The depth of molecular response achieved with tyrosine kinase inhibitor (TKI) therapy in patients with chronic myeloid leukemia (CML) may improve the long-term outcome. Among patients who achieve complete cytogenetic response (CCyR), those who do not achieve a major molecular response (MMR) have a seven-year event free survival (EFS) of 50% compared to 85% for those who achieved MMR and 95% for those with MR4.5.[1] There is also a trend for improved relative survival for those with the deepest response.[2] Furthermore, those with the deepest responses might be eligible for elective treatment discontinuation.[3] Hence, improving the depth of molecular response has become an important goal of therapy in CML.
Hypermethylation of abl, bcr, cadherin-13, and p15, among others, have been reported in CML and is associated with worse outcomes.[4–9] Preclinical studies have demonstrated synergy between imatinib and hypomethylating agents like decitabine.[10,11] Hypomethylating agents have been studied in patients with advanced CML and in patients who have failed TKIs, and has shown clinical activity as a single agent and in combination with TKIs.[12,13] We hypothesized that adding low-dose azacytidine (AZA) to a TKI may improve the depth of molecular response in CML patients with CCyR.
We conducted a single arm open label phase I/II trial to evaluate the toxicity and efficacy of low-dose AZA in CML patients with CCyR who have persistent molecular minimal residual disease (MRD, detected with quantitative real time polymerase chain reaction) while receiving TKIs (NCT01460498). For the phase I portion of the study, patients were eligible for enrollment regardless of the level of BCR-ABL transcript. For phase II part of the study, patient needed to have detectable BCR-ABL transcript levels on two consecutive measurements one month apart, either increased by any value for patients who had never achieved MMR, or increased by at least 1-log leading to loss of MMR. Adult patients with BCR/ABL-positive CML and ECOG performance status ≤2 who had CCyR were eligible for this trial. The European LeukemiaNet criteria were used for staging and response assessment.[14] Patients needed to be on TKI therapy for at least 18 months, and stable TKI dose for at least 6 months prior to enrollment. Patients received AZA 50 mg/m2/day subcutaneously or intravenously for three days every four weeks. Patients continued on the same dose of the TKI they were receiving up to the time of enrollment. The study was halted after three patients were enrolled because of slow accrual.
The enrolled patients included one man and two women, aged 56, 56, and 72 years, respectively. The baseline characteristics and outcomes are shown in Table 1. Two patients had been initially diagnosed in chronic phase (CP) and one in accelerated phase (AP) by blast percentage and cytogenetic clonal evolution (CE). Two patients had initially received therapy with imatinib and later switched to dasatinib 100 mg daily after 36.5 and 30.4 months, respectively, one for transformation to AP by CE criteria, and one for unknown reasons (medication change before coming to our institution). The other patient was initially on dasatinib for 6.4 months and was later changed to imatinib 400 mg twice daily prior to referral to our institution due to concerns about drug absorption given her prior history of gastric bypass surgery. All patients confirmed (verbally) full adherence to treatment prior to enrollment.
Table 1.
Baseline characteristics and outcomes in the three patients.
| Variables | Patient 1 | Patient 2 | Patient 3 | Reference ranges | Units |
|---|---|---|---|---|---|
| Age | 56 | 56 | 72 | Years | |
| Sex | M | F | F | ||
| Stage at initial diagnosis | CP | CP | APa | ||
| Variables prior to enrollment on trial | |||||
| Hemoglobin | 131 | 98 | 105 | 140–180 for men, 95–133 for women | g/L |
| Absolute neutrophil count | 2.4 | 1.76 | 0.82 | 1.7–7.3 | ×109/L |
| Platelet count | 175 | 97 | 110 | 140–440 | ×109/L |
| ABL kinase domain mutation analysis | No mutations identified | No mutations identified | Testing canceled due to MRD of 0.448 IS | ||
| Bone marrow morphology | Trilineage hematopoiesis, no evidence of CML | Cellular marrow (50%) with trilineage hematopoiesis, no evidence of CML | Cellular (30%) marrow with dyspoietic trilineage hematopoiesis; 1% blasts | ||
| Conventional cytogenetics (% of normal metaphases) | 100 | 100 | 90% (2 metaphases with trisomy 8) | ||
| Treatment related variables | |||||
| Duration on frontline TKI | 36.5 | 6.4 | 30.4 | Months | |
| Duration on second line TKI | 52.7 | 23 | 31.5 | Months | |
| Duration on single agent TKI | 89.2 | 29.4 | 61.9 | Months | |
| Duration on TKI and AZA | 37.1 | 27.0 | 25.0 | Months | |
| No. of cycles of AZA | 38 | 27 | 19 | ||
| Duration of cycles | 28 (26–43) | 28 (27–41) | 35 (27–63) | Days (range) | |
| BCR-ABL transcript level at enrollment | 1.7255 | 1.2495 | 0.1085 | International Scale | |
| Outcome related variables | |||||
| BCR-ABL transcript levels at last follow up | 0.0032 | 0.0032 | 0.0032 | International Scale | |
| Time to achieve sustained MMR | 13.5 | 10.1 | 0.9 | Months | |
| Duration of MMR | 23.6 | 17.0 | 24.0 | Months | |
| Time to achieve MR4.5 | 19.8 | 27.0 | 4.4 | Months | |
| Total duration of MR4.5 | 15.2 | NA | 13.6 | Months | |
| Variables while on AZA-TKI combinationb | |||||
| Hemoglobin | 125 (116–132) | 104 (91–109) | 94 (86–102) | 140–180 for men, 95–133 for women | g/L |
| Absolute neutrophil count | 2.13 (1.57–3.74) | 1.7 (1.31–2.7) | 0.64 (0.34–1.31) | 1.7–7.3 | ×109/L |
| Platelet count | 206 (123–289) | 147 (86–172) | 140 (107–176) | 140–440 | ×109/L |
| Bone marrow morphology at six months | Cellular marrow with adequate trilineage maturation | Cellular marrow (30–40%) with mild erythroid hyperplasia. No morphologic evidence of CML | Hypocellular marrow (15–20%) with mild dysgranulopoiesis and mild dyserythropoiesisc | ||
| Conventional cytogenetics at 6 months (% of normal metaphases) | 100 | 100 | 100c | ||
By blasts and clonal evolution criteria.
Where applicable, values measured at the end of the cycle; expressed as median and range.
At nine months.
NA: not applicable.
The enrolled patients have received 38, 27, and 19 cycles of AZA. All continue on therapy: two at the starting dose of AZA 50 mg/m2 and one at a reduced dose (due to recurrent neutropenia) of 25 mg/m2 for three days every six weeks. The latter patient also developed grade one myalgia, grade one chest pain, and subconjunctival hemorrhage. She also had mild anemia and moderate neutropenia before starting AZA; grade 1/2 anemia and grade 1–4 neutropenia has persisted throughout the course of treatment. A total of 18 adverse events (AE) were recorded, five of them related to AZA and all grade 1 or 2 (myalgia, constipation, gastrointestinal hemorrhage, edema, and chest pain, one each). Hematologic toxicities were confounded by preexisting cytopenias prior to the start of AZA. Patient 3 had mild dysplastic features on bone marrow biopsy prior to, and throughout the clinical trial. She also had trisomy 8 in 1/20 and 2/20 meta-phases in two consecutive bone marrow samples prior to starting on clinical trial. The cytogenetic abnormality resolved, but she continued to have mild dysplastic features. There have been no serious AEs.
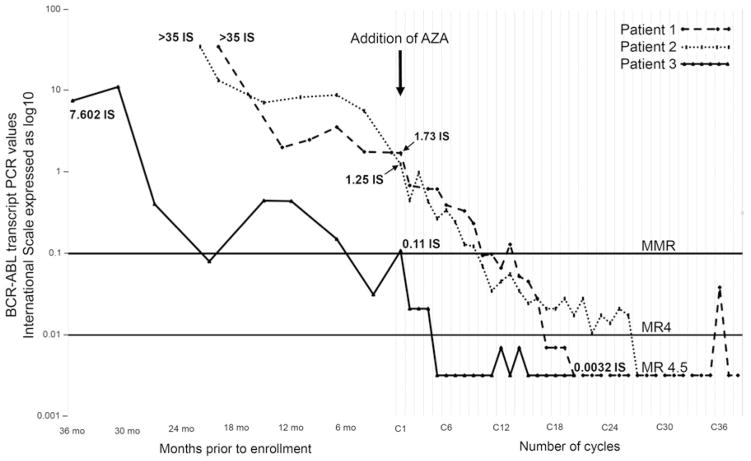
All three patients had detectable MRD with transcript levels of 1.73 IS, 1.25 IS, and 0.11 IS, after 89, 29, and 62 months on TKI, respectively. Addition of low dose AZA caused steady decline in MRD (Figure 1). All patients achieved MMR after a median of 10.1 months (range 0.9–13.5 months). At the time of this analysis, all patients continue to maintain MMR for 23.6, 17.0, and 24.0 months. All patients achieved MR4.5 after 19.8, 27.0, and 4.4 months on study. One patient achieved MR4.5 on last follow-up while the other two patients continue to be in MR4.5 for total duration of 15.2 and 13.6 months, respectively.
Figure 1.
Trends of BCR-ABL transcripts prior to enrollment and while on clinical trial receiving AZA-TKI.
The major limitation of our study was small sample size due to slow accrual. The need for injections was the main reason that discouraged patients to enroll. Due to the low enrollment, the maximum tolerated dose for AZA when used in combination with a TKI could not be determined. However, the dose tested appeared safe in this limited sample. Our patients had persistent molecular MRD despite being on TKIs for over 2–7 years, and achieved sustained MMR and MR4.5 after addition of AZA. These trends in the transcript levels are encouraging and suggest a possible role of hypomethylating agents in treating molecular MRD in patients in whom TKI alone has not been sufficient to achieve MR4.5. Other similar strategies using a TKI in combination with another agent for suboptimal responders are being investigated. These include arsenic trioxide (NCT01397734), ruxolitinib (NCT01751425), BL-8040 (NCT02115672), and hydroxy-chloroquine (NCT01227135). The actual efficacy and safety of AZA in this setting cannot be properly assessed with the patients reported here. However, given these preliminary observations, this approach merits further investigation. The availability of oral AZA [15] may make it more appealing for patients to consider such an approach.
Acknowledgments
Funding
This study was sponsored in part by Celgene, and by the MD Anderson Cancer Center Support Grant CA016672 (PI: Dr. Ronald DePinho) and Award Number P01 CA049639 (PI: Dr. Richard Champlin) from the National Cancer Institute.
Footnotes
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT01460498.
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at http://dx.doi.org/10.1080/10428194.2016.1207764.
References
- 1.Verma D, Kantarjian H, Shan J, et al. Sustained complete molecular response to imatinib in chronic myeloid leukemia (CML): a target worth aiming and achieving? Blood. 2009;114:505. [Google Scholar]
- 2.Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–e193. doi: 10.1016/S2352-3026(15)00048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahon F-X, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 4.Zion M, Ben-Yehuda D, Avraham A, et al. Progressive de novo DNA methylation at the bcr-abl locus in the course of chronic myelogenous leukemia. Proc Natl Acad Sci USA. 1994;91:10722–10726. doi: 10.1073/pnas.91.22.10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Issa JPJ, Kantarjian H, Mohan A, et al. Methylation of the ABL1 promoter in chronic myelogenous leukemia: lack of prognostic significance. Blood. 1999;93:2075–2080. [PubMed] [Google Scholar]
- 6.Nguyen TT, Mohrbacher AF, Tsai YC, et al. Quantitative measure of c-abl andp15 methylation in chronic myelogenous leukemia: biological implications. Blood. 2000;95:2990–2992. [PubMed] [Google Scholar]
- 7.Mizuno S, Chijiwa T, Okamura T, et al. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97:1172–1179. doi: 10.1182/blood.v97.5.1172. [DOI] [PubMed] [Google Scholar]
- 8.Ge X-Q, Tanaka K, Mansyur A, et al. Possible prediction of myeloid and lymphoid crises in chronic myelocytic leukemia at onset by determining the methylation status of the major breakpoint cluster region. Cancer Genet Cytogenet. 2001;126:102–110. doi: 10.1016/s0165-4608(00)00394-0. [DOI] [PubMed] [Google Scholar]
- 9.Roman-Gomez J, Castillejo JA, Jimenez A, et al. Cadherin-13, a mediator of calcium-dependent cell-cell adhesion, is silenced by methylation in chronic myeloid leukemia and correlates with pretreatment risk profile and cytogenetic response to interferon alfa. J Clin Oncol. 2003;21:1472–1479. doi: 10.1200/JCO.2003.08.166. [DOI] [PubMed] [Google Scholar]
- 10.La Rosee P, O’Dwyer M, Druker B. Insights from pre-clinical studies for new combination treatment regimens with the Bcr-Abl kinase inhibitor imatinib mesylate (gleevec/glivec) in chronic myelogenous leukemia: a translational perspective. Leukemia. 2002;16:1213–1219. doi: 10.1038/sj.leu.2402555. [DOI] [PubMed] [Google Scholar]
- 11.Rosee PL, Johnson K, Corbin AS, et al. In vitro efficacy of combined treatment depends on the underlying mechanism of resistance in imatinib-resistant Bcr-Abl–positive cell lines. Blood. 2004;103:208–215. doi: 10.1182/blood-2003-04-1074. [DOI] [PubMed] [Google Scholar]
- 12.Issa JPJ, Gharibyan V, Cortes J, et al. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer. 2003;98:522–528. doi: 10.1002/cncr.11543. [DOI] [PubMed] [Google Scholar]
- 14.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelo-monocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29:2521–2527. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]