Abstract
Background & Aims
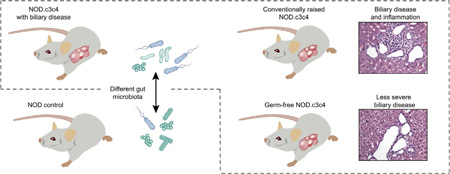
A strong association between human inflammatory biliary diseases and gut inflammation has led to the hypothesis that gut microbes and lymphocytes activated in the intestine play a role in biliary inflammation. The NOD.c3c4 mouse model develops spontaneous biliary inflammation in extra- and intra-hepatic bile ducts. We aimed to clarify the role of the gut microbiota in the biliary disease of NOD.c3c4 mice.
Methods
We sampled cecal content and mucosa from conventionally raised (CONV-R) NOD.c3c4 and NOD control mice, extracted DNA and performed 16S rRNA sequencing. NOD.c3c4 mice were rederived into a germ free (GF) facility and compared with CONV-R NOD.c3c4 mice. NOD.c3c4 mice were also co-housed with NOD mice and received antibiotics from weaning.
Results
The gut microbial profiles of mice with and without biliary disease were different both before and after rederivation (unweighted UniFrac-distance). GF NOD.c3c4 mice had less distended extra-hepatic bile ducts than CONV-R NOD.c3c4 mice, while antibiotic treated mice showed reduction of biliary infarcts. GF animals also showed a reduction in liver weight compared with CONV-R NOD.c3c4 mice, and this was also observed in antibiotic treated NOD.c3c4 mice. Co-housing of NOD and NOD.c3c4 mice indicated that the biliary phenotype was neither transmissible nor treatable by co-housing with healthy mice.
Conclusions
NOD.c3c4 and NOD control mice show marked differences in the gut microbiota. Germ free NOD.c3c4 mice develop a milder biliary affection compared with conventionally raised NOD.c3c4 mice. Our findings suggest that the intestinal microbiota contributes to disease in this murine model of biliary inflammation.
Keywords: germ free, microbiota, NOD.c3c4, PBC, PSC
LAY SUMMARY
Mice with liver disease have a gut microflora (microbiota) that differs substantially from normal mice. When these mice, that under normal circumstances spontaneously develops disease in their bile ducts, are raised in an environment devoid of bacteria, the disease in the bile ducts diminishes. Overall this clearly indicates that the bacteria in the gut (the gut microbiota) influences the liver disease in these mice.
Graphical Abstract
INTRODUCTION
NOD.c3c4 mice spontaneously develop biliary inflammation in intra-hepatic and extra-hepatic bile ducts [1]. The NOD.c3c4 model is developed on the NOD background [2], a genetic background with increased susceptibility to autoimmune phenotypes similar to what is seen in human biliary diseases [3], and the regular NOD mice develop diabetes [4]. In contrast, NOD.c3c4 mice do not develop diabetes [2]. The NOD.c3c4 mouse has been used as a model of the human biliary disease primary biliary cirrhosis (PBC) as it develops autoantibodies and lymphocytic infiltrates similar to PBC [2]. The pathogenesis in NOD.c3c4 mice is not completely clarified, but the biliary disease is considered to be immune mediated [5]. The NOD.c3c4 mouse is the only known mouse model that spontaneously develops dilatation and inflammation of the common bile duct [6]. These features are hallmarks of the human biliary disease primary sclerosing cholangitis (PSC) and as such this mouse model can also be used to model aspects of PSC.
Inflammatory biliary diseases are strongly associated with gut diseases [7,8]. This clinical association has led to the hypothesis that gut microbes and lymphocytes activated in the intestine play a role in biliary inflammation [7]. That gut microbiota could play an important role in bile duct disease is experimentally supported by studies showing that small bowel bacterial overgrowth in rats leads to bile duct inflammation that can be treated with antibiotics [9,10]. From human cholangiopathies it has recently been demonstrated in several independent studies that patients with PSC have a different gut microbiota compared to healthy individuals [11–14] and in line with this, manipulation of the gut microbiota using antibiotics in PSC patients has shown beneficial effects [15]. A recent study demonstrated that germ free (GF) multidrug resistance knock out (Mdr2−/−) mice developed more severe liver disease compared with conventionally raised (CONV-R) Mdr2−/− mice [16]. The Mdr2−/− mouse is a common mouse model of PSC [6], as it develops periductal fibrosis due to regurgitation of bile into portal tracts [17]. The NOD.c3c4 model contrasts the Mdr2−/− model as the biliary disease of the NOD.c3c4 model is largely immune driven, but with minimal fibrosis [1].
In the present study, we explored the role of the intestinal microbiota in the biliary inflammation observed in NOD.c3c4 mice. We found significant differences between the gut microbiota of NOD.c3c4 mice and NOD control mice. In experiments with rederivation into a GF environment we found that GF NOD.c3c4 mice were protected from biliary disease compared with CONV-R NOD.c3c4 mice. Also, when NOD.c3c4 mice were treated with antibiotics we saw a milder liver phenotype corroborating the GF results. Collectively, the present results suggest that intestinal bacteria contribute to disease in this murine model of biliary inflammation.
MATERIALS AND METHODS
Mice
NOD.c3c4 and NOD mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All CONV-R mice were housed in a Minimal Disease Unit (MDU) at the animal facility at Oslo University Hospital Rikshospitalet, Oslo, Norway.
NOD.c3c4 and NOD mice housed in the MDU facility, were harvested at 10 weeks of age and cecal content and mucosa was sampled. NOD.c3c4 and NOD strains were then rederived into a new MDU facility by caesarean sections, and after three generations sampling of cecal content and mucosa was repeated. These rederived NOD.c3c4 mice were then rederived as axenic mice at the Core Facility for Germfree Research at Karolinska Institutet, Stockholm, Sweden by caesarean sections, housed in a GF environment and regularly monitored to ensure their GF status. GF and CONV-R NOD.c3c4 mice were sampled at 9 weeks and 18 weeks of age. GF NOD mice were housed at a GF facility at the University of Gothenburg, Sweden. In co-housing experiments age- and gender-matched CONV-R NOD.c3c4 mice and NOD mice were co-housed in a MDU facility from the age of 4 weeks (after weaning) for 4 weeks. CONV-R NOD.c3c4 mice were treated with non-absorbable antibiotics; Ampicillin 1.0 g/l (Bristol-Myers Squibb, Solna, Sweden) and Neomycin 0,5 g/l (Fisher Scientific, Geel Belgium) in drinking water from weaning for 4 weeks. The number of animals in each experiment was determined by power calculations and experience from similar experiments.
All animal experiments were approved by the Norwegian National Animal Research Authority (project license no FOTS 6809/14) and/or the Ethics Committee on Animal Care and Use in Gothenburg and Stockholm, Sweden. The animal experiments were performed in accordance with the European Directive 2010/63/EU and The Guide for the Care and Use of Laboratory Animals, 8th edition (NRC 2011, National Academic Press). All mice had ad libitum access to water and standard rodent diet.
Tissue collection and extraction of primary lymphocytes from liver
Mice at the indicated age were sacrificed and weight of the mice and weight of the liver, spleen and cecum were registered. Dilatation of the common bile duct (CBDD) was measured. Collection of blood, serum, liver tissue, and extraction of primary lymphocytes from perfused livers were also performed as described in the Supplementary Material. Cecal content and mucosal samples were taken from the cecum with sterile equipment, and immediately snap-frozen in liquid nitrogen and later stored at −80°C until DNA extraction.
DNA extraction
DNA from cecal content or 15–20 mm of cecal tissue was extracted as previously described [18], and a more detailed description included in the Supplementary Material.
Library preparations, sequencing and bioinformatic processing
Library preparations and 16S rRNA sequencing of the V4 region were performed at BGI (Shenzhen, China), on the Illumina MiSeq platform (San Diego, CA, USA). The Quantitative Insights Into Microbial Ecology (QIIME) platform (version 1.8.0) [19], was used for further bioinformatic processing using closed-reference operational taxonomic unit (OTU) mapping to the Greengenes database [20]. Detailed methods are included in the Supplementary Material.
RNA isolation, reverse transcription and quantitative real-time PCR
Total RNA from snap-frozen liver tissue was isolated, and reverse transcription and quantitative real-time PCR was performed as described in the Supplementary Material. Detailed primer information is provided in Supplementary Table 1. The relative expression of each sample was first normalised to the expression of the reference gene (beta-actin (Actb)), and then normalised to the average expression in samples from CONV-R NOD.c3c4 mice, and the data were analysed according to the 2−ΔΔCT method.
Flow cytometry
Following preparation of single-cell suspensions, the cells were incubated with anti-mouse CD16/32 clone 93 (BioLegend, San Diego, CA, USA) for blocking of Fc-receptors to avoid non-specific binding. Lymphocytes were stained with FITC anti-mouse TCRβ, clone H57-597 (BD Biosciences, Franklin Lakes, NJ, USA) for an hour. Flow cytometric analysis was performed using a BD FACS Verse flow cytometer (BD Biosciences). The results were analysed in FlowJo version 9.5.3 (TreeStar, Ashland, OR, USA). Detailed anti-body information is stated in Supplementary Table 2.
Histology and scoring
Liver tissue and snap frozen cecal tissue were fixed in 4% formalin in room temperature, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E), or Sirius red staining, and scored in a blinded fashion (Supplementary Fig. 1). Detailed methods are included in the Supplementary Material.
Immunohistochemistry
Immunohistochemical staining of CD3 (T cell marker), Ly6G (neutrophil marker), α-smooth muscle actin (α-SMA, myofibroblast marker) and Mac-2 (macrophage marker) was performed as described in the Supplementary Material, and detailed anti-body information is stated in Supplementary Table 2.
Biochemistry
Alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (ALP) were measured in serum using a ADVIA 1800 (Siemens, Munich, Germany) at The Central Laboratory, Norwegian School of Veterinary Science (Oslo, Norway).
Statistical analysis
Unless stated otherwise all values are presented as means ± SEM. Statistical significance was calculated with unpaired Student's t-test for variables meeting requirements of normal distribution or Mann-Whitney U test for variable not meeting the requirements for normal distribution using GraphPad Prism version 5.0 b (GraphPad Software, La Jolla, CA). Statistical analyses on relative taxa abundances were done using the R statistical software environment (version 3.1.2, https://www.R-project.org/), using the Mann-Whitney U test, and calculations based on beta diversity (unweighted UniFrac) were done using the anosim function in QIIME (version 1.8.0). Relative abundance ratios were calculated by dividing the mean relative abundance of each bacterial taxon in each category.
RESULTS
Bacterial communities in NOD.c3c4 and NOD mice
We first explored differences in the gut microbiota of mice with and without biliary inflammation by comparing the microbial profiles in the cecal mucosa and cecal content of NOD and NOD.c3c4 mice at 10 weeks of age (n = 4–5 in each group). The experiments were performed before the onset of diabetes in the NOD mice (Supplementary Table 3). The gut microbiota in NOD.c3c4 and NOD control mice showed marked difference in their total bacterial community, both in the cecal content and mucosa (Fig. 1A), and the phenotype of the mice explained 41.2% of the variation of the bacterial community in the cecal content. To further explore whether these differences could be replicated in another environment and to rule out potential cage effects, NOD and NOD.c3c4 mice were rederived into a new MDU facility by caesarean section. The extent of the global differences in both mucosa and cecal content was similar in the new facility (Fig. 1B). Bacterial diversity and richness were not different in the two strains in any of the experiments (Fig. 1C). At the genus-level, the abundances of multiple bacterial taxa were significantly different between the NOD.c3c4 and NOD mice, both in cecal content (p <0.05, Table 1) and mucosa (p <0.05, Supplementary Table 4), in both experiments.
Fig. 1. NOD.c3c4 mice have a distinct global bacterial composition compared with NOD control mice.
Principal coordinate plot based on unweighted UniFrac distances illustrating separation of the NOD (n = 4–5) and NOD.c3c4 mice (n = 5) in cecal content and mucosa (A) before (p <0.01) and (B) after rederivation into another conventional animal facility (p <0.02). (C) Intra-individual diversity measured by Shannon Diversity Index in cecal content of NOD (n = 4–5) and NOD.c3c4 mice (n = 5) was similar. Data in (A) and (B) compared using the anosim function in QIIME. Data in (C) are presented as mean ± SEM, unpaired Student's t-test was used for comparison.
Table 1. Bacterial taxa that differ between NOD.c3c4 and control mice in cecal content.
In the first experiment (“Before rederivation”), several taxa on the genus level were different between NOD.c3c4 and control mice in cecal content. To examine these results in a new environment and rule out potential cage effects, we rederived the mice into another conventional animal facility, and again identified differentiating genera between the phenotypes. Data analysed using Mann-Whitney U test. Relative abundance ratios were calculated by dividing the mean relative abundance of each bacterial taxon in each category.
| Taxa enriched in NOD.c3c4 mice | Before rederivation | After rederivation into another conventional animal facility |
||||
|---|---|---|---|---|---|---|
| Relative abundance ratio |
Relative abundance ratio |
|||||
| Order | Family | Genus | NOD.c3c4/NOD | p-value | NOD.c3c4/NOD | p-value |
| Bacteroidales | S24.7 | [unknown] | 0.57 | 0.032 | 0.64 | 0.41 |
| Clostridiales | Ruminococcaceae | Oscillospira | 1.92 | 0.032 | 1.04 | 0.90 |
| Clostridiales | Christensenellaceae | [unknown] | 3.39 | 0.016 | 1.13 | 0.56 |
| Erysipelotrichales | Erysipelotrichaceae | Allobaculum | 0.01 | 0.016 | 1.77 | 0.81 |
| Anaeroplasmatales | Anaeroplasmataceae | Anaeroplasma | 6.57 | 0.016 | 3.08 | 0.73 |
| YS2 | [unknown] | [unknown] | 2212.32 | 0.011 | 5.93 | 0.23 |
| Deferribacterales | Deferribacteraceae | Mucispirillum | 0.04 | 0.011 | 1.23 | 0.73 |
| Gemellales | Gemellaceae | [unknown] | 84.21 | 0.011 | 3.00 | 1.00 |
| Clostridiales | Lachnospiraceae | Anaerostipes | 0.00 | 0.0097 | 4.77 | 0.35 |
| Clostridiales | Lachnospiraceae | Coprococcus | 0.22 | 0.0079 | 0.66 | 0.90 |
| Clostridiales | Ruminococcaceae | [unknown] | 2.11 | 0.0079 | 1.21 | 0.29 |
| Clostridiales | Ruminococcaceae | Ruminococcus | 5.95 | 0.0079 | 1.05 | 1.00 |
| Clostridiales | Mogibacteriaceae | [unknown] | 0.47 | 0.0079 | 0.97 | 1.00 |
| Verrucomicrobiales | Verrucomicrobiaceae | Akkermansia | 1281.86 | 0.0079 | 1.79 | 0.37 |
| Clostridiales | Clostridiaceae | Clostridium | 2.07 | 0.46 | 33.93 | 0.020 |
| Bacteroidales | Porphyromonadaceae | Parabacteroides | 0.42 | 0.66 | 0.47 | 0.032 |
| Clostridiales | Lachnospiraceae | Dorea | 1.06 | 0.84 | 5.40 | 0.032 |
| Burkholderiales | Burkholderiaceae | Burkholderia | 0.92 | 1.00 | 0.00 | 0.042 |
Significant values (p <0.05) in bold.
Severity of biliary disease is attenuated in germ free NOD.c3c4 mice
To evaluate the impact of the intestinal microbiota on the biliary disease in NOD.c3c4 mice, we rederived the NOD.c3c4 mice that had been rederived into a new MDU facility (hereafter denoted CONV-R NOD.c3c4) into a GF facility (germ free animals denoted GF NOD.c3c4).
The body weight of GF NOD.c3c4 mice (n = 9–11) was reduced compared with the weight of CONV-R NOD.c3c4 mice (n = 9–11) (Fig. 2A) at 9 and 18 weeks of age. The livers of 9 and 18 weeks old GF NOD.c3c4 mice weighed significantly less than the livers of age- and gender-matched CONV-R NOD.c3c4 mice, both in absolute numbers (Supplementary Fig. 2A), and as percentage of body weight (Fig. 2B). To explore whether this was a general phenomenon in the NOD mouse strain we compared liver weights of NOD mice raised in a GF and a conventional facility (n = 5–9 in each group). The decrease in liver weight seen in GF NOD.c3c4 was present to a lesser degree in GF NOD mice (Fig. 2C, Supplementary Fig. 2B) pointing to a phenotypic specific effect in NOD.c3c4 mice. The extra-hepatic bile duct dilatation seen in 9 weeks old NOD.c3c4 was significantly reduced in GF NOD.c3c4 mice (p = 0.0043, Fig. 2D) and a similar trend was seen in 18 weeks old mice (p = 0.13, Fig. 2D). There was no recovery of a more normal phenotype in the CONV-R animals at 18 weeks as compared to 9 weeks (data not shown). No extrahepatic bile duct dilatation was detected in NOD control mice (data not shown).
Fig. 2. Germ free (GF) NOD.c3c4 mice have lower liver weight and less distended common bile ducts than conventionally raised (CONV-R) NOD.c3c4 mice.
(A) Total body weight and (B) liver weight as percentage of body weight of CONV-R and GF NOD.c3c4 mice at 9 (n = 9 in each group) and 18 weeks (w) of age (n = 11 in each group). (C) Liver weight as percentage of body weight of CONV-R and GF NOD mice at 9 (GF: n = 9, CONV-R: n = 5) and 18 w of age (GF: n = 4, CONV-R: n = 5). (D) Log-transformed common bile duct dilatation (CBDD) of CONV-R and GF NOD.c3c4 mice at 9 (n = 9 in each group) and 18 w of age (n = 11 in each group). Data are presented as mean ± SEM, unpaired Student's t-test was used for comparison. *p <0.05, **p <0.01, ***p <0.001.
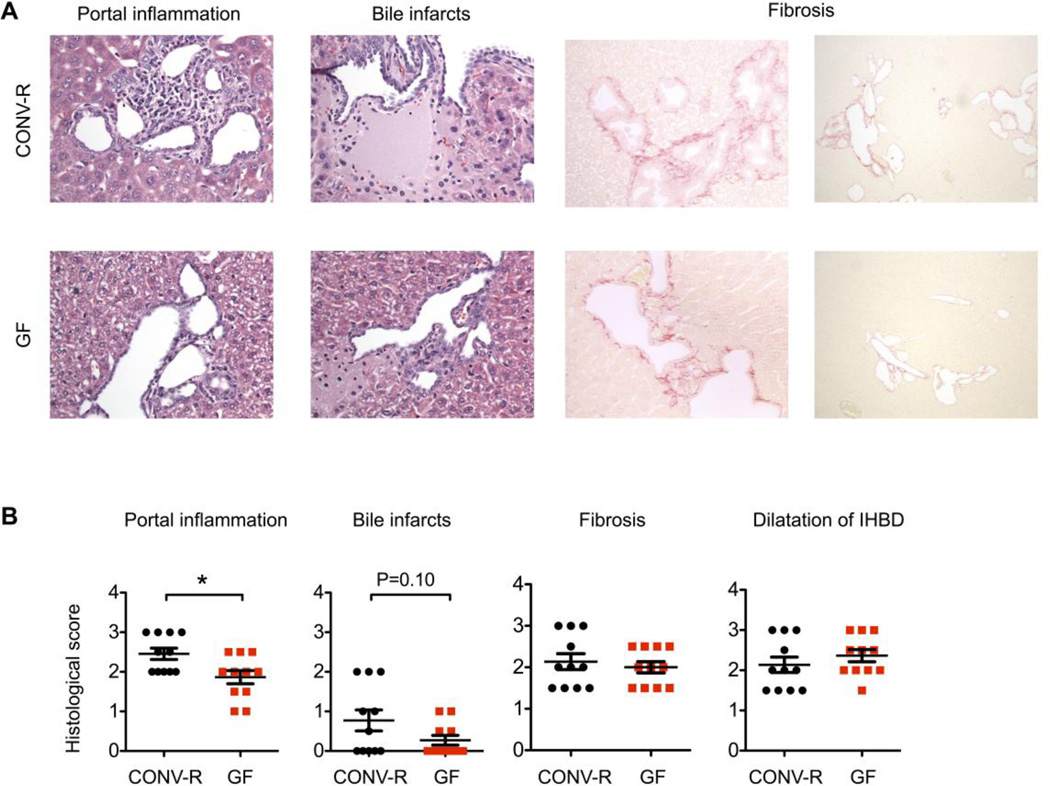
At 18 weeks of age the biliary inflammation was more pronounced in CONV-R NOD.c3c4 mice and CONV-R NOD.c3c4 mice displayed significantly larger infiltrates around the intrahepatic bile ducts than GF mice at this age (p = 0.014, Fig. 3A and B). The percentages of T cells in livers of conventionally raised NOD and NOD.c3c4 mice were similar (Supplementary Fig. 3, [1]), so to further assess the disease we evaluated histology and the biliary infiltrates. We further investigated the portal infiltrates and demonstrated that CONV-R NOD.c3c4 mice had a higher count of CD3, Ly6G and Mac-2 positive cells around their bile ducts at 18 weeks of age compared with GF NOD.c3c4 (Fig 4A and 4B). At 9 weeks of age we observed a tendency towards larger portal infiltrates and a significantly higher count of Ly6G positive cells around the bile ducts of CONV-R NOD.c3c4 mice compared to GF mice (Supplementary Fig. 4A and B). The 9 and 18 weeks old CONV-R NOD.c3c4 mice also exhibited a tendency to larger biliary infarcts (Fig. 3A and B, Supplementary Fig. 4A and B). A trend towards lower ALT serum levels was demonstrated in the GF NOD.c3c4 mice compared with CONV-R mice at 9 and 18 weeks of age (Supplementary Fig. 5A). In contrast, AST and ALP values were more elevated in the GF NOD.c3c4 mice in both age groups (Supplementary Fig. 5B and C). As expected, the spleen of GF NOD.c3c4 mice weighed less, while their cecum was enlarged and weighed significantly more, than those of CONV-R mice (Supplementary Fig. 5D). We were unable to detect any difference in the degree of liver fibrosis in GF and CONV-R NOD.c3c4 mice (Supplementary Fig. 6). There were no signs of inflammation in cecal tissue of neither CONV-R nor GF NOD.c3c4 mice (Supplementary Fig. 7).
Fig. 3. Germ free (GF) NOD.c3c4 mice have reduced inflammatory portal infiltrates compared with conventionally raised (CONV-R) NOD.c3c4 mice at 18 weeks of age.
(A) H&E (40×) and Sirius red stained sections (40×, 10×) illustrating portal inflammation, bile infarcts and fibrosis. (B) Liver pathology in GF (n = 11) and CONV-R (n = 11) NOD.c3c4 mice scored on parameters portal inflammation, bile infarcts, fibrosis and dilatation of intrahepatic bile ducts (IHBD). Data are presented as mean ± SEM, unpaired Student's t-test was used for comparison. *p <0.05.
Fig. 4. Germ free (GF) NOD.c3c4 mice have less CD3, Ly6G and Mac-2 positive cells around their bile ducts compared with conventionally raised (CONV-R) NOD.c3c4 mice.
(A) CD3, Ly6G and Mac-2 positive cells around bile ducts (indicated by arrows) in 18 weeks old CONV-R (n = 11) and GF (n = 11) NOD.c3c4 mice (40×). (B) Mean CD3/ Ly6G/ Mac-2 positive cell count from 6 different areas with bile ducts of CONV-R and GF NOD.c3c4 mice. CD3, Ly6G and Mac-2 are makers of T cells, neutrophils and macrophages, respectively. Data are presented as mean ± SEM, for CD3+ count unpaired Student's t-test was used for Ly6G+ and Mac-2+ count Mann-Whitney U test was used for comparison due to a non-normal data distribution. *p <0.05, ***p <0.001.
The biliary phenotype of NOD.c3c4 mice is not transmissible
To explore whether it was possible to transmit the biliary disease phenotype of NOD.c3c4 mice to the NOD control mice, we co-housed NOD.c3c4 mice and NOD control mice for four weeks from weaning (four weeks of age). We were unable to identify a transmittable phenotype, and NOD.c3c4 co-housed with NOD control mice developed biliary disease to the same degree as NOD.c3c4 mice housed with their litter mates (Supplementary Fig. 8).
Antibiotics affects the liver phenotype in NOD.c3c4 mice
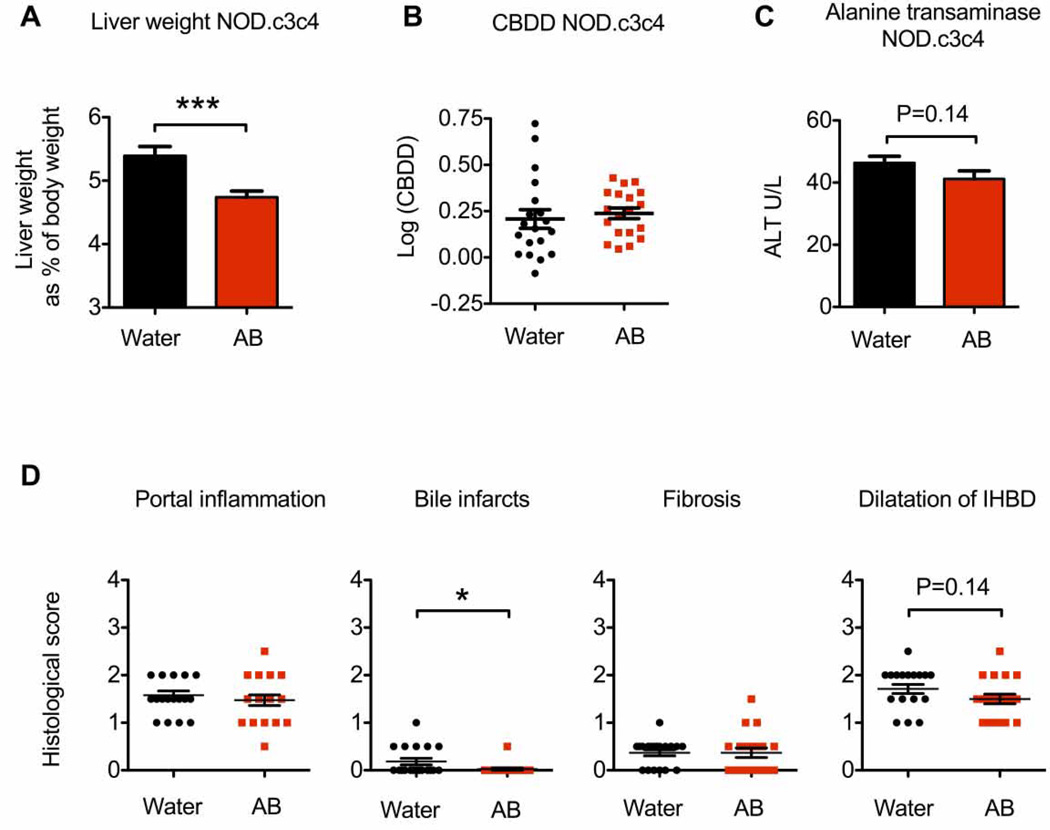
Given the amelioration of biliary disease observed in GF NOD.c3c4 mice, we hypothesised that treatment with non-absorbable antibiotics from weaning would protect the CONV NOD.c3c4 mice against the biliary phenotype. Similar to GF NOD.c3c4 mice NOD.c3c4 mice treated with antibiotics had livers that weighed significantly less than the livers of age- and gender-matched control NOD.c3c4 mice and a tendency to lower ALT levels in their serum (Fig. 5A and 5C), but there was no difference in CBDD between antibiotic treated and control mice (Fig. 5B). Antibiotic treated NOD.c3c4 mice had less biliary infarcts compared to control NOD.c3c4, but no difference was observed in other histological parameters such as portal inflammation (Fig. 5D).
Fig. 5. Antibiotic treated (AB) NOD.c3c4 mice have lower liver weight and less biliary infarcts compared with NOD.c3c4 mice receiving normal drinking water (control).
(A) Liver weight as percentage of body weight of AB NOD.c3c4 (n = 19) and control NOD.c3c4 (n = 19) mice. AB NOD.c3c4 mice received treatment for 4 weeks from weaning. (B) Log-transformed common bile duct dilatation (CBDD) and (C) alanine transaminase (ALT) levels measured in serum of AB and control NOD.c3c4 mice. (D) Liver pathology scored on parameters portal inflammation, bile infarcts, fibrosis and dilatation of intrahepatic bile ducts (IHBD). The data represents results from two pooled, independent experiments. Data are presented as mean ± SEM, unpaired Student's t-test was used for comparison. *p <0.05, ***p <0.001.
DISCUSSION
In the present study we demonstrate that there are clear differences in the gut microbiota in NOD.c3c4 mice with biliary disease and NOD control mice. Furthermore, when raised in a GF environment, NOD.c3c4 mice develop less biliary disease compared with CONV-R NOD.c3c4 mice. Likewise, treatment with non-absorbable antibiotics in NOD.c3c4 mice partially dampens the liver phenotype. Our findings implicate the gut microbiota as a contributor to biliary disease in the NOD.c3c4 mouse model.
We observed distinct differences in the global bacterial community between mice with and without biliary disease. The results represent a parallel to the human biliary disease, where the gut microbiota of both stool and mucosa is characterised by distinct differences from healthy individuals [11–14]. Differences in gut microbiota have not been well explored in other human biliary diseases than PSC or murine models of biliary inflammation, but induction of cholestasis in mice by means of bile duct ligation, does not seem to alter either global bacterial composition or diversity [21]. This could indicate that the differences in bacterial composition demonstrated develop at an earlier time point in life and that a later induction of cholestasis will not contribute to a global shift in the microbiota. To rule out potential cage effects as far as possible, the mice were rederived into a new conventional animal facility. Although we cannot formally rule out that new cage effects can establish after rederivation, we consider it as unlikely that the differences observed are driven by cage effects as the differences in global bacterial communities were demonstrated in two different environments. The use of NOD mice as controls could be challenging as NOD mice develop diabetes and this can potentially affect the microbiota [22], but the NOD mice used in the present analysis of gut microbial profiles were harvested before the development of diabetes as confirmed by blood glucose measurements.
Following the identification of differences in the bacterial communities we hypothesised that GF NOD.c3c4 would have an altered biliary phenotype. Indeed, the GF NOD.c3c4 mice were protected from biliary disease in terms of several clinical and pathological features. The common bile duct dilatation was less pronounced in GF NOD.c3c4 mice and we demonstrated the development of larger portal infiltrates and higher counts of CD3, Ly6G and Mac-2 positive cells around the bile ducts of CONV-R mice. Several factors could potentially contribute to the observed differences in biliary phenotype of CONV-R and GF mice. First of all, the total amount of bile acids is reduced in feces and serum of GF mice, and the composition of bile acids vary greatly between GF and CONV-R mice, also in the liver [23]. Secondly, the immune system and lymphocyte populations of GF mice are also known to be altered with mucosal accumulation of natural killer T cells and absence of mucosal associated invariant T cells in GF mice [24,25]. This likely can affect an inflammatory disease phenotype, nevertheless, it is striking that several components of the biliary inflammation are altered in a GF environment. In line with the observation in GF animals, treatment of NOD.c3c4 mice with non-absorbable antibiotics after weaning resulted in reduced liver weight and less biliary infarcts. Using antibiotics to deplete the microbiota constitutes a less controlled situation than using GF animals [26], nevertheless the biliary disease of NOD.c3c4 mice was dampened although not cured with antibiotics. Since co-housing of animals has been shown to affect the development of non-alcoholic fatty liver disease (NAFLD) [27] and colitis [28] in other animal models, we investigated whether this was the case for the biliary phenotype of NOD.c3c4 mice. These experiments clearly demonstrated no sign of transmissibility or protection from biliary disease. Taken together the results from the GF, antibiotics and co-housing experiments indicate that the majority of effects of the microbiota is initiated before weaning during the time when the host immune system is developed and tolerance to environmental exposures is achieved [29].
Another murine model of PSC is the Mdr2−/− mouse. In contrast to our findings, a recent study by Tabibian et al. [16] demonstrated that GF Mdr2−/− mice developed more severe liver disease measured by ALP, AST and bilirubin in serum, as well as more severe fibrosis compared with CONV-R Mdr2−/− mice. Aggravation of liver fibrosis is also seen in thioacetamide and carbon tetrachloride (CCl4) treated GF mice compared with thioacetamide - and CCl4-treated CONV-R mice [30], while in models of acute hepatic injury such as acetaminophen- and alcohol-induced hepatotoxicity mice raised in GF facilities seem to be protected from hepatic disease [31,32]. In NOD.c3c4 mice a minimal degree of fibrosis is seen, and this could suggest that liver disease models that are more driven by fibrosis develop an aggravated phenotype in the absence of commensal bacteria, while the liver disease of other murine models, displaying features of lipid accumulation or portal inflammation, is ameliorated in a germ free environment.
Our histological analysis and measurements of CBDD demonstrated an ameliorated phenotype in GF NOD.c3c4 mice, and there was a trend towards lower levels of the liver specific enzyme ALT in GF mice. ALP and AST serum levels on the contrary were elevated in GF mice. The use of ALP serum levels as a marker of biliary disease in mice has been somewhat debated [33,34] as the ALP levels measured in mice seem to be mostly bone-derived ALP isoforms, some intestinal ALP isoforms and close to no liver ALP, and it also varies between different mouse strains [35]. Intestinal ALP activity is increased in GF mice [36] and this could explain some of the discrepancies seen in our serum measurements.
In conclusion, we have demonstrated that there are distinct differences in the gut microbiota between mice with and without biliary disease, and that the biliary disease of NOD.c3c4 mice is reduced in a germ free environment and affected by non-absorbable antibiotics. This indicates that commensal bacteria can contribute to biliary inflammation.
Supplementary Material
Acknowledgments
The authors wish to thank Anne Pharo, Eva Kristine Klemsdal Henriksen, Tonje Bjørnetrø, Hege Dahlen Sollid and Liv Wenche Thorbjørnsen at NoPSC Research Center, Aurelija Abraityte at the Research Institute for Internal Medicine, and Hege G. Russnes and Ellen Hellesylt at Department of Pathology, Oslo University Hospital, for great assistance and technical help. We also wish to thank Johanna Aspsäter at Department of Microbiology, Karolinska Institutet, Stockholm for help with our germ free mouse colony. We are grateful to Prof Erik Schrumpf for critical reading of our manuscript and to Prof Tore Midtvedt for valuable input on our project.
Financial support: The study was supported by the South Eastern Norway Regional Health Authority (project number 2012024), the Norwegian Research Council (project number 240787/F20), US National Institutes of Health (DK44319 to R.S.B.), PSC Partners and the Norwegian PSC Research Center.
Abbreviations
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
- IBD
inflammatory bowel disease
- GF
germ free
- Mdr2−/−
multidrug resistance knockout
- CONV-R
conventionally raised
- MDU
minimal disease unit
- CBDD
common bile duct dilatation
- H&E
hematoxylin and eosin
- ALT
alanine transaminase
- AST
aspartate transaminase
- ALP
alkaline phosphatase
- CCl4
carbon tetrachloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Author´s contributions: E.M., E.S. and M.K. designed the experiments. E.S., L.V. and M.K. performed the experiments. E.S., M.K. and K.H. performed data analysis. T.U.G. and F.B. provided control material. V.A. administered the GF mouse colony. J.B. assisted with sequencing. H.M.R. evaluated histological slides. E.M., J.H., T.H.K., F.B., J.B. and R.S.B. supervised different experiments and contributed with new ideas throughout the project. E.S., M.K. and E.M. drafted the manuscript. All authors revised the manuscript and approved the final version.
REFERENCES
- 1.Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koarada S, Wu Y, Fertig N, Sass DA, Nalesnik M, Todd JA, et al. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–2323. doi: 10.4049/jimmunol.173.4.2315. [DOI] [PubMed] [Google Scholar]
- 3.Folseraas T, Liaskou E, Anderson CA, Karlsen TH. Genetics in PSC: what do the “risk genes” teach us? Clin Rev Allergy Immunol. 2015;48:154–164. doi: 10.1007/s12016-014-8417-z. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 5.Chuang Y-H, Ridgway WM, Ueno Y, Gershwin ME. Animal models of primary biliary cirrhosis. Clin Liver Dis. 2008;12:333–347. doi: 10.1016/j.cld.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollheimer MJ, Fickert P. Animal models in primary biliary cirrhosis and primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2015;48:207–217. doi: 10.1007/s12016-014-8442-y. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi PJ, Adams DH. Mucosal immunity in liver autoimmunity: a comprehensive review. J Autoimmun. 2013;46:97–111. doi: 10.1016/j.jaut.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Kummen M, Schrumpf E, Boberg KM. Liver abnormalities in bowel diseases. Best Pract Res Clin Gastroenterol. 2013;27:531–542. doi: 10.1016/j.bpg.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Lichtman SN, Keku J, Clark RL, Schwab JH, Sartor RB. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology. 1991;13:766–772. [PubMed] [Google Scholar]
- 10.Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414–423. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- 11.Rühlemann MC, Heinsen F-A, Zenouzi R, Lieb W, Franke A, Schramm C. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut. 2016 doi: 10.1136/gutjnl-2016-312180. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016 doi: 10.1136/gutjnl-2015-311004. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kummen M, Holm K, Anmarkrud JA, Nygård S, Vesterhus M, Høivik ML, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2016 doi: 10.1136/gutjnl-2015-310500. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Quraishi MN, Sergeant M, Kay G, Iqbal T, Chan J, Constantinidou C, et al. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut. 2016 doi: 10.1136/gutjnl-2016-311915. [DOI] [PubMed] [Google Scholar]
- 15.Tabibian JH, Weeding E, Jorgensen RA, Petz JL, Keach JC, Talwalkar JA, et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2013;37:604–612. doi: 10.1111/apt.12232. [DOI] [PubMed] [Google Scholar]
- 16.Tabibian JH, O’Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology. 2016;63:185–196. doi: 10.1002/hep.27927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Uronis JM, Arthur JC, Keku T, Fodor A, Carroll IM, Cruz ML, et al. Gut microbial diversity is reduced by the probiotic VSL#3 and correlates with decreased TNBS-induced colitis. Inflamm Bowel Dis. 2011;17:289–297. doi: 10.1002/ibd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg R, Toft MF, August B, Hansen AK, Hansen CHF. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. 2016;7:68–74. doi: 10.1080/19490976.2015.1127463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29:1043–1055. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Possamai LA, McPhail MJ, Khamri W, Wu B, Concas D, Harrison M, et al. The role of intestinal microbiota in murine models of acetaminophen-induced hepatotoxicity. Liver Int. 2015;35:764–773. doi: 10.1111/liv.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canesso MCC, Lacerda NL, Ferreira CM, Gonçalves JL, Almeida D, Gamba C, et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol. 2014;14:240. doi: 10.1186/s12866-014-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krones E, Erwa W, Trauner M, Fickert P. Serum alkaline phosphatase levels accurately reflect cholestasis in mice. Hepatology. 2015;62:981–983. doi: 10.1002/hep.27622. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi PJ, Weston CJ, Webb GJ, Newsome PN, Hirschfield GM, Adams DH. Serum alkaline phosphatase in multidrug resistance 2 (Mdr2−/−) knockout mice is strain specific. Hepatology. 2016;63:346. doi: 10.1002/hep.27874. [DOI] [PubMed] [Google Scholar]
- 35.Halling Linder C, Englund UH, Narisawa S, Millán JL, Magnusson P. Isozyme profile and tissue-origin of alkaline phosphatases in mouse serum. Bone. 2013;53:399–408. doi: 10.1016/j.bone.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yolton DP, Stanley C, Savage DC. Influence of the indigenous gastrointestinal microbial flora on duodenal alkaline phosphatase activity in mice. Infect Immun. 1971;3:768–773. doi: 10.1128/iai.3.6.768-773.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.