Abstract
The ubiquitin proteasome pathway (UPP) is essential for removing abnormal proteins and preventing accumulation of potentially toxic proteins within the neuron. UPP dysfunction occurs with normal aging and is associated with abnormal accumulation of protein aggregates within neurons in neurodegenerative diseases. Ischemia disrupts UPP function and thus may contribute to UPP dysfunction seen in the aging brain and in neurodegenerative diseases. Ubiquitin carboxy-terminal hydrolase L1 (UCHL1), an important component of the UPP in the neuron, is covalently modified and its activity inhibited by reactive lipids produced after ischemia. As a result, degradation of toxic proteins is impaired which may exacerbate neuronal function and cell death in stroke and neurodegenerative diseases. Preserving or restoring UCHL1 activity may be an effective therapeutic strategy in stroke and neurodegenerative diseases.
Keywords: ubiquitin, ubiquitin proteasome pathway (UPP), neurodegenerative disease, ubiquitin carboxy-terminal hydrolase L1(UCHL1), cerebral ischemia, aging
1. Ubiquitin proteasome pathway (UPP) in normal brain function
In order to maintain brain function, neurons must survive for the life of the organism since neuronal mitosis is complete in most neurons by birth. This poses unique challenges for the neuron and requires efficient systems for maintaining cellular integrity, cellular repair and removing potential toxins from the cell (Ciechanover and Kwon, 2015; Jara et al., 2013). These toxins include abnormally folded or aggregated proteins (Haass and Selkoe, 2007). Neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) are characterized by the accumulation of abnormal protein aggregates within the neurons (Ciechanover and Kwon, 2015; Sulistio and Heese, 2016). One of the major mechanisms by which the neuron removes abnormal proteins is the ubiquitin proteasome pathway (UPP) (Huang and Figueiredo-Pereira, 2010; Schwartz and Ciechanover, 2009). Without continued effective operation of the UPP, accumulation of toxic intracellular proteins may threaten the viability of the neuron (Ciechanover and Kwon, 2015). Thus, the life or death of the aging neuron depends upon continued efficient removal of abnormal protein trash by the UPP.
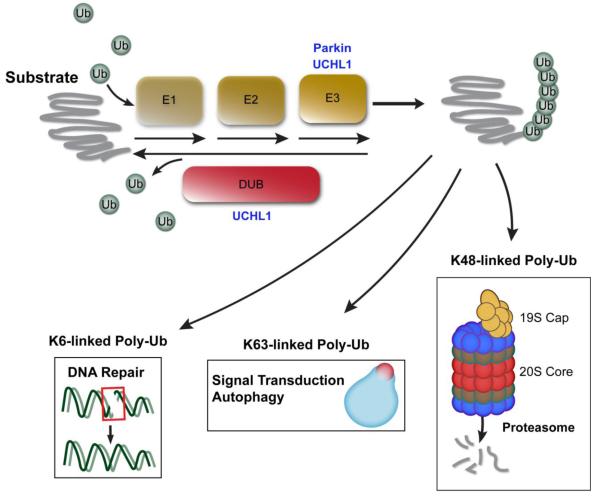
The UPP serves to identify, label and transport damaged or misfolded proteins to the proteasome where abnormal proteins are degraded (Schwartz and Ciechanover, 2009). The UPP has several components which include ubiquitin, the 26S proteasome, ubiquitin activating enzyme(E1), ubiquitin conjugating enzyme (E2), ubiquitin ligating enzyme (E3), and deubiquitinating enzymes (DUBs) (Gadhave et al., 2016; McNaught et al., 2001). Ubiquitin (Ub), a 8.5 kD protein with 76-amino acids, is activated by E1 and then transferred to E2 before it is conjugated to the substrate proteins by E3 ligase (McNaught et al., 2001; Moore et al., 2003; Scheffner et al., 1995). Additional ubiquitin molecules are linked to the initial ubiquitin, forming poly-ubiquitin (poly-Ub) chains. The poly-Ub chains associated with protein degradation via the proteasome are formed by linkage of Ub at its lysine 48 amino acid (K48) (Ciechanover and Kwon, 2015; Huang and Figueiredo-Pereira, 2010; Komander, 2009). K48-poly-Ub-linked proteins are then transported to the proteasome via interactions with cytoskeletal proteins and other enzymes (Finley, 2009). Prior to entering the proteasome, Ub is removed from the substrate proteins, by way of deubiquitinating enzymes (DUBs) (Kim et al., 2003). DUBs are grouped into five different classes, which include 1) ubiquitin C-terminal hydrolases (UCHs, such as UCHL1, UCHL3 and UCHL5) ; 2) ubiquitin-specific proteases (USPs, such as USP7, USP9x and USP14); 3) ovarian tumor-like proteases; 4) JAB1/MPN/Mov34 metalloproteases and 5) Machado-Jakob disease proteases (Komander et al., 2009; Todi and Paulson, 2011). This allows Ub to be recycled and reused for transport of additional damaged proteins. Once deubiquitinated, the abnormal protein may enter the proteasome where it is proteolyzed to amino acids (McNaught et al., 2001; Moore et al., 2003). The 26S proteasome consists of the 19S regulatory particle (which is responsible for substrate recognition, deubiquitination and unfolding ) and the 20S core particle which degrades substrate proteins (Fig. 1) (Baumeister et al., 1998; Braun et al., 1999; DeMartino and Slaughter, 1999; Glickman et al., 1998; Walz et al., 1998). Thus, normal UPP function is a primary method resulting in misfolded or damaged protein removal from the neuron, helping assure normal neuronal function and preventing accumulation of abnormal proteins.
Figure 1.
Ubiquitin Proteasome Pathway. Ubiquitin is activated and sequentially linked to the protein to form a poly-ubiquitin chain, targeting the substrate for: degradation, autophagy and cell signaling transduction.
Ligation of Ub to proteins may have other functions in addition to the UPP (Kulathu and Komander, 2012). For example, poly-Ub chains may also be formed by conjugation at lysine 63 (K63) and other lysine sites (K6, K11, K27,K29 and K33), but these linkages are associated with other cellular functions such as signal transduction, DNA repair and autophagy (Erpapazoglou et al., 2014; Moore et al., 2003; Nathan et al., 2013; Silva et al., 2015). Ubiquitination of proteins may play an important role in axonal transport; mutations in or disruption of UCHL1 results in axonal abnormalities and disruption of motor function (Korhonen and Lindholm, 2004; Mukoyama et al., 1989). Protein ubiquitination also may play an important role in transport of synaptosomes to the cell membrane (Chen et al., 2011b; Yin et al., 2015).
2. The role of the UPP in aging and neurodegenerative diseases
2.1 UPP and aging
In order to preserve the function and viability of the neuron throughout its lifespan, it is essential to degrade and remove abnormal potentially toxic proteins. In addition, neurons are unique in that they contain axons and dendrites that do not include all of the proteolytic components of the cell body. Thus, abnormal proteins located in axons and dendrites must be transported to the cell body for disposal by the proteasome and other systems such as the lysosome and autophagy (Yerbury et al., 2016). 20S and 26S proteasome substrate degradation activity is significantly decreased in aged brains compared to young rat brains (Giannini et al., 2013; Keller et al., 2000; Tydlacka et al., 2008). Hence, a decline in UPP function may be a component of normal aging and may be an important factor in the increased incidence of common neurodegenerative diseases associated with accumulation of abnormal proteins in the brain.
2.2 UPP and Parkinson’s disease (PD)
The pathological hallmark of PD is the presence of abnormal protein aggregations, also known as Lewy bodies, containing α-synuclein and other proteins within the cytoplasm of neurons (Goedert and Spillantini, 1998; Goedert et al., 2013; Spillantini et al., 1998). Many of these proteins are ubiquitinated suggesting that they were destined for degradation via the UPP (Hasegawa et al., 2002; McNaught et al., 2001; Moore et al., 2003). The aggregates also contain proteasome components and cytoskeletal elements associated with the UPP and are termed aggresomes (Choi et al., 2004). These observations suggest that the protein inclusions found in PD may result from failed attempts by the UPP to remove abnormal neuronal proteins.
Additional support for the hypothesis that UPP dysfunction is important in the pathogenesis of PD comes from the identification of several mutations in genes that may have a role in the neuronal UPP in patients with familial PD. α-synuclein is the most prominent component of the Lewy body and a mutation has been associated with increased aggregation of this protein and PD (Engelender, 2008). α-synuclein aggregates have been shown to inhibit proteasome function (Ghee et al., 2000; McNaught et al., 2001; Tanaka et al., 2001). Parkin (PARK2), another gene mutated in familial PD, is an E3-ubiquitin ligase, which plays an important role in selecting proteins for ubiquitination (Imai et al., 2001; Shimura et al., 2000; Zhang et al., 2000). UCHL1 (PARK5), is a prevalent neuronal protein that hydrolyzes Ub from proteins prior to their entry into the proteasome and also ligates Ub to its target proteins (Liu et al., 2002; Nishikawa et al., 2003; Wintermeyer et al., 2000). Thus, UCHL1 may also play an important role in the neuronal UPP. DJ1 (PARK7) is poly-ubiquitinated by Parkin (Olzmann and Chin, 2008; Olzmann et al., 2007). A mutation in DJ1 results in abnormal folding of the protein making it prone to aggregation and accumulation in aggresomes (Olzmann et al., 2004; Olzmann et al., 2008). Therefore, a number of the mutations that produce familial PD are associated with abnormalities in the UPP.
2.3 UPP and Alzheimer’s disease (AD)
AD is characterized by aggregation of amyloid β (Aβ) and tau proteins. Aβ oligomers accumulate within neurons and there are extracellular accumulations of high molecular weight deposits of Aβ peptides in the fibular form (Deger et al., 2015; Gadhave et al., 2016). Aβpeptides also form diffuse plaques consisting of beta sheets of Aβthat are resistant to degradation (Ciechanover and Kwon, 2015). AD is also associated with cerebral amyloid angiopathy, where Aβ accumulates in the media and adventitia of small and medium sized arteries in the brain. Aβ inhibits the function of the UPP and promotes the formation and accumulation of hyperphosphorylated tau, a highly soluble microtubule-binding protein (Ciechanover and Kwon, 2015; Gadhave et al., 2016; Haass and Selkoe, 2007). Hyperphosphorylated and misfolded tau accumulates at both pre- and post- synaptic terminals in brains of patients with AD (Brandt et al., 2005; Goedert et al., 1998; Spillantini and Goedert, 1998; Tai et al., 2012). Abnormally hyperphosphorylated and misfolded tau accumulates in the cell body of neurons, is associated with neurofibulary tangles and impairs axonal transport and neuronal function (Gadhave et al., 2016). Tau also accumulates in dendritic processes and axons and is present in neuritic plaques (Avila et al., 2013; Brandt et al., 2005).
The UPP may play an important role in the degradation of both abnormally folded or aggregated tau and Aβ. Proteasome activity is decreased in the brain of early phase AD patients (Keck et al., 2003; Keller et al., 2000; Lopez Salon et al., 2000; Sulistio and Heese, 2016). Downregulation of E1 and E2 enzymes and proteasome subunits have been detected in AD brains (Lopez Salon et al., 2000). In addition, oligomers of Aβhave been reported to disrupt proteasome function by directly binding to the 20S proteasome and inhibiting its proteolytic activity (Gregori et al., 1995; Gregori et al., 1997; Oh et al., 2005; Tseng et al., 2008). Moreover, polymerized tau, but not soluble tau, can also significantly inhibit proteasome activity (Keck et al., 2003; Lam et al., 2000; Yen, 2011). In turn, impaired proteasome activity may account for decreased clearance of both hyperphosphorylated tau and Aβsheets (Mawuenyega et al., 2010; Sulistio and Heese, 2016). Thus, the UPP may be disrupted by a number of mechanisms in AD leading to accumulation of both tau and Aβ.
2.4 UPP and other neurodegenerative diseases
Dysfunction of the UPP has also has been associated with other neurodegenerative diseases. In prion diseases, the constitutive form of the cellular prion protein (PrPc) is converted into a pathogenic scrapie prion protein (PrPsc), which is not a good substrate for the UPP (Ciechanover and Kwon, 2015). The PrPsc is enriched in β-sheets and tends to aggregate. In addition, PrPsc binds to and blocks the 20S proteasome, impairing proteasomal degradation (Deriziotis et al., 2011). Therefore, it has been hypothesized that UPP dysfunction may underlie prion diseases such as Creutzfeldt-Jacob Disease (Hooper, 2003; Ma et al., 2002).
Abnormal aggregates of ubiquitinated SOD1 are found in neurons bearing the mutation of SOD1 associated with familial amyotrophic lateral disease (ALS). Consequently, UPP dysfunction has been hypothesized to contribute to the pathogenesis of ALS (Cleveland and Liu, 2000). Recently, conditional knock-out mice of the proteasome subunit Rpt3 in a motor neuron-specific manner (Rpt3-CKO) exhibited locomotor dysfunction accompanied by progressive motor neuron loss and gliosis. Moreover, diverse ALS-linked proteins, including TAR DNA-binding protein 43 kDa (TDP-43), fused in sarcoma (FUS), ubiquilin 2, and optineurin were mislocalized or accumulated in motor neurons, together with other typical ALS hallmarks such as basophilic inclusion bodies(Tashiro et al., 2012). Therefore, impairment of proteasome activity may play an important role in the pathogenesis of ALS.
Huntington’s disease (HD) is caused by expanded polyglutamine (polyQ) repeats in the N-terminal region of mutant huntingtin gene (mHtt). The resultant abnormal protein mHtt is resistant to degradation by the UPP and autophagy (Chai et al., 1999). mHtt may also inhibit proteasome activity either directly by clogging the 20S proteasome core or indirectly through mitochondrial dysfunction and ATP depletion (Ortega and Lucas, 2014). mHtt forms aggregates in both intra-neuron and extracellular spaces (Landles and Bates, 2004). Mutant Htt has been found to be ubiquitinated, suggesting that its aggregates form as a result of UPP failure (Kalchman et al., 1996). Additionally, abnormal ubiquitin-reactive neurites have been observed in HD brains (Cammarata et al., 1993; Ciechanover and Kwon, 2015). Increasing UPP function by overexpressing PA28γ, a 26S proteasome activator, has been shown to improve cell survival of HD-derived neurons (Seo et al., 2007). Accordingly, UPP dysfunction may contribute to the pathogenesis of HD.
2.5 UPP dysfunction after cerebral ischemia
Dysfunction of the UPP is also exhibited after cerebral ischemia (Liu et al., 2010; Luo et al., 2013). Protein aggregates accumulate in neurons destined to die after transient cerebral ischemia (Ge et al., 2007; Hu et al., 2000; Luo et al., 2013). There is depletion of free Ub and an increase in ubiquitinated (Ub-) proteins after transient focal ischemia suggesting that there is impairment of the UPP after ischemia (Gubellini et al., 1997; Vannucci et al., 1998). The 26S proteasome undergoes selective dissociation and inhibition after ischemia. Furthermore, 26S proteasome ChTL activity is decreased after forebrain ischemia in the gerbil (Asai et al., 2002). Additionally, the proteasome, including the 19S subunit, are found in intracellular protein aggregates after cerebral ischemia (Ge et al., 2007). Proteasome activity is decreased after hypoxia in neurons of aged rats (Gozal et al., 2003). Thus, inhibition of UPP function after ischemia contributes to aggregation of Ub-proteins within vulnerable neurons.
3. Interrelation between UPP dysfunction in ischemia, aging and neurodegenerative diseases
UPP dysfunction may play an important role in the aging neuron and may underlie the accumulation of abnormal proteins in neurodegenerative diseases. Epidemiological studies indicate that vascular risk factors, white matter hyper-intensities and strokes increase the incidence of cognitive impairment and AD (Jefferson et al., 2015; Nelson et al., 2016; Zlokovic, 2011). Hypertension, diabetes and hyperlipidemia are associated with small vessel cerebrovascular disease associated with micro infarctions and small strokes, often referred to as “lacunes” (Pantoni, 2010). In addition to infarctions, small vessel disease may also result in oligemia: less severe hypoperfusion that results in mild ischemia but not cell death and infarction (Iadecola, 2003; Koike et al., 2010; Saito and Ihara, 2016; Schneider et al., 2007; Shang et al., 2016) . Obstructive sleep apnea and diabetes are common in the elderly and are associated with transient hypoxia and hypopglycemia that may also contribute to brain injury and insults to the UPP (Balfors and Franklin, 1994; Gozal, 2013; Gozal et al., 2003; Iadecola, 2003). Thus, the epidemiological association between vascular risk factors, stroke and AD may be due to the effects of chronic ischemia on the UPP resulting in impaired clearance of abnormal proteins (Gupta and Iadecola, 2015; Hohman et al., 2015; Jefferson et al., 2015; Kapasi and Schneider, 2016).
Cerebral ischemia may impair UPP function via several mechanisms (Ahmad et al., 2014). Cerebral ischemia results in production of reactive oxygen species (ROS) generated via induction of free radical generating enzymes and mitochondrial dysfunction (Chen et al., 2011a; Ruszkiewicz and Albrecht, 2015). ROS generated after cerebral ischemia include nitric oxide (NO) and superoxide, which have a large array of potential protein targets (Chung, 2010; Chung et al., 2005; Shang and Taylor, 2011). Proteins modified by ROS may gain toxic functions and contribute to protein aggregation (Uehara, 2007). The UPP is an important mechanism by which these abnormal proteins are removed from the neuron (Davies and Shringarpure, 2006; Grune et al., 2005). A fully functional UPP is required for cells to manage oxidative stress and the function of the UPP is also regulated by cellular redox status as sustained oxidative stress can interfere with ubiquitin enzymes and proteasome activity (Shang and Taylor, 2011).
Cerebral ischemia also results in the formation of a number of reactive lipid species, including isoprostanes, levuglandins, and cyclopentenone prostaglandins (CyPgs) (Figueiredo-Pereira et al., 2014; Liu et al., 2013a; Liu et al., 2013c). Many of these reactive lipids contain reactive carbonyl moieties which can readily adduct cysteine in a number of proteins (Figueiredo-Pereira et al., 2014; Ishii and Uchida, 2004; Kim et al., 2007; Koharudin et al., 2010; Kondo et al., 2002; Li et al., 2004; Liu et al., 2015a; Liu et al., 2011; Ogburn and Figueiredo-Pereira, 2006; Yamamoto et al., 2011). Prostaglandin (PG) J2 and 15-deoxy-Δ12, 14 -prostaglandin J2 are CyPgs formed via nonenzymatic degradation of PGD2, the most common prostaglandin in brain (Liu et al., 2013b). These carbonyl moieties containing CyPgs are produced in ischemic brain (Liu et al., 2013a; Liu et al., 2013c). CyPgs disrupt the function of the UPP by inhibiting the activity of the 26S proteasome and by other proteasome-independent mechanisms (Figueiredo-Pereira et al., 2014; Kondo et al., 2002; Wang et al., 2006). CyPgs induce accumulation of ubiquitinated proteins and induce cell death in neurons. In addition, CyPgs exacerbate neuronal cell death due to hypoxia in vitro (Liu et al., 2011). Infusion of PGJ2 into brain induces accumulation of protein aggregates immunoreactive for ubiquitin and α-synuclein in dopaminergic neurons that closely resembles some of the pathology found in the brains of patients with PD (Ogburn et al., 2006; Pierre et al., 2009). The generation of reactive lipids in elderly patients with microvascular disease, hypoxia due to sleep apnea and other comorbidities may contribute to UPP dysfunction and the pathogenesis of neurodegenerative diseases.
4. Ubiquitin C-terminal hydrolase L1 (UCHL1) and the UPP
4.1 Role in neuronal function
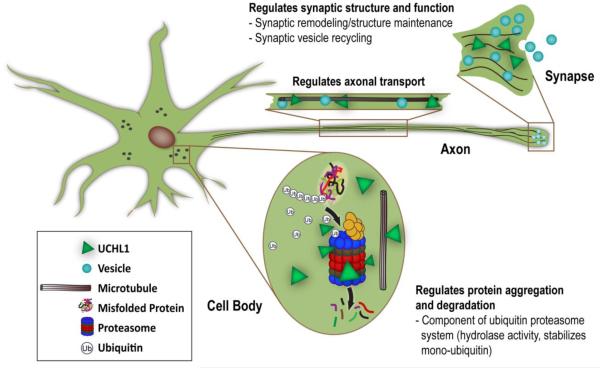
UCHL1 is highly expressed in brain, composing > 1% of brain protein and may play an important role in the neuronal UPP (Larsen et al., 1996). It is selectively expressed in neurons, suggesting that it may play a role in additional neuron-specific activities. UCHL1 has both ubiquitin E3 ligase and hydrolase activities (Liu et al., 2002). Besides hydrolyzing Ub from a poly-Ub chain for recycling, UCHL1 also ligates Ub onto specific proteins, tagging these proteins for transport for proteasomal degradation and other functions. Furthermore, UCHL1 binds to mono-Ub and stabilizes ubiquitin to maintain an available ubiquitin pool in the cell (Cartier et al., 2009). UCHL1 may also play an important role in regulating other neuronal processes in addition to the UPP (Fig. 2). Unfolded UCHL1 also may inhibit autophagy under pathological conditions (Hussain et al., 2013; Kabuta et al., 2008a) UCHL1 closely interacts with proteins of the neuronal cytoskeleton and may regulate axonal transport and maintain axonal integrity (Liu et al., 2002; Sakurai et al., 2008). Mutations or deletion of UCHL1 results in axonal and dendritic pathology, particularly effecting motor systems (Bilguvar et al., 2013; Chen et al., 2010; Kikuchi et al., 1990). UCHL1 also regulates synaptic function and long-term potentiation (LTP) under normal and pathological conditions and may play an important role in memory function (Gong et al., 2006).
Figure 2.
Physiologic functions of ubiquitin carboxy-terminal hydrolase 1 (UCHL1). Besides its role in the UPP, UCHL1also regulates synaptic structure and function, protein aggregation and degradation, and axonal transport in the neuron.
4.2 UCHL1 dysfunction in neurodegenerative diseases
UCHL1 dysfunction has been associated with a number of neurodegenerative diseases including PD, AD and ALS (Setsuie and Wada, 2007). UCHL1 is a major component in Lewy bodies associated with PD and neurofibrillary tangles observed in AD (Choi et al., 2004; Maraganore et al., 2004). UCHL1 levels are down-regulated in idiopathic PD as well as AD brains as UCHL1 is a major target of oxidative damage, which is extensively modified by carbonyl formation, methionine oxidation, and cysteine oxidation (Choi et al., 2004; Liu et al., 2011; Lombardino et al., 2005).
UCHL1 co-aggregates with α-synuclein in Lewy bodies, the intra-neural fibrillary aggregate that is the histological hallmark of PD. A familial mutation in UCHL1, namely UCHL1I93M (PARK 5), has been associated with PD (Maraganore et al., 2004; Wintermeyer et al., 2000). Compared to wild-type UCHL1, UCHL1I93M exhibits structural changes, increased insolubility and aberrant interactions with multiple proteins such as tubulin (Kabuta et al., 2008a; Kabuta et al., 2008b). Expression of UCHL1I93M in cells inhibits chaperone-mediated autophagy and increases α-synuclein, a protein whose accumulation is associated with neurotoxicity and the development of PD (Kabuta et al., 2008a). Besides its soluble form, UCHL1 also exists in a membrane-associated form (UCHL1(M)) which has been shown to correlate with intracellular levels of α-synuclein: decreased UCHL1(M) reduces α -synuclein levels and increases cell viability (Liu et al., 2009). Furthermore, Kim et al recently discovered that over-expression of an N-terminal 11 amino acid truncated UCHL1 (NT-UCHL1) decreases cellular ROS levels and protects cells from H2O2- and rotenone- induced cell death. NT UCHL1-expressing transgenic mice are less susceptible to degeneration of nigrostriatal dopaminergic neurons seen in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD (Kim et al., 2014).
Deposition of Aβ to form neuritic plaques in the brain is the pathological hallmark of AD. UCHL1 may play an important role in β-secretase degradation and Aβ deposition. UCHL1 also interacts with APP and regulates Aβ production (Gong et al., 2006). UCHL1 increases free ubiquitin levels and accelerates the lysosomal degradation of APP by promoting its ubiquitination. UCHL1 null mice have higher levels of brain Aβ , and overexpression of UCHL1 slows the deposition of Aβ, inhibits neuritic plaque formation and cognitive decline in a mouse model of AD (Zhang et al., 2014). Hyperphosphorylated microtubule-associated protein tau is the major component of neurofibrillary tangles, which is another pathological feature of AD. Choi et al reported that soluble UCHL1 protein levels are inversely proportional to the number of tangles in AD brains (Choi et al., 2004). Recently, it has been shown that the expression of UCHL1 can affect the levels of phosphorylated tau: phosphorylated tau increased after knockdown of UCHL1, and phosphorylated tau decreased when UCHL1 was overexpressed (Zhao et al., 2014).
UCHL1 null mice have extensive abnormalities in motor pathways including loss of dendrites of motor neurons and motor abnormalities (Kikuchi et al., 1990; Saigoh et al., 1999). Recessive loss of UCHL1 function caused by a homozygous missense mutation (UCHL1GLU7ALA), leads to early-onset progressive neurodegeneration in humans including childhood onset blindness, cerebellar ataxia, nystagmus, dorsal column dysfunction, and spasticity with upper motor neuron dysfunction (Bilguvar et al., 2013). These and other observations have led to the hypothesis that UCHL1 may be important in the pathogenesis of ALS. In addition, UCHL1 expression was significantly decreased in motor neurons derived from patients of spinal muscular atrophy (SMA), an inherited neuromuscular disease primarily characterized by degeneration of spinal motor neurons (Fuller et al., 2015). Corticospinal motor neurons are susceptible to ER stress and display early, selective, progressive, and profound degeneration in the absence of normally functioning UCHL1 (Genc et al., 2016; Jara et al., 2015).
4.3 UCHL1 and cerebral ischemia
Cerebral ischemia generates the formation of a number of reactive lipids, including carbonyl-containing species such as CyPgs (Ahmad et al., 2014; Figueiredo-Pereira et al., 2014). CyPgs can covalently modify the cysteine amino acid at 152 (C152) of UCHL1, reducing its activity and unfolding the enzyme resulting in protein aggregation (Kondo et al., 2002; Liu et al., 2015b). In addition, UCHL1 may also be covalently modified at C220 resulting in an insoluble membrane-bound form of the protein (Liu et al., 2009). Down-regulation of endogenous UCHL1 in mouse N2a neuroblastoma cells increases cell death induced by oxygen-glucose deprivation (Shen et al., 2006). UCHL1 activity protects primary neuronal cultures from hypoxia in vitro and transduction of neurons with a TAT-UCHL1 fusion protein protects neurons from hypoxic injury (Liu et al., 2011). Mutation of the C152 site prevents reactive lipids from binding to UCHL1 and protects neurons from neurite injury and cell death induced by CyPgs (Liu et al., 2015b). Thus, reactive lipids produced as a result of cerebral ischemia may disrupt UCHL1 function and exacerbate ischemic injury.
These findings suggest that the normal function of UCHL1 may be inhibited by cerebral ischemia, including chronic causes of cerebral ischemia associated with human aging such as cerebral small vessel disease and chronic hypoxia due to obstructive sleep apnea (Shang et al., 2016). UCHL1 undergoes extensive carbonyl/oxidative modification in sporadic PD thought to be the consequence of oxidative stress (Choi et al., 2004). These data suggest that modification by reactive lipid species does occur in vivo and may be associated with Parkinson’s and other neurodegenerative diseases.
4.4 Therapeutic Approaches
The observation that reduced UCHL1 activity due to modification of UCHL1 by reactive lipids provides a new potential therapeutic target in diseases associated with decreased UCHL1 function. One approach is to replace UCHL1 activity in brain. The prothrombin domain of the HIV capsid protein trans-activator of transcription (TAT) confers the ability of HIV to readily enter neurons (Gong et al., 2006; Hill et al., 2012; Kim et al., 2014; Liu et al., 2011). By fusing the TAT with UCHL1, the resultant protein (TATUCHL1) readily enters neurons even when administered systemically. TAT-UCHL1 reverses deficits in long term potentiation in hippocampal slices from a mouse model of AD, and systemic administration of TAT-UCHL1 improves memory function (Gong et al., 2006). Thus, TAT-UCHL1 treatment has the potential to be applied to patients with neurodegenerative diseases including AD and PD. Because UCHL1 may play an important role in axonal integrity and axonal transport, administration of TAT-UCHL1 could also be useful in other diseases where white matter and axonal function is important in recovery such as TBI and stroke (Gong et al., 2016; Kim et al., 2014). The identification of the C152 site as a critical binding site for reactive lipids provides a strategy for rational design of agents that would compete for binding of reactive lipids at this site. The feasibility of this approach is demonstrated by the development of agents that compete with reactive lipid ligands of PPAR γ (Seargent et al., 2004).
5. Conclusions
UPP function is impaired in the aging brain. Neurodegenerative diseases are associated with the accumulation of toxic proteins, which may be in part due to dysfunction of the UPP. Cerebral ischemia results in UPP dysfunction and may contribute to UPP dysfunction found in the aging brain and in neurodegenerative diseases. Modification of UCHL1 by reactive lipids produced after cerebral ischemia may be an important mechanism by which the UPP is disrupted in the aging brain. Preventing modification of UCHL1 by reactive lipids or restoring UCHL1 activity are novel therapeutic strategies for age-related, stroke and neurodegenerative diseases.
Highlights.
The UPP is essential for removing abnormal proteins from the neuron
UPP dysfunction occurs in normal aging and neurodegenerative diseases
UPP disruption by ischemia may contribute to the risk of neurodegenerative diseases
UCHL1 is inhibited after ischemia resulting in UPP dysfunction
Restoring UCHL1 activity is a novel therapeutic approach for these disorders
Acknowledgements
This work was supported by the National Institutes of Health NINDS R01NS37549, 2015 (SHG). The authors thank Marie Rose for figure preparation and editorial assistance.
Footnotes
6. Disclosure/Conflict of Interest
The authors declare no conflict of interest. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah A, Liu H, Graham SH. Inflammation in ischemic stroke: mechanisms, consequences and possible drug targets. CNS & neurological disorders drug targets. 2014;13:1378–1396. doi: 10.2174/1871527313666141023094720. [DOI] [PubMed] [Google Scholar]
- Asai A, Tanahashi N, Qiu JH, Saito N, Chi S, Kawahara N, Tanaka K, Kirino T. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:705–710. doi: 10.1097/00004647-200206000-00009. [DOI] [PubMed] [Google Scholar]
- Avila J, de Barreda EG, Pallas-Bazarra N, Hernandez F. Tau and neuron aging. Aging and disease. 2013;4:23–28. [PMC free article] [PubMed] [Google Scholar]
- Balfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. American journal of respiratory and critical care medicine. 1994;150:1587–1591. doi: 10.1164/ajrccm.150.6.7952619. [DOI] [PubMed] [Google Scholar]
- Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- Bilguvar K, Tyagi NK, Ozkara C, Tuysuz B, Bakircioglu M, Choi M, Delil S, Caglayan AO, Baranoski JF, Erturk O, Yalcinkaya C, Karacorlu M, Dincer A, Johnson MH, Mane S, Chandra SS, Louvi A, Boggon TJ, Lifton RP, Horwich AL, Gunel M. Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3489–3494. doi: 10.1073/pnas.1222732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochimica et biophysica acta. 2005;1739:331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Braun BC, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, Finley D, Schmidt M. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nature cell biology. 1999;1:221–226. doi: 10.1038/12043. [DOI] [PubMed] [Google Scholar]
- Cammarata S, Caponnetto C, Tabaton M. Ubiquitin-reactive neurites in cerebral cortex of subjects with Huntington's chorea: a pathological correlate of dementia? Neuroscience letters. 1993;156:96–98. doi: 10.1016/0304-3940(93)90448-t. [DOI] [PubMed] [Google Scholar]
- Cartier AE, Djakovic SN, Salehi A, Wilson SM, Masliah E, Patrick GN. Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7857–7868. doi: 10.1523/JNEUROSCI.1817-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Koppenhafer SL, Shoesmith SJ, Perez MK, Paulson HL. Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Human molecular genetics. 1999;8:673–682. doi: 10.1093/hmg/8.4.673. [DOI] [PubMed] [Google Scholar]
- Chen F, Sugiura Y, Myers KG, Liu Y, Lin W. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1636–1641. doi: 10.1073/pnas.0911516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants & redox signaling. 2011a;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Bhattacharyya BJ, Hanna J, Minkel H, Wilson JA, Finley D, Miller RJ, Wilson SM. Ubiquitin homeostasis is critical for synaptic development and function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011b;31:17505–17513. doi: 10.1523/JNEUROSCI.2922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. The Journal of biological chemistry. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- Chung KK. Modulation of pro-survival proteins by S-nitrosylation: implications for neurodegeneration. Apoptosis : an international journal on programmed cell death. 2010;15:1364–1370. doi: 10.1007/s10495-010-0464-1. [DOI] [PubMed] [Google Scholar]
- Chung KK, Dawson TM, Dawson VL. Nitric oxide, S-nitrosylation and neurodegeneration. Cell Mol Biol (Noisy-le-grand) 2005;51:247–254. [PubMed] [Google Scholar]
- Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Experimental & molecular medicine. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Liu J. Oxidation versus aggregation - how do SOD1 mutants cause ALS? Nature medicine. 2000;6:1320–1321. doi: 10.1038/82122. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Shringarpure R. Preferential degradation of oxidized proteins by the 20S proteasome may be inhibited in aging and in inflammatory neuromuscular diseases. Neurology. 2006;66:S93–96. doi: 10.1212/01.wnl.0000192308.43151.63. [DOI] [PubMed] [Google Scholar]
- Deger JM, Gerson JE, Kayed R. The interrelationship of proteasome impairment and oligomeric intermediates in neurodegeneration. Aging cell. 2015;14:715–724. doi: 10.1111/acel.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino GN, Slaughter CA. The proteasome, a novel protease regulated by multiple mechanisms. The Journal of biological chemistry. 1999;274:22123–22126. doi: 10.1074/jbc.274.32.22123. [DOI] [PubMed] [Google Scholar]
- Deriziotis P, Andre R, Smith DM, Goold R, Kinghorn KJ, Kristiansen M, Nathan JA, Rosenzweig R, Krutauz D, Glickman MH, Collinge J, Goldberg AL, Tabrizi SJ. Misfolded PrP impairs the UPS by interaction with the 20S proteasome and inhibition of substrate entry. The EMBO journal. 2011;30:3065–3077. doi: 10.1038/emboj.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S. Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease. Autophagy. 2008;4:372–374. doi: 10.4161/auto.5604. [DOI] [PubMed] [Google Scholar]
- Erpapazoglou Z, Walker O, Haguenauer-Tsapis R. Versatile roles of k63-linked ubiquitin chains in trafficking. Cells. 2014;3:1027–1088. doi: 10.3390/cells3041027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Rockwell P, Schmidt-Glenewinkel T, Serrano P. Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Frontiers in molecular neuroscience. 2014;7:104. doi: 10.3389/fnmol.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annual review of biochemistry. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller HR, Mandefro B, Shirran SL, Gross AR, Kaus AS, Botting CH, Morris GE, Sareen D. Spinal Muscular Atrophy Patient iPSC-Derived Motor Neurons Have Reduced Expression of Proteins Important in Neuronal Development. Frontiers in cellular neuroscience. 2015;9:506. doi: 10.3389/fncel.2015.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadhave K, Bolshette N, Ahire A, Pardeshi R, Thakur K, Trandafir C, Istrate A, Ahmed S, Lahkar M, Muresanu DF, Balea M. The ubiquitin proteasomal system: a potential target for the management of Alzheimer's disease. Journal of cellular and molecular medicine. 2016;20:1392–1407. doi: 10.1111/jcmm.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Luo Y, Liu CL, Hu B. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke; a journal of cerebral circulation. 2007;38:3230–3236. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc B, Jara JH, Schultz MC, Manuel M, Stanford MJ, Gautam M, Klessner JL, Sekerkova G, Heller DB, Cox GA, Heckman CJ, DiDonato CJ, Ozdinler PH. Absence of UCHL 1 function leads to selective motor neuropathy. Annals of clinical and translational neurology. 2016;3:331–345. doi: 10.1002/acn3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghee M, Fournier A, Mallet J. Rat alpha-synuclein interacts with Tat binding protein 1, a component of the 26S proteasomal complex. Journal of neurochemistry. 2000;75:2221–2224. doi: 10.1046/j.1471-4159.2000.0752221.x. [DOI] [PubMed] [Google Scholar]
- Giannini C, Kloss A, Gohlke S, Mishto M, Nicholson TP, Sheppard PW, Kloetzel PM, Dahlmann B. Poly-Ub-substrate-degradative activity of 26S proteasome is not impaired in the aging rat brain. PloS one. 2013;8:e64042. doi: 10.1371/journal.pone.0064042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman MH, Rubin DM, Coux O, Wefes I, Pfeifer G, Cjeka Z, Baumeister W, Fried VA, Finley D. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell. 1998;94:615–623. doi: 10.1016/s0092-8674(00)81603-7. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Hasegawa M, Smith MJ, Spillantini MG. Intraneuronal filamentous tau protein and alpha-synuclein deposits in neurodegenerative diseases. Biochemical Society transactions. 1998;26:463–471. doi: 10.1042/bst0260463. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. Lewy body diseases and multiple system atrophy as alpha-synucleinopathies. Molecular psychiatry. 1998;3:462–465. doi: 10.1038/sj.mp.4000458. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nature reviews. Neurology. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Gong B, Radulovic M, Figueiredo-Pereira ME, Cardozo C. The Ubiquitin-Proteasome System: Potential Therapeutic Targets for Alzheimer's Disease and Spinal Cord Injury. Frontiers in molecular neuroscience. 2016;9:4. doi: 10.3389/fnmol.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D. CrossTalk proposal: the intermittent hypoxia attending severe obstructive sleep apnoea does lead to alterations in brain structure and function. The Journal of physiology. 2013;591:379–381. doi: 10.1113/jphysiol.2012.241216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, Qiang F, Brittian KR. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. Journal of neurochemistry. 2003;86:1545–1552. doi: 10.1046/j.1471-4159.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- Gregori L, Fuchs C, Figueiredo-Pereira ME, Van Nostrand WE, Goldgaber D. Amyloid beta-protein inhibits ubiquitin-dependent protein degradation in vitro. The Journal of biological chemistry. 1995;270:19702–19708. doi: 10.1074/jbc.270.34.19702. [DOI] [PubMed] [Google Scholar]
- Gregori L, Hainfeld JF, Simon MN, Goldgaber D. Binding of amyloid beta protein to the 20 S proteasome. The Journal of biological chemistry. 1997;272:58–62. doi: 10.1074/jbc.272.1.58. [DOI] [PubMed] [Google Scholar]
- Grune T, Merker K, Jung T, Sitte N, Davies KJ. Protein oxidation and degradation during postmitotic senescence. Free radical biology & medicine. 2005;39:1208–1215. doi: 10.1016/j.freeradbiomed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Bisso GM, Ciofi-Luzzatto A, Fortuna S, Lorenzini P, Michalek H, Scarsella G. Ubiquitin-mediated stress response in a rat model of brain transient ischemia/hypoxia. Neurochemical research. 1997;22:93–100. doi: 10.1023/a:1027389623767. [DOI] [PubMed] [Google Scholar]
- Gupta A, Iadecola C. Impaired Abeta clearance: a potential link between atherosclerosis and Alzheimer's disease. Frontiers in aging neuroscience. 2015;7:115. doi: 10.3389/fnagi.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nature reviews. Molecular cell biology. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM, Trojanowski JQ, Mann D, Iwatsubo T. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. The Journal of biological chemistry. 2002;277:49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- Hill MD, Martin RH, Mikulis D, Wong JH, Silver FL, Terbrugge KG, Milot G, Clark WM, Macdonald RL, Kelly ME, Boulton M, Fleetwood I, McDougall C, Gunnarsson T, Chow M, Lum C, Dodd R, Poublanc J, Krings T, Demchuk AM, Goyal M, Anderson R, Bishop J, Garman D, Tymianski M. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. The Lancet. Neurology. 2012;11:942–950. doi: 10.1016/S1474-4422(12)70225-9. [DOI] [PubMed] [Google Scholar]
- Hohman TJ, Samuels LR, Liu D, Gifford KA, Mukherjee S, Benson EM, Abel T, Ruberg FL, Jefferson AL. Stroke risk interacts with Alzheimer's disease biomarkers on brain aging outcomes. Neurobiology of aging. 2015;36:2501–2508. doi: 10.1016/j.neurobiolaging.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper NM. Could inhibition of the proteasome cause mad cow disease? Trends in biotechnology. 2003;21:144–145. doi: 10.1016/S0167-7799(03)00026-X. [DOI] [PubMed] [Google Scholar]
- Hu BR, Martone ME, Jones YZ, Liu CL. Protein aggregation after transient cerebral ischemia. J Neurosci. 2000;20:3191–3199. doi: 10.1523/JNEUROSCI.20-09-03191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Figueiredo-Pereira ME. Ubiquitin/proteasome pathway impairment in neurodegeneration: therapeutic implications. Apoptosis : an international journal on programmed cell death. 2010;15:1292–1311. doi: 10.1007/s10495-010-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Feldman AL, Das C, Ziesmer SC, Ansell SM, Galardy PJ. Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Mol Cell Biol. 2013;33:1188–1197. doi: 10.1128/MCB.01389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Cerebrovascular effects of amyloid-beta peptides: mechanisms and implications for Alzheimer's dementia. Cellular and molecular neurobiology. 2003;23:681–689. doi: 10.1023/A:1025092617651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Ishii T, Uchida K. Induction of reversible cysteine-targeted protein oxidation by an endogenous electrophile 15-deoxy-delta12,14-prostaglandin J2. Chemical research in toxicology. 2004;17:1313–1322. doi: 10.1021/tx049860+. [DOI] [PubMed] [Google Scholar]
- Jara JH, Frank DD, Ozdinler PH. Could dysregulation of UPS be a common underlying mechanism for cancer and neurodegeneration? Lessons from UCHL1. Cell biochemistry and biophysics. 2013;67:45–53. doi: 10.1007/s12013-013-9631-7. [DOI] [PubMed] [Google Scholar]
- Jara JH, Genc B, Cox GA, Bohn MC, Roos RP, Macklis JD, Ulupinar E, Ozdinler PH. Corticospinal Motor Neurons Are Susceptible to Increased ER Stress and Display Profound Degeneration in the Absence of UCHL1 Function. Cereb Cortex. 2015;25:4259–4272. doi: 10.1093/cercor/bhu318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Hohman TJ, Liu D, Haj-Hassan S, Gifford KA, Benson EM, Skinner JS, Lu Z, Sparling J, Sumner EC, Bell S, Ruberg FL. Adverse vascular risk is related to cognitive decline in older adults. Journal of Alzheimer's disease : JAD. 2015;44:1361–1373. doi: 10.3233/JAD-141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuta T, Furuta A, Aoki S, Furuta K, Wada K. Aberrant interaction between Parkinson disease-associated mutant UCH-L1 and the lysosomal receptor for chaperone-mediated autophagy. The Journal of biological chemistry. 2008a;283:23731–23738. doi: 10.1074/jbc.M801918200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuta T, Setsuie R, Mitsui T, Kinugawa A, Sakurai M, Aoki S, Uchida K, Wada K. Aberrant molecular properties shared by familial Parkinson's disease-associated mutant UCH-L1 and carbonyl-modified UCH-L1. Human molecular genetics. 2008b;17:1482–1496. doi: 10.1093/hmg/ddn037. [DOI] [PubMed] [Google Scholar]
- Kalchman MA, Graham RK, Xia G, Koide HB, Hodgson JG, Graham KC, Goldberg YP, Gietz RD, Pickart CM, Hayden MR. Huntingtin is ubiquitinated and interacts with a specific ubiquitin-conjugating enzyme. The Journal of biological chemistry. 1996;271:19385–19394. doi: 10.1074/jbc.271.32.19385. [DOI] [PubMed] [Google Scholar]
- Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer's disease, and dementia in older persons. Biochimica et biophysica acta. 2016;1862:878–886. doi: 10.1016/j.bbadis.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck S, Nitsch R, Grune T, Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. Journal of neurochemistry. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98:149–156. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Mukoyama M, Yamazaki K, Moriya H. Axonal degeneration of ascending sensory neurons in gracile axonal dystrophy mutant mouse. Acta neuropathologica. 1990;80:145–151. doi: 10.1007/BF00308917. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jeong JE, Baek JY, Jeong J, Kim S, Kim YM, Kim Y, Nam JH, Huh SH, Seo J, Jin BK, Lee KJ. N-terminal truncated UCH-L1 prevents Parkinson's disease associated damage. PloS one. 2014;9:e99654. doi: 10.1371/journal.pone.0099654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim JY, Meng Z, Wang LH, Liu F, Conrads TP, Burke TR, Veenstra TD, Farrar WL. 15-deoxy-Delta12,14-prostaglandin J2 inhibits transcriptional activity of estrogen receptor-alpha via covalent modification of DNA-binding domain. Cancer research. 2007;67:2595–2602. doi: 10.1158/0008-5472.CAN-06-3043. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park KC, Chung SS, Bang O, Chung CH. Deubiquitinating enzymes as cellular regulators. Journal of biochemistry. 2003;134:9–18. doi: 10.1093/jb/mvg107. [DOI] [PubMed] [Google Scholar]
- Koharudin LM, Liu H, Di Maio R, Kodali RB, Graham SH, Gronenborn AM. Cyclopentenone prostaglandin-induced unfolding and aggregation of the Parkinson disease-associated UCH-L1. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6835–6840. doi: 10.1073/pnas.1002295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-{beta} The American journal of pathology. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D. The emerging complexity of protein ubiquitination. Biochemical Society transactions. 2009;37:937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nature reviews. Molecular cell biology. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, Sasaki S, Iwata M, Noguchi N, Uchida K. 15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7367–7372. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen L, Lindholm D. The ubiquitin proteasome system in synaptic and axonal degeneration: a new twist to an old cycle. The Journal of cell biology. 2004;165:27–30. doi: 10.1083/jcb.200311091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulathu Y, Komander D. Atypical ubiquitylation - the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nature reviews. Molecular cell biology. 2012;13:508–523. doi: 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- Lam YA, Pickart CM, Alban A, Landon M, Jamieson C, Ramage R, Mayer RJ, Layfield R. Inhibition of the ubiquitin-proteasome system in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9902–9906. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landles C, Bates GP. Huntingtin and the molecular pathogenesis of Huntington's disease. Fourth in molecular medicine review series. EMBO reports. 2004;5:958–963. doi: 10.1038/sj.embor.7400250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CN, Price JS, Wilkinson KD. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: identification of two active site residues. Biochemistry. 1996;35:6735–6744. doi: 10.1021/bi960099f. [DOI] [PubMed] [Google Scholar]
- Li Z, Melandri F, Berdo I, Jansen M, Hunter L, Wright S, Valbrun D, Figueiredo-Pereira ME. Delta12-Prostaglandin J2 inhibits the ubiquitin hydrolase UCH-L1 and elicits ubiquitin-protein aggregation without proteasome inhibition. Biochemical and biophysical research communications. 2004;319:1171–1180. doi: 10.1016/j.bbrc.2004.05.098. [DOI] [PubMed] [Google Scholar]
- Liu C, Gao Y, Barrett J, Hu B. Autophagy and protein aggregation after brain ischemia. Journal of neurochemistry. 2010;115:68–78. doi: 10.1111/j.1471-4159.2010.06905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Chen J, Li W, Rose ME, Shinde SN, Balasubramani M, Uechi GT, Mutus B, Graham SH, Hickey RW. Protein disulfide isomerase as a novel target for cyclopentenone prostaglandins: implications for hypoxic ischemic injury. The FEBS journal. 2015a;282:2045–2059. doi: 10.1111/febs.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li W, Ahmad M, Miller TM, Rose ME, Poloyac SM, Uechi G, Balasubramani M, Hickey RW, Graham SH. Modification of ubiquitin-C-terminal hydrolase-L1 by cyclopentenone prostaglandins exacerbates hypoxic injury. Neurobiology of disease. 2011;41:318–328. doi: 10.1016/j.nbd.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li W, Ahmad M, Rose ME, Miller TM, Yu M, Chen J, Pascoe JL, Poloyac SM, Hickey RW, Graham SH. Increased generation of cyclopentenone prostaglandins after brain ischemia and their role in aggregation of ubiquitinated proteins in neurons. Neurotoxicity research. 2013a;24:191–204. doi: 10.1007/s12640-013-9377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li W, Rose ME, Hickey RW, Chen J, Uechi GT, Balasubramani M, Day BW, Patel KV, Graham SH. The point mutation UCH-L1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity: implications for post-ischemic neuronal injury. Cell death & disease. 2015b;6:e1966. doi: 10.1038/cddis.2015.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li W, Rose ME, Pascoe JL, Miller TM, Ahmad M, Poloyac SM, Hickey RW, Graham SH. Prostaglandin D2 toxicity in primary neurons is mediated through its bioactive cyclopentenone metabolites. Neurotoxicology. 2013b;39:35–44. doi: 10.1016/j.neuro.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Rose ME, Miller TM, Li W, Shinde SN, Pickrell AM, Poloyac SM, Graham SH, Hickey RW. COX2-derived primary and cyclopentenone prostaglandins are increased after asphyxial cardiac arrest. Brain research. 2013c;1519:71–77. doi: 10.1016/j.brainres.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, Logan T, Lansbury PT., Jr. Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4635–4640. doi: 10.1073/pnas.0806474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino AJ, Li XC, Hertel M, Nottebohm F. Replaceable neurons and neurodegenerative disease share depressed UCHL1 levels. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8036–8041. doi: 10.1073/pnas.0503239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Salon M, Morelli L, Castano EM, Soto EF, Pasquini JM. Defective ubiquitination of cerebral proteins in Alzheimer's disease. Journal of neuroscience research. 2000;62:302–310. doi: 10.1002/1097-4547(20001015)62:2<302::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Luo T, Park Y, Sun X, Liu C, Hu B. Protein misfolding, aggregation, and autophagy after brain ischemia. Translational stroke research. 2013;4:581–588. doi: 10.1007/s12975-013-0299-5. [DOI] [PubMed] [Google Scholar]
- Ma J, Wollmann R, Lindquist S. Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science. 2002;298:1781–1785. doi: 10.1126/science.1073725. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, Lesnick TG, Elbaz A, Chartier-Harlin MC, Gasser T, Kruger R, Hattori N, Mellick GD, Quattrone A, Satoh J, Toda T, Wang J, Ioannidis JP, de Andrade M, Rocca WA. UCHL1 is a Parkinson's disease susceptibility gene. Annals of neurology. 2004;55:512–521. doi: 10.1002/ana.20017. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. Failure of the ubiquitinproteasome system in Parkinson's disease. Nature reviews. Neuroscience. 2001;2:589–594. doi: 10.1038/35086067. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Dawson VL, Dawson TM. Role for the ubiquitin-proteasome system in Parkinson's disease and other neurodegenerative brain amyloidoses. Neuromolecular medicine. 2003;4:95–108. doi: 10.1385/NMM:4:1-2:95. [DOI] [PubMed] [Google Scholar]
- Mukoyama M, Yamazaki K, Kikuchi T, Tomita T. Neuropathology of gracile axonal dystrophy (GAD) mouse. An animal model of central distal axonopathy in primary sensory neurons. Acta neuropathologica. 1989;79:294–299. doi: 10.1007/BF00294664. [DOI] [PubMed] [Google Scholar]
- Nathan JA, Kim HT, Ting L, Gygi SP, Goldberg AL. Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? The EMBO journal. 2013;32:552–565. doi: 10.1038/emboj.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer's disease. Biochimica et biophysica acta. 2016;1862:887–900. doi: 10.1016/j.bbadis.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Li H, Kawamura R, Osaka H, Wang YL, Hara Y, Hirokawa T, Manago Y, Amano T, Noda M, Aoki S, Wada K. Alterations of structure and hydrolase activity of parkinsonism-associated human ubiquitin carboxyl-terminal hydrolase L1 variants. Biochemical and biophysical research communications. 2003;304:176–183. doi: 10.1016/s0006-291x(03)00555-2. [DOI] [PubMed] [Google Scholar]
- Ogburn KD, Bottiglieri T, Wang Z, Figueiredo-Pereira ME. Prostaglandin J2 reduces catechol-O-methyltransferase activity and enhances dopamine toxicity in neuronal cells. Neurobiology of disease. 2006;22:294–301. doi: 10.1016/j.nbd.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Ogburn KD, Figueiredo-Pereira ME. Cytoskeleton/endoplasmic reticulum collapse induced by prostaglandin J2 parallels centrosomal deposition of ubiquitinated protein aggregates. The Journal of biological chemistry. 2006;281:23274–23284. doi: 10.1074/jbc.M600635200. [DOI] [PubMed] [Google Scholar]
- Oh S, Hong HS, Hwang E, Sim HJ, Lee W, Shin SJ, Mook-Jung I. Amyloid peptide attenuates the proteasome activity in neuronal cells. Mechanisms of ageing and development. 2005;126:1292–1299. doi: 10.1016/j.mad.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Brown K, Wilkinson KD, Rees HD, Huai Q, Ke H, Levey AI, Li L, Chin LS. Familial Parkinson's disease-associated L166P mutation disrupts DJ-1 protein folding and function. The Journal of biological chemistry. 2004;279:8506–8515. doi: 10.1074/jbc.M311017200. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Chin LS. Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy. 2008;4:85–87. doi: 10.4161/auto.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann JA, Li L, Chin LS. Aggresome formation and neurodegenerative diseases: therapeutic implications. Curr Med Chem. 2008;15:47–60. doi: 10.2174/092986708783330692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann JA, Li L, Chudaev MV, Chen J, Perez FA, Palmiter RD, Chin LS. Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6. The Journal of cell biology. 2007;178:1025–1038. doi: 10.1083/jcb.200611128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega Z, Lucas JJ. Ubiquitin-proteasome system involvement in Huntington's disease. Frontiers in molecular neuroscience. 2014;7:77. doi: 10.3389/fnmol.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet. Neurology. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Pierre SR, Lemmens MA, Figueiredo-Pereira ME. Subchronic infusion of the product of inflammation prostaglandin J2 models sporadic Parkinson's disease in mice. Journal of neuroinflammation. 2009;6:18. doi: 10.1186/1742-2094-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszkiewicz J, Albrecht J. Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochemistry international. 2015;88:66–72. doi: 10.1016/j.neuint.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Saigoh K, Wang YL, Suh JG, Yamanishi T, Sakai Y, Kiyosawa H, Harada T, Ichihara N, Wakana S, Kikuchi T, Wada K. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nature genetics. 1999;23:47–51. doi: 10.1038/12647. [DOI] [PubMed] [Google Scholar]
- Saito S, Ihara M. Interaction between cerebrovascular disease and Alzheimer pathology. Current opinion in psychiatry. 2016;29:168–173. doi: 10.1097/YCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Sekiguchi M, Zushida K, Yamada K, Nagamine S, Kabuta T, Wada K. Reduction in memory in passive avoidance learning, exploratory behaviour and synaptic plasticity in mice with a spontaneous deletion in the ubiquitin C-terminal hydrolase L1 gene. The European journal of neuroscience. 2008;27:691–701. doi: 10.1111/j.1460-9568.2008.06047.x. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Nuber U, Huibregtse JM. Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature. 1995;373:81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annual review of pharmacology and toxicology. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- Seargent JM, Yates EA, Gill JH. GW9662, a potent antagonist of PPARgamma, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARgamma agonist rosiglitazone, independently of PPARgamma activation. British journal of pharmacology. 2004;143:933–937. doi: 10.1038/sj.bjp.0705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Sonntag KC, Kim W, Cattaneo E, Isacson O. Proteasome activator enhances survival of Huntington's disease neuronal model cells. PloS one. 2007;2:e238. doi: 10.1371/journal.pone.0000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsuie R, Wada K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochemistry international. 2007;51:105–111. doi: 10.1016/j.neuint.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free radical biology & medicine. 2011;51:5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Yamashita T, Zhai Y, Nakano Y, Morihara R, Fukui Y, Hishikawa N, Ohta Y, Abe K. Strong Impact of Chronic Cerebral Hypoperfusion on Neurovascular Unit, Cerebrovascular Remodeling, and Neurovascular Trophic Coupling in Alzheimer's Disease Model Mouse. Journal of Alzheimer's disease : JAD. 2016;52:113–126. doi: 10.3233/JAD-151126. [DOI] [PubMed] [Google Scholar]
- Shen H, Sikorska M, Leblanc J, Walker PR, Liu QY. Oxidative stress regulated expression of ubiquitin Carboxyl-terminal Hydrolase-L1: role in cell survival. Apoptosis : an international journal on programmed cell death. 2006;11:1049–1059. doi: 10.1007/s10495-006-6303-8. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nature genetics. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Silva GM, Finley D, Vogel C. K63 polyubiquitination is a new modulator of the oxidative stress response. Nature structural & molecular biology. 2015;22:116–123. doi: 10.1038/nsmb.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends in neurosciences. 1998;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- Sulistio YA, Heese K. The Ubiquitin-Proteasome System and Molecular Chaperone Deregulation in Alzheimer's Disease. Molecular neurobiology. 2016;53:905–931. doi: 10.1007/s12035-014-9063-4. [DOI] [PubMed] [Google Scholar]
- Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. The American journal of pathology. 2012;181:1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, V LD, Dawson TM, Ross CA. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Human molecular genetics. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- Tashiro Y, Urushitani M, Inoue H, Koike M, Uchiyama Y, Komatsu M, Tanaka K, Yamazaki M, Abe M, Misawa H, Sakimura K, Ito H, Takahashi R. Motor neuron-specific disruption of proteasomes, but not autophagy, replicates amyotrophic lateral sclerosis. The Journal of biological chemistry. 2012;287:42984–42994. doi: 10.1074/jbc.M112.417600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todi SV, Paulson HL. Balancing act: deubiquitinating enzymes in the nervous system. Trends in neurosciences. 2011;34:370–382. doi: 10.1016/j.tins.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM. Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiology of aging. 2008;29:1607–1618. doi: 10.1016/j.neurobiolaging.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tydlacka S, Wang CE, Wang X, Li S, Li XJ. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13285–13295. doi: 10.1523/JNEUROSCI.4393-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T. Accumulation of misfolded protein through nitrosative stress linked to neurodegenerative disorders. Antioxidants & redox signaling. 2007;9:597–601. doi: 10.1089/ars.2006.1517. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Mummery R, Hawkes RB, Rider CC, Beesley PW. Hypoxia-ischemia induces a rapid elevation of ubiquitin conjugate levels and ubiquitin immunoreactivity in the immature rat brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:376–385. doi: 10.1097/00004647-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Walz J, Erdmann A, Kania M, Typke D, Koster AJ, Baumeister W. 26S proteasome structure revealed by three-dimensional electron microscopy. Journal of structural biology. 1998;121:19–29. doi: 10.1006/jsbi.1998.3958. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aris VM, Ogburn KD, Soteropoulos P, Figueiredo-Pereira ME. Prostaglandin J2 alters pro-survival and pro-death gene expression patterns and 26 S proteasome assembly in human neuroblastoma cells. The Journal of biological chemistry. 2006;281:21377–21386. doi: 10.1074/jbc.M601201200. [DOI] [PubMed] [Google Scholar]
- Wintermeyer P, Kruger R, Kuhn W, Muller T, Woitalla D, Berg D, Becker G, Leroy E, Polymeropoulos M, Berger K, Przuntek H, Schols L, Epplen JT, Riess O. Mutation analysis and association studies of the UCHL1 gene in German Parkinson's disease patients. Neuroreport. 2000;11:2079–2082. doi: 10.1097/00001756-200007140-00004. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Takase K, Kishino J, Fujita M, Okamura N, Sakaeda T, Fujimoto M, Yagami T. Proteomic identification of protein targets for 15-deoxy-Delta(12,14)-prostaglandin J2 in neuronal plasma membrane. PloS one. 2011;6:e17552. doi: 10.1371/journal.pone.0017552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SS. Proteasome degradation of brain cytosolic tau in Alzheimer's disease. International journal of clinical and experimental pathology. 2011;4:385–402. [PMC free article] [PubMed] [Google Scholar]
- Yerbury JJ, Ooi L, Dillin A, Saunders DN, Hatters DM, Beart PM, Cashman NR, Wilson MR, Ecroyd H. Walking the tightrope: proteostasis and neurodegenerative disease. Journal of neurochemistry. 2016;137:489–505. doi: 10.1111/jnc.13575. [DOI] [PubMed] [Google Scholar]
- Yin P, Tu Z, Yin A, Zhao T, Yan S, Guo X, Chang R, Zhang L, Hong Y, Huang X, Zhou J, Wang Y, Li S, Li XJ. Aged monkey brains reveal the role of ubiquitin-conjugating enzyme UBE2N in the synaptosomal accumulation of mutant huntingtin. Human molecular genetics. 2015;24:1350–1362. doi: 10.1093/hmg/ddu544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Cai F, Zhang S, Song W. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) delays Alzheimer's progression in vivo. Scientific reports. 2014;4:7298. doi: 10.1038/srep07298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZB, Wu L, Xiong R, Wang LL, Zhang B, Wang C, Li H, Liang L, Chen SD. MicroRNA-922 promotes tau phosphorylation by downregulating ubiquitin carboxy-terminal hydrolase L1 (UCHL1) expression in the pathogenesis of Alzheimer's disease. Neuroscience. 2014;275:232–237. doi: 10.1016/j.neuroscience.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature reviews. Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]