot be discounted because the flavors they used did not stimulate taste or texture.
Third, Naim et al. (25,26) used food flavors that were preferred to plain food by naive rats, but these preferences may have waned with chronic exposure. One could argue that the extended association of a flavor with the metabolic effects of chow might lead to the flavor losing hedonic value, and so blunting its effects on body weight. But here, we found that strong preferences persisted even after the mice had consumed the diets with added sucralose and/or mineral oil as their only food for 6 or 9 weeks. One reason may be that we used palatability agents that are probably innately preferred. Preferences for sweetness are very likely innate [(36); review (4)]; whether the oral response to mineral oil is also innate is less clear, although two hallmarks of an innate response have been demonstrated: mineral oil stimulates ingestive responses early in life (1), and it supports sham ingestion (23).
Fourth, Naim et al. (26) manipulated both flavor and variety; rats were presented with several flavored foods both concurrently and successively. In contrast, here the focus was on manipulating taste without variety, although Experiment 1 included a group that received chow containing sucralose alternating daily with chow containing mineral oil. The reason to give animals variety is they might habituate or adapt to a single flavor (i.e., develop sensory-specific satiety). However, this clearly was not the case for sucralose or mineral oil, because foods containing these additives were strongly preferred even by mice that had consumed them for 6 or 9 weeks.
Our goal was to use nonnutritive additives that would be among the most strongly preferred by mice. To accomplish this, we chose sucralose because C57BL/6J mice have strong preferences for sucralose solutions [(3,11); unlike rats (5,35)]. Sucralose has advantages over other artificial sweeteners in that it is easily available, and it has a less pronounced aftertaste than does saccharin (12). It has been argued that artificial sweeteners mixed with food (as opposed to water) are not particularly palatable stimuli to rats (24). However, our mice had preferences scores for sucralose-containing chow or high-fat diet that were consistently very strong, sometimes 100%; such high preferences are very rare, in our experience.
To our knowledge, the laboratory mouse's preferences for mineral oil in chow have not been assessed previously, although house mice appear to like it added to oatmeal (32). Early work, sometimes cited as evidence that the oiliness or greasiness imparted by mineral oil leads to chronic hyperphagia in rats, actually used a mixture of mineral oil and corn oil (8–10), which confounds interpretation by changing the type and proportion of dietary fat.
Sucralose stimulates T1R2+T1R3 receptors, including those in the gut and pancreas [e.g., (18,20); review (13)], and it may alter gut bacteria that can influence metabolism [reviews (27,34)]. Our results suggest that these postingestive actions do not influence body weight, although they may be responsible for our finding that mice fed the high-fat diet with sucralose had less body fat than did those fed plain high-fat diet. This conclusion is limited because it is based on only the single dose of sucralose our mice ingested (~300 mg/kg/day), which on a per-kilogram basis is far above corresponding human intakes (~380 12-oz cans of diet cola/day). But more generally, there appears to be a disconnection between the substantial in vitro and minimal in vivo actions of sucralose [review (13)]. One important difference is that in vitro, sucralose is generally given alone whereas here, it was always ingested with food. Food has metabolic effects that likely dwarf those of sucralose, perhaps rendering it ineffectual.
Our findings do not support the adage that “if it tastes good, it must be bad for you.” We suspect the confusion derives from two sources. First, when good-tasting foods are initially made available, they are eaten in large quantities. However, it is wrong to infer that these acute increases in energy intake accumulate with repetition to influence body weight. Second, most palatable foods are also nutrient-dense, and so any effects attributed to “good taste” may actually be due to the high macronutrient density, a known cause of obesity (30). Appreciating that good taste is not inevitably linked to obesity raises a glimmer of hope for the food industry: Good taste can be used to attract consumers to nutrient-sparse foods without concern that this will adversely influence body weight.
Highlights.
Mice had extremely strong preferences for chow with added sucralose and/or mineral oil
Mice fed chow with added sucralose and/or mineral oil did not become obese
Mice fed plain or sucralose-sweetened high-fat diet gained body weight equally
Good-tasting foods do not necessarily cause obesity
ACKNOWLEDGEMENTS
Financial support was provided by Monell Institutional Funds. Use of the Bruker Minispec was supported by funding from NIH–NIDCD Grant P30 DC-011735. We thank Dr. Bruce Kimball for his helpful comments on an earlier draft of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ackroff K, Vigorito M, Sclafani A. Fat appetite in rats: the response of infant and adult rats to nutritive and non-nutritive oil emulsions. Appetite. 1990;15:171–188. doi: 10.1016/0195-6663(90)90018-4. [DOI] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp GK. Why do we like sweet taste: A bitter tale? Physiol Behav. 2016;164:432–437. doi: 10.1016/j.physbeh.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bello NT, Hajnal A. Male rats show an indifference-avoidance response for increasing concentrations of the artificial sweetener sucralose. Nutr Res. 2005;25:693–699. doi: 10.1016/j.nutres.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besnard P, Passilly-Degrace P, Khan NA. Taste of fat: A sixth taste modality? Physiol Rev. 2016;96:151–176. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 7.Boakes RA, Kendig MD, Martire SI, Rooney KB. Sweetening yoghurt with glucose, but not with saccharin, promotes weight gain and increased fat pad mass in rats. Appetite. 2016;105:114–128. doi: 10.1016/j.appet.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Carlisle HJ, Stellar E. Caloric regulation and food preference in normal, hyperphagic, and aphagic rats. J Comp Physiol Psychol. 1969;69:107–114. doi: 10.1037/h0027925. [DOI] [PubMed] [Google Scholar]
- 9.Corbit JD, Stellar E. Palatability, food intake, and obesity in normal and hyperphagic rats. J Comp Physiol Psychol. 1964;58:63–67. doi: 10.1037/h0039787. [DOI] [PubMed] [Google Scholar]
- 10.Coscina DV, Lacombe S, Chambers JW, Dixon L, Nobrega JN. Intake of greasy diets in hypothalamic obesity: a re-assessment. Appetite. 1989;13:15–24. doi: 10.1016/0195-6663(89)90023-8. [DOI] [PubMed] [Google Scholar]
- 11.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 12.Dess NK. Saccharin's aversive taste in rats: evidence and implications. Neurosci Biobeh Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 13.Fernstrom JD. Non-nutritive sweeteners and obesity. Annu Rev Food Sci Technol. 2015;6:119–136. doi: 10.1146/annurev-food-022814-015635. [DOI] [PubMed] [Google Scholar]
- 14.Gamble KR, Smith DW. Discrimination of “odorless” mineral oils alone and as diluents by behaviorally trained mice. Chem Senses. 2009;34:559–563. doi: 10.1093/chemse/bjp036. [DOI] [PubMed] [Google Scholar]
- 15.Gentile RL. The role of taste preference in the eating behavior of the albino rat. Physiol Behav. 1970;5:311–316. doi: 10.1016/0031-9384(70)90103-4. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton CL. Rat's preference for high fat diets. J Comp Physiol Psychol. 1964;58:459–460. doi: 10.1037/h0047142. [DOI] [PubMed] [Google Scholar]
- 17.Khan NA, Besnard P. Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta. 2009;1791:149–155. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Kojima I, Nakagawa Y, Ohtsu Y, Medina A, Nagasawa M. Sweet taste-sensing receptors expressed in pancreatic beta-cells: Sweet molecules act as biased agonists. Endocrinol Metab (Seoul) 2014;29:12–19. doi: 10.3803/EnM.2014.29.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis-Sylvestre J, Giachetti I, Le Magnen J. Sensory versus dietary factors in cafeteria-induced overweight. Physiol Behav. 1984;32:901–905. doi: 10.1016/0031-9384(84)90275-0. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–739. doi: 10.1152/ajpgi.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattes RD. Is there a fatty acid taste? Ann Rev Nutr. 2009;29:305–327. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCrory MA, Burke A, Roberts SB. Dietary (sensory) variety and energy balance. Physiol Behav. 2012;107:576–583. doi: 10.1016/j.physbeh.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Mindell S, Smith GP, Greenberg D. Corn oil and mineral oil stimulate sham feeding in rats. Physiol Behav. 1990;48:283–287. doi: 10.1016/0031-9384(90)90314-t. [DOI] [PubMed] [Google Scholar]
- 24.Mook DG. Saccharin preference in the rat: Some unpalatable findings. Psych Rev. 1974;81:475–490. doi: 10.1037/h0037238. [DOI] [PubMed] [Google Scholar]
- 25.Naim M, Brand JG, Christensen CM, Kare MR, Van Buren S. Preference of rats for food flavors and texture in nutritionally controlled semi-purified diets. Physiol Behav. 1986;37:15–21. doi: 10.1016/0031-9384(86)90377-x. [DOI] [PubMed] [Google Scholar]
- 26.Naim M, Brand JG, Kare MR, Carpenter RG. Energy intake, weight gain and fat deposition in rats fed flavored, nutritionally controlled diets in a multichoice (“cafeteria”) design. J Nutr. 1985;115:1447–1458. doi: 10.1093/jn/115.11.1447. [DOI] [PubMed] [Google Scholar]
- 27.Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol Behav. 2016;164:488–493. doi: 10.1016/j.physbeh.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Porikos KP, Koopmans HS. The effect of non-nutritive sweeteners on body weight in rats. Appetite. 1988;11(Suppl 1):12–15. [PubMed] [Google Scholar]
- 29.Ramirez I. Stimulation of energy intake and growth by saccharin in rats. J Nutr. 1990;120:123–133. doi: 10.1093/jn/120.1.123. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez I, Tordoff MG, Friedman MI. Dietary obesity and hyperphagia: What causes them? Physiol Behav. 1989;45:163–168. doi: 10.1016/0031-9384(89)90180-7. [DOI] [PubMed] [Google Scholar]
- 31.Rolls BJ, van Duijvenvoorde PM, Rowe EA. Variety in the diet contributes to the development of obesity in the rat. Physiol Behav. 1983;31:21–27. doi: 10.1016/0031-9384(83)90091-4. [DOI] [PubMed] [Google Scholar]
- 32.Rowe FP, Bradfield A, Redfern R. Food preferences of wild house-mice (Mus musclus L) J Hyg. 1974;73:473–478. doi: 10.1017/s0022172400042819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitou K, Yoneda T, Mizushige T, Asano H, Okamura M, et al. Contribution of gustation to the palatability of linoleic acid. Physiol Behav. 2009;96:142–148. doi: 10.1016/j.physbeh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Schiffman SS, Rother KI. Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J Toxicol Environ Health B Crit Rev. 2013;16:399–451. doi: 10.1080/10937404.2013.842523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sclafani A, Clare RA. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem Senses. 2004;29:523–528. doi: 10.1093/chemse/bjh055. [DOI] [PubMed] [Google Scholar]
- 36.Steiner JE. The gusto-facial response: Observation on normal and anencephalic newborn infants. In: Bosmas JF, editor. 4th Symposium on Oral sensation and Perception; Washington, DC: US Government Printing Office; 1973. [PubMed] [Google Scholar]
- 37.Strouthes A. Saccharin eating in undeprived and hungry rats. Animal Learn Behav. 1977;5:42–46. [Google Scholar]
- 38.Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav Neurosci. 2009;123:772–780. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122:161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- 40.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wene JD, Barnwell GM, Mitchell DS. Flavor preferences, food intake, and weight gain in baboons (Papio sp.) Physiol Behav. 1982;28:569–573. doi: 10.1016/0031-9384(82)90155-x. [DOI] [PubMed] [Google Scholar]
- 42.Yoneda T, Saitou K, Mizushige T, Matsumura S, Manabe Y, et al. The palatability of corn oil and linoleic acid to mice as measured by short-term two-bottle choice and licking tests. Physiol Behav. 2007;91:304–309. doi: 10.1016/j.physbeh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Zylan KD, Brown SD. Effect of stress and food variety on food intake in male and female rats. Physiol Behav. 1996;59:165–169. doi: 10.1016/0031-9384(95)02039-x. [DOI] [PubMed] [Google Scholar]