Abstract
Epigenetic modifications to cytosine are known to alter transcriptional states and deregulate gene expression in cancer, embryonic development, and most recently in neurodegeneration. To test the hypothesis that global levels of cytosine modification are altered throughout the progression of Alzheimer’s disease, 5-methylcytosine (5-mC) and 5-hydroxmethylcytosine (5-hmC) were quantified using gas chromatography/mass spectrometry (GC/MS) and stable labeled internal standards of cytosine, 5-mC, and 5-hmC. Cytosine modifications were quantified in DNA extracted from tissue specimens of four brain regions (cerebellum, inferior parietal lobe, superior and middle temporal gyrus, and hippocampus/parahippocampal gyrus) of cognitively normal control (NC) subjects and subjects with mild cognitive impairment (MCI), preclinical Alzheimer’s disease (PCAD), late onset Alzheimer’s disease (LOAD), frontotemporal lobar degeneration (FTLD) and dementia with Lewy bodies (DLB). Repeated measures analyses of the data show significant alterations in 5-mC and 5-hmC in early stages of Alzheimer’s disease (PCAD and MCI), as well as FTLD and DLB subjects, across multiple regions of the brain. These data suggest alterations in epigenetic regulation of genes may play an early role in the progression of AD as well as other types of neurodegeneration.
Keywords: Alzheimer’s disease, epigenetic, 5-hydroxymethylcytosine, 5-methylcytosine
Graphical abstract
Cytosine modifications can alter transcriptional states and may play a role in Alzheimer’s disease (AD). DNA from AD affected brain regions was analyzed using gas chromatography mass spectrometry for normal aging, preclinical AD (PCAD), mild cognitive impairment (MCI), late onset AD (LOAD), frontotemporal lobar degeneration (FTLD), and dementia with Lewy bodies (DLB) subjects. Alterations in 5-methylcytosine (5-mC) and 5-hydroxymethylcytosine (5-hmC) were seen in early stages of AD, indicating a possible role of epigenetic modification in AD progression.
Introduction
Familial Alzheimer’s disease (AD) has been linked to mutations in key genes involved in amyloid beta processing, including amyloid precursor protein (APP), presenilin-1 (PS1), and presenilin-2 (PS2) (Selkoe 1997). While risk factors, such as advanced age and the ε4 allele of Apolipoprotein E (APOE) (Corder et al. 1993), have been discovered for the most common form of AD, sporadic AD, no known cause or discovered mechanism has been elucidated, limiting understanding of the disease progression or development of successful therapeutic options to halt or slow degeneration (Selkoe 1997, Walsh & Selkoe 2004, Chakrabarti et al. 2015). The main pathologic markers of AD include senile plaques (SPs) comprised of amyloid β peptide and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau fibrils. These neuropathologic lesions are thought to accumulate in brain regions years before memory loss, cognitive impairments, or alterations in reasoning present as clinical symptoms of dementia (Jack et al. 2010). As pathology progresses throughout the brain, clinical symptoms worsen, ultimately leading to dependent care, decreased cognition, and shortened lifespan (Fargo 2014). According to criteria from the National Institute on Aging and the Alzheimer’s Association, the three stages of AD progression include preclinical AD (PCAD), mild cognitive impairment due to AD (MCI), and dementia due to AD (Montine et al. 2012). In general, PCAD subjects exhibit no clinical deficits on cognitive test scores compared to normal control (NC) subjects, but have increased SP and NFT pathology in brain regions associated with AD (Schmitt et al. 2000). In contrast, MCI subjects show cognitive decline but do not meet the clinical criteria for dementia, and also show advanced pathology in AD affected brain regions (Petersen et al. 2006).
In the absence of gene mutations that promote pathology and cognitive deficits during the progression of AD, alternative methods of dysregulation of normal biological mechanisms may hold the key to understanding the progression of neurodegeneration in sporadic AD (Zawia et al. 2009, Bihaqi et al. 2012). Epigenetic modifications to DNA can alter transcription and gene expression in embryonic development and normal cellular function, as well as to deregulate gene expression mechanisms in cancer (You & Jones 2012) and thus may potentially play a role in the pathogenesis of AD.
Methylation of cytosine by DNA methyl transferase can alter gene function by regulating transcriptional processes (Nabel et al. 2012). Typically, increased 5-methylcytosine (5-mC) in genomic regions is associated with gene silencing and deregulated transcription (Jjingo et al. 2012). However, oxidation of 5-mC by the ten-eleven translocase (TET) family of enzymes to 5-hydroxymethylcytosine (5-hmC) (Tahiliani et al. 2009) is thought to have the opposite effect, increasing transcription and upregulating gene expression (Mellen et al. 2012, James et al. 2014). Due to the seemingly opposed functions of 5-mC and 5-hmC, distinguishing and quantifying each epigenetic mark is essential to understanding the potential biological significance epigenetic modifications to cytosine play in normal cellular function and in disease. Although 5-mC and 5-hmC levels have been studied extensively in aging and cancer research (Szulwach et al. 2011, Johnson et al. 2012, Olkhov-Mitsel & Bapat 2012, Lister et al. 2013), interest in neurodegenerative diseases such as AD has been recent. While some studies show increased levels of 5-mC and/or 5-hmC in AD (Bradley-Whitman & Lovell 2013, Coppieters et al. 2014, Mastroeni 2016), others show decreased epigenetic marks (Mastroeni et al. 2010, Chouliaras et al. 2013, Condliffe et al. 2014) or no changes of either mark in AD (Lashley et al. 2014). These conflicting data for global quantification (Mastroeni et al. 2010, Bradley-Whitman & Lovell 2013, Chouliaras et al. 2013, Condliffe et al. 2014, Coppieters et al. 2014, Lashley et al. 2014) and genome-wide studies (Bakulski et al. 2012, De Jager et al. 2014, Lunnon et al. 2014, Humphries et al. 2015) may be attributed to variations in quantification techniques as well as tissue processing procedures. Many of these studies use antibodies as the method of detection, where non-specific binding and antigen retrieval could potentially cause errors in quantification of analyte signal, or bisulfite sequencing techniques, which cannot distinguish between 5-mC and 5-hmC. Quantification of 5-mC and 5-hmC in neurodegenerative diseases is essential to elucidating the possible role of altered gene regulation via epigenetic mechanisms in the progression of AD.
To test the hypothesis that global levels of 5-mC and 5-hmC are altered in brain regions susceptible to AD pathology throughout the progression of AD using a quantitative method, gas chromatography mass spectrometry (GC/MS) was used. Two advantages of GC/MS for quantification of analytes are the availability of isotopically labeled standards, which can be used as internal standards throughout processing and analysis, as well as specific chromatographic separation of DNA bases (Crain & McCloskey 1983, Romerio et al. 2005, Rossella et al. 2009, Tang et al. 2012). GC/MS paired with stable labeled standards of each modified cytosine base was used to analyze DNA from subjects across the spectrum of AD progression (PCAD, MCI, late onset AD [LOAD]), as well as in frontotemporal lobar degeneration (FTLD) and dementia with Lewy bodies (DLB). DNA was extracted from SP and NFT vulnerable (superior and middle temporal gyrus – SMTG, inferior parietal lobe –IPL, and hippocampus/parahippocampal gyrus – HPG) and non-vulnerable (cerebellum – CER) brain regions. Alterations in epigenetic modifications in each subject group were compared to (frequency) age-matched cognitively NC subjects. Quantification of changes in epigenetic marks in neurodegenerative disease subjects compared to NC tissue may provide insight into how hydroxymethylation and methylation of DNA affect the progression of AD and other types of dementia.
Materials and methods
Reagents
Proteinase K, BioUltra Phenol with 8-hydroxyquinoline, chloroform, 3-methyl-1-butanol, cytosine, 5-methylcytosine, formic acid, and pyridine were purchased from Sigma Aldrich (St. Louis, MO, USA), while BSTFA-TMCS, 99:1 was purchased from Supelco, a supplier of Sigma Aldrich. 5-hydroxymethylcytosine was purchased from MP Biomedical (Santa Ana, CA, USA). [2-13C, 1, 3- 15N2] cytosine was purchased from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA, USA). [2-13C, 1, 3- 15N2] 5-hydroxymethylcytosine was purchased from Moravek Biochemicals Inc. (Brea, CA, USA), and 5-methyl-d3-cytosine-6-d1 was purchased from CDN Isotopes (Pointe-Claire, Quebec, Canada).
Subject data
Tissue samples were obtained from short (<4 hours) post mortem interval (PMI) autopsies of PCAD, MCI, LOAD, FTLD/DLB, and (frequency) age-matched NC subjects through the Neuropathology Core of the University of Kentucky Alzheimer’s Disease Center (UK-ADC), in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and Institutional review board approved protocols. All participants or their legally authorized representatives provided written informed consent. Annual neuropsychological testing and biannual physical examinations were completed for all subjects in the UK-ADC followed longitudinally. All subject diagnoses were based on UK-ADC postmortem consensus conferences between neurologists, neuropsychologists, and social workers, combining neuropathological and clinical data. NC subjects showed no evidence of memory decline and fell within the normal range for mental status test battery exams, comprising 14 neuropsychological tests (Schmitt et al. 2000), and showed only age-associated histopathological changes and low Braak stage scores (0-II). The UK-ADC describes PCAD subjects as those who meet intermediate or high National Institute of Aging-Reagan Institute (NIA-RI) criteria (Hyman & Trojanowski 1997) for advanced AD pathologic changes at autopsy, frequent to moderate neuritic plaque scores according to the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) (Mirra et al. 1991), Braak staging scores between III and VI, but with normal range scores for psychometric test scores when corrected for age and education (Schmitt et al. 2000). MCI subjects showed increased neuropathology burden compared to NC subjects, with Braak stage scores between II-V, increased neuritic plaque density in neocortical regions and increased NFT density in entorhinal cortex, hippocampus, and amygdala, as well as increased memory impairment upon clinical cognition and memory exams, specifically objective memory test impairment (based on word list learning task exams), but do not meet criteria for clinical diagnosis of dementia (Markesbery et al. 2006, Petersen et al. 2006). LOAD subjects meet CERAD and NIA-RI guidelines for intermediate or high likelihood of AD, based on advanced neuropathology (Braak scores of V-VI) and decreased cognition and memory impairment for clinical diagnosis of dementia and probable AD (McKhann et al. 1984, Hyman & Trojanowski 1997). Evaluation of AD-related pathologic markers including Braak NFT stage scoring (Braak & Braak 1995) and counts of diffuse and neuritic plaques, as well as NFTs, were determined by the UK-ADC Neuropathology Core. Global cognitive status was determined based on Mini Mental State Examination (MMSE) scores (Folstein et al. 1975) for all subjects. provided by the UK-ADC. For the current study, MMSE scores were from the last exam before autopsy, given 1.4 ± 1.6 years prior to death. Each subject’s APOE genotype was available. At autopsy, tissue from the superior and middle temporal gyrus (SMTG), hippocampus/parahippocampal gyrus (HPG), inferior parietal lobe (IPL), and cerebellum (CER) were flash frozen in liquid nitrogen and stored at −80°C until processed for analysis. Average frozen storage time of tissue specimens used in this analysis was 7.2 ± 3.7 yrs.
DNA isolation
Frozen samples of brain tissue were homogenized in 1 mL of digestion buffer (0.5% sodium dodecyl sulfate, 0.05M Tris-HCl, 0.1M Na2EDTA) and 0.5 mg/mL proteinase K solution and incubated overnight at 56 °C as described previously (Wang et al. 2005, Wang et al. 2006). Following digestion, 5M NaCl was added to a final concentration of 0.08 M and the samples vortexed. A series of three 1mL phenol/8-hydroxyquinoline extractions was followed by three 1 mL 24:1 chloroform:isoamyl alcohol extractions. NaCl was again added to the supernatant along with 1.5 mL of cold ethanol to precipitate DNA. The solution was hand mixed and precipitated overnight at −20°C. To remove salt and other impurities, the DNA pellet was washed with 60% ethanol three times, dried and suspended in deionized water after 15 min incubation at 40°C. Concentration and purity of DNA samples were measured using the ND1000 NanoDrop UV-Vis Spectrophotometer. The inclusion of EDTA and 8-hydroxyquinoline should help minimize the potential risk of artefactual oxidation.
Gas chromatography mass spectrometry
Internal Standard (IS) Solutions
Commercially available stable labeled standards [2-13C, 1, 3- 15N2] cytosine, [2-13C, 1, 3- 15N2] 5-hydroxymethylcytosine, and 5-methyl-d3-cytosine-6-d1 were used to analyze epigenetic marks using GC/MS. To prepare stock solutions, approximately 5 mg of each standard was dissolved in 2.5 mL of sterile double distilled water, and the concentration of the internal standard (IS) solution determined before each use by measuring the absorbance at 270 nm for cytosine and 5-hmC (Wyatt & Cohen 1953) and 274nm for 5-mC (Wyatt 1951), in triplicate using a Genesys 10uv Thermo Spectronic UV-Vis Spectrophotometer. To compare labeled and unlabeled standard retention times and fragmentation, unlabeled cytosine, 5-mC, and 5-hmC were purchased and analyzed.
Acid Hydrolysis
40 μg of DNA were added to 5mL conical glass vials and lyophilized. To hydrolyze the bases, 250 μL 90% formic acid was added. Air was evacuated from samples using a 60mL syringe and 20 gauge needle through a Teflon seal and samples were heated for 30 min using a sand bath set to 140°C. After cooling for 10 min at −80°C, standards were added to the vials which were then frozen at −80°C for at least 2 h. Samples were then lyophilized overnight.
Derivatization of DNA Bases
To volatilize bases, 250 μL of a 50:50 solution of pyridine/BSTFA:TMCS was added to the sample vial and the air evacuated as previously described (Wang et al. 2006). The samples were vortexed every 30 min for 2 h at room temperature to ensure the reaction went to completion and all bases were derivatized. The vials were then dried under nitrogen gas for 2 h. Fresh BSTFA:TMCS was added to each vial to suspend DNA bases in 20 μL of solvent. The sample solution was transferred to a GC/MS autosampler insert and capped.
GC/MS Method
Derivatized bases were separated using an Agilent 7890A GC on an Agilent HP-5MSI ultra inert capillary column (0.25 mm diameter, 0.25 micron film thickness, 30 m length). To analyze the sample, 1 μL was injected using a splitless mode with ultrapure helium carrier gas (99.999%) at an inlet pressure of 11.8 psi under a constant flow. The temperature program for separation was: 2 min hold at 100°C, ramp one: 5°C/min to 125°C, ramp two: 0.75°C/min to 134.75°C, ramp three: 0.3°C/min to 137.15°C, ramp four: 10°C/min to 307.15°C and held at 307.15°C for 2 min to make the total run time 47 min per sample. Retention times for the standards were 17 min for cytosine, 19 min for 5-mC, and 36 min for 5-hmC.
Samples were introduced into the mass spectrometer (Agilent 5975C inert XL EI/CI MSD with Triple Axis Detector) from the GC column, with an injector temperature maintained at 250°C and the ion source at 230°C. Data were collected in selective ion monitoring mode specific for m/z ratios of the base peak for IS and unlabeled cytosine derivatives. The IS m/z ratios monitored were 257 for 13C, 15N-cytosine, 258 for d4-5-mC, and 360 for 13C, 15N-5-hmC. The unlabeled cytosine derivatives were monitored at m/z ratios of 254 for cytosine and 5-mC, and 357 for 5-hmC at each respective retention time. The integrated area of the signal for each analyte was normalized with respect to the IS signal and corrected based on instrument response for each standard via the standard curve. Sample values were calculated as pmol modification/mg DNA, taking into account the 20:1 dilution factor (20 μL resuspended: 1 μL injection) and 0.04 mg DNA analyzed for each sample.
Serial dilutions (1:3) of each stable labeled IS were used to analyze instrument response curves over a range of 1.5 pmol/μL to 375 pmol/μL of cytosine base injected in five replicate measures prior to tissue experiments. Concentration range was based on average amounts of cytosine, 5-mC, and 5-hmC in 1 μL injections of processed DNA as described above. LOD (limit of detection) and LOQ (limit of quantification) were calculated based on standard deviation of blank measurements and the slope of the standard curves.
Statistical analysis
Sample sizes were determined based on tissue specimen availability, but no formal power analysis was utilized. To limit the potential for batch differences, DNA samples were randomized by diagnosis and brain region and processed in sets of 10 for acid hydrolysis and derivatization steps. To minimize subjective bias when quantifying results, data were recorded as subject case number with no indication of subject diagnosis.
To test significant differences in subject demographic data among disease groups, ANOVA was used for measures with normal distributions (PMI and age), while ANOVA on Ranks was used for testing significant differences in non-normally distributed data (MMSE scores, Braak stage scores, and pathologic burden counts). In the event the omnibus test was significant, Dunn’s post-hoc analysis was performed. Statistical analysis testing of subject demographic data was analyzed using SigmaPlot version 13.0®.
General linear regression models (GLM) were used to test associations between epigenetic marks (5-mC and 5-hmC) in brain regions (SMTG, HPG, IPL) with mean brain region-specific NP and NFT counts. A series of six regression models was fitted to the data, one for each dependent variable (NP and NFT counts) in each of the three brain regions. Models simultaneously estimated the association between 5-mC and 5-hmC and NP/NFT counts while adjusting for age at death and gender. GLMs were also used to test associations with epigenetic marks (5-mC and 5-hmC) with global cognition scores (MMSE) near death, adjusting for age at death and gender.
To determine significant differences in cytosine, 5-mC, and 5-hmC levels across brain regions (CER, IPL, SMTG, HPG) and across subject groups (NC, PCAD, MCI, LOAD, FTLD/DLB), a repeated measures analysis with brain regions as the repeated factor within subjects was used. Separate models were fitted for each epigenetic mark (cytosine, 5-mC, and 5-hmC). To account for correlation within the four measurements taken on the same subject, an unstructured covariance matrix was used. Models tested the main effect of subject group and brain region, as well as their two-way interaction, and adjusted for age at death and gender. The two-way interaction between subject group and brain region was not significant in any of the models and was not retained. GLMs and repeated measures analysis was performed in SAS version 9.4®.
Results
GC/MS method: cytosine, 5-mC, and 5-hmC
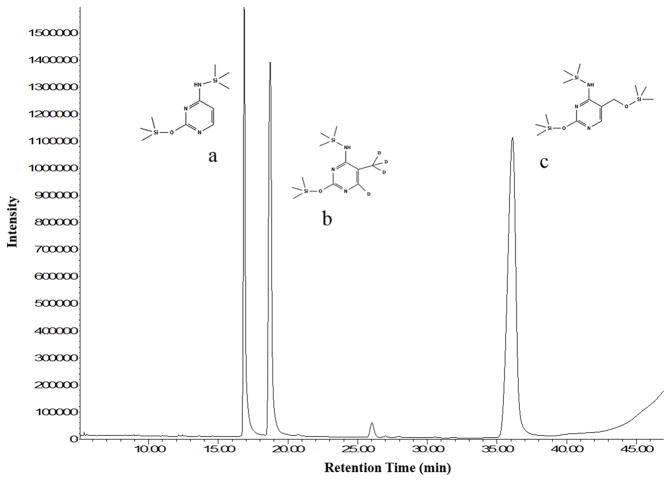
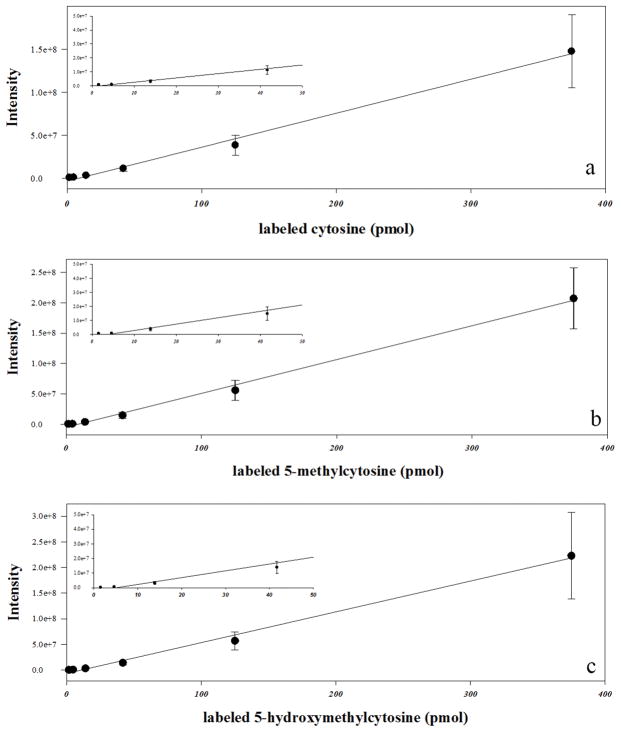
To analyze epigenetic modifications to cytosine using GC/MS, stable labeled standards of cytosine, 5-mC, and 5-hmC (Fig. 1) were used. Characterization of each standard was carried out using m/z ratios and retention times of commercially available labeled vs unlabeled standards. Mass spectra were compared to database references when available (Wishart et al. 2007, Wishart et al. 2009, Wishart et al. 2013). Analysis of stable labeled cytosine derivatives showed high percentage of labeling: cytosine (99.8%), 5-mC (99.3%), and 5-hmC (99.8%). While several m/z ratios were used to identify each peak, the most abundant ion (base ion) was used to analyze each labeled standard. The base ion m/z ratios were 257 for cytosine ([M-H]+), 258 for 5-mC ([M-CH3]+), and 360 for 5-hmC ([M]+). LOQ were calculated for each labeled standard (cytosine, 3.4 pmol; 5-mC, 0.4 pmol; 5-hmC, 0.8 pmol), and calibration curves throughout the 1.5 – 375 pmol/μL concentration range analyzed showed linear responses (R2 > 0.99) for each standard (Fig. 2).
Fig. 1. Total ion chromatogram of internal standards.
Commercially available stable labeled standards of (a) [2-13C, 1, 3-15N2] cytosine, (b) 5-methyl-d3-cytosine-6-d1, and (c) [2-13C, 1, 3-15N2] 5-hydroxymethylcytosine were derivatized for analysis using GC/MS. Standards are shown with tri-methyl silyl groups.
Fig. 2. Standard response curves for GC/MS method.
Over a concentration range of 1–375 pmol/μL of internal standard injected, all curves show a linear fit (R2 > 0.994) in 1:3 serial dilution series. Each point represents mean ± SD (pmol/μL) of replicate measures (n=5) for six concentrations of labeled cytosine (a), 5-mC (b), and 5-hmC (c). Inserts highlight lower region of the standard curves, between 1–50 pmol, for each cytosine base.
Subject demographic analysis
To correct for potential age associated epigenetic modifications in brain tissue, NC specimens were matched for age. To determine if epigenetic modifications were AD specific, diseased control (FTLD/DLB) tissue specimens were also analyzed (Table 1). No significant differences were observed for PMI or age among the subject groups. As expected, significant differences were detected in Braak staging scores for PCAD, MCI, and LOAD subjects compared to NC (p < 0.05). Taking into account a nondemented pool (NC, PCAD, and MCI) of subjects compared to LOAD, Braak stage scores remain significantly higher in LOAD with a median Braak stage score of VI [interquartile range: V-VI] compared to the nondemented pooled subjects with a median Braak stage score of III [interquartile range: II – IV] using Mann-Whitney Rank Sum test (p < 0.05). No difference was observed for Braak staging scores between NC and FTLD/DLB subjects. MMSE scores were significantly decreased in LOAD and for FTLD/DLB subjects (p < 0.05) compared to NC subjects. APOE genotype data from the UK-ADC are listed in Table 1 with total number of subjects per group with the specific ε4 allele.
Table 1.
Demographic data for each subject group. Mean ± SD of age and post-mortem interval (PMI) and median and interquartile range of Mini-Mental State Exam (MMSE) scores. Number of subjects with the ε4 allele of APOE for each subject group also shown. Overall significance tested using ANOVA or ANOVA on Ranks, with post-hoc analysis testing using Dunn’s analysis (*p < 0.05). CER – cerebellum, HPG – hippocampus/parahippocampal gyrus, SMTG – superior and middle temporal gyrus, IPL – inferior parietal lobe.
| NC (N=10) | PCAD (N=8) | MCI (N=11) | LOAD (N=10) | FTLD/DLB (N=11) | |
|---|---|---|---|---|---|
| Subject Demographics | |||||
| Age (years) | 82.8 ± 8.9 | 85.4 ± 6.6 | 89.5 ± 6.4 | 82.5 ± 8.4 | 80.7 ± 12.8 |
| Gender | 5W/5M | 6W/2M | 8W/3M | 5W/5M | 3W/8M |
| PMI (h) | 2.7 ± 0.9 | 3.0 ± 0.5 | 2.6 ± 0.5 | 2.8 ± 0.8 | 3.6 ± 1.1 |
| MMSE Score | 29.5 [28–30] | 30 [28.25–30] | 28 [26–28] | 16* [3–25.5] | 17* [1.5–26.5] |
| APOE-ε4 allele | 1 | 2 | 5 | 6 | 3 |
Quantitative neuropathology data was obtained from the UK-ADC. Regional NP and NFT counts represent arithmetic means of individual plaques and tangles calculated from five of the most severely affected fields within the brain regions analyzed (SMTG, HPG, IPL) as described by Nelson et al. (Nelson et al. 2007). NP and NFT counts for three brain regions were compared using ANOVA on Ranks between subject groups. The omnibus test was significant (p < 0.05) for all NP and NFT counts between the groups in each brain region (Table 2). For significant differences within the groups, Dunn’s post-hoc analysis was used to determine which disease group showed significantly increased burden of pathological counts compared to NC. For NP counts, all brain regions in LOAD subjects were significantly increased compared to NC (SMTG, p = 0.026; IPL, p = 0.024; HPG, p = 0.021). NFT counts in the SMTG (p < 0.001) and IPL (p < 0.001) of LOAD were significantly higher compared to NC subjects.
Table 2.
Braak scores and pathology counts for each subject group. Median and interquartile range of Braak staging score and neuritic plaque (NP) and neurofibrillary tangle (NFT) counts in three brain regions were compared to NC subjects. To determine significant differences between the pathology groups and NC, omnibus significance was tested using ANOVA on Ranks, with post-hoc analysis testing using Dunn’s analysis compared to NC (*p < 0.05). HPG – hippocampus/parahippocampal gyrus, IPL – inferior parietal lobe, SMTG – superior and middle temporal gyrus.
| NC | PCAD | MCI | LOAD | FTLD/DLB | |
|---|---|---|---|---|---|
| Pathology Burden | |||||
| Braak Stage | II [0-II] | IV* [III-V] | III* [II-V] | VI* [V-VI] | II [0-III] |
| NP – HPG | 0 | 0 [0–1] | 0.3 [0–5.0] | 2.8* [0.9–4.1] | 0 [0–0.8] |
| NP – SMTG | 0 [0–5.0] | 10.3 [7.5–19.5] | 5.6 [2.3–12.4] | 16.4* [6.1–30.8] | 3.4 [0–17.8] |
| NP – IPL | 0 [0–7.2] | 13.1 [7.4–31.4] | 6 [2–14.3] | 19.8* [8.6–30.9] | 5.8 [1.6–13.2] |
| NFT – HPG | 3.2 [0.7–6.8] | 2.6 [0.6–8.9] | 24.3 [2.3–78.9] | 20.7 [10.6–50.8] | 1.8 [0–4] |
| NFT – SMTG | 0 | 0.7 [0.1–1.5] | 1.2 [0.5–9.4] | 18.2* [11.0–24.6] | 0 [0–0.8] |
| NFT – IPL | 0 | 0.5 [0.2–1.3] | 0 [0–5.8] | 15.1* [9.6–25.6] | 0 [0–0.4] |
Complete sets of CER, HPG, SMTG, and IPL tissue specimens were analyzed for NC, PCAD, MCI, LOAD, and FTLD/DLB subjects with exceptions for PCAD (HPG, n=5), MCI (HPG, n=5), and FTLD/DLB (HPG, n=6; SMTG, n=10). Total number of subjects (N) in each pathology group is represented in Table 1.
Association of pathologic burden and cognition scores versus epigenetic marks
In the SMTG, 5-mC was significantly associated with mean NP count (t = −2.45, p = 0.021) but not NFT count. In contrast, 5-hmC was not associated with either measure. In the IPL and HPG, neither epigenetic mark (5-mC and 5-hmC) was associated with mean NP or NFT count. Similarly, MMSE scores near death were not associated with 5-mC and 5-hmC levels in any brain region.
Quantification of cytosine derivatives in SMTG, IPL, HPG, and CER
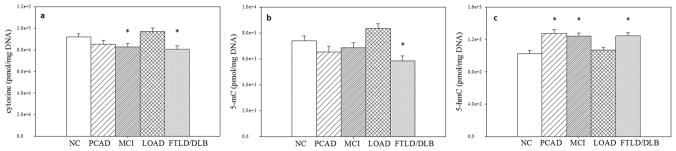
Cytosine, 5-mC, and 5-hmC were quantified as pmol per mg DNA (Table 3), calculated from the signal intensity of the sample compared to the IS and the instrument response of the standard curve for each base, taking into account a dilution factor of 20:1 (20 μL solution: 1 μL injected), as well as the amount of DNA analyzed for each tissue specimen. Cytosine levels did not differ by brain region (p = 0.133), but were significantly different by subject group (p = 0.0018). Compared to NC, MCI cases had significantly lower mean levels (t = −2.10, p = 0.042), as did FTLD/DLB cases (t = −2.61, p = 0.012). There was no significant difference between NC and LOAD (p = 0.2178) or PCAD (p = 0.1494) subjects (Fig. 3). Compared to LOAD, MCI cases had significantly lower mean levels of cytosine (t = −3.29, p = 0.002), as did PCAD (t = −2.60, p = 0.0128) and FTLD/DLB (t = −3.83, p = 0.0004) cases.
Table 3.
Mean ± SD (pmol/mg DNA) of cytosine, 5-mC, and 5-hmC for each subject group by brain region as determined by GC/MS. Values rounded to whole numbers for simplicity. HPG – hippocampus/parahippocampal gyrus, IPL – inferior parietal lobe, SMTG – superior and middle temporal gyrus, CER - cerebellum.
| HPG | SMTG | IPL | CER | |
|---|---|---|---|---|
| cytosine | ||||
| NC | 95056 ± 21963 | 84034 ± 13661 | 99634 ± 21729 | 91869 ± 16517 |
| PCAD | 81788 ± 25440 | 73660 ± 13319 | 94545 ± 25434 | 94247 ± 38731 |
| MCI | 74496 ± 14108 | 77930 ± 12408 | 87374 ± 18830 | 81711 ± 23190 |
| LOAD | 94926 ± 16336 | 96526 ± 20066 | 107957 ± 30112 | 85145 ± 16431 |
| FTLD/DLB | 94971 ± 27815 | 82071 ± 10903 | 74392 ± 10121 | 74896 ± 14954 |
| 5-methylcytosine | ||||
| NC | 6139 ± 2037 | 7020 ± 1568 | 8088 ± 3852 | 9563 ± 2269 |
| PCAD | 7735 ± 3121 | 5486 ± 657 | 6799 ± 2556 | 8115 ± 3580 |
| MCI | 5386 ± 1288 | 6794 ± 1262 | 7256 ± 1738 | 7009 ± 2718 |
| LOAD | 6027 ± 1494 | 8787 ± 1958 | 8901 ± 4296 | 8950 ± 2651 |
| FTLD/DLB | 6336 ± 2131 | 5580 ± 1016 | 5512 ± 1503 | 5935 ± 1613 |
| 5-hydroxymethylcytosine | ||||
| NC | 751 ± 135 | 915 ± 234 | 1135 ± 258 | 1302 ± 260 |
| PCAD | 1065 ± 136 | 1316 ± 321 | 1252 ± 193 | 1474 ± 257 |
| MCI | 828 ± 210 | 1345 ± 202 | 1280 ± 264 | 1474 ± 302 |
| LOAD | 787 ± 224 | 1111 ± 215 | 1082 ± 297 | 1251 ± 249 |
| FTLD/DLB | 1147 ± 226 | 1218 ± 167 | 1126 ± 327 | 1540 ± 217 |
Fig. 3. Global levels of cytosine, 5-mC, and 5-hmC throughout brain regions.
Adjusted mean ± SEM (pmol/mg DNA) of cytosine (a), 5-mC (b), and 5-hmC (c) throughout brain regions for each subject group. Statistical significance reported is for comparison to NC values based on repeated measures analysis model (*p < 0.05).
For 5-mC, there were significant differences by brain region (p = 0.0054) and subject group (p = 0.0002). In the CER, 5-mC levels were significantly higher compared to HPG (t = 3.51, p = 0.0011) and SMTG (t = 2.79, p = 0.0079). 5-mC was significantly lower in the HPG compared to IPL (t = −2.30, p = 0.0265) but not the SMTG (p = 0.1006). Compared to NC, 5-mC was significantly lower in FTLD/DLB (t = −3.01, p = 0.0011), but not in PCAD (p = 0.1244), MCI (p = 0.3043), or LOAD (p = 0.0529) cases. Compared to LOAD, 5-mC levels were significantly lower in PCAD (t = −3.38, p = 0.0016), MCI (t = −2.93, p = 0.0054), and FTLD/DLB (t = −4.99, p < 0.0001) subjects.
As with 5-mC, 5-hmC levels were significantly different by both brain region (p < 0.0001) and subject group (p < 0.0001). In the CER, 5-hmC levels were significantly higher compared to HPG (t = 10.73, p < 0.0001), SMTG (t = 5.19, p < 0.0001) and IPL (t = 4.17, p = 0.0001). Levels of 5-hmC were significantly lower in the HPG compared to SMTG (t = −6.28, p < 0.0001) and the IPL (t = −4.52, p < 0.0001). Compared to NC, levels of 5-hmC were significantly higher in PCAD (t = 4.43, p < 0.0001), MCI (t = 4.09, p =0.0002), and FTLD/DLB (t = 4.21, p = 0.0001) subjects, but not LOAD (p = 0.4286). Compared to LOAD, 5-hmC levels were significantly higher in PCAD (t = 3.70, p = 0.0006), MCI (t = 3.31, p = 0.0019), and FTLD/DLB (t = 3.39, p = 0.0015) cases.
Effect of AD specific epigenetic marks on brain regions
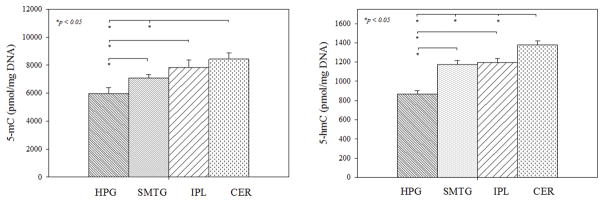
To determine if alterations in epigenetic marks in brain regions susceptible to AD pathology are specific to AD progression, FTLD/DLB subjects were removed from the repeated measures analysis and significance between brain regions of NC, PCAD, MCI and LOAD subjects was tested. In the CER, levels of 5-mC were significantly higher compared to HPG (p = 0.0002) and SMTG (p = 0.0075). In the HPG, 5-mC levels were significantly decreased compared to SMTG (p = 0.0342) and IPL (p = 0.0057). Levels of 5-hmC were significantly elevated in the CER compared to HPG (p < 0.0001), SMTG (p = 0.0003), and the IPL (p = 0.0079). In the HPG, 5-hmC levels were significantly lower compared to the SMTG (p < 0.0001) and the IPL (p < 0.0001) (Fig. 4).
Fig. 4. AD specific epigenetic marks in HPG, SMTG, IPL, and CER.
Adjusted mean ± SEM (pmol/mg DNA) of 5-mC and 5-hmC were averaged across normal control (NC), preclinical Alzheimer’s disease (PCAD), mild cognitive impairment due to Alzheimer’s disease (MCI), and late onset Alzheimer’s disease (LOAD) subject groups and compared to each brain region based on repeated measures analysis model (*p < 0.05).
Discussion
Epigenetic modifications to cytosine are known to be potentially causal in the progression of disease phenotypes, specifically in tumor inhibition suppression and tumor growth stimulation in various types of cancer (You & Jones 2012). In AD, however, it remains controversial whether epigenetic marks change in affected brain regions or if changes merely reflect age related epigenetic alterations. It was the aim of this study to measure these epigenetic marks using a more specific technique, mass spectrometry, instead of the semi-quantitative method of immunochemistry where antigen retrieval and non-specific antibody binding have the potential to cloud results (Yuan 2014, Celik 2015). To determine epigenetic changes in brain regions associated with AD throughout the progression of the disease, modifications to cytosine were analyzed using GC/MS and stable labeled standards of each cytosine base (Fig. 1). After characterizing each stable labeled standard with commercially available synthetic non-labeled standards, calibration curves were analyzed (Fig. 2) and LOQ calculated (see Results) to produce a sensitive and selective method to quantify cytosine, 5-mC, and 5-hmC in DNA from several disease states (PCAD, MCI, LOAD, and FTLD/DLB) and cognitively normal subjects.
Based on our data, epigenetic modifications to cytosine are globally altered in early stages of AD throughout the brain, as well as in other types of neurodegeneration, specifically FTLD and DLB subjects (Fig. 3). This finding is in agreement with current literature suggesting dysregulation of epigenetic mechanisms are relevant to neurological disorders including dementia, neurological psychosis, and genetic diseases (Amir et al. 1999, Schanen 2006, Rao et al. 2012, Veerappan et al. 2013, Wang et al. 2014, Sherwani & Khan 2015).
In AD, levels of 5-hmC were significantly elevated in PCAD and MCI subjects across the four brain regions analyzed compared to NC. While 5-mC levels were not significantly different in any stage of AD progression compared to NC, levels of 5-mC were marginally significant in LOAD (p = 0.0529). When compared to LOAD levels, epigenetic marks in PCAD and MCI subjects show decreased cytosine and 5-mC and increased 5-hmC. This suggests that global levels of epigenetic marks are altered early in AD progression and return to what could be considered “basal levels” at the final stage of the disease. Epigenetic marks may play a role early in AD, but after rampant neurodegeneration and cell death, global changes of epigenetic marks may have less of an impact on the brain as a whole. In analyzing epigenetic marks in specific brain regions, levels of 5-mC and 5-hmC were elevated in the CER compared to HPG, SMTG, and IPL levels in the progression of AD (Fig. 4). This is consistent with previous studies of elevated epigenetic marks in human cerebellum samples (Illingworth et al. 2015), (Lunnon et al. 2016). The differences in 5-mC and 5-hmC levels between brain regions may reflect changes in cell type distributions. Brain regions susceptible to AD pathology later in the progression of the disease have increased global levels of 5-mC and 5-hmC compared to regions of the brain affected earlier (Fig. 4); however, further study is needed to elucidate the biological significance of these brain region specific altered levels of epigenetic marks.
Alterations to cytosine modifications are thought to affect gene transcription where methylation of cytosine leads to gene repression, while hydroxymethylation is thought to have the opposite effect. However, it has been suggested that specific genetic locations of cytosine modification may be more relevant to transcription changes than global methylation/hydroxymethylation levels (Song et al. 2005, Nabel et al. 2012, Wen et al. 2014, Wen & Tang 2014, Breiling & Lyko 2015). Several studies have shown altered gene expression in the progression of AD pathology over multiple brain regions in human and animal models (Blalock et al. 2004, Liang et al. 2008, Avramopoulos et al. 2011, Twine et al. 2011, Karch et al. 2012, Berchtold et al. 2013, Berchtold et al. 2014, Cummings et al. 2015, Sekar et al. 2015). Bossers et al. studied genome-wide expression profiles in the medial frontal gyrus of varying stages of AD pathology (Braak stages 0-VI) in 49 clinically well-studied subjects. In early stages of disease pathology, expression of synaptic activity and plasticity genes increased, but as AD pathology progressed, expression levels decreased. Alongside these pathways, decreased expression in cell differentiation/proliferation, transcriptional regulation, and inflammation pathways was present early in AD, with increased expression of genes in these pathways as AD pathology progressed (Bossers et al. 2010). The repression and upregulation of specific genes throughout the progression of AD could be the result of disease specific epigenetic modifications, however, more research is needed to understand these complex mechanisms.
Epigenetic drift is a natural component of the aging process as well as a factor in age-related diseases, where genes relevant to aging mechanisms have altered transcription (Fraga et al. 2005, Bihaqi et al. 2012, Brunet & Berger 2014, Jung et al. 2015). To account for age effects, AD subjects were age-matched with cognitively normal control subjects, as well as correcting for age at death and gender in the repeated measures analysis. This study shows that alterations in 5-mC and 5-hmC in the brain are not merely age effects, but may be an indication of neurodegeneration and disease.
To correlate pathologic burden with epigenetic marks, regression models were used and showed 5-mC levels negatively correlated with NP count in the SMTG, however no other model was significant for 5-mC or 5-hmC. This is likely due to the small sample sizes between the subject groups and that these reflect global relationships between pathology counts and epigenetic marks, while other studies show correlations between 5-mC and AD pathology in specific gene studies (Chibnik et al. 2015, Levine et al. 2015).
While it is unclear if storage time and storage conditions impact cytosine oxidation, it is unlikely for tissue snap-frozen in liquid nitrogen at autopsy and stored at −80°C until used for analysis. In cell culture, the production of 5-hmC was dependent on TET enzyme activity in the presence of reactive oxygen species and oxidizing compounds (Coulter et al. 2013, Minor et al. 2013, Kang et al. 2014, Zhao et al. 2014). Thus, if TET is required for conversion of 5-mC to 5-hmC, the severity of the cold storage conditions should inhibit enzyme activity, making enzymatic oxidation of 5-mC at −80°C unlikely. Extended PMIs may affect oxidation of DNA bases, where TET could be residually active, however it has been shown in rat brains with PMIs between 0–96 h, there was no effect on global or site-specific levels of 5-mC or 5-hmC (Gross et al. 2016). Although we cannot fully rule out effects of storage conditions on 5-hmC levels in the frozen tissue specimens analyzed in this study, there was no correlation between length of storage time and measured 5-hmC level among the subject groups in our analysis (data not shown).
In order to study nucleic acids in tissue samples using GC/MS, DNA must be extracted chemically from tissue specimens, cytosine bases freed from the phosphate backbone of the DNA, and bases derivatized to increase volatility and enable detection by GC/MS. While artefactual oxidation of nucleic acids, typically 8-oxoguanine, has been suggested to occur during chemical DNA isolation and derivatization (Bruskov et al. 2002, Ravanat et al. 2002) previous studies have demonstrated no significant difference in the levels of oxidized nucleic acids when prepared with a phenol:chloroform extraction or a more gentle “salting-out” approach (Rehman et al. 2000, Wang et al. 2006). To further minimize artefactual oxidation, several precautions were implemented during sample preparation, including the chelation of free radical generating trace metals (Fe/Cu) by Na2EDTA during tissue digestion, as well as subsequent DNA extraction in the presence of 8-hydroxyquinoline. Additionally, previous studies have demonstrated that derivatization of nucleic acids is the most vulnerable step to artefactual oxidation (Hamberg & Zhang 1995, Ravanat et al. 1995), specifically in the presence of atmospheric oxygen and at elevated temperatures. To minimize these effects in the current study, derivatization was carried out in evacuated tubes at room temperature, reducing the risk of artefactual oxidation (Dizdaroglu & Gajewski 1990, Jenner et al. 1998, Rehman et al. 2000).
Despite the incorporation of precautionary measures implemented during sample preparation and derivatization, it remains unclear if the same measures are effective in the prevention of artefactual oxidation of 5-mC. However, chelation of iron by Na2EDTA during tissue digestion should minimize the iron-dependent enzymatic activity of TET1, thus minimizing further oxidation of 5-mC during tissue digestion. Madugundu et al. demonstrated that TET-independent hydroxyl-radical-oxidation of 5-mC to 5-hmC is minimal in oligonucleotide duplexes subjected to lethal doses of ionizing radiation (Madugundu et al. 2014), suggesting that under current isolation conditions artefactual oxidation of 5-mC should be minimal. Furthermore, the absence of detectable levels of 5-hmC during GC/MS analysis of commercially available 5-mC standard subjected to derivatization procedures demonstrates the efficacy of the experimental conditions in preventing artefactual oxidation with respect to base derivatization.
While studies of global levels of epigenetic changes give valuable insight into modified regions of the brain vulnerable to AD pathology, gene level studies of epigenetic marks as they relate to transcriptional activity would give targets of possible gene mechanisms that lead to neurodegeneration and dysfunction in these vulnerable brain regions. As research progresses, the cost of genome-wide mapping of 5-hmC and 5-mC will decrease, opening doors to map the entire epigenome and discover specific gene targets that could be epigenetically modified in AD.
In summary, global levels of cytosine, 5-mC, and 5-hmC were measured in tissue from four brain regions across the spectrum of AD using stable labeled cytosine standards and GC/MS. While epigenetic drift is a known factor in aging, these data show changes in epigenetic marks are not only due to age, but are significantly altered in early stages of AD progression, which could be an indicator of neurodegenerative disease. These marks are also not specific to AD, but are altered in FTLD and DLB. Alterations in epigenetic marks in key brain regions associated with disease progression could play a role in deregulated gene transcription and aid to the progressive nature of neurodegenerative diseases. While this study highlights global epigenetic changes throughout the progression of the disease in key brain regions, it gives no information about the epigenetic state of specific genes. Further study of epigenetic modifications of target genes in brain regions associated with AD could lead to a better understanding of the mechanism of dysfunction in neurodegeneration and the discovery of potential therapeutic targets.
Acknowledgments
This work was supported by National Institute of Health grants 5P01-AG05119 and P30-AG028383, as well as a grant from Alltech Biotechnology. The authors thank Dr. Peter Nelson, Dr. Steven Scheff, Ms. Sonya Anderson, and Ms. Ela Patel from the UK-ADC Clinical and Neuropathology Cores, and Mr. Timothy Shannon from the Data Management and Statistics Core for subject data, and Ms. Paula Thomason for editorial assistance.
Abbreviations
- AD
Alzheimer’s disease
- PCAD
preclinical Alzheimer’s disease
- MCI
mild cognitive impairment
- LOAD
late onset AD
- NC
normal control
- FTLD
frontotemporal lobar degeneration
- DLB
dementia with Lewy bodies
- APP
amyloid precursor protein
- PS1
presenilin 1
- PS2
presenilin 2
- APOE
apolipoprotein E
- Aβ
amyloid beta
- SP
senile plaque
- NP
neuritic plaque
- NFT
neurofibrillary tangle
- 5-mC
5-methylcytosine
- 5-hmC
5-hydroxymethylcytosine
- TET
ten eleven translocase
- GC/MS
gas chromatography mass spectrometry
- SMTG
superior and middle temporal gyrus
- IPL
inferior parietal lobe
- HPG
hippocampus/parahippocampal gyrus
- CER
cerebellum
- BSTFA-TMCS
N,O, Bis(trimethylsilyl)trifluoroacetamide-Trimethylchlorosilane
- PMI
post mortem interval
- MMSE
Mini Mental State Examination
- IS
internal standard
- m/z
mass to charge ratio
Footnotes
conflict of interest statement
The authors report no conflict of interest in the research or data described in this article.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature genetics. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Avramopoulos D, Szymanski M, Wang R, Bassett S. Gene expression reveals overlap between normal aging and Alzheimer’s disease genes. Neurobiol Aging. 2011;32:2319, e2327–2334. doi: 10.1016/j.neurobiolaging.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakulski KM, Dolinoy DC, Sartor MA, Paulson HL, Konen JR, Lieberman AP, Albin RL, Hu H, Rozek LS. Genome-wide DNA methylation differences between late-onset Alzheimer’s disease and cognitively normal controls in human frontal cortex. Journal of Alzheimer’s disease: JAD. 2012;29:571–588. doi: 10.3233/JAD-2012-111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol Aging. 2013;34:1653–1661. doi: 10.1016/j.neurobiolaging.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Sabbagh MN, Beach TG, Kim RC, Cribbs DH, Cotman CW. Brain gene expression patterns differentiate mild cognitive impairment from normal aged and Alzheimer’s disease. Neurobiol Aging. 2014;35:1961–1972. doi: 10.1016/j.neurobiolaging.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihaqi SW, Schumacher A, Maloney B, Lahiri DK, Zawia NH. Do epigenetic pathways initiate late onset Alzheimer disease (LOAD): towards a new paradigm. Current Alzheimer research. 2012;9:574–588. doi: 10.2174/156720512800617982. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossers K, Wirz KT, Meerhoff GF, Essing AH, van Dongen JW, Houba P, Kruse CG, Verhaagen J, Swaab DF. Concerted changes in transcripts in the prefrontal cortex precede neuropathology in Alzheimer’s disease. Brain: a journal of neurology. 2010;133:3699–3723. doi: 10.1093/brain/awq258. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- Bradley-Whitman MA, Lovell MA. Epigenetic changes in the progression of Alzheimer’s disease. Mechanisms of ageing and development. 2013;134:486–495. doi: 10.1016/j.mad.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiling A, Lyko F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics & chromatin. 2015;8:24. doi: 10.1186/s13072-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Berger SL. Epigenetics of aging and aging-related disease. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69(Suppl 1):S17–20. doi: 10.1093/gerona/glu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruskov VI, Malakhova LV, Masalimov ZK, Chernikov AV. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic acids research. 2002;30:1354–1363. doi: 10.1093/nar/30.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik S. Understanding the complexity of antigen retrieval of DNA methylation for immunofluorescence-based measurement and an approach to challenge. Journal of immunological methods. 2015;416:1–16. doi: 10.1016/j.jim.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Khemka VK, Banerjee A, Chatterjee G, Ganguly A, Biswas A. Metabolic Risk Factors of Sporadic Alzheimer’s Disease: Implications in the Pathology, Pathogenesis and Treatment. Aging and disease. 2015;6:282–299. doi: 10.14336/AD.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibnik LB, Yu L, Eaton ML, Srivastava G, Schneider JA, Kellis M, Bennett DA, De Jager PL. Alzheimer’s loci: epigenetic associations and interaction with genetic factors. Annals of clinical and translational neurology. 2015;2:636–647. doi: 10.1002/acn3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouliaras L, Mastroeni D, Delvaux E, et al. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol Aging. 2013;34:2091–2099. doi: 10.1016/j.neurobiolaging.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe D, Wong A, Troakes C, et al. Cross-region reduction in 5-hydroxymethylcytosine in Alzheimer’s disease brain. Neurobiol Aging. 2014;35:1850–1854. doi: 10.1016/j.neurobiolaging.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters N, Dieriks BV, Lill C, Faull RL, Curtis MA, Dragunow M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol Aging. 2014;35:1334–1344. doi: 10.1016/j.neurobiolaging.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Coulter JB, O’Driscoll CM, Bressler JP. Hydroquinone increases 5-hydroxymethylcytosine formation through ten eleven translocation 1 (TET1) 5-methylcytosine dioxygenase. The Journal of biological chemistry. 2013;288:28792–28800. doi: 10.1074/jbc.M113.491365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain PF, McCloskey JA. Analysis of modified bases in DNA by stable isotope dilution gas chromatography-mass spectrometry: 5-methylcytosine. Analytical biochemistry. 1983;132:124–131. doi: 10.1016/0003-2697(83)90434-7. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Liu W, Portelius E, et al. First effects of rising amyloid-beta in transgenic mouse brain: synaptic transmission and gene expression. Brain: a journal of neurology. 2015;138:1992–2004. doi: 10.1093/brain/awv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Srivastava G, Lunnon K, et al. Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nature neuroscience. 2014;17:1156–1163. doi: 10.1038/nn.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M, Gajewski E. Selected-ion mass spectrometry: assays of oxidative DNA damage. Methods in enzymology. 1990;186:530–544. doi: 10.1016/0076-6879(90)86147-n. [DOI] [PubMed] [Google Scholar]
- Fargo K. 2014 Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2014;10:e47–92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JA, Nagy C, Lin L, Bonneil E, Maheu M, Thibault P, Mechawar N, Jin P, Turecki G. Global and Site-Specific Changes in 5-Methylcytosine and 5-Hydroxymethylcytosine after Extended Post-mortem Interval. Frontiers in genetics. 2016;7:120. doi: 10.3389/fgene.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Zhang LY. Quantitative determination of 8-hydroxyguanine and guanine by isotope dilution mass spectrometry. Analytical biochemistry. 1995;229:336–344. doi: 10.1006/abio.1995.1422. [DOI] [PubMed] [Google Scholar]
- Humphries CE, Kohli MA, Nathanson L, Whitehead P, Beecham G, Martin E, Mash DC, Pericak-Vance MA, Gilbert J. Integrated whole transcriptome and DNA methylation analysis identifies gene networks specific to late-onset Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2015;44:977–987. doi: 10.3233/JAD-141989. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. Journal of neuropathology and experimental neurology. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Illingworth RS, Gruenewald-Schneider U, De Sousa D, et al. Inter-individual variability contrasts with regional homogeneity in the human brain DNA methylome. Nucleic acids research. 2015;43:732–744. doi: 10.1093/nar/gku1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet. Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Shpyleva S, Melnyk S, Pavliv O, Pogribny IP. Elevated 5-hydroxymethylcytosine in the Engrailed-2 (EN-2) promoter is associated with increased gene expression and decreased MeCP2 binding in autism cerebellum. Translational psychiatry. 2014;4:e460. doi: 10.1038/tp.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner A, England TG, Aruoma OI, Halliwell B. Measurement of oxidative DNA damage by gas chromatography-mass spectrometry: ethanethiol prevents artifactual generation of oxidized DNA bases. The Biochemical journal. 1998;331(Pt 2):365–369. doi: 10.1042/bj3310365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jjingo D, Conley AB, Yi SV, Lunyak VV, Jordan IK. On the presence and role of human gene-body DNA methylation. Oncotarget. 2012;3:462–474. doi: 10.18632/oncotarget.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AA, Akman K, Calimport SR, Wuttke D, Stolzing A, de Magalhaes JP. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation research. 2012;15:483–494. doi: 10.1089/rej.2012.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Jin SG, Zhang X, Xiong W, Gogoshin G, Rodin AS, Pfeifer GP. Longitudinal epigenetic and gene expression profiles analyzed by three-component analysis reveal down-regulation of genes involved in protein translation in human aging. Nucleic acids research. 2015;43:e100. doi: 10.1093/nar/gkv473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KA, Piao MJ, Kim KC, Kang HK, Chang WY, Park IC, Keum YS, Surh YJ, Hyun JW. Epigenetic modification of Nrf2 in 5-fluorouracil-resistant colon cancer cells: involvement of TET-dependent DNA demethylation. Cell death & disease. 2014;5:e1183. doi: 10.1038/cddis.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, Goate AM. Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PloS one. 2012;7:e50976. doi: 10.1371/journal.pone.0050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley T, Gami P, Valizadeh N, Li A, Revesz T, Balazs R. Alterations in global DNA methylation and hydroxymethylation are not detected in Alzheimer’s disease. Neuropathology and applied neurobiology. 2014 doi: 10.1111/nan.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Bennett DA, Horvath S. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging. 2015 doi: 10.18632/aging.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WS, Dunckley T, Beach TG, et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiological genomics. 2008;33:240–256. doi: 10.1152/physiolgenomics.00242.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunnon K, Hannon E, Smith RG, et al. Variation in 5-hydroxymethylcytosine across human cortex and cerebellum. Genome Biol. 2016;17:27. doi: 10.1186/s13059-016-0871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunnon K, Smith R, Hannon E, et al. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer’s disease. Nature neuroscience. 2014;17:1164–1170. doi: 10.1038/nn.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madugundu GS, Cadet J, Wagner JR. Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic acids research. 2014;42:7450–7460. doi: 10.1093/nar/gku334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Archives of neurology. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic changes in Alzheimer’s disease: decrements in DNA methylation. Neurobiol Aging. 2010;31:2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni DCL, Van den Hove D, Nolz J, Rutten B, Delvaux E, Coleman P. Increased 5-hydroxymethylation levels in the sub ventricular zone of the Alzheimer’s brain. Neuroepigenetics. 2016;6:26–31. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mellen M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. The Journal of biological chemistry. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta neuropathologica. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel CS, Manning SA, Kohli RM. The curious chemical biology of cytosine: deamination, methylation, and oxidation as modulators of genomic potential. ACS chemical biology. 2012;7:20–30. doi: 10.1021/cb2002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. Journal of neuropathology and experimental neurology. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhov-Mitsel E, Bapat B. Strategies for discovery and validation of methylated and hydroxymethylated DNA biomarkers. Cancer medicine. 2012;1:237–260. doi: 10.1002/cam4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Archives of neurology. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Rao JS, Keleshian VL, Klein S, Rapoport SI. Epigenetic modifications in frontal cortex from Alzheimer’s disease and bipolar disorder patients. Translational psychiatry. 2012;2:e132. doi: 10.1038/tp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanat JL, Douki T, Duez P, et al. Cellular background level of 8-oxo-7,8-dihydro-2′-deoxyguanosine: an isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up. Carcinogenesis. 2002;23:1911–1918. doi: 10.1093/carcin/23.11.1911. [DOI] [PubMed] [Google Scholar]
- Ravanat JL, Turesky RJ, Gremaud E, Trudel LJ, Stadler RH. Determination of 8-oxoguanine in DNA by gas chromatography--mass spectrometry and HPLC--electrochemical detection: overestimation of the background level of the oxidized base by the gas chromatography--mass spectrometry assay. Chemical research in toxicology. 1995;8:1039–1045. doi: 10.1021/tx00050a007. [DOI] [PubMed] [Google Scholar]
- Rehman A, Jenner A, Halliwell B. Gas chromatography-mass spectrometry analysis of DNA: optimization of protocols for isolation and analysis of DNA from human blood. Methods in enzymology. 2000;319:401–417. doi: 10.1016/s0076-6879(00)19038-x. [DOI] [PubMed] [Google Scholar]
- Romerio AS, Fiorillo G, Terruzzi I, Senesi P, Testolin G, Battezzati A. Measurement of DNA methylation using stable isotope dilution and gas chromatography-mass spectrometry. Analytical biochemistry. 2005;336:158–163. doi: 10.1016/j.ab.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Rossella F, Polledri E, Bollati V, Baccarelli A, Fustinoni S. Development and validation of a gas chromatography/mass spectrometry method for the assessment of genomic DNA methylation. Rapid communications in mass spectrometry: RCM. 2009;23:2637–2646. doi: 10.1002/rcm.4166. [DOI] [PubMed] [Google Scholar]
- Schanen NC. Epigenetics of autism spectrum disorders. Human molecular genetics. 2006;15(Spec No 2):R138–150. doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Sekar S, McDonald J, Cuyugan L, et al. Alzheimer’s disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol Aging. 2015;36:583–591. doi: 10.1016/j.neurobiolaging.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- Sherwani SI, Khan HA. Role of 5-hydroxymethylcytosine in neurodegeneration. Gene. 2015;570:17–24. doi: 10.1016/j.gene.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Song F, Smith JF, Kimura MT, Morrow AD, Matsuyama T, Nagase H, Held WA. Association of tissue-specific differentially methylated regions (TDMs) with differential gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3336–3341. doi: 10.1073/pnas.0408436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Li Y, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nature neuroscience. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Gao XD, Wang Y, Yuan BF, Feng YQ. Widespread existence of cytosine methylation in yeast DNA measured by gas chromatography/mass spectrometry. Analytical chemistry. 2012;84:7249–7255. doi: 10.1021/ac301727c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine NA, Janitz K, Wilkins MR, Janitz M. Whole transcriptome sequencing reveals gene expression and splicing differences in brain regions affected by Alzheimer’s disease. PloS one. 2011;6:e16266. doi: 10.1371/journal.pone.0016266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerappan CS, Sleiman S, Coppola G. Epigenetics of Alzheimer’s disease and frontotemporal dementia. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2013;10:709–721. doi: 10.1007/s13311-013-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wang F, Fischhaber PL, Guo C, Tang TS. Epigenetic modifications as novel therapeutic targets for Huntington’s disease. Epigenomics. 2014;6:287–297. doi: 10.2217/epi.14.19. [DOI] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Journal of neurochemistry. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. Journal of neurochemistry. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Wen L, Li X, Yan L, et al. Whole-genome analysis of 5-hydroxymethylcytosine and 5-methylcytosine at base resolution in the human brain. Genome Biol. 2014;15:R49. doi: 10.1186/gb-2014-15-3-r49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Tang F. Genomic distribution and possible functions of DNA hydroxymethylation in the brain. Genomics. 2014 doi: 10.1016/j.ygeno.2014.08.020. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic acids research. 2013;41:D801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Knox C, Guo AC, et al. HMDB: a knowledgebase for the human metabolome. Nucleic acids research. 2009;37:D603–610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Tzur D, Knox C, et al. HMDB: the Human Metabolome Database. Nucleic acids research. 2007;35:D521–526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt GR. Recognition and estimation of 5-methylcytosine in nucleic acids. The Biochemical journal. 1951;48:581–584. doi: 10.1042/bj0480581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt GR, Cohen SS. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. The Biochemical journal. 1953;55:774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan BF. 5-methylcytosine and its derivatives. Advances in clinical chemistry. 2014;67:151–187. doi: 10.1016/bs.acc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Lahiri DK, Cardozo-Pelaez F. Epigenetics, oxidative stress, and Alzheimer disease. Free radical biology & medicine. 2009;46:1241–1249. doi: 10.1016/j.freeradbiomed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Yang Y, Wang X, et al. Redox-active quinones induces genome-wide DNA methylation changes by an iron-mediated and Tet-dependent mechanism. Nucleic acids research. 2014;42:1593–1605. doi: 10.1093/nar/gkt1090. [DOI] [PMC free article] [PubMed] [Google Scholar]