Abstract
As human life expectancy rises, the aged population will increase. Aging is accompanied by changes in tissue structure, often resulting in functional decline. For example, aging within blood vessels contributes to a decrease in blood flow to important organs, potentially leading to organ atrophy and loss of function. In the central nervous system, cerebral vascular aging can lead to loss of the integrity of the blood-brain barrier, eventually resulting in cognitive and sensorimotor decline. One of the major of types of cognitive dysfunction due to chronic cerebral hypoperfusion is vascular cognitive impairment and dementia (VCID). In spite of recent progress in clinical and experimental VCID research, our understanding of vascular contributions to the pathogenesis of VCID is still very limited. In this review, we summarize recent findings on VCID, with a focus on vascular age-related pathologies and their contribution to the development of this condition.
Keywords: blood-brain barrier, hypoperfusion, vascular aging, white matter
Introduction
Age-related dementia is an irreversible condition with remarkable progressive cognitive decline. Each year, there are 7.7 million new dementia diagnoses (Iadecola, 2013). In the United States, the financial burden of dementia has already surpassed that of cancer and heart disease (Hurd et al., 2013). Vascular cognitive impairment and dementia (VCID) accounts for at least 20% cases of dementia, being second only to Alzheimer's disease (AD). As with AD, the incidence and severity of VCID is recognized as an urgent public health crisis (Gorelick et al., 2011). The risk for VCID rises with age, doubling every ~5.3 years (Jorm and Jolley, 1998). Thus, the prevalence of VCID is expected to increase exponentially with the aging of the US population.
VCID is a type of cognitive disorder induced by vascular abnormalities. The major hemodynamic alteration in this condition is a chronic and significant decrease in cerebral blood flow (CBF) (Sabayan et al., 2012; Scheel et al., 1999). Diverse types of pathology have been reported in VCID, including atherosclerosis, arteriolosclerosis, infarcts, white matter (WM) changes, and microbleeds. Despite much scientific progress in VCID research during the past few decades, many controversies and unanswered questions still remain (T O'Brien and Thomas, 2015). In this review, we will describe the impact of vascular aging on cerebrovascular function and the contribution of vasculopathy to cognitive impairment and dementia.
1. Cerebrovascular biology and aging
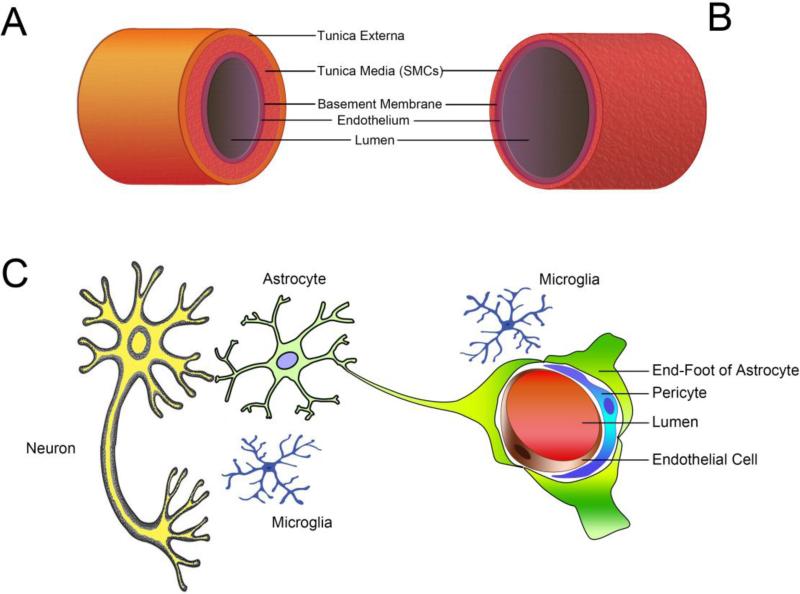
The brain vasculature consists of two blood supply systems. The major one of these is the internal carotid artery system, which carries approximately 70% of total CBF. The second is the vertebral artery system, which accounts for 30% of total CBF. These two systems converge to form the Willis circle, from which the main cerebral arteries branch out (Fig. 1A). A dense network of arterioles (Fig. 1B) is formed within the pia mater before penetration into the cortex (Willie et al., 2014b). The deeper WM is irrigated by long branches of the cerebral vascular supply, and is therefore more vulnerable to ischemic damage (Iadecola, 2013). The pial arterioles are generally considered to be the key regulators of CBF. The neurovascular unit (NVU) is a defined structure formed within brain tissue composed of the cerebral microvascular endothelium, astrocyte endfeet, pericytes, neurons, and extracellular matrix (Fig. 1C). During the process of aging, the cerebral vasculature and other components of the NVU undergo multiple changes that predispose the brain to neurovascular diseases, including VCID.
Fig. 1. Anatomical structures of the cerebral vasculature.
(A) Brain artery branch from the circle of Willis. The vessel wall contains three layers. The tunica externa is composed of connective tissue and elastic fibers. The tunica media is a thick layer of smooth muscle cells that control vessel tone. In the inner layer, the tunica intima consists of a monolayer of endothelial cells that rest on the basement membrane. (B) Brain arteries branch into a dense network of arterioles within the pia mater. These arterioles lack the tunica externa but contain the thinner media layer. As the major switch that controls the CBF, these arterioles are exposed to both blood in the lumen and the CSF rushing through the subarachnoid space. (C) The structure of the NVU is unique to the brain. Pericytes are contractile cells sitting outside the endothelial monolayer. The endothelial cells and neurons are connected by astrocyte endfeet. Microglial cells migrate around the NVU. Complex cell-cell interactions exist within the NVU and are integrated to control CBF and cellular function. SMCs: smooth muscle cells.
1.1 Specialized features of the cerebral vessels
Like all other vessels, cerebral vessels permit the diffusion of gas, form a physical barrier, act as a gatekeeper and participant in inflammatory process, and promote or prevent thrombosis under special circumstances (Hainsworth et al., 2015). In addition to these conventional properties shared by all vessels, cerebral vessels also exhibit some specialized functions.
The leading structural feature of cerebral vessels is the formation of a blood-brain barrier (BBB). The BBB is composed of tight junctions (TJ) and adherens junctions between endothelial cells, as well as other cellular components, such as the basal membrane, pericytes, and astrocyte endfeet (Fig. 1C) (Abbott et al., 2010). As the major interface between the blood and the neural environment, the BBB prevents harmful molecules from entering the brain tissue from the circulation. Multiple studies have reported increased BBB permeability in dementia, which contributes to neurodegeneration and functional decline (Alafuzoff et al., 1987; Farrall and Wardlaw, 2009; Schreiber et al., 2013; Skoog et al., 1998; Zlokovic, 2008).
Cerebral vessels also play an active role in transporting specific molecules between the blood and brain tissue. Even glucose—the critical fuel for brain metabolism—is unable to enter the brain without the facilitation of transport proteins located in the endothelial luminal membrane (Hainsworth et al., 2015). This transport capacity of the cerebral endothelium is supported by a unique transcriptional profile, with overexpression of specific transporter genes compared to other types of vessels in the periphery (Demarest et al., 2012; Enerson and Drewes, 2006; Macdonald et al., 2010; Ohtsuki et al., 2014). These genes encode carrier-mediated transporters, active efflux transporters, or receptor-mediated transporters (Pardridge, 2005). Cerebral vessels continuously scan the contents of circulating blood, actively select beneficial materials, and facilitate their entry into the brain (mainly via transporters), while simultaneously blocking the passage of detrimental materials. Thus, neuronal function and survival both rely on the structural and functional integrity of cerebral vessels. In addition, brain vessels play active roles in removing neurotoxic molecules from the interstitial fluid, including amyloid-β (Aβ) (Zlokovic, 2011). Disturbance of the normal cerebral vasculature not only reduces the supply of oxygen and nutrients, but also results in the accumulation and deposition of harmful molecules in the brain.
Another distinct feature of cerebral vessels is their participation in the regulation of CBF. Major arteries and small arterioles, and even the capillaries contribute to some degree to regulation of CBF (Argaw et al., 2009; Baumbach and Heistad, 1988). This function is based on a high sensitivity to carbon dioxide (CO2), which is unique to cerebral vasculature (Ainslie et al., 2005), and is carried out by vascular smooth muscle cells in the arterial and arteriole walls (Fig. 1A), as well as pericytes within the NVU (Fig. 1C), due to their contractile properties (Hall et al., 2014). Accounting for only 2% of total body weight, the cerebral circulation disproportionately carries about 12-20% of cardiac output, and consumes 20-25% of total oxygen and glucose (Williams and Leggett, 1989). This disproportionate distribution indicates that the brain is the most critical organ, consuming large amounts of the blood supply and with superior sensitivity to alterations in CBF alteration. Decreases in CBF therefore contribute to the pathogenesis of VCID (De Vis et al., 2015; del Ser et al., 1990; Scheel et al., 1999; Schuff et al., 2009).
The blood flow of the WM is supplied by long, penetrating arterioles that lack anastomotic branches (Blinder et al., 2013). In addition, the WM receives less blood flow—only about two thirds of the flow in grey matter (Harris and Attwell, 2012). This difference probably reflects the fewer numbers of synapses in WM, based on the observation that the brain uses most of the available energy to supply ionic pumps that maintain the ionic gradients for proper synaptic activity (Harris et al., 2012). Therefore, these vascular features render WM prone to injury in ischemia and during energy deficiency. In addition, the deep WM and basal ganglia are irrigated by arterioles arising directly from the circle of Willis and its proximal branches, which are more susceptible to mechanical stress imposed by hypertension and arterial stiffness (Scuteri et al., 2011).
1.2 Mechanisms of CBF regulation
Mechanisms of CBF regulation can be classified into three broad categories: (1) metabolic regulation of CBF, (2) cerebral autoregulation, and (3) autonomic regulation of CBF.
1.2.1 Metabolic regulation
A significant increase in the pressure of arterial carbon dioxide (PaCO2) leads to vascular dilation and increases in CBF. As mentioned previously, the entire cerebral vasculature is highly sensitive to PaCO2 throughout large arteries, ranging from the internal carotid arteries and vertebral arteries (Willie et al., 2012), intracranial arteries (Giller et al., 1993; Willie et al., 2011; Willie et al., 2014a), to the small pial arterioles (Wolff and Lennox, 1930), and even the capillaries of the parenchyma (Binks et al., 2008; Mandell et al., 2008; Nöth et al., 2008; Piechnik et al., 2008). Among these structures, the pial arterioles are thought to be the major site of resistance modulation (Fig. 1B). It is worth noting that some cerebral vessels such as the pial arterioles also respond to metabolic materials in the cerebrospinal fluid (CSF) (Willie et al., 2014b) as they are anatomically immersed in CSF in the subarachnoid space. Remarkably, pial arteries can dilate up to 40% upon an increase of CO2 in both the blood and CSF (Kontos et al., 1977; Wolff and Lennox, 1930).
1.2.2. Cerebral autoregulation
Cerebral autoregulation functions to buffer CBF in response to blood pressure. It was previously widely believed that ‘in all physiological conditions, a rise in arterial pressure accelerates the CBF, and a fall slackens it (Bayliss et al., 1895)’. However, this concept was revised by Lassen and colleagues (Lassen, 1959). When these authors graphed average blood pressure against CBF, a plateau region emerged wherein the CBF appears to remain constant despite a wide range in blood pressure (60-150 mmHg). These results were based on between-subject measurements in different patients with various diseases. In 2012, Tan and colleagues conducted a within-subject analysis of 43 healthy volunteers, where CBF and blood pressure were measured simultaneously. They demonstrated that minor fluctuations in the plateau region remained within 10 mmHg, which suggests that CBF has effective buffering capacity (Tan, 2012). Aging-related hypertension and increased pulsatility disrupt normal cerebral autoregulation by affecting the function of baroceptors (Ogawa et al., 1996) and subject the aging brain to hypoperfusion (van Beek et al., 2008). This may increase vulnerability to age-related pathologies such as neurodegenerative disorders.
1.2.3 Autonomic regulation of CBF
The entire cerebral vasculature is innervated by sympathetic and parasympathetic fibers. Three layers of nerve plexi have been described. The most superficial is a perivascular layer arranged longitudinally and superficially to the adventitia. Within the adventitia, there is a dense perivascular plexus. And a deep perivascular plexus extends along the transverse axis at the adventitial-medial border (Tan, 2012). In general, excitation in the sympathetic nervous system decreases CBF and excitation in parasympathetic nerves increases CBF (Ainslie, 2009; Cassaglia et al., 2008; Mayhan et al., 1987; Tzeng et al., 2010).
1.3 Cerebrovascular aging and mechanisms
Healthy cerebrovascular function is defined as the coordinated regulation of CBF so that it meets neuronal requirements (Bolduc et al., 2013). Aging leads to complex vascular phenotypic changes that render the brain prone to diseases, especially VCID, regardless of the existence of traditional risk factors (Ungvari et al., 2010). Multiple pathophysiological processes participate in accelerated aging and aging-related cerebrovascular disorders, as described further below.
1.3.1 Arterial stiffness
Arterial stiffness is a major feature of vascular aging. The stiffness of large conduit arteries increases both pulse wave velocity and pulse pressure, leading to an increase in systolic pressure and decrease in diastolic pressure. Alterations in blood flow dynamics result in the expression of mechanosensitive genes, which induce vascular remodeling, oxidative stress, and pro-atherogenic changes in the vascular wall (Ungvari et al., 2010). Upregulation of the renin-angiotensin system (Spinetti et al., 2004; Wang et al., 2007; Wang et al., 2005) and elevation in advanced glycation end products (Semba et al., 2009; Semba et al., 2015) also contribute to arterial wall stiffness.
1.3.2 Endothelial replicative senescence
Cellular senescence arrests the proliferation of mitotically competent cells, leading to permanent withdrawal from the cell cycle and is recognized as an example of evolutionary antagonistic pleiotropy (Campisi, 2003), preventing organs from developing cancer early in the reproductive years while promoting age-related phenotypes and pathologies (Chinta et al., 2015). In addition, senescent cells acquire a distinct phenotype known as the senescence-associated secretory phenotype and secrete multiple inflammatory cytokines, growth factors, and proteases that contribute to aging and the development of aging-related diseases (Chinta et al., 2015; Coppé et al., 2010). These detrimental factors are likely to function as paracrine mediators, altering neighboring cells and modulating the internal milieu. Senescent cells are known to accumulate with age in a variety of tissues (Erusalimsky and Kurz, 2005; Herbig and Sedivy, 2006; Jeyapalan et al., 2007; KISHI, 2004). Studies on cultured endothelial cells suggest that oxidative stress is a major stimulus to induce endothelial senescence (Erusalimsky, 2009). In the aged brain, cellular senescence is associated with impaired cognition and an increase in pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6 (Bachstetter et al., 2011). However, the relationship between cellular senescence and dementia remains poorly understood.
1.3.3 Microvascular rarefaction
Rarefaction of the microvasculature has been reported in some regions of the brain, especially the hippocampus and the residual vessels show structural alterations that are associated with the development of VCID (Riddle et al., 2003; Sonntag et al., 1997). The causes for microvascular rarefaction are not fully understood. A higher rate of endothelial apoptosis is observed in aged rodents (Csiszar et al., 2007b; Csiszar et al., 2004; Pearson et al., 2008) and non-human primates (Asai et al., 2000). Oxidative stress and chronic inflammation may contribute to endothelial apoptosis. Another reason for microvascular rarefaction may be related to circulating endothelial progenitor cells. Dysfunction in and decreased numbers of circulating endothelial progenitor cells have been reported with aging (Chang et al., 2007; Heiss et al., 2005; Zhang et al., 2009), and this is associated with WM changes and cognitive impairments (Hajjar et al., 2016; Hayakawa et al., 2013).
1.3.4 Narrowing of the vascular lumen
The major causes of luminal narrowing are atherosclerosis in large vessels and arteriosclerosis in small vessels, characterized by deposition of atherosclerotic plaques (Jellinger, 2008, 2013; Yurdagul et al., 2016). The accumulation of toxic proteins in the vessel walls also causes narrowing of the vascular lumen. Examples of such proteins include 1) mutant Notch3 in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and 2) high-temperature requirement A serine peptidase 1 (HTRA1) in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) (Fukutake, 2011; Hara et al., 2009; Henshall et al., 2015; Joutel et al., 1996). Luminal narrowing causes chronic cerebral hypoperfusion, ischemia, and oxidative stress, eventually leading to cognitive impairments and dementia.
1.3.5 Oxidative stress in inflammation
Although the mechanisms underlying vascular aging are not fully understood, oxidative stress and inflammation may play important roles. Excessive oxidative stress contributes to vascular aging in both laboratory animals (Csiszar et al., 2007a; Hamilton et al., 2001; Sun et al., 2004; Ungvari et al., 2010; Van Der Loo et al., 2000) and humans (Donato et al., 2007; Jablonski et al., 2007), and this may increase the risk of vascular diseases such as stroke, VCID, and coronary heart disease. The age-related increase in reactive oxygen species (ROS) may at least partly be attributed to the increased activity of nicotinamide adenine dinucleotide phosphate-oxidase in vessel walls (Adler et al., 2003; Csiszar et al., 2007a; Donato et al., 2007; Jacobson et al., 2007; Van Der Loo et al., 2000). Nitric oxide (NO) is a critical vasodilation factor that contributes to CBF regulation; it can be inactivated by high levels of superoxides, resulting in significant cerebral vasomotor dysfunction (Ungvari et al., 2008). In addition to regulation of the CBF, NO also confers vascular protective effects, including inhibition of platelet aggregation, inhibition of endothelial apoptosis, preservation of endothelial progenitor cells, and anti-inflammatory properties (Ungvari et al., 2010). These benefits of NO are all compromised by ROS. Furthermore, proxynitrite, the oxidative product of NO, causes severe cytotoxicity, leading to cellular death not only in cerebral vessels, but other cell types as well, including neurons (Pacher et al., 2007).
Increasing evidence suggests that mitochondrial oxidative stress plays an important role in aging-induced cerebral vascular dysfunction (Springo et al., 2015). Mitochondrial-derived H2O2 activates nuclear factor (NF)-κB, leading to a pro-inflammatory shift in endothelial gene profiles (Ungvari et al., 2007). In addition, oxidative stress damages mitochondrial DNA, further contributing to the pathogenesis of dementia (Bonanni et al., 2015; Coppedè and Migliore, 2015).
Both experimental and clinical data demonstrate that aging is associated with continuously low-grade inflammation, rendering the vasculature vulnerable to atherosclerosis, the major vasculopathy in VCID (Csiszar et al., 2008; Franceschi et al., 2000; Nilsson et al., 2015; T O'Brien and Thomas, 2015). As discussed above, oxidative stress induces vascular inflammation in experimental aging models (Csiszar et al., 2003, 2004; Pearson et al., 2008; Ungvari et al., 2007; Wang et al., 2007). In humans, diverse inflammatory factors are positively correlated with age, and this is independent of conventional risk factors (Bruunsgaard et al., 2000; Miles et al., 2008). These inflammatory factors include, tumor necrosis factor (TNF)-α, IL-6, and IL-1β, which are associated with increased risk of developing VCID (Zuliani et al., 2007). High levels of pro-inflammatory factors contribute to a pro-inflammatory microenvironment, which facilitates vascular dysfunction and promotes endothelial apoptosis, mainly through activation of NF-κB (Arenas et al., 2006; Donato et al., 2008; Donato et al., 2007; Ungvari et al., 2007; Zou et al., 2006).
2. Vascular pathophysiology underlying VCID—how does vascular aging lead to neural disorders?
Decreased CBF is the major cerebral hemodynamic alteration in VCID (Sabayan et al., 2012; Scheel et al., 1999). Diverse vascular and cerebral pathologies have been reported in VCID, including atherosclerosis, arteriolosclerosis, infarcts, WM changes, and microbleeds. In this section, we will classify and describe vasculopathies associated with VCID.
2.1. Diverse cerebrovascular pathologies underlying VCID
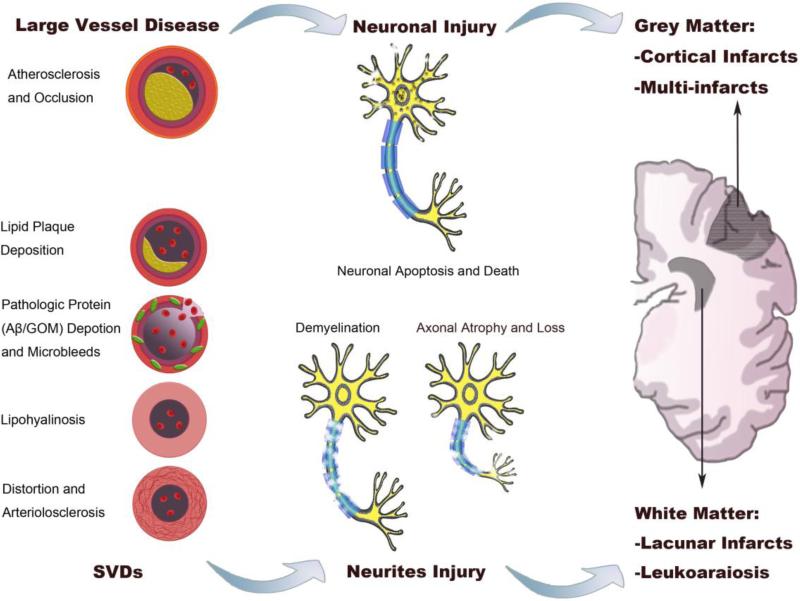
As summarized in Fig. 2, multiple cerebrovascular pathologies can cause VCID. Pathologies that cause reduction in global CBF such as atherosclerosis and arterial stenosis can induce VCID. If the CBF reduction is severe and persistent, stroke and infarcts may develop (Moskowitz et al., 2010).
Fig. 2. Vascular and neural pathology underlying VCID.
The left panel represents diverse vascular pathologies in VCID. Large vessel diseases, including atherosclerosis and arterial occlusion, cause neuronal death (middle upper panel) or cerebral infarction. SVDs (left lower panel) typically cause neuritic injuries (middle lower panel), such as demyelination and axonal injuries. According to neuroimaging studies, VCID lesions can be observed in both gray and white matters (right panel). Among the lesions, cortical infarcts (typically multi-infarcts) are common in post-stroke VCID, while lacunar infarcts and leukoaraiosis are mainly located at periventricular regions. SVDs: small vessel diseases; Aβ: amyloid-β; GOM: granular osmiophilic material.
The most prevalent vascular lesions associated with VCID are small vessel diseases (SVDs) (Hainsworth et al., 2015; Jellinger, 2013; Pantoni, 2010). Diverse vascular pathologies can cause SVDs, which are composed of atherosclerotic plaques in small vessels, deposition of hyaline in the vascular wall (lipohyalinosis), and vessel wall fibrosis, resulting in microvascular stiffening and distortion (arteriolosclerosis) (Fig. 2) (Thal et al., 2012).
Deposition of Aβ in cerebral vessels, termed cerebral amyloid angiopathy (CAA), is associated with VCID. The major risk factor for CAA is advanced age (Charidimou et al., 2012). The amyloid accumulation occurs in the media and adventitia, resulting in degeneration of vascular smooth muscle cells and pericytes (Thal et al., 2012). Both ischemic and hemorrhagic lesions can be observed in CAA. Another type of SVD that leads to VCID is CADASIL, in which the vascular lesions are related to accumulation of granular osmiophilic material that contains the mutant Notch 3 protein (Cognat et al., 2014; Joutel et al., 1996; Paquet et al., 2010).
2.2. Vascular gray matter injuries
Selective neuronal death and cerebral infarction are the major pathologies in gray matter injury, and their usual cause is cerebral ischemia (Fig. 2). Selective neuronal death occurs in vulnerable regions such as hippocampal CA1 and layer 3 and 5 of the cerebral cortex (Won et al., 2012; Zhang et al., 2013). Reductions in global CBF such as those caused by heart disease or carotid artery stenosis/occlusion can induce neuronal death and cognitive impairments (Marshall et al., 2012). There is general consensus that the cognitive impairments in this case are caused by cumulative brain tissue damage (Gelber et al., 2012; Sacco et al., 2013). Cerebral infarction is typically caused by occlusion of arterioles and consequent acute ischemia. If the lesion is located in regions responsible for cognitive function, such as the frontal lobe and thalamus, it will induce a “strategic” infarct, leading to cognitive dysfunction (Lanna et al., 2008).
2.3. Vascular WM injuries
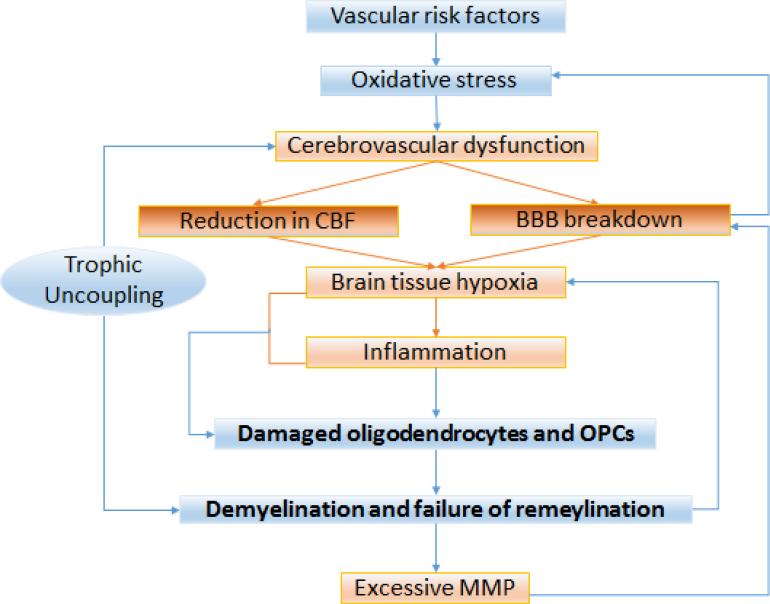
Myelinated WM tracts are responsible for long-range connectivity, inter-hemispheric synchronization, neurotrophic effects through axonal transport, neuroplasticity, and learning. Disruption of the WM has profound effects on the precision of information transfer (Nave, 2010). WM lesions are associated with global reduction of CBF and cardiovascular risk factors, especially hypertension, diabetes, smoking, and hyperlipidemia (Burton et al., 2004; Polvikoski et al., 2010; Udaka et al., 2002; Yoshizaki et al., 2008). Compared to gray matter injuries, the mechanisms underlying vascular WM injuries are more complex, and multiple vascular-oriented pathogenic factors collaborate with each other to contribute to its pathogenesis (Fig. 3).
Fig. 3. Pathophysiology of WM injury in VCID.
Vascular risk factors such as aging, hypertension, and diabetes result in systemic oxidative stress, which may induce cerebrovascular disorders. Two major consequences of cerebrovascular dysfunction are CBF reduction and BBB breakdown, leading to brain tissue hypoxia and inflammation. WM is particularly vulnerable to hypoxia, leading to damaged oligodendrocytes and OPCs. Loss of trophic coupling facilitates demyelination and interrupts remyelination, thereby predisposing axons to atrophy. Due to increased energy consumption during the action potential, tissue hypoxia is exacerbated. Injured OPCs secrete MMP, which amplifies BBB breakdown, leading to a feed-forward cycle that is vascular-oriented and culminates in WM injury.
2.3.1. BBB disruption
A meta-analysis of BBB permeability in 1953 individuals demonstrated that BBB permeability increases with aging, even in healthy humans (Farrall and Wardlaw, 2009). Furthermore, patients with AD and leukoaraiosis exhibit even greater BBB dysfunction. The leading mechanism responsible for BBB disruption is the excessive production of matrix metalloproteinases (MMPs), due to chronic hypoxia and inflammation induced by age-related vascular changes. MMPs facilitate the degradation of TJ proteins and the basement membrane. In addition, even normal aging can lead to pericyte loss, which results in further BBB disruption and microvascular degeneration (Bell et al., 2010).
BBB breakdown leads to the accumulation of neurotoxic materials in cerebral vessels or the brain parenchyma. For example, the accumulation of plasma proteins such as immunoglobulin and albumin can lead to brain edema and suppress capillary blood flow (Bell et al., 2010), whereas thrombin causes neurotoxicity and cognitive impairments (Mhatre et al., 2004). Furthermore, plasmin has been shown to catalyze the degradation of laminin (Chen and Strickland, 1997), and fibrin can accelerate neurovascular damage (Paul et al., 2007). In extreme cases, red blood cells leak into brain tissue, resulting in the deposition of hemoglobin-derived neurotoxic molecules such as iron, which contributes to ROS generation through Fenton chemistry (Zhong et al., 2008).
2.3.2. Hypoxia and hypoperfusion
WM is more vulnerable to CBF reduction compared to gray matter due to reduced vascular perfusion. This increased vulnerability is exemplified by the ‘intracerebral steal’ phenomenon, whereby hypercapnia or vasodilators decrease CBF in the periventricular WM due to vasodilation of upstream vessels that ‘steal’ the blood flow of the WM (Mandell et al., 2008). Systemic vascular aging such as hypertension and stiffness impair cerebrovascular autoregulation and decrease resting CBF, and are strong predictors of WM lesions (Jennings et al., 2005; Matsushita et al., 1994; Tarumi et al., 2011; Webb et al., 2012).
A decrease in CBF also exists in otherwise normal-appearing WM, suggesting that hypoperfusion precedes cognitive dysfunction (O'Sullivan et al., 2002). Mild hypoperfusion can impair protein synthesis, which is necessary for synaptic plasticity during learning and memory consolidation (Iadecola, 2004). Severe hypoperfusion causes failure to form action potentials (Kalaria, 2010) and alters acid-base balance and electrolyte concentrations, inducing neuronal/axonal edema and the accumulation of neurotoxic proteins (Aβ and glutamate), all of which lead to WM injury (Kalaria, 2010). Multiple studies have shown that ischemia-hypoxia increase the activity of two critical enzymes for Aβ production: β-secretase and γ-secretase (Li et al., 2009; Sun et al., 2006), and decreased levels of the Aβ degrading enzyme neprilysin (Wang et al., 2011).
2.3.3. Oxidative stress and neuroinflammation
Oxidative stress and chronic neuroinflammation may be present within WM lesions and are strongly associated with VCID, as indicated by the elevation of oxidative markers and inflammatory factors in this condition (Morrison et al., 2010; Yates et al., 2012; Zhang et al., 2014). In addition, activated microglia and astrocytes are present in WM lesions, which might exacerbate oxidative stress (Simpson et al., 2007). Therapeutic scavenging of ROS and anti-inflammatory approaches have been used to suppress inflammation and attenuate WM injuries in rodent models (Ma et al., 2015; Ueno et al., 2009; Wakita et al., 2008).
2.3.4. Disturbance of trophic coupling
Within the NVU (Fig. 1C), endothelial cells play a supportive role by secreting multiple growth factors. The most well studied of these are vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) (Nakahashi et al., 2000). VEGF has diverse functions, including angiogenesis, axonal guidance, and the migration of oligodendrocytes and oligodendrocyte precursor cells (OPCs) (Carmeliet and de Almodovar, 2013; Quaegebeur et al., 2011). In the adult brain, BDNF guides the migration of neuroblasts along blood vessels (Snapyan et al., 2009), and promotes the proliferation and survival of oligodendrocytes (Arai and Lo, 2009). Aging induces endothelial apoptosis (Csiszar et al., 2007b; Csiszar et al., 2004; Pearson et al., 2008) and microvascular rarefaction (Riddle et al., 2003; Sonntag et al., 1997), thereby impairing the normal supportive function of the vessel walls, which contributes to the malfunction of neurons and oligodendrocytes.
2.3.5. Demyelination and failure of remyelination
Hypoperfusion and BBB breakdown, together with an oxidative and inflammatory milieu, can lead to demyelination (Iadecola, 2004; Iadecola, 2013). Demyelination slows down action potentials and can lead to axonal loss (Fig. 3) (Franklin and Ffrench-Constant, 2008). This may be attributed to a decrease in axonal supportive factors derived from oligodendrocytes, such as insulin-like growth factor (Wilkins et al., 2003). Demyelination also exposes the axon to a deleterious microenvironment in WM, as the interstitial space is then filled with cytokines and ROS.
In damaged WM, there may be compensatory attempts to remyelinate axons, but most of these efforts fail (Franklin and Ffrench-Constant, 2008; Jonsson et al., 2012). The following factors are responsible for this failure: 1) Oligodendrocytes and OPCs are extremely vulnerable to hypoxia and oxidative stress in ischemic WM (Back et al., 2011; Fernando et al., 2006) (French et al., 2009); 2) withdrawal of trophic support from endothelial cells reduces remyelination capacity (Arai and Lo, 2009); 3) failure of OPC maturation prevents myelination. These factors may be the result of oxidative stress (French et al., 2009), dysregulation of Wnt signal transduction (Fancy et al., 2011), and excessive hyaluronan cleavage products (Preston et al., 2013). Finally, OPCs can also produce MMP9 and induce early BBB breakdown (Seo et al., 2013).
3. Age-related VCID
3.1 Risk factors of VCID
Several factors increase the risk of VCID and can be classified into two categories. The first category contributes to or accelerates vascular aging, and includes hypertension, smoking, heart disease, diabetes, obesity, hypercholesterolemia and hyperhomocysteinemia (de Bruijn and Ikram, 2014; Jackson and Sudlow, 2006; Kalaria, 2010). The second category is specific to the brain and increases the risk of cognitive impairments. It includes aging, female gender, and lower education (Pendlebury and Rothwell, 2009; Wiesmann et al., 2013). Stroke and depression also increase the risk of VCID (Diniz et al., 2013; Thomas et al., 2004).
3.2 Subtypes of VCID
According to a most widely applied VCID classification scheme, seven subtypes with specific imaging changes have been described (T O'Brien and Thomas, 2015). (1) Multi-infarct dementia: multiple cortical infarcts; (2) small vessel dementia (subcortical vascular dementia): lacunes and extensive WM lesions; (3) strategic infarct dementia: infarct in strategic locations such as the thalamus; (4) hypoperfusion dementia: watershed infarcts, WM lesions; (5) hemorrhagic dementia: hemorrhagic changes, which may or may not be associated with amyloid angiopathy; (6) hereditary vascular dementia (CADASIL and CARASIL): multiple lacunes and WM lesions, with disruption of temporal lobe WM; (7) AD with cardiovascular diseases: combination of vascular changes and atrophy, mainly affecting the medial temporal lobe.
In the current review, we classify VCID based on etiology and describe experimental models that attempt to simulate the clinical syndrome.
3.2.1. Ischemic VCID
Ischemic VCID is characterized by a reduction in CBF and accounts for the majority of VCID cases. Both large and small vessel diseases contribute to ischemic VCID. In large vessel diseases, reduced CBF leads to cerebral hypoperfusion, which further induces ischemic injury and oxidative stress in gray and white matters. Loss of neurons and neuronal dysfunction underlie severe cognitive abnormalities. In addition, WM injury is observed in VCID due to large vessel diseases, which can present as diffuse WM rarefaction, vacuolation, demyelination, axonal atrophy and loss, and lacunar infarcts multiple infarcts, or enlarged perivascular spaces (Fig. 2) (Fernando et al., 2006; Liu and Zhang, 2012; Shibata et al., 2004; Yoshizaki et al., 2008). These WM alterations can occur in isolation or, more commonly, coexist within the same brain (T O'Brien and Thomas, 2015).
Accumulating data suggest that SVDs cause the majority of VCID cases (Erkinjuntti et al., 2004; Raz et al., 2016; Roman et al., 2010). The brain pathologies in SVDs include hyalinization of vessels, expansion of the perivascular space, and pallor of adjacent perivascular myelin, with astrocytic gliosis (Kalaria, 2012). BBB dysfunction is often seen as an early change in SVDs, and exhibits increased permeability to inflammatory factors such as IL-1, IL-6, and TNFα (Iemolo et al., 2009; Reuter et al., 2015), which penetrate the brain and exacerbate the encephalopathy associated with edema (Chen et al., 2011; Park et al., 2010). The neuropathology of SVD is driven by severe stenosis and microvessel occlusion, which induces WM ischemia and multiple lacunar infarcts in subcortical regions (Raz et al., 2016).
SVDs are a group of heterogeneous diseases, and based on etiology, are generally classified as sporadic or hereditary. Sporadic SVDs are age-related diseases, with progressive arteriopathy/arteriolopathy, subcortical infarcts, and WM disease (Craggs et al., 2014), and with hypertension as a major risk factor. Binswanger's disease, or subcortical leukoencephalopathy, is a type of SVD associated with typical arteriosclerotic leukoencephalopathy within deep WM (Cai et al., 2015). Arterial hardening leads to luminal narrowing or occlusion, resulting in chronic ischemia and multiple infarcts within the WM (Rosenberg et al., 2014). MMPs play an important role in the pathogenesis of Binswanger's disease, due to their contribution to the opening of the BBB, demyelination, and oligodendrocyte degeneration (Rosenberg, 2016).
Hereditary SVDs include CADASIL and CARASIL. CADASIL is characterized by mutations in the Notch3 gene (Joutel et al., 1996). The Notch3 pathway plays an important role in cell growth, apoptosis, and differentiation (Bianchi et al., 2006; Henshall et al., 2015). Mutations in Notch3 lead to the accumulation of granular osmiophilic materials in vessel walls and the apoptosis of vascular smooth muscle cells, profoundly impacting vessel contraction and CBF regulation (Henshall et al., 2015). Aβ1-42 levels are elevated in CADASIL patients, which may also contribute to further pathogenesis (Paquet et al., 2010). CARASIL is associated with mutations in the HATRA1 gene, which lead to a decrease in protease activity and failure to repress TGF-β family signaling. Hyperactivity of TGF-β signaling contributes to vascular fibrosis and a decline in CBF (Hara et al., 2009; Lan et al., 2013).
3.2.2. Hemorrhagic VCID
Both major and minor cerebral hemorrhages lead to increased risk of developing VCID. Intracerebral hemorrhages (ICH) account for ~10–20% of all strokes, and increase morbidity and mortality (Feigin et al., 2009; Mozaffarian et al., 2016). As in ischemic stroke, cognitive decline in ICH occurs at two different stages following stroke: the acute-subacute stage and the chronic stage. Traditional vascular risk factors contribute to ICH, with hypertension being the most significant (Ferket et al., 2014; O'Donnell et al., 2010). Indeed, hypertension increases the risk of ICH by two fold (Jackson and Sudlow, 2006). As one might expect, the underlying pathologies correlate with specific anatomical locations of the hemorrhage (Fazekas et al., 1999; Woo et al., 2002; Xiong et al., 2016). ICH is thought to be the result of SVD in up to 85% of hemorrhagic stroke patients, due to hypertensive arteriopathy-induced rupture of small arterioles in non-lobar regions, such as the basal ganglia, thalamus, cerebellum and brainstem (Kremer et al., 2015). However, a fairly large proportion of lobar ICHs result from rupture of small and medium-sized arteries in the elderly, due to accumulation of amyloid-beta in both cortical blood vessels and leptomeninges (Kremer et al., 2015). Consistent with this view, CAA accounts for the majority of lobar ICH in the elderly (Greenberg, 1998; Hernandez-Guillamon et al., 2012; Knudsen et al., 2001), and is significantly associated with lobar and cerebellar ICH but not with deep ICH (Samarasekera et al., 2012; Yamada, 2015).
Cerebral microbleeds (CMBs), defined as chronic hemorrhagic microvascular lesions or microangiopathy in the brain, also contribute to VCID. Although traditionally considered to be clinically silent (Greenberg et al., 2009; Lei et al., 2013), CMBs are present in 17% to 46% of patients with cognitive impairments (Cordonnier et al., 2006). Thus, CMBs are regarded as an independent factor for cognitive deficits (Lei et al., 2013; Miwa et al., 2014; Pasquini et al., 2016). As one might expect, CMBs are also related to traditional vascular risk factors (Seshadri et al., 2004). For example, an association of deep CMBs with hypertensive vasculopathy and lobar CMBs with CAA has been identified through positron emission tomography (Greenberg et al., 2009; Gurol et al., 2012; Romero et al., 2014; Wiegman et al., 2014). Histopathologic studies indicate that CMBs are related to structural angiopathy and account for hypertensive arteriopathy, vascular β-amyloid deposition, and concomitant microstructural damage of surrounding tissue (Werring et al., 2010). As in ICH, the etiology of CMBs is thought to vary depending on the topographical location of the injury. CMBs that are located in deep brain tissue are related to hypertensive vasculopathy, whereas those at the cortico-subcortical boundaries of the cerebral lobes are related to CAA (Auer and Sutherland, 2005; Greenberg et al., 2009; Vernooij et al., 2008; Werring et al., 2010).
3.2.3. Post-stroke dementia
Stroke is the second leading cause of cognitive dysfunction, and it increases the risk of cognitive impairment by five folds (Merino, 2002; Qu et al., 2015; Srikanth et al., 2003). Recent clinical studies indicate that VCID occurs in about 25-30% of elderly stroke survivors (Allan et al., 2011; Savva et al., 2010). Not only may cognitive decline occur immediately following the onset of stroke (Nakase et al., 2013; Nys et al., 2007), but progressive cognitive disorders might also set in after the acute phase has passed (Altieri et al., 2004; Douiri et al., 2013). Post-stroke dementia may encompass all types of cognitive dysfunction, and the specific symptoms depend on stroke location, type, volume, and number. As each cortical lobe controls distinct cognitive functions, stroke in regions such as the hippocampus and temporal lobe leads to acute cognitive impairments more frequently than stroke in other regions such as the occipital lobe. In addition, stroke-mediated cognitive deficits are more strongly associated with lesions that affect the dominant hemisphere (Kalaria et al., 2016). The risk factors for post-stroke dementia are multifactorial, and include advanced age, genetic traits, low educational status, stroke severity, presence of diabetes, multiple infarcts, prior transient ischemic attack or recurrent stroke, and depressive illness (Gregoire et al., 2012; Jacquin et al., 2012; Kalaria et al., 2016; Kim et al., 2012; Leys et al., 2005; Lin et al., 2003; Yang et al., 2015).
As the cognitive decline after stroke has a close relationship with the overlapping pathology of cerebrovascular disease and dementia, the mechanisms accounting for post-stroke dementia are complex (Deramecourt and Pasquier, 2014; Iadecola, 2013). Some of the most important causes of post-stroke dementia are lesions of neuroanatomical structures and cerebral vessels (Sun et al., 2014). In post-stroke dementia, a series of events, such as energy failure, excitoxicity, calcium overload, inflammation, and oxidative stress lead to microvascular damage, vasogenic edema, BBB disruption, hemostatic activation, pro-inflammatory immune responses, and cell death in the NVU (Kalaria et al., 2016). Furthermore, neuronal death following stroke is largely attributed to a continuum of apoptosis–necrosis (Kalaria et al., 2016). These stroke-related processes subsequently impair the neurological function of the brain and contribute to cognitive decline.
WM injury is an integral part of stroke and consists of activated microglia, astrocytosis, shrunken oligodendrocytes, myelin rarefaction, and axonal abnormalities and degeneration (Fernando et al., 2006; Ihara et al., 2010; Polvikoski et al., 2010). WM injury contributes to cognitive decline in stroke and cerebral SVD, and its severity is correlated with the degree of cognitive impairment (Pantoni, 2010; Sun et al., 2014). Although the pathophysiological mechanisms underlying WM lesions after stroke remain elusive, recent studies indicate that the presence and severity of WM hyperintensities on T2-weighted or FLAIR magnetic resonance images are predictive of post-stroke dementia (Burton et al., 2004; Ihle-Hansen et al., 2011; Kalaria et al., 2016; Leys et al., 2005; Sachdev et al., 2007).
CMBs are not uncommon in the general elderly population and their prevalence increases with age (Koennecke, 2006; Sveinbjornsdottir et al., 2008; Wiegman et al., 2014). CMBs are now accepted as the manifestation of cerebral small vessel pathologies, including hypertensive SVD and CAA (Poels et al., 2010; Vernooij et al., 2008; Werring et al., 2010). Higher numbers of CMBs are associated with poorer cognitive function according to numerous clinical studies (Miwa et al., 2014; Poels et al., 2012; Wiegman et al., 2014). Multiple CMBs or mixed CMBs independently indicate higher risk of post-stroke dementia (Miwa et al., 2014).
4. Animal models of VCID
Despite an impressive degree of progress in VCID research in the past several decades, the pathogenesis and underlying mechanisms of VCID remain poorly understood. In order to understand VCID at a mechanistic level, it is essential to use experimental models (Helman and Murphy, 2016; Madigan et al., 2016). In the next section, we will describe the most frequently used animal models for VCID research (Table 1).
Table 1.
Animal models for VCID
| Type of VCID | Models | References | |
|---|---|---|---|
| Ischemic VCID | Cortical Infarcts | 2VO/BCCAo | (Lee et al., 2015; Soria et al., 2013; Zhang et al., 2014b) |
| 4VO | (Kim et al., 2011; Neto et al., 2005; Pulsinelli and Buchan, 1988; Pulsinelli and Brierley, 1979) | ||
| UCCAO | (Yoshizaki et al., 2008; Zhao et al., 2014) | ||
| BCAS | (Nishio et al., 2010; Shibata et al., 2004) | ||
| Subcortical Infarcts | Cerebral emboli | (Purandare et al., 2012; Zhang et al., 2013) | |
| Cholesterol crystals | (Wang et al., 2012; Zhang et al., 2013) | ||
| Micro-sphere | (Miyake et al., 1993; Takagi and Takeo, 2003) | ||
| Notch3 mutation | (Serlin et al., 2011; Wallays et al., 2011a) | ||
| Risk factors induced VCID | High fat diet | (Miyake et al., 1993; Morrison et al., 2010) | |
| Hypertension with SHR | (Yamaguchi et al., 1994a; Yamori et al., 1976) | ||
| Diabetes | (Alvarez et al., 2009; Serlin et al., 2011) | ||
| Hypercholesterolemia | (Bell et al., 2012; Nishitsuji et al., 2011; Rodriguez et al., 2013) | ||
| Diet induced-hyperhomosysteinemia | (Fuso et al., 2012; Sudduth et al., 2013; Troen et al., 2008) | ||
| Hemorrhagic VCID | ICH | Intraparenchymal bacterial collagenase (Type IV-S) | (MacLellan et al., 2009) |
| Intraparenchymal bacterial collagenase (Type VII-S) | (Hartman et al., 2009) | ||
| CAA | Tg2576 mice treated with angiotensin II and L-NG-nitroarginine methyl ester | (Passos et al., 2016) | |
| Tg2576 mice treated with dipyridamole-supplemented high-fat diet | (Fisher et al., 2011) | ||
| APP23 mice | (Winkler et al., 2001) | ||
4.1. Ischemic VCID models
4.1.1. Large vessel occlusion models
Large vessel occlusion models are achieved by occluding major cerebral arteries, such as the common carotid artery (CCA) by itself or in combination with the vertebral arteries. Depending on the number of cerebral arteries occluded, there are four types of animal models that mimic VCID induced by large vessel disorders. All of them lead to chronic, relatively mild cerebral ischemia, with significant decline in CBF (Helman and Murphy, 2016; Venkat et al., 2015).
Unilateral CCA occlusion (UCCAO)
In this model, one CCA is permanently occluded, typically in mice (Yoshizaki et al., 2008). This model induces WM rarefaction and decreased synaptic plasticity, and is accompanied by significant neuroinflammatory responses. However, the resultant neurodegeneration is relatively mild.
Two-vessel occlusion (2VO), also known as bilateral common carotid artery occlusion (BCCAO): 2 VO is induced by permanently occluding both CCAs, and is the most frequently used model of VCID (Liu and Zhang, 2012; Ma et al., 2015; Ni et al., 1995; Shibata et al., 2004; Soria et al., 2013; Wakita et al., 2002; Zhao et al., 2014). Mice cannot tolerate sudden occlusion of both CCAs. Therefore, this model is only used in rats, including spontaneously hypertensive rats (Yamaguchi et al., 1994; Yamori et al., 1976). 2VO causes chronic cerebral hypoperfusion, especially in the forebrain, leading to early BBB disruption, WM rarefaction, axonal and myelin damage, hippocampal and cortical neuron damage, as well as neuroinflammation.
Bilateral CCA stenosis (BCAS)
This model is developed for VCID research in mice, and includes symmetric and asymmetric occlusion of the two CAAs. In symmetric models, microcoils (Nishio et al., 2010; Shibata et al., 2004) or ameroid constrictors (Hattori et al., 2014a; Hattori et al., 2014b) are placed around the CAAs. When ameroid constrictors are used, the CCAs will be gradually narrowed and eventually occluded around 3-4 weeks after surgery. Asymmetric BCAS is created by applying a microcoil around one CCA and an ameroid constrictor around another CCA (Hattori et al., 2015). In these models, WM lesions and rarefactions are induced in the corpus callosum, with significant inflammation, gliosis, and BBB disruption. The models are also associated with delayed hippocampal damage and atrophy.
Four-vessel occlusion (4VO)
4VO for VCID differs from a global ischemia model and is induced by a sequential three-stage occlusion of the vertebral arteries and CCAs. Both vertebral arteries are occluded, followed by occluding one CCA, and then followed by occluding another CCA, with inter-stage intervals of 7 days (Neto et al., 2005). This model induces cognitive impairments as well as neurodegeneration in the hippocampi and retina.
4.1.2. Small vessel occlusion models
To mimic SVDs, microemboli can be injected into the internal carotid artery or middle cerebral artery, which can induce multiple infarcts in the cortex and subcortical structures. The microemboli are either cholesterol crystals/microspheres, or microbeads/thrombi, or thrombus fragments, with a size range of 40–100 μm (Rapp et al., 2008; Shih et al., 2013; Venkat et al., 2015). It has been reported that the occlusion of a single penetrating vessel can lead to microinfarcts and cognitive impairments (Shih et al., 2013). Rapp and colleagues (Rapp et al., 2008) compared the effects of embolus types in the induction of infarcts, and found that the infarcts induced by cholesterol crystals are smaller than those induced by thrombus fragments of the same size, especially in subcortical tissue. With thrombus fragments, astrocytic and microglial activation were limited within the infarct sites, whereas with cholesterol crystals, microglial activation and BBB damage were widespread.
Given that CADASIL is caused by Notch3 mutations, the TgPAC-Notch3R169C mouse has been introduced as a preclinical CADASIL model and is characterized by progressive, age-related cerebral WM pathology (Cognat et al., 2014). In this model, loss of oligodendrocytes occurs before axonal injury, and the myelin damage is progressive and segmental. Intramyelinic edema is present as an early and conspicuous feature of cerebral WM changes, resulting in poor clearance of myelin debris. Another mouse model for CADASIL uses an Arg170Cys substitution in murine Notch3, mimicking the Arg169Cys mutation in CADASIL patients (Wallays et al., 2011). In this knock-in mouse model, Notch3Arg170Cys can be expressed unperturbed, allowing for direct comparison to wildtype littermates. In addition, arteriopathy develops in both the brain and peripheral sites (tail) with granular osmiophilic material deposition in blood vessels. Histological analyses of these mouse brains demonstrate many similarities with the typical features of CADASIL in human beings.
It is worth noting that these ischemic models were predominantly applied in young or middle animals in majority of the studies, and the results obtained from young animals may not reflect the impacts of cerebrovascular aging on VCID. Although the researches on cognitive decline in aged animals are common (Krause et al., 2008; Murchison et al., 2009; Bobkova et al., 2015; Gocmez et al., 2016; Smith et al., 2015), the literature on the effects of aging on ischemic VCID is very rare. In a study comparing the different effects of hypoperfusion on between young and aged rats, it is reported the aged rats showed continuous hippocampal CBF decrease, spatial memory deficiency and CA1 neuronal death, which are companied by increased astrocytic response (Dela Torre et al., 1992). A recent study shows that post-stroke cognitive impairment presents in both young and aged mice; however, aged mice demonstrate more sever memory decline. Underlying mechanisms include CBF reduction, increased myelin loss and reduced M2 phenotype of microglia/macrophage (Suenaga et al., 2015). The shared findings of these two studies are the more severe cognitive impairment and reduction of CBF in aged animals, probably due to the aging of cerebral vasculature. Apparently, more integrated and systematic researches on aged animals are needed to clarify the effect of cerebrovascular aging on VCID.
4.2 Hemorrhagic VCID models
Compared to the ischemic VCID models, hemorrhagic VCID models are difficult to establish and less frequently used. ICH models are generally induced by intraparenchymal injection of bacterial collagenase or blood (MacLellan et al., 2008). Some studies have established cognitive disorders after ICH in rats (Hartman et al., 2009; MacLellan et al., 2009), which can be recognized as hemorrhagic VCID models. However, induction of ICH in otherwise healthy animals is not necessarily representative of spontaneous ICH in humans. Thus, hypertension-related and CAA-related spontaneous ICH animal models have been introduced to mimic clinical scenarios, albeit to a limited degree.
Transgenic mice with both human renin and angiotensinogen genes may be useful to mimic chronic hypertension (Merrill et al., 1996). Based on the hypertensive model, double transgenic animals treated with a high-salt diet or N-nitro-L-arginine methyl ester develop severe hypertension with symptomatic ICH (Ahmad, 1997; Iida et al., 2005; Smeda, 1989). Furthermore, transgenic animal models are widely used in CAA-related spontaneous ICH. For example, APP23 transgenic mice contain β-amyloid precursor protein (APP) expressing human APP with the Swedish mutation (Winkler et al., 2001), Tg2576 transgenic mice express human APP 695 with the Swedish mutation (Fisher et al., 2011), APP Dutch mice express AβDutch40 (Herzig et al., 2004), and Tg-SwDl mice harbor Swedish and vasculotropic Dutch and lowa mutations (Davis et al., 2004). All these transgenic models exhibit spontaneous ICH.
5. Summary
As a common age-related disease, VCID is attracting increasing attention due to its ever-increasing prevalence and significant economic and social burden. Age-related vascular pathology and CBF dysregulation contribute to the pathogenesis of VCID. Although several animal models and behavioral tests have been used to mimic and assess VCID, significant progress has not been made and the definition, diagnostic criteria, and treatments are still debated. Therefore, a more fruitful collaboration across basic science, translational, and clinical approaches is urgently needed to establish effective treatment strategies for VCID patients.
Highlights.
Cognitive impairment and dementia are age-related health burden.
Neurovascular aging predisposes the brain to reduced blood flow and long-term ischemic injuries, leading to VCID.
Diverse pathology underlies VCID, including neuronal loss and white matter injury. Microinfarcts and leukoaraiosis are common.
Age-related VCID can be classified into ischemic, hemorrhagic and post-stroke subtypes.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NS092810 to F.Z.; and NS093539 to R.K.L), and the Natural Science Foundation of China (81271276 to F.Z.). We thank Patricia Strickler for secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiology of disease. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD (P) H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285:H1015–H1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- Ahmad S. Angiotensin receptor antagonists delay nitric oxide-deficient stroke in stroke-prone rats. Eur J Pharmacol. 1997;333:39–45. doi: 10.1016/s0014-2999(97)01089-3. [DOI] [PubMed] [Google Scholar]
- Ainslie PN. Have a safe night: intimate protection against cerebral hyperperfusion during REM sleep. Journal of Applied Physiology. 2009;106:1031–1033. doi: 10.1152/japplphysiol.00091.2009. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Ashmead JC, Ide K, Morgan BJ, Poulin MJ. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. The Journal of physiology. 2005;566:613–624. doi: 10.1113/jphysiol.2005.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafuzoff I, Adolfsson R, Grundke-Iqbal I, Winblad B. Blood-brain barrier in Alzheimer dementia and in non-demented elderly. Acta neuropathologica. 1987;73:160–166. doi: 10.1007/BF00693782. [DOI] [PubMed] [Google Scholar]
- Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, O'Brien JT, Kalaria RN. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134:3716–3727. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri M, Di Piero V, Pasquini M, Gasparini M, Vanacore N, Vicenzini E, Lenzi GL. Delayed poststroke dementia: a 4-year follow-up study. Neurology. 2004;62:2193–2197. doi: 10.1212/01.wnl.0000130501.79012.1a. [DOI] [PubMed] [Google Scholar]
- Arai K, Lo EH. An Oligovascular Niche: Cerebral Endothelial Cells Promote the Survival and Proliferation of Oligodendrocyte Precursor Cells. Journal of Neuroscience. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-α antagonism. American Journal of Physiology-Heart and Circulatory Physiology. 2006;290:H1259–H1263. doi: 10.1152/ajpheart.00990.2005. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proceedings of the National Academy of Sciences. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai K, Kudej RK, Shen Y-T, Yang G-P, Takagi G, Kudej AB, Geng Y-J, Sato N, Nazareno JB, Vatner DE. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- Auer RN, Sutherland GR. Primary intracerebral hemorrhage: pathophysiology. Can J Neurol Sci. 2005;32(Suppl 2):S3–12. [PubMed] [Google Scholar]
- Bachstetter AD, Xing B, de Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ. Microglial p38a MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Ab). J Neuroinflammation. 2011;8:79–79. doi: 10.1186/1742-2094-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Kroenke CD, Sherman LS, Lawrence G, Gong X, Taber EN, Sonnen JA, Larson EB, Montine TJ. White Matter Lesions Defined by Diffusion Tensor Imaging in Older Adults. Annals of Neurology. 2011;70:465–476. doi: 10.1002/ana.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 1988;12:89–95. doi: 10.1161/01.hyp.12.2.89. [DOI] [PubMed] [Google Scholar]
- Bayliss W, Hill L, Gulland GL. On intra-cranial pressure and the cerebral circulation: Part I. Physiological; Part II. Histological. The Journal of physiology. 1895;18:334. doi: 10.1113/jphysiol.1895.sp000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S, Dotti MT, Federico A. Physiology and pathology of notch signalling system. J Cell Physiol. 2006;207:300–308. doi: 10.1002/jcp.20542. [DOI] [PubMed] [Google Scholar]
- Binks AP, Cunningham VJ, Adams L, Banzett RB. Gray matter blood flow change is unevenly distributed during moderate isocapnic hypoxia in humans. Journal of Applied Physiology. 2008;104:212–217. doi: 10.1152/japplphysiol.00069.2007. [DOI] [PubMed] [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc V, Thorin-Trescases N, Thorin E. Endothelium-dependent control of cerebrovascular functions through age: exercise for healthy cerebrovascular aging. American Journal of Physiology-Heart and Circulatory Physiology. 2013;305:H620–H633. doi: 10.1152/ajpheart.00624.2012. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Frazzini V, Thomas A, Onofrj M. Mitochondrial involveMent in neurodegenerative deMentia. The Functions, Disease-Related Dysfunctions, and Therapeutic Targeting of Neuronal Mitochondria. 2015:280. [Google Scholar]
- Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen B. Ageing, tumour necrosis factor - alpha (TNF - α) and atherosclerosis. Clinical & Experimental Immunology. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, Kenny RA, O'Brien J, Stephens S, Bradbury M, Rowan E, Kalaria R, Firbank M, Wesnes K, Ballard C. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004;35:1270–1275. doi: 10.1161/01.STR.0000126041.99024.86. [DOI] [PubMed] [Google Scholar]
- Cai Z, Wang C, He W, Tu H, Tang Z, Xiao M, Yan LJ. Cerebral small vessel disease and Alzheimer's disease. Clin Interv Aging. 2015;10:1695–1704. doi: 10.2147/CIA.S90871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Experimental gerontology. 2003;38:5–11. doi: 10.1016/s0531-5565(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, de Almodovar CR. VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cellular and Molecular Life Sciences. 2013;70:1763–1778. doi: 10.1007/s00018-013-1283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassaglia P, Griffiths R, Walker A. Sympathetic withdraw augments cerebral blood flow during acute hypercapnia in sleeping lambs. Sleep. 2008;31:1729–1734. doi: 10.1093/sleep/31.12.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin S.-e., Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1α stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. Journal of Neurology Neurosurgery and Psychiatry. 2012;83:124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Experimental gerontology. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognat E, Cleophax S, Domenga-Denier V, Joutel A. Early white matter changes in CADASIL: evidence of segmental intramyelinic oedema in a pre-clinical mouse model. Acta Neuropathol Commun. 2014;2:49. doi: 10.1186/2051-5960-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J-P, Patil CK, Rodier F, Krtolica A, Beauséjour CM, Parrinello S, Hodgson JG, Chin K, Desprez P-Y, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppedè F, Migliore L. DNA damage in neurodegenerative diseases. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2015;776:84–97. doi: 10.1016/j.mrfmmm.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–1360. doi: 10.1212/01.wnl.0000210535.20297.ae. [DOI] [PubMed] [Google Scholar]
- Craggs LJ, Yamamoto Y, Deramecourt V, Kalaria RN. Microvascular pathology and morphometrics of sporadic and hereditary small vessel diseases of the brain. Brain Pathology. 2014;24:495–509. doi: 10.1111/bpa.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. American Journal of Physiology-Heart and Circulatory Physiology. 2007a;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. 378 Vasculoprotective effects of anti-TNFalfa treatment in aging. The American journal of pathology. 2007b;379:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. The FASEB journal. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiological genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. Journal of Applied Physiology. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- de Bruijn RF, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer's disease. BMC medicine. 2014;12:130. doi: 10.1186/s12916-014-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vis J, Hendrikse J, Bhogal A, Adams A, Kappelle L, Petersen E. Age - related changes in brain hemodynamics; A calibrated MRI study. Human brain mapping. 2015;36:3973–3987. doi: 10.1002/hbm.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Ser T, Bermejo F, Portera A, Arredondo J, Bouras C, Constantinidis J. Vascular dementia: a clinicopathological study. Journal of the neurological sciences. 1990;96:1–17. doi: 10.1016/0022-510x(90)90052-o. [DOI] [PubMed] [Google Scholar]
- Dela Torre J, Fortin T, Park G, Butler K, Kozlowski P, Pappas B, De Socarraz H, Saunders J, Richard M. Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain research. 1992;582:186–195. doi: 10.1016/0006-8993(92)90132-s. [DOI] [PubMed] [Google Scholar]
- Demarest T, Murugesan N, Macdonald JA, Pachter JS. Endothelial Cell Heterogeneity of Blood–Brain Barrier Gene Expression: Analysis by LCM/qRT-PCR. Expression Profiling in Neuroscience. 2012:63–75. [Google Scholar]
- Deramecourt V, Pasquier F. Neuronal substrate of cognitive impairment in post-stroke dementia. Brain. 2014;137:2404–2405. doi: 10.1093/brain/awu188. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. The British Journal of Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct Evidence of Endothelial Oxidative Stress With Aging in Humans Relation to Impaired Endothelium-Dependent Dilation and Upregulation of Nuclear Factor-κB. Circulation research. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995-2010. Stroke. 2013;44:138–145. doi: 10.1161/STROKEAHA.112.670844. [DOI] [PubMed] [Google Scholar]
- Enerson BE, Drewes LR. The rat blood–brain barrier transcriptome. Journal of Cerebral Blood Flow & Metabolism. 2006;26:959–973. doi: 10.1038/sj.jcbfm.9600249. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Roman G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke. 2004;35:1010–1017. doi: 10.1161/01.STR.0000120731.88236.33. [DOI] [PubMed] [Google Scholar]
- Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. Journal of Applied Physiology. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Experimental gerontology. 2005;40:634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Fancy SPJ, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJM, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nature Neuroscience. 2011;14:1009–U1099. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood–brain barrier: ageing and microvascular disease– systematic review and meta-analysis. Neurobiology of aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- Ferket BS, van Kempen BJ, Wieberdink RG, Steyerberg EW, Koudstaal PJ, Hofman A, Shahar E, Gottesman RF, Rosamond W, Kizer JR, Kronmal RA, Psaty BM, Longstreth WT, Jr., Mosley T, Folsom AR, Hunink MG, Ikram MA. Separate prediction of intracerebral hemorrhage and ischemic stroke. Neurology. 2014;82:1804–1812. doi: 10.1212/WNL.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG, Function MRCC, Ageing Neuropathology Study G. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Fisher M, Vasilevko V, Passos GF, Ventura C, Quiring D, Cribbs DH. Therapeutic modulation of cerebral microhemorrhage in a mouse model of cerebral amyloid angiopathy. Stroke. 2011;42:3300–3303. doi: 10.1161/STROKEAHA.111.626655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, de Luca M, Ottaviani E, de Benedictis G. Inflamm - aging: an evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87:3076–3087. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutake T. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): from discovery to gene identification. J Stroke Cerebrovasc Dis. 2011;20:85–93. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Fuso A, Nicolia V, Ricceri L, Cavallaro RA, Isopi E, Mangia F, Fiorenza MT, Scarpa S. S-adenosylmethionine reduces the progress of the Alzheimer-like features induced by B-vitamin deficiency in mice. Neurobiology of aging. 2012;33:1482, e1481–1482, e1416. doi: 10.1016/j.neurobiolaging.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: Epidemiologic and Neuropathologic Research on Cognitive Impairment. Current Alzheimer Research. 2012;9:664–672. doi: 10.2174/156720512801322618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–742. [PubMed] [Google Scholar]
- Gorelick P, Scuteri A, Black S, Decarli C, Greenberg S, Iadecola C, Launer L, Laurent S, Lopez O, Nyenhuis D. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology. 1998;51:690–694. doi: 10.1212/wnl.51.3.690. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM, Microbleed Study G. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire SM, Smith K, Jager HR, Benjamin M, Kallis C, Brown MM, Cipolotti L, Werring DJ. Cerebral microbleeds and long-term cognitive outcome: longitudinal cohort study of stroke clinic patients. Cerebrovasc Dis. 2012;33:430–435. doi: 10.1159/000336237. [DOI] [PubMed] [Google Scholar]
- Gurol ME, Dierksen G, Betensky R, Gidicsin C, Halpin A, Becker A, Carmasin J, Ayres A, Schwab K, Viswanathan A, Salat D, Rosand J, Johnson KA, Greenberg SM. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. 2012;79:320–326. doi: 10.1212/WNL.0b013e31826043a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth AH, Oommen AT, Bridges LR. Endothelial cells and human cerebral small vessel disease. Brain Pathology. 2015;25:44–50. doi: 10.1111/bpa.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Goldstein FC, Waller EK, Moss LD, Quyyumi A. Circulating Progenitor Cells is Linked to Cognitive Decline in Healthy Adults. The American journal of the medical sciences. 2016;351:147–152. doi: 10.1016/j.amjms.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Hara K, Shiga A, Fukutake T, Nozaki H, Miyashita A, Yokoseki A, Kawata H, Koyama A, Arima K, Takahashi T, Ikeda M, Shiota H, Tamura M, Shimoe Y, Hirayama M, Arisato T, Yanagawa S, Tanaka A, Nakano I, Ikeda S, Yoshida Y, Yamamoto T, Ikeuchi T, Kuwano R, Nishizawa M, Tsuji S, Onodera O. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360:1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D. The Energetics of CNS White Matter. Journal of Neuroscience. 2012;32:356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic Energy Use and Supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Hartman R, Lekic T, Rojas H, Tang J, Zhang JH. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Enmi J, Kitamura A, Yamamoto Y, Saito S, Takahashi Y, Iguchi S, Tsuji M, Yamahara K, Nagatsuka K, Iida H, Ihara M. A novel mouse model of subcortical infarcts with dementia. J Neurosci. 2015;35:3915–3928. doi: 10.1523/JNEUROSCI.3970-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Kitamura A, Nagatsuka K, Ihara M. A Novel Mouse Model of Ischemic Carotid Artery Disease. Plos One. 2014a.;9 doi: 10.1371/journal.pone.0100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Kitamura A, Tsuji M, Nagatsuka K, Ihara M. Motor and cognitive impairment in a mouse model of ischemic carotid artery disease. Neuroscience Letters. 2014b;581:1–6. doi: 10.1016/j.neulet.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Miyamoto N, Seo JH, Pham LDD, Kim KW, Lo EH, Arai K. High - mobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. Journal of neurochemistry. 2013;125:273–280. doi: 10.1111/jnc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. Journal of the American College of Cardiology. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Helman AM, Murphy MP. Vascular cognitive impairment: Modeling a critical neurologic disease in vitro and in vivo. Biochim Biophys Acta. 2016;1862:975–982. doi: 10.1016/j.bbadis.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall TL, Keller A, He L, Johansson BR, Wallgard E, Raschperger E, Mae MA, Jin S, Betsholtz C, Lendahl U. Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler Thromb Vasc Biol. 2015;35:409–420. doi: 10.1161/ATVBAHA.114.304849. [DOI] [PubMed] [Google Scholar]
- Herbig U, Sedivy JM. Regulation of growth arrest in senescence: telomere damage is not the end of the story. Mechanisms of ageing and development. 2006;127:16–24. doi: 10.1016/j.mad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guillamon M, Delgado P, Penalba A, Rodriguez-Luna D, Molina CA, Rovira A, Alvarez-Sabin J, Boada M, Montaner J. Plasma beta-amyloid levels in cerebral amyloid angiopathy-associated hemorrhagic stroke. Neurodegener Dis. 2012;10:320–323. doi: 10.1159/000333811. [DOI] [PubMed] [Google Scholar]
- Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Sturchler-Pierrat C, Burki K, van Duinen SG, Maat-Schieman ML, Staufenbiel M, Mathews PM, Jucker M. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. New England Journal of Medicine. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nature Reviews Neuroscience. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo F, Duro G, Rizzo C, Castiglia L, Hachinski V, Caruso C. Pathophysiology of vascular dementia. Immun Ageing. 2009;6:13. doi: 10.1186/1742-4933-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Polvikoski TM, Hall R, Slade JY, Perry RH, Oakley AE, Englund E, O'Brien JT, Ince PG, Kalaria RN. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer's disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–589. doi: 10.1007/s00401-009-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle-Hansen H, Thommessen B, Wyller TB, Engedal K, Oksengard AR, Stenset V, Loken K, Aaberg M, Fure B. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement Geriatr Cogn Disord. 2011;32:401–407. doi: 10.1159/000335361. [DOI] [PubMed] [Google Scholar]
- Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke. 2005;36:1253–1258. doi: 10.1161/01.str.0000167694.58419.a2. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. Journal of Applied Physiology. 2007;103:1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Sudlow CL. Is hypertension a more frequent risk factor for deep than for lobar supratentorial intracerebral haemorrhage? J Neurol Neurosurg Psychiatry. 2006;77:1244–1252. doi: 10.1136/jnnp.2006.089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H1344–H1350. doi: 10.1152/ajpheart.00413.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin A, Aboa-Eboule C, Rouaud O, Osseby GV, Binquet C, Durier J, Moreau T, Bonithon-Kopp C, Giroud M, Bejot Y. Prior transient ischemic attack and dementia after subsequent ischemic stroke. Alzheimer Dis Assoc Disord. 2012;26:307–313. doi: 10.1097/WAD.0b013e3182420b2c. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Morphologic diagnosis of “vascular dementia” - a critical update. J Neurol Sci. 2008;270:1–12. doi: 10.1016/j.jns.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mechanisms of ageing and development. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M, Zetterberg H, Rolstad S, Edman A, Gouw AA, Bjerke M, Lind K, Blennow K, Pantoni L, Inzitari D, Wallin A. Low Cerebrospinal Fluid Sulfatide Predicts Progression of White Matter Lesions - The LADIS Study. Dementia and Geriatric Cognitive Disorders. 2012;34:61–67. doi: 10.1159/000341576. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jolley D. The incidence of dementia A meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutrition Reviews. 2010;68:S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN. Cerebrovascular Disease and Mechanisms of Cognitive Impairment Evidence From Clinicopathological Studies in Humans. Stroke. 2012;43:2526–2534. doi: 10.1161/STROKEAHA.112.655803. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862:915–925. doi: 10.1016/j.bbadis.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Oh MY, Jang MS, Han MK, Lee J, Lee J, Kang Y, Yu KH, Lee BC, Kim S, Yoon BW, Bae HJ. Medial temporal atrophy and memory dysfunction in poststroke cognitive impairment-no dementia. J Clin Neurol. 2012;8:43–50. doi: 10.3988/jcn.2012.8.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISHI S. Functional aging and gradual senescence in zebrafish. Annals of the New York Academy of Sciences. 2004;1019:521–526. doi: 10.1196/annals.1297.097. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171. doi: 10.1212/01.wnl.0000194266.55694.1e. [DOI] [PubMed] [Google Scholar]
- Kontos H, Wei E, Raper A, Patterson J. Local mechanism of CO2 action of cat pial arterioles. Stroke. 1977;8:226–229. doi: 10.1161/01.str.8.2.226. [DOI] [PubMed] [Google Scholar]
- Kremer PH, Jolink WM, Kappelle LJ, Algra A, Klijn CJ, Smart, Groups ES. Risk Factors for Lobar and Non-Lobar Intracerebral Hemorrhage in Patients with Vascular Disease. PLoS One. 2015;10:e0142338. doi: 10.1371/journal.pone.0142338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan TH, Huang XQ, Tan HM. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. 2013;22:401–407. doi: 10.1016/j.carpath.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Lanna ME, Madeira DM, Alves G, Alves CE, Valente LE, Laks J, Engelhardt E. Vascular dementia by thalamic strategic infarct. Arq Neuropsiquiatr. 2008;66:412–414. doi: 10.1590/s0004-282x2008000300027. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiological reviews. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Lei C, Lin S, Tao W, Hao Z, Liu M, Wu B. Association between cerebral microbleeds and cognitive function: a systematic review. J Neurol Neurosurg Psychiatry. 2013;84:693–697. doi: 10.1136/jnnp-2012-303948. [DOI] [PubMed] [Google Scholar]
- Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Aβ generation by altering β- and γ-cleavage of APP. Neurobiology of Aging. 2009;30:1091–1098. doi: 10.1016/j.neurobiolaging.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lin RT, Tai CT, Hsieh CL, Hsiao SF, Liu CK. Prediction of poststroke dementia. Neurology. 2003;61:343–348. doi: 10.1212/01.wnl.0000078891.27052.10. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang J. Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci. 2012;122:494–499. doi: 10.3109/00207454.2012.686543. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang J, Hou WW, Wu XH, Liao RJ, Chen Y, Wang Z, Zhang XN, Zhang LS, Zhou YD, Chen Z, Hu WW. Early treatment of minocycline alleviates white matter and cognitive impairments after chronic cerebral hypoperfusion. Sci Rep. 2015;5:12079. doi: 10.1038/srep12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JA, Murugesan N, Pachter JS. Endothelial cell heterogeneity of blood - brain barrier gene expression along the cerebral microvasculature. Journal of neuroscience research. 2010;88:1457–1474. doi: 10.1002/jnr.22316. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Langdon KD, Churchill KP, Granter-Button S, Corbett D. Assessing cognitive function after intracerebral hemorrhage in rats. Behav Brain Res. 2009;198:321–328. doi: 10.1016/j.bbr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Madigan JB, Wilcock DM, Hainsworth AH. Vascular Contributions to Cognitive Impairment and Dementia Topical Review of Animal Models. Stroke. 2016;47:1953–1959. doi: 10.1161/STROKEAHA.116.012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Kassner A, Fisher JA, Mikulis DJ. Selective reduction of blood flow to white matter during hypercapnia corresponds with leukoaraiosis. Stroke. 2008;39:1993–1998. doi: 10.1161/STROKEAHA.107.501692. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Festa JR, Cheung YK, Chen R, Pavol MA, Derdeyn CP, Clarke WR, Videen TO, Grubb RL, Adams HP, Powers WJ, Lazar RM. Cerebral hemodynamics and cognitive impairment: baseline data from the RECON trial. Neurology. 2012;78:250–255. doi: 10.1212/WNL.0b013e31824365d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T. Periventricular White-Matter Lucency and Cerebral Blood-Flow Autoregulation in Hypertensive Patients. Hypertension. 1994;23:565–568. doi: 10.1161/01.hyp.23.5.565. [DOI] [PubMed] [Google Scholar]
- Mayhan W, Werber A, Heistad D. Protection of cerebral vessels by sympathetic nerves and vascular hypertrophy. Circulation. 1987;75:I107–112. [PubMed] [Google Scholar]
- Merino JG. Dementia after stroke: high incidence and intriguing associations. Stroke. 2002;33:2261–2262. [PubMed] [Google Scholar]
- Merrill DC, Thompson MW, Carney CL, Granwehr BP, Schlager G, Robillard JE, Sigmund CD. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre M, Nguyen A, Kashani S, Pham T, Adesina A, Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiology of Aging. 2004;25:783–793. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196:298–305. doi: 10.1016/j.atherosclerosis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Miwa K, Tanaka M, Okazaki S, Yagita Y, Sakaguchi M, Mochizuki H, Kitagawa K. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology. 2014;83:646–653. doi: 10.1212/WNL.0000000000000692. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics, C. Stroke Statistics, S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. Febs Letters. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- Nakase T, Sasaki M, Yoshioka S, Ikeda Y, Suzuki A. Risk of cognitive impairment in acute phase of intracerebral haemorrhage. Int J Stroke. 2013;8:E15. doi: 10.1111/ijs.12104. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Neto CJBF, Paganelli RA, Benetoli A, Lima KCM, Milani H. Permanent, 3-stage, 4-vessel occlusion as a model of chronic and progressive brain hypoperfusion in rats: a neurohistological and behavioral analysis. Behavioural brain research. 2005;160:312–322. doi: 10.1016/j.bbr.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Ni JW, Matsumoto K, Li HB, Murakami Y, Watanabe H. Neuronal damage and decrease of central acetylcholine level following permanent occlusion of bilateral common carotid arteries in rat. Brain Res. 1995;673:290–296. doi: 10.1016/0006-8993(94)01436-l. [DOI] [PubMed] [Google Scholar]
- Nilsson PM, Olsen MH, Laurent S. Early Vascular Aging (EVA): New Directions in Cardiovascular Protection. Academic Press; 2015. [Google Scholar]
- Nishio K, Ihara M, Yamasaki N, Kalaria RN, Maki T, Fujita Y, Ito H, Oishi N, Fukuyama H, Miyakawa T. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke. 2010;41:1278–1284. doi: 10.1161/STROKEAHA.110.581686. [DOI] [PubMed] [Google Scholar]
- Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. Journal of Biological Chemistry. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöth U, Kotajima F, Deichmann R, Turner R, Corfield DR. Mapping of the cerebral vascular response to hypoxia and hypercapnia using quantitative perfusion MRI at 3 T. NMR in biomedicine. 2008;21:464–472. doi: 10.1002/nbm.1210. [DOI] [PubMed] [Google Scholar]
- Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23:408–416. doi: 10.1159/000101464. [DOI] [PubMed] [Google Scholar]
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S, investigators I. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SCR, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Asanuma M, Tanaka K, Hirata H, Kondo Y, Goto M, Kawauchi M, Ogura T. Long-term time course of regional changes in cholinergic indices following transient ischemia in the spontaneously hypertensive rat brain. Brain Res. 1996;712:60–68. doi: 10.1016/0006-8993(95)01446-2. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Hirayama M, Ito S, Uchida Y, Tachikawa M, Terasaki T. Quantitative targeted proteomics for understanding the blood–brain barrier: towards pharmacoproteomics. Expert review of proteomics. 2014;11:303–313. doi: 10.1586/14789450.2014.893830. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Paquet C, Jouvent E, Mine M, Vital A, Hugon J, Chabriat H, Gray F. A cortical form of CADASIL with cerebral Aβ amyloidosis. Acta neuropathologica. 2010;120:813–820. doi: 10.1007/s00401-010-0758-y. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Molecular biology of the blood-brain barrier. Molecular biotechnology. 2005;30:57–69. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010;482:235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- Pasquini M, Benedictus MR, Boulouis G, Rossi C, Dequatre-Ponchelle N, Cordonnier C. Incident Cerebral Microbleeds in a Cohort of Intracerebral Hemorrhage. Stroke. 2016;47:689–694. doi: 10.1161/STROKEAHA.115.011843. [DOI] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. Journal of Experimental Medicine. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell metabolism. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. The Lancet Neurology. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Piechnik SK, Chiarelli PA, Jezzard P. Modelling vascular reactivity to investigate the basis of the relationship between cerebral blood volume and flow under CO 2 manipulation. Neuroimage. 2008;39:107–118. doi: 10.1016/j.neuroimage.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- Polvikoski TM, van Straaten EC, Barkhof F, Sulkava R, Aronen HJ, Niinisto L, Oinas M, Scheltens P, Erkinjuntti T, Kalaria RN. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–2078. doi: 10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M, Gong X, Su WP, Matsumoto SG, Banine F, Winkler C, Foster S, Xing RB, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Back SA, Sherman LS. Digestion products of the PH20 hyaluronidase inhibit remyelination. Annals of Neurology. 2013;73:266–280. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Zhuo L, Li N, Hu Y, Chen W, Zhou Y, Wang J, Tao Q, Hu J, Nie X, Zhan S. Prevalence of post-stroke cognitive impairment in china: a community-based, cross-sectional study. PLoS One. 2015;10:e0122864. doi: 10.1371/journal.pone.0122864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaegebeur A, Lange C, Carmeliet P. The Neurovascular Link in Health and Disease: Molecular Mechanisms and Therapeutic Implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Rapp JH, Hollenbeck K, Pan XM. An experimental model of lacunar infarction: embolization of microthrombi. J Vasc Surg. 2008;48:196–200. doi: 10.1016/j.jvs.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. Journal of Cerebral Blood Flow & Metabolism. 2016;36:172–186. doi: 10.1038/jcbfm.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter B, Rodemer C, Grudzenski S, Meairs S, Bugert P, Hennerici MG, Fatar M. Effect of simvastatin on MMPs and TIMPs in human brain endothelial cells and experimental stroke. Transl Stroke Res. 2015;6:156–159. doi: 10.1007/s12975-014-0381-7. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing research reviews. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez GA, Burns MP, Weeber EJ, Rebeck GW. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learning & memory. 2013;20:256–266. doi: 10.1101/lm.030031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC, Salloway S, Black SE, Royall DR, Decarli C, Weiner MW, Moline M, Kumar D, Schindler R, Posner H. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41:1213–1221. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix Metalloproteinase-Mediated Neuroinflammation in Vascular Cognitive Impairment of the Binswanger Type. Cell Mol Neurobiol. 2016;36:195–202. doi: 10.1007/s10571-015-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke. 2014;45:1531–1538. doi: 10.1161/STROKEAHA.113.004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabayan B, Jansen S, Oleksik AM, van Osch MJ, van Buchem MA, van Vliet P, de Craen AJ, Westendorp RG. Cerebrovascular hemodynamics in Alzheimer's disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing research reviews. 2012;11:271–277. doi: 10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MSV, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, Council AHAS, Anesthesia CCS, Radiology CC, Nursing CCS, Prevention CE, Dis CPV, Metab CNPA. An Updated Definition of Stroke for the 21st Century A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Chen X, Joscelyne A, Wen W, Altendorf A, Brodaty H. Hippocampal size and dementia in stroke patients: the Sydney stroke study. J Neurol Sci. 2007;260:71–77. doi: 10.1016/j.jns.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83:275–281. doi: 10.1136/jnnp-2011-300371. [DOI] [PubMed] [Google Scholar]
- Savva GM, Stephan BC, Alzheimer's Society Vascular Dementia Systematic Review, G. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- Scheel P, Puls I, Becker G, Schöning M. Volume reduction in cerebral blood flow in patients with vascular dementia. The Lancet. 1999;354:2137. doi: 10.1016/S0140-6736(99)04016-7. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Bueche CZ, Garz C, Braun H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease? - New insights from a rat model. Experimental & translational stroke medicine. 2013;5:4. doi: 10.1186/2040-7378-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, Miller BL, Kramer JH, Jagust WJ, Chui HC. Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimer's & Dementia. 2009;5:454–462. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl–lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. American journal of hypertension. 2009;22:74–79. doi: 10.1038/ajh.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Sun K, Schwartz AV, Varadhan R, Harris TB, Satterfield S, Garcia M, Ferrucci L, Newman AB. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. Journal of hypertension. 2015;33:797–803. doi: 10.1097/HJH.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Miyamoto N, Hayakawa K, Pham LDD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. Journal of Clinical Investigation. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, D'Agostino RB, DeCarli C. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci. 2013;16:55–63. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JE, Fernando MS, Clark L, Ince PG, Matthews F, Forster G, O'Brien JT, Barber R, Kalaria RN, Brayne C, Shaw PJ, Lewis CE, Wharton SB. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathology and Applied Neurobiology. 2007;33:410–419. doi: 10.1111/j.1365-2990.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries C, Blennow K. A population study on blood-brain barrier function in 85-year-olds Relation to Alzheimer's disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- Smeda JS. Hemorrhagic stroke development in spontaneously hypertensive rats fed a North American, Japanese-style diet. Stroke. 1989;20:1212–1218. doi: 10.1161/01.str.20.9.1212. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature Guides Migrating Neuronal Precursors in the Adult Mammalian Forebrain via Brain-Derived Neurotrophic Factor Signaling. Journal of Neuroscience. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in Cerebral Microvasculature with Age Are Associated with the Decline in Growth Hormone and Insulin-Like Growth Factor 1*. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Soria G, Tudela R, Márquez-Martín A, Camón L, Batalle D, Muñoz-Moreno E, Eixarch E, Puig J, Pedraza S, Vila E. The ins and outs of the BCCAo model for chronic hypoperfusion: a multimodal and longitudinal MRI approach. PloS one. 2013;8:e74631. doi: 10.1371/journal.pone.0074631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth VK, Thrift AG, Saling MM, Anderson JF, Dewey HM, Macdonell RA, Donnan GA, Community-Based Prospective Study of Nonaphasic English-Speaking, S. Increased risk of cognitive impairment 3 months after mild to moderate first-ever stroke: a Community-Based Prospective Study of Nonaphasic English-Speaking Survivors. Stroke. 2003;34:1136–1143. doi: 10.1161/01.STR.0000069161.35736.39. [DOI] [PubMed] [Google Scholar]
- Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. Journal of Cerebral Blood Flow & Metabolism. 2013;33:708–715. doi: 10.1038/jcbfm.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. American Journal of Physiology-Heart and Circulatory Physiology. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, Lopez OL, van Buchem MA, Jonsson PV, Gudnason V, Launer LJ. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T O'Brien J, Thomas A. Vascular dementia. The Lancet. 2015;386:1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- Tan CO. Defining the characteristic relationship between arterial pressure and cerebral flow. Journal of Applied Physiology. 2012;113:1194–1200. doi: 10.1152/japplphysiol.00783.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Shah F, Tanaka H, Haley AP. Association Between Central Elastic Artery Stiffness and Cerebral Perfusion in Deep Subcortical Gray and White Matter. American Journal of Hypertension. 2011;24:1108–1113. doi: 10.1038/ajh.2011.101. [DOI] [PubMed] [Google Scholar]
- Thal DR, Grinberg LT, Attems J. Vascular dementia: Different forms of vessel disorders contribute to the development of dementia in the elderly brain. Experimental Gerontology. 2012;47:816–824. doi: 10.1016/j.exger.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Kalaria RN, T O'Brien J. Depression and vascular disease: what is the relationship? Journal of affective disorders. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- Troen AM, Chao W-H, Crivello NA, D'Anci KE, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. Cognitive impairment in folate-deficient rats corresponds to depleted brain phosphatidylcholine and is prevented by dietary methionine without lowering plasma homocysteine. The Journal of nutrition. 2008;138:2502–2509. doi: 10.3945/jn.108.093641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng Y-C, Willie CK, Atkinson G, Lucas SJ, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension. 2010;56:268–273. doi: 10.1161/HYPERTENSIONAHA.110.152066. [DOI] [PubMed] [Google Scholar]
- Udaka F, Sawada H, Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci. 2002;977:411–415. doi: 10.1111/j.1749-6632.2002.tb04845.x. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T. Edaravone Attenuates White Matter Lesions through Endothelial Protection in a Rat Chronic Hypoperfusion Model. Neuroscience. 2009;162:317–327. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008;13:5056–5070. doi: 10.2741/3064. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, De Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- Van Der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T. Enhanced peroxynitrite formation is associated with vascular aging. The Journal of experimental medicine. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- Wakita H, Ruetzler C, Illoh KO, Chen Y, Takanohashi A, Spatz M, Hallenbeck JM. Mucosal tolerization to E-selectin protects against memory dysfunction and white matter damage in a vascular cognitive impairment model. Journal of Cerebral Blood Flow and Metabolism. 2008;28:341–353. doi: 10.1038/sj.jcbfm.9600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, McGeer PL. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924:63–70. doi: 10.1016/s0006-8993(01)03223-1. [DOI] [PubMed] [Google Scholar]
- Wallays G, Nuyens D, Silasi-Mansat R, Souffreau J, Callaerts-Vegh Z, Van Nuffelen A, Moons L, D'Hooge R, Lupu F, Carmeliet P, Collen D, Dewerchin M. Notch3 Arg170Cys knock-in mice display pathologic and clinical features of the neurovascular disorder cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Arterioscler Thromb Vasc Biol. 2011;31:2881–2888. doi: 10.1161/ATVBAHA.111.237859. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Jiang L-Q, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Spinetti G, Jiang L-Q, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. The American journal of pathology. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang D, Zhang X, Li T, Li J, Tang Y, Le W. Hypoxia-induced down-regulation of neprilysin by histone modification in mouse primary cortical and hippocampal neurons. PLoS One. 2011;6:e19229. doi: 10.1371/journal.pone.0019229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AJS, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased Cerebral Arterial Pulsatility in Patients With Leukoaraiosis Arterial Stiffness Enhances Transmission of Aortic Pulsatility. Stroke. 2012;43:2631–+.. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Wiegman AF, Meier IB, Schupf N, Manly JJ, Guzman VA, Narkhede A, Stern Y, Martinez-Ramirez S, Viswanathan A, Luchsinger JA, Greenberg SM, Mayeux R, Brickman AM. Cerebral microbleeds in a multiethnic elderly community: demographic and clinical correlates. J Neurol Sci. 2014;345:125–130. doi: 10.1016/j.jns.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Kiliaan AJ, Claassen JA. Vascular aspects of cognitive impairment and dementia. Journal of Cerebral Blood Flow & Metabolism. 2013;33:1696–1706. doi: 10.1038/jcbfm.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Leggett R. Reference values for resting blood flow to organs of man. Clinical Physics and Physiological Measurement. 1989;10:187. doi: 10.1088/0143-0815/10/3/001. [DOI] [PubMed] [Google Scholar]
- Willie C, Cowan E, Ainslie P, Taylor C, Smith K, Sin P, Tzeng Y. Neurovascular coupling and distribution of cerebral blood flow during exercise. Journal of neuroscience methods. 2011;198:270–273. doi: 10.1016/j.jneumeth.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Willie C, Macleod D, Shaw A, Smith K, Tzeng Y, Eves N, Ikeda K, Graham J, Lewis N, Day T. Regional brain blood flow in man during acute changes in arterial blood gases. The Journal of physiology. 2012;590:3261–3275. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, Smith KJ, Day TA, Ray LA, Lewis NC, Bakker A, Macleod DB, Ainslie PN. Regional cerebral blood flow in humans at high altitude: gradual ascent and 2 wk at 5,050 m. Journal of applied physiology. 2014a;116:905–910. doi: 10.1152/japplphysiol.00594.2013. [DOI] [PubMed] [Google Scholar]
- Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. The Journal of physiology. 2014b;592:841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold KH, Tolnay M, Staufenbiel M, Jucker M. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21:1619–1627. doi: 10.1523/JNEUROSCI.21-05-01619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff H, Lennox W. The effect on pial vessels of variations in the oxygen and carbon dioxide content of the blood. Arch. Neurol. & Psychiat. 1930;23:1097–1120. [Google Scholar]
- Won SJ, Yoo BH, Kauppinen TM, Choi BY, Kim JH, Jang BG, Lee MW, Sohn M, Liu J, Swanson RA, Suh SW. Recurrent/moderate hypoglycemia induces hippocampal dendritic injury, microglial activation, and cognitive impairment in diabetic rats. J Neuroinflammation. 2012;9:182. doi: 10.1186/1742-2094-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, Shukla R, Pancioli AM, Jauch EC, Menon AG, Deka R, Carrozzella JA, Moomaw CJ, Fontaine RN, Broderick JP. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190–1195. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- Xiong L, Reijmer YD, Charidimou A, Cordonnier C, Viswanathan A. Intracerebral hemorrhage and cognitive impairment. Biochim Biophys Acta. 2016;1862:939–944. doi: 10.1016/j.bbadis.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. 2015;17:17–30. doi: 10.5853/jos.2015.17.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sugimachi K, Nakano K, Fujimoto M, Takahashi M, Chikugo T, Ogawa H. Memory deficit accompanying cerebral neurodegeneration after stroke in stroke-prone spontaneously hypertensive rats (SHRSP). Acta Neurochir Suppl (Wien) 1994;60:200–202. doi: 10.1007/978-3-7091-9334-1_54. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Horie R, Handa H, Sato M, Fukase M. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke. 1976;7:46–53. doi: 10.1161/01.str.7.1.46. [DOI] [PubMed] [Google Scholar]
- Yang J, Wong A, Wang Z, Liu W, Au L, Xiong Y, Chu WW, Leung EY, Chen S, Lau C, Chan AY, Lau AY, Fan F, Ip V, Soo Y, Leung T, Ho CL, Wong LK, Mok VC. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimers Dement. 2015;11:16–23. doi: 10.1016/j.jalz.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of Metabolic Syndrome on Cognition and Brain A Selected Review of the Literature. Arteriosclerosis Thrombosis and Vascular Biology. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210:585–591. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Yurdagul A, Jr., Finney AC, Woolard MD, Orr AW. The arterial microenvironment: the where and why of atherosclerosis. Biochem J. 2016;473:1281–1295. doi: 10.1042/BJ20150844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HA, Gao M, Chen B, Shi L, Wang Q, Yu X, Xuan Z, Gao L, Du G. Evaluation of hippocampal injury and cognitive function induced by embolization in the rat brain. Anat Rec (Hoboken) 2013;296:1207–1214. doi: 10.1002/ar.22715. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu B, Nie K, Jia Y, Yu J. Effects of acupuncture on declined cerebral blood flow, impaired mitochondrial respiratory function and oxidative stress in multi-infarct dementia rats. Neurochem Int. 2014;65:23–29. doi: 10.1016/j.neuint.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Herbert B-S, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-α via the p38 mitogen-activated protein kinase pathway. The FASEB Journal. 2009;23:1358–1365. doi: 10.1096/fj.08-110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Gu J.-h., Dai C.-l., Liu Q, Iqbal K, Liu F, Gong C-X. Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice. Frontiers in aging neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong ZH, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nature Neuroscience. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim M-S, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61:232–244. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Ranzini M, Guerra G, Rossi L, Munari M, Zurlo A, Volpato S, Atti A, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer's disease or vascular dementia. Journal of psychiatric research. 2007;41:686–693. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]