Abstract
Telomeres are repeating DNA at chromosome ends. Telomere length (TL) declines with age in most human tissues, and shorter TL is thought to accelerate senescence. In contrast, older men have sperm with longer TL; correspondingly, older paternal age at conception (PAC) predicts longer TL in offspring. This PAC-effect could be a unique form of transgenerational genetic plasticity that modifies somatic maintenance in response to cues of recent ancestral experience. The PAC-effect has not been examined in any non-human mammals.
Objectives
Here we examine the PAC-effect in chimpanzees (Pan troglodytes). The PAC-effect on TL is thought to be driven by continual production of sperm—the same process that drives increased de novo mutations with PAC. Since chimpanzees have both greater sperm production and greater sperm mutation rates with PAC than humans, we predict that the PAC-effect on TL will be more pronounced in chimpanzees. Additionally we examine whether PAC predicts TL of grandchildren.
Materials and Methods
TL were measured using qPCR from DNA from blood samples from 40 captive chimpanzees and 144 humans.
Results
Analyses showed increasing TL with PAC in chimpanzees (p=0.009) with a slope six times that in humans (p=0.026). No associations between TL and grandpaternal ages were found in humans or chimpanzees—although statistical power was low.
Discussion
These results suggest that sperm production rates across species may be a determinant of the PAC-effect on offspring TL. This raises the possibility that sperm production rates within species may influence the TL passed on to offspring.
Keywords: aging, adaptive intergenerational plasticity, epigenetics, life history, evolution
Introduction
Telomeres are repeating DNA sequences at the ends of chromosomes that protect and buffer genes from nucleotide loss as cells divide (Blackburn and Gall 1978). In many human tissues, telomere lengths (TL) are shortened by cellular proliferation and as a result TL declines with age (Ishii et al. 2006; Kimura et al. 2008a; Olovnikov 1971; Watson 1972). As cell replication generally requires a minimal TL, shortened TL is thought to contribute to senescence (Harley 1991). Consistent with this, people with shorter telomeres have reduced survival (Bakaysa et al. 2007; Cawthon et al. 2003; Ehrlenbach et al. 2009; Fitzpatrick et al. 2011; Honig et al. 2006; Kimura et al. 2008b; Martin-Ruiz et al. 2006).
While it is well established that TL shortens with age in most proliferating tissues in humans (e.g. Ishii et al. 2006; Kimura et al. 2008a), sperm TL is an exception—older men have sperm with longer telomeres (Allsopp et al. 1992; Baird et al. 2006; Kimura et al. 2008a). This may be explained by the fact that telomerase (an enzyme that extends TL) activity is high in the testes (Wright et al. 1996; Zalenskaya and Zalensky 2002) and by the apparent selective loss of sperm progenitor cells with shorter TL with age (Hjelmborg et al. 2015; Kimura et al. 2008a). Consistent with the fact that offspring inherit half their chromosomes from sperm, offspring of older men tend to have longer telomeres (De Meyer et al. 2007; Kimura et al. 2008a; Unryn et al. 2005) and one study has shown that this effect persists across at least two generations (Eisenberg et al. 2012). In contrast, because the pool of ova is established in utero, TL in ova are thought to be stable with age, and there is no evidence for a maternal age effect on TL in offspring (e.g. Arbeev et al. 2011; Kimura et al. 2008a).
The multigenerational effect of PAC on descendants TL supports the notion that this could represents a mechanism of adaptive intergenerational plasticity in the pace of aging and senescent functional decline (Eisenberg et al. 2012; Eisenberg and Kuzawa 2013; Eisenberg 2011; Kuzawa and Eisenberg 2014). Notably, as paternal ancestors delay reproduction, longer TL will be passed to offspring, which could allow lifespan to be extended as lineages survive to reproduce at older ages. Having been born to an older father could signal that that individual is likely to grow up in social and ecological contexts within which mortality rates are low and reproduction is likely to occur later in life, thus placing more of a premium on a durable long-lived body. By integrating information about the average age at reproduction across multiple generations of ancestors, the paternal age effect on TL could allow a unique form of transgenerational genetic plasticity that modifies physiologic function in response to a relatively stable cue of recent ancestral experience and behavior (Eisenberg et al. 2012; Eisenberg 2011).
While this paternal age at conception (PAC) effect is one of the few consistent predictors of TL in humans, there are only two studies we are aware of to test whether a PAC-effect occurs in non-humans—both in non-mammals. The first examined only 12 individual sand lizards (Lacerta agilis) and found that having older PAC predicted shorter TL (Olsson et al. 2011). The second examined 204 European shag (Phalacrocorax aristotelis) chicks and found no association of TL with paternal age (Heidinger et al. 2015). Here we examine whether the PAC-effect is evident in captive chimpanzees (Pan troglodytes), our closest living relatives.
The mechanistic biology of the PAC-effect leads to the prediction that chimpanzees should show a greater PAC-effect than humans, while the adaptive intergenerational inertia hypothesis leads to less clear predictions. Like humans, chimpanzees show an increase in genome-wide de novo mutation rate with PAC, but the rate in chimpanzees is estimated to be 50% greater than humans for each increased year of paternal age (Venn et al. 2014). This is thought to be driven by a more promiscuous mating system that has selected for increased sperm competition and a 3.4× more massive testis (body weight adjusted) in chimpanzees than humans to enable greater sperm production (Venn et al. 2014; Wong 2014). Since the PAC-effects on TL and on mutation rates are thought to be similarly driven by the proliferative process of sperm production, we predict that the PAC-effect on TL will also be greater in chimpanzees than humans.
Alternatively, it has been suggested that the PAC-effect on TL is an adaptive intergenerational signaling mechanism that depends on intergenerational stability in experienced environments between male ancestors and direct descendants (Eisenberg 2011). Humans have elaborate culture and accumulation of social and material resources which are transmitted to offspring (e.g. Smith et al. 2010). This could create greater intergenerational stability in experienced human environments than chimpanzees. On this basis we would expect the PAC-effect on TL to be more strongly selected for in humans, and for humans thus to have a greater PAC-effect on TL than chimpanzees. However, humans also show dramatic behavioral diversity over time and space which might equate to less intergenerational stability and the PAC-effect being less strongly selected for in humans than chimpanzees.
Here we use previously reported TL data from chimpanzees and humans (Cawthon et al. 2003; Tackney et al. 2014) to compare the PAC-effect in 40 female chimpanzees with 144 humans. As a secondary aim we attempt to replicate the previously observed transmission of the PAC-effect across multiple generations—particularly whether grandfather age at conception of parents predicts grandchildren’s TL (Eisenberg et al. 2012) in a subset of these chimpanzees and humans with known or estimated dates of birth of grandparents.
Materials and Methods
Samples
The samples and laboratory analysis have been described in detail previously (Cawthon et al. 2003; Tackney et al. 2014). Briefly, blood was drawn from female chimpanzees during routine health checks of captive populations at the Southwest National Primate Research Center hosted by the Texas Biomedical Research Institute (formerly Southwest Foundation for Biomedical Research) in San Antonio, Texas and at the Yerkes National Primate Research Center at Emory University Atlanta, Georgia. Samples were chosen for the purposes of a previous study to maximize the age range of the chimpanzee population (6.2–56.7 years). For the human samples, TL measured from blood samples from the Utah CEPH collection (northern and western European descent). The human data analyzed here consisted of unrelated females picked to match the age range of the chimpanzee samples (Tackney et al. 2014), and unrelated males and females over the age of 60 years old previously reported in a survival analysis (Cawthon et al. 2003). If first degree relatives were found between these two combined humans datasets at least one of the relatives were excluded so that no known first degree relatives remained.
Dates of birth were retrieved from ancestors to calculate PAC. Dates of birth were only available or estimable for a subset of individuals (indicated by n values in Table 1). Eighteen chimpanzee fathers, four mothers, seven paternal grandfathers, and four maternal grandfathers did not have known birth dates, but had their dates of birth estimated using standard age estimation procedures (Goodall 1968; Goodall 1983; Goodall 1986) and were retained in the analyses here. The additional error introduced by estimated birth dates should act to bias against our primary hypothesis by attenuating any associations between PAC and TL in chimpanzees.
Table 1.
Description statistics. Limited to individuals for which paternal age at conception is available.
| Human | Chimp | |||||
|---|---|---|---|---|---|---|
| n | mean (sd) | range (diff) | n | mean (sd) | range (diff) | |
| age | 144 | 61.3 (22.0) | 7.4–97.3 (89.9) | 40 | 24.4 (11.4) | 6.2–56.7 (50.5) |
| PACa | 144 | 32.4 (8.2) | 19.7–62.9 (43.2) | 40 | 17.7 (6.4) | 8–37 (29) |
| pGPACb | 120 | 34.1 (9.7) | 21.1–65.6 (44.5) | 7 | 19.3 (3.7) | 14–23 (9) |
| mGPACc | 89 | 36.2 (11.5) | 17.9–69.5 (51.6) | 9 | 19 (10.0) | 11–37 (26) |
paternal age at conception;
paternal grandfather age at conception;
maternal grandfather age at conception
We note that the term paternal age at conception (PAC) is used to refer to the PAC-effect on TL and for analyses here. In fact, in the analyses paternal ages at birth are actually utilized, but since gestation duration contributes very little to the variation in PAC and the biologically important effect is thought to occur at conception, we use the term PAC here.
Statistical methods
Hypotheses were tested with multivariate linear regressions with robust standard errors run in Stata 11.2. Power analyses were conducted with the ‘powerreg’ command in Stata. Significance was defined as p<0.05.
Laboratory analysis
Human DNA was phenol–chloroform or GentraSystems PureGene extracted from whole blood while chimpanzee samples were extracted using Qiagen QIAamp DNA Blood Mini Kits. PCR reactions were set up as described previously (Cawthon et al. 2003; Tackney et al. 2014). Since the coefficient of variation (CV) has recently been recognized to be an invalid statistic to assess TL measurement reliability, we instead use the intraclass correlation coefficient (ICC) (Eisenberg in press; Verhulst et al. 2015) which estimates the percent of variation attributable to individuals versus to measurement error. Individual and average ICCs were calculated using a two-way random effects model to calculate absolute agreement between the averages of the same samples run in triplicate on different runs with the ICC command in Stata 14.1. Individual and average ICC values correspond to ICC(A,1) and ICC(A,k) in McGraw and Wong (1996). Individual ICC gives an estimate of the reliability of measures of samples analyzed on one run (in triplicate), while average ICC gives an estimate of the reliability of the average TL estimate of a sample measured across multiple runs. 42 of the human samples were run separately in triplicate on two separate runs and had an individual ICC of 0.93 (95% CI: 0.87–0.96) and average ICC of 0.96 (95% CI 0.93–0.98). 35 of the chimpanzee samples were run separately in triplicate on four separate runs and had an individual ICC of 0.83 (95% CI: 0.75–0.91) and average ICC of 0.95 (95% CI: 0.92–0.98). The overlapping confidence intervals of ICC measures between humans and chimpanzees and near identical average ICC estimates suggests that the TL measurement error is similar in both species. While conventional rules of thumb suggest that these ICC values are excellent (Cicchetti 1994), we are not aware of any other reports of ICC values in the telomere biology literature to compare these to.
Results
Averages ages, PAC, paternal grandfather ages at father’s conception (pGPAC) and maternal grandfathers age at mothers’ conception (mGPAC) for the human and chimpanzee samples are show in Table 1. Paternal age did not significantly differ between chimpanzees from the two different colonies (t=−0.38, p=0.703). We note that the average chimpanzee PACs in our captive sample (mean=17.7, 95% CI: 15.6–19.7) is lower than has been found in a wild population (mean=24.08, 95% CI: 23.83–24.34; Langergraber et al. 2012). The chimpanzee sample is all female while the human sample contains 92 females and 52 males (63.9% female).
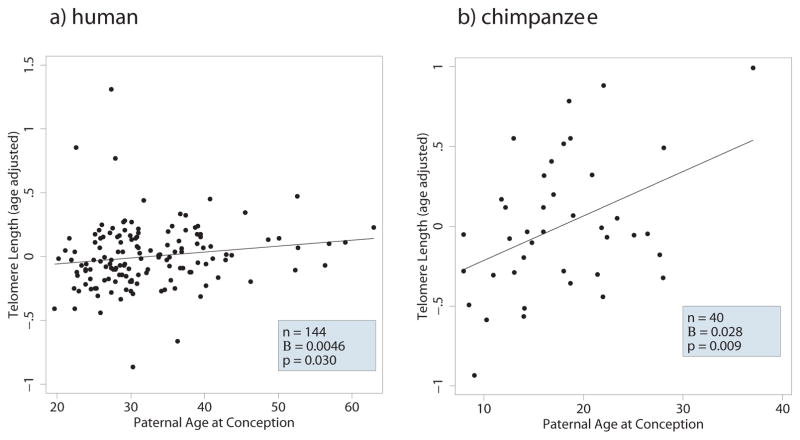
A multivariate regression model including both chimpanzees and humans together in the same analysis and controlling for age and sex shows a significant PAC-effect (Table 2; p = 0.016). Further, the PAC-effect is significantly greater in chimpanzees than humans (p = 0.026). The differences in PAC-effects in chimpanzees and humans are illustrated in Figure 1. The estimates from Figure 1 suggests a six fold greater PAC-effect in chimpanzees than humans. The PAC-effects on TL did not show a curvilinear relationship in humans or in chimpanzees (quadratic term p values>0.891).
Table 2.
Linear regression to evaluate paternal age at conception effects on telomere lengths in chimps and humans. β values with p-values in parentheses.
| chimp | 1.47*** (0.000) |
| age | −0.0063*** (0.000) |
| chimp X age | −0.013* (0.011) |
| male | 0.0099 (0.764) |
| PAC | 0.0049* (0.016) |
| chimp X PAC | 0.023* (0.026) |
| Y-intercept | 1.34*** (0.000) |
|
| |
| N | 184 |
| adj. R2 | 0.867 |
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001
Figure 1.
Paternal and grandpaternal ages at conception and descendant telomere lengths in chimpanzees and humans
Unlike previous work with partially overlapping data (Tackney et al. 2014), our results show a significantly greater age decline in TL in chimpanzees than humans (p=0.011). However, when the analysis is restricted to only include humans that match the age range of chimpanzees (<57), no significant difference in age related decline were observed between humans and chimpanzees (p=0.432).
Associations of pGPAC and mGPAC with TL in humans and chimpanzees are reported in Table 3. There were no significant effects of pGPAC or mGPAC in either humans or chimpanzees. However, all beta coefficients were in the expected positive direction. There was a near significant effect of mGPAC in humans. While also not significant, chimpanzee betas are larger than in humans. Assuming the previous effect sizes observed for pGPAC in Eisenberg et al (2012), with the sample sizes available here we had 5.4% power to detect this effect as different from zero in chimpanzees and 34.5% power in humans. Assuming the pGPAC-effect is six times larger in chimpanzees than previously observed in humans (as observed for the PAC-effect above), this would increase our power to 7.3%
Table 3.
Linear regressions to evaluate grandpaternal age at conception effects on telomere lengths in chimps and humans. β values with p-values in parentheses.
| (1) Human | (2) Human | (3) Chimp | (4) Chimp | |
|---|---|---|---|---|
| age | −0.0074*** (0.000) | −0.0056* (0.019) | −0.028* (0.030) | −0.020 (0.123) |
| PAC | 0.0039* (0.044) | 0.0065** (0.001) | 0.053* (0.012) | 0.0072 (0.866) |
| pGPAC | 0.0025 (0.247) | 0.047 (0.338) | ||
| mGPAC | 0.0025+ (0.081) | 0.014 (0.366) | ||
| Y-intercept | 1.37*** (0.000) | 1.16*** (0.000) | 2.00 (0.128) | 2.94* (0.023) |
|
| ||||
| N | 120 | 89 | 7 | 9 |
| adj. R2 | 0.272 | 0.123 | 0.685 | 0.366 |
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001
Discussion
These results are the first examination of the PAC-effect on TL in a non-human mammal and the second study to look for the PAC-effect across more than one generation. Consistent with predictions that a greater sperm production rate should lead to a more rapid increase in sperm TL with age, we find that chimpanzees have a significant, six fold greater PAC-effect on TL than humans. We were unable to replicate with significance a GPAC-effect reported previously (Eisenberg et al. 2012), but this is not surprising given the low statistical power we had to detect these effects. Still, it is noteworthy that all GPAC-effects were in the expected direction and that chimpanzee GPAC associations showed considerably (but non-significantly) greater slopes than humans.
A previous study using an overlapping dataset to the one utilized here showed no difference in the age related decline in TL between humans and chimpanzees (Tackney et al. 2014), while the current analysis shows a greater age related decline in chimpanzees. This is likely due to the expanded human sample which is not closely age matched with the chimpanzees as in the previous analysis (Tackney et al. 2014). Accordingly, matching the age ranges between chimpanzee and humans eliminated the association. Inclusion of more elderly humans causing a lower estimated age related decline in TL is consistent with past studies which have suggested that the rate of decline in TL decreases with age in humans (possibly due to survival bias effects, reviewed in Eisenberg 2011; Lapham et al. 2015). Nonetheless, it is possible the greater PAC-effect in chimpanzees is reflective of some underlying factor which causes faster changes in TL in chimpanzees than humans. However, in chimpanzees the PAC-effect is 150% of the magnitude of the age related decline while in humans the PAC-effect is only 79% of the magnitude of the age decline in TL (based on estimates from Table 2). This suggests that even accounting for possible ‘faster’ changes in TL in chimpanzees, the PAC-effect is still relatively greater in chimpanzees than humans.
One factor which might help explain the findings here stems from the fact that chimpanzees have approximately half the body mass of humans (Smith and Jungers 1997). Somatic telomerase activity has been found to decrease with body mass across mammalian species (Gomes et al. 2011; Gorbunova and Seluanov 2009). However, in almost all mammalian species larger than 10 kg, somatic telomerase activity is undetectable, and thus shows no relationship with body mass across these larger species (Gomes et al. 2011). Further, no examined primate species showed detectable somatic telomerase activity in the Gomes et al (2011) analysis (although the replicative capacity of the prosimian Lemur catta has been shown to be qualitatively greater than anthropoid primates (Steinert et al. 2002)). For telomerase activity to explain the findings of this paper, telomerase activity would need to effect sperm telomere lengthening with age. Little is known about how testicular telomerase activity varies across species. There is limited evidence that the Macaca fasicularis (crab-eating monkey) show less testicular telomerase activity than either M. fuscata (Japanese Monkey) or M. mulatta (rhesus) (Kakuo et al. 1999). However, M. fasicularis is about half as massive as M. fuscata or M. mulatta—the opposite of expected if larger bodied primates had lower testicular telomerase activity.
This paper, and most other TL PAC-effect work, have interpreted associations as reflecting longitudinal lengthening of TL in sperm as males age. However, it is possible that these associations reflect selection effects—such as healthier males with longer constitutive TL being more likely to father offspring at later ages and/or birth order effects. These sorts of selection effects are unlikely for several reasons. First, telomerase activity (which extends TL) is high in the testes (Achi et al. 2000; Bekaert et al. 2004; Fradiani et al. 2004; Gardner et al. 2007; Kim et al. 1994; Wright et al. 1996; Yashima et al. 1998) and the distribution of sperm TLs is consistent with selective attrition of sperm progenitor cells with shorter TL with age (Hjelmborg et al. 2015; Kimura et al. 2008a)—both providing mechanisms for TL extension with age in sperm. Second, the PAC-effect is linear and consistent, whether looking at the TL of offspring of various aged fathers, or sperm TL of various aged men (Eisenberg et al. 2012; Kimura et al. 2008a). Third, the PAC-effect is not attenuated by adjustment for socioeconomic status, birth order and other likely confounders (Eisenberg et al. 2012; Prescott et al. 2012). Fourth, selection effects would likely vary across cultures and species, yet the PAC effect has been demonstrated from cohorts of people from the US, Canada, UK, Denmark, and the Philippines (Eisenberg et al. 2012; Kimura et al. 2008a; Unryn et al. 2005; Wojcicki et al. 2016) and in this article in chimpanzees. Since chimpanzees do not mate monogamously nor do chimpanzee males exhibit parental care, birth-order effects are unlikely to manifest similarly in chimpanzees as in humans. Finally, within the same men sperm TL shows a cross-sectional increase with age while blood shows a cross-sectional decrease (Aston et al. 2012)—inconsistent with constitutively longer telomeres predicting increased probability of donating sperm with age. While more definitive illumination of this issue would require repeat longitudinal collection of sperm samples in men as they age or sibling based analyses, these convergent evidence strongly suggest that sperm TL does in fact increase with age within males and that they do so to a much greater degree in chimpanzees than in humans.
The adaptive significance, if any, of the greater PAC-effect on TL in chimpanzees than in humans is not entirely clear. Sperm production rate and testis size is thought to be influenced largely by the degree of selection for sperm competition based on different mating systems (Short 2001). It may be that the PAC-effect on TL is best viewed as an evolutionary spandrel, changing across species primarily due to selection for different sperm production rates. Alternatively, it is possible that the PAC-effect on TL is selectively modulated based on the degree to which it effectively acts as an intergenerational predictive adaptive response. That is, if paternal age is more strongly correlated with offspring’s likelihood of living to a late age in a species than we would expect that the PAC-effect would be more strongly selective for. For example, it might be that in chimpanzees, maintaining social ranks and concomitant likelihood of successful siring of offspring at late ages (Newton-Fisher et al. 2010; Wroblewski et al. 2009) is more dependent on later life physiological status and health than in humans. To the extent that TL influences physiological status, than their might be stronger selection on PAC in chimpanzees as a modulator of investments into maintaining a durable soma versus other energetic allocations.
The results in this paper suggest that greater sperm production rates, as indexed by larger testis sizes may predict greater increases in sperm TL with age. However, this study only compared two species, and it is possible that these differences are driven by many other factors which distinguish these species. In order to more rigorously test this hypothesis, we suggest careful targeted sampling of species for variation in testis size and for phylogenetic relationships to maximize statistical power (Arnold et al. 2010). Finally, since testis size varies within species (Simmons et al. 2004) and testis size can be influenced even in adulthood by sex hormones (Hembree et al. 2009) it is possible that environmental influences and physiological status may modify testis size and thereby sperm production rates and the TL that men pass on to their descendants.
Acknowledgments
Grant sponsorship: Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, R24 HD042828, to the Center for Studies in Demography & Ecology at the University of Washington; National Science Foundation (grant number BCS 0717886 to Hawkes); Contract grant sponsor: National Institutes of Health (grant number P51 RR000165 to Yerkes National Primate Research Center; grant numbers P5 1RR013986 and OD P51 OD011133 to Southwest National Primate Research Center).
Thanks to Hilary Bethancourt for help with merging data and feedback on study design; Amy Klegarth and two anonymous reviewers for feedback on the manuscript; Cori Mar for statistical advice; Dr. Silvia Smith for assistance with logistics early in this project and the keepers, veterinarians, and biological material procurement staff at both Yerkes and Southwest National Primate Research Centers. Access to the Utah CEPH DNA samples was provided by Dr. Mark Leppert. Finally, the authors are in debt to the laboratory of Dr. Vicente Planelles, and in particular Dr. Alberto Bosque, who generously provided time and space in their BSL-2 research facilities for the chimpanzee DNA extractions and the storage of primate blood samples. Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, R24 HD042828, to the Center for Studies in Demography & Ecology at the University of Washington; National Science Foundation (grant number BCS 0717886 to Hawkes); Contract grant sponsor: National Institutes of Health (grant number P51 RR000165 to Yerkes National Primate Research Center; grant numbers P5 1RR013986 and OD P51 OD011133 to Southwest National Primate Research Center). DTAE conceived of this study, conducted all statistical analyses and wrote the manuscript. JT conducted most telomere length measurements. RMC supervised all telomere length measurements. CTC led sample and data acquisition from the primate centers. KH ran the original study which generated the chimpanzee telomere length measurements.
Works cited
- Achi MV, Ravindranath N, Dym M. Telomere Length in Male Germ Cells Is Inversely Correlated with Telomerase Activity. 2000;63:591–598. doi: 10.1095/biolreprod63.2.591. [DOI] [PubMed] [Google Scholar]
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeev KG, Hunt SC, Kimura M, Aviv A, Yashin AI. Leukocyte telomere length, breast cancer risk in the offspring: The relations with father’s age at birth. Mech Ageing Dev. 2011;132(4):149–153. doi: 10.1016/j.mad.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C, Nunn CL, Dean CA, Associate Editor, Mark AM., Editor Phylogenetic Targeting of Research Effort in Evolutionary Biology. The American Naturalist. 2010;176(5):601–612. doi: 10.1086/656490. [DOI] [PubMed] [Google Scholar]
- Aston KI, Hunt SC, Susser E, Kimura M, Factor-Litvak P, Carrell D, Aviv A. Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans. Mol Hum Reprod. 2012;18(11):517–522. doi: 10.1093/molehr/gas028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DM, Britt-Compton B, Rowson J, Amso NN, Gregory L, Kipling D. Telomere instability in the male germline. Hum Mol Genet. 2006;15(1):45–51. doi: 10.1093/hmg/ddi424. [DOI] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6(6):769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Bekaert S, Derradji H, Baatout S. Telomere biology in mammalian germ cells and during development. Dev Biol. 2004;274(1):15–30. doi: 10.1016/j.ydbio.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6(4):284. [Google Scholar]
- De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Van Criekinge W, De Backer GG, Gillebert TC, Van Oostveldt P, Bekaert S, Asklepios I. Paternal age at birth is an important determinant of offspring telomere length. Hum Mol Genet. 2007;16(24):3097–3102. doi: 10.1093/hmg/ddm271. [DOI] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38(6):1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Telomere Length Measurement Validity: The Coefficient of Variation (CV) is invalid and cannot be used to compare qPCR and Southern Blot telomere length measurement techniques. Int J Epidemiol. doi: 10.1093/ije/dyw191. in press. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci. 2012;109(26):10251–10256. doi: 10.1073/pnas.1202092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Kuzawa CW. Commentary: The evolutionary biology of the paternal age effect on telomere length. Int J Epidemiol. 2013;42(2):462–465. doi: 10.1093/ije/dyt027. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA. An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011;23(2):149–167. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradiani PA, Ascenzioni F, Lavitrano M, Donini P. Telomeres and telomerase activity in pig tissues. Biochimie. 2004;86(1):7–12. doi: 10.1016/j.biochi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Gardner JP, Kimura M, Chai W, Durrani JF, Tchakmakjian L, Cao X, Lu X, Li G, Peppas AP, Skurnick J, et al. Telomere dynamics in macaques and humans. J Gerontol A Biol Sci Med Sci. 2007;62(4):367–374. doi: 10.1093/gerona/62.4.367. [DOI] [PubMed] [Google Scholar]
- Gomes NMV, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, Austad SN, Venditti C, Pagel M, Shay JW, et al. Comparative Biology of Mammalian Telomeres: Hypotheses on Ancestral States and the Roles of Telomeres in Longevity Determination. Aging Cell. 2011;10(5):761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Animal behaviour monographs. 1968;1:161–311. [Google Scholar]
- Goodall J. Population dynamics during a 15 year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Zeitschrift für Tierpsychologie. 1983;61(1):1–60. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: patterns of behavior. Cambridge, Mass: Belknap Press of Harvard University Press; 1986. p. 673. 678 p. of plates p. [Google Scholar]
- Gorbunova V, Seluanov A. Coevolution of telomerase activity and body mass in mammals: from mice to beavers. Mech Ageing Dev. 2009;130(1–2):3–9. doi: 10.1016/j.mad.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256(2–6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Heidinger BJ, Herborn KA, Granroth-Wilding HMV, Boner W, Burthe S, Newell M, Wanless S, Daunt F, Monaghan P. Parental age influences offspring telomere loss. Funct Ecol. 2015 n/a-n/a. [Google Scholar]
- Hembree WC, Cohen-Kettenis P, Waal HAD-vd, Gooren LJ, Walter J, Meyer I, Spack NP, Tangpricha V, Montori VM. Endocrine Treatment of Transsexual Persons: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2009;94(9):3132–3154. doi: 10.1210/jc.2009-0345. [DOI] [PubMed] [Google Scholar]
- Hjelmborg JB, Dalgård C, Mangino M, Spector T, Halekoh U, Möller S, Kimura M, Horvath K, Kark JD, Christensen K. Paternal age and telomere length in twins: the germ stem cell selection paradigm. Aging Cell. 2015 doi: 10.1111/acel.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon-4 and dementia. Ann Neurol. 2006;60(2):181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Ishii A, Nakamura K, Kishimoto H, Honma N, Aida J, Sawabe M, Arai T, Fujiwara M, Takeuchi F, Kato M, et al. Telomere shortening with aging in the human pancreas. Exp Gerontol. 2006;41(9):882–886. doi: 10.1016/j.exger.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Kakuo S, Asaoka K, Ide T. Human is a unique species among primates in terms of telomere length. Biochem Biophys Res Commun. 1999;263(2):308–314. doi: 10.1006/bbrc.1999.1385. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PLC, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, Cupples A, Hunkin JL, Gardner JP, Lu X, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008a;4(2):e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel JW, Aviv A, Christensen K. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008b;167(7):799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Eisenberg DTA. The Long Reach of History: Intergenerational Pathways to Plasticity in Human Lifespan. In: Weinstein M, Lane MA, editors. Sociality, hierarchy, health comparative biodemography : a collection of papers. Washington, DC: The National Academies Press; 2014. pp. 65–94. [PubMed] [Google Scholar]
- Langergraber KE, Prüfer K, Rowney C, Boesch C, Crockford C, Fawcett K, Inoue E, Inoue-Muruyama M, Mitani JC, Muller MN, et al. Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proc Natl Acad Sci. 2012;109(39):15716–15721. doi: 10.1073/pnas.1211740109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapham K, Kvale MN, Lin J, Connell S, Croen LA, Dispensa BP, Fang L, Hesselson S, Hoffmann TJ, Iribarren C, et al. Automated Assay of Telomere Length Measurement and Informatics for 100, 000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics. 2015 doi: 10.1534/genetics.115.178624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30. [Google Scholar]
- Newton-Fisher NE, Thompson ME, Reynolds V, Boesch C, Vigilant L. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am J Phys Anthropol. 2010;142(3):417–428. doi: 10.1002/ajpa.21241. [DOI] [PubMed] [Google Scholar]
- Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201(6):1496–1499. [PubMed] [Google Scholar]
- Olsson M, Pauliny A, Wapstra E, Uller T, Schwartz T, Blomqvist D. Sex Differences in Sand Lizard Telomere Inheritance: Paternal Epigenetic Effects Increases Telomere Heritability and Offspring Survival. PLoS ONE. 2011;6(4):e17473. doi: 10.1371/journal.pone.0017473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J, Du M, Wong JY, Han J, De Vivo I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum Reprod. 2012;27(12):3622–3631. doi: 10.1093/humrep/des314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short RV. THE TESTIS: THE WITNESS OF THE MATING SYSTEM, THE SITE OF MUTATION AND THE ENGINE OF DESIRE. In: Reeve ECR, Black I, editors. Encyclopedia of genetics. London ; Chicago: Fitzroy Dearborn; 2001. pp. 559–563. [Google Scholar]
- Simmons LW, Firman RC, Rhodes G, Peters M. Human sperm competition: testis size, sperm production and rates of extrapair copulations. Anim Behav. 2004;68(2):297–302. [Google Scholar]
- Smith E, Hill K, Marlowe F, Nolin D, Wiessner P, Gurven M, Bowles S, Mulder M, Hertz T, Bell A. Wealth transmission and inequality among hunter gatherers. Curr Anthropol. 2010;51(1) doi: 10.1086/648530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Jungers WL. Body mass in comparative primatology. J Hum Evol. 1997;32(6):523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- Steinert S, White DM, Zou Y, Shay JW, Wright WE. Telomere biology and cellular aging in nonhuman primate cells. Exp Cell Res. 2002;272(2):146–152. doi: 10.1006/excr.2001.5409. [DOI] [PubMed] [Google Scholar]
- Tackney J, Cawthon RM, Coxworth JE, Hawkes K. Blood cell telomere lengths and shortening rates of chimpanzee and human females. Am J Hum Biol. 2014;26(4):452–460. doi: 10.1002/ajhb.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unryn BM, Cook LS, Riabowol KT. Paternal age is positively linked to telomere length of children. Aging Cell. 2005;4(2):97–101. doi: 10.1111/j.1474-9728.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- Venn O, Turner I, Mathieson I, de Groot N, Bontrop R, McVean G. Strong male bias drives germline mutation in chimpanzees. Science. 2014;344(6189):1272–1275. doi: 10.1126/science.344.6189.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Susser E, Factor-Litvak PR, Simons MJ, Benetos A, Steenstrup T, Kark JD, Aviv A. Commentary: The reliability of telomere length measurements. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wojcicki JM, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E. Early exclusive breastfeeding is associated with longer telomeres in Latino preschool children. The American Journal of Clinical Nutrition. 2016 doi: 10.3945/ajcn.115.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. Covariance between Testes Size and Substitution Rates in Primates. Mol Biol Evol. 2014;31(6):1432–1436. doi: 10.1093/molbev/msu091. [DOI] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim Behav. 2009;77(4):873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashima K, Maitra A, Rogers BB, Timmons CF, Rathi A, Pinar H, Wright WE, Shay JW, Gazdar AF. Expression of the RNA component of telomerase during human development and differentiation. Cell Growth Differ. 1998;9(9):805–813. [PubMed] [Google Scholar]
- Zalenskaya IA, Zalensky AO. Telomeres in Mammalian Male germline Cells. In: Kwang WJ, editor. Int Rev Cytol. Academic Press; 2002. pp. 37–67. [DOI] [PubMed] [Google Scholar]