Abstract
Introduction
The burden of inflammatory bowel disease (IBD) in the older population is increasing. Older-onset disease is associated with reduced use of immunosuppressive medications. In addition, older patients may be more vulnerable to the effect of disease-related symptoms, and consequently may experience worse health-related quality of life (HRQoL) compared to younger patients.
Methods
This prospective study included a cohort of patients with Crohn’s disease (CD) and ulcerative colitis (UC) recruited from a single center. All patients completed the short inflammatory bowel disease questionnaire (SIBDQ) and the short form-12 questionnaire (SF-12) yielding general physical (PCS) and mental component scale subscores (MCS). Patients older than age 60 years were compared to those younger than age 60 using multivariable regression analysis.
Results
Our study included 1,607 patients, among whom 186 were older than age 60 at the time of assessment. Older patients were more likely to have isolated colonic disease and less likely to use immunosuppressive therapy. On multivariable analysis, older IBD patients had higher SIBDQ (2.34, 95% confidence interval (CI) 0.82 – 3.87) and SF12 mental subscores (3.78, 95% CI 2.26 – 5.30) but lower physical HRQoL (−1.80, 95% CI −3.21 to −0.38). There was no difference in the SIBDQ and PCS scores between older patients with newly diagnosed IBD or with established disease.
Conclusion
Older age was associated with modestly higher SIBDQ and mental HRQoL but lower physical HRQoL. Comprehensive care of the older IBD patient should include assessment of factors impairing physical quality of life to ensure appropriate interventions.
Keywords: quality of life, elderly, older age, inflammatory bowel disease
INTRODUCTION
Inflammatory bowel diseases (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, relapsing conditions of the gastrointestinal tract affecting an estimated 1.6 million individuals in the United States1. With a peak incidence between the second and fourth decades of life, IBD is associated with significant morbidity including in many the need for lifelong immunosuppression, hospitalizations, and surgery1–5. In addition, the physical, emotional, and psychosocial impact of IBD poses substantial burden on patients’ personal health experience adversely affecting their health-related quality of life (HRQoL)6–8.
With aging of the population and a negligible impact of IBD on mortality, the burden of IBD in the elderly is increasing9–15. Prior studies have examined phenotypic differences between older-onset IBD and younger onset disease, concluding that the former is associated with more frequent isolated colonic involvement, and fewer stricturing and penetrating complications9–18. Comparison of the disease severity between the two groups has yielded more conflicting results with similar or higher rates of surgery in older IBD patients, but less frequent use of immunosuppression and biologic therapies11, 16, 19–21. The reduced use of immunosuppressive agents in spite of similar surgical rates (suggesting perhaps disease severity is not different between younger and older patients) raises the concern for potential under-treatment of active disease in older patients owing to concerns about side effects of medications. One can hypothesize that this hesitation in escalating medical therapy may result in inadequate control of symptoms, which, together with a diminished physical reserve, may lead to more profound impairments of HRQoL in the older IBD patient. Few previous studies have compared general and disease-specific quality of life between older and younger IBD populations. Yet examining this difference could help accurately assess patients’ subjective perception of their health state and suggest approaches to age-appropriate healthcare planning and personalized management of this life-long disease.
Consequently, we performed this study in a large well-characterized prospectively recruited IBD cohort: (1) to compare the IBD-specific HRQoL in older IBD patients compared to younger patients; (2) to analyze differences in general quality of life between the two populations; and (3) to define the impact of disease activity on quality of life among older and younger IBD patients with the hypothesis that older patients may experience more negative consequences of persistent disease activity.
METHODS
Study Population
The study included patients recruited into the Prospective Registry in IBD Study at Massachusetts General Hospital (PRISM). This is an ongoing prospective cohort of adult patients diagnosed with CD, UC, or IBD-unspecified (IBDU) receiving care at the Massachusetts General Hospital Crohn’s and Colitis center22–25. Upon obtaining informed consent, a detailed questionnaire assessing disease and treatment related information was completed by interview with a trained clinical research coordinator and confirmed by review of the medical records. The older IBD cohort was defined as individuals of at least 60 years of age at the time of assessment. This group was further subdivided into older IBD patients with newly diagnosed disease and those who were diagnosed before age 60 years and had long-standing established disease. Younger IBD patients less than 60 years of age comprised the control population.
Covariates and Outcomes
Detailed information was collected about age of onset, disease location and behavior (in CD) and extent (in UC) according to the Montreal classification26, and past and current medical and surgical history. Laboratory values at the time of enrollment included a measurement of hemoglobin, C-reactive protein and erythrocyte sedimentation rate within two weeks of the enrollment visit. Each patient completed either the Harvey Bradshaw index (HBI)27 in CD or simple clinical colitis activity index (SCCAI)28 in UC and IBDU.
The two primary outcomes for this study were IBD-specific and general quality of life scores. IBD-specific QOL was assessed using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ)29. This questionnaire assesses quality of life over the previous week by scoring responses to 10 questions on a scale of 1 through 7; a total score of 70 represents perfect HRQoL. General quality of life was assessed using the short form-12 questionnaire (SF-12)30, 31. This 12-item questionnaire provides responses which can be combined, weighted, and scored to create two meta-scores, the physical health composite scale score (PCS) and the mental health composite scale score (MCS), each ranging from 0 to 10030. Higher values indicate a better perception of the general health. Both MCS and PCS have a mean of 50 and a standard deviation of 10 in the general US population.
Statistical Analysis
Continuous variables were summarized using means and standard deviations and compared using the t-test. Categorical variables were expressed as proportions and compared using the chi-square test with the Fisher’s exact modification where appropriate. Univariate linear regression with SIBDQ as the outcome was performed to identify predictive variables. Multivariable regression models adjusting for these covariates and those previously described to impact HRQoL were used to examine the independent effect of older age on quality of life. A two-sided p-value < 0.05 indicated statistical significance. Similar analyses were repeated for the SF-12 PCS and MCS scores separately. For the primary analysis, both scores were modeled as a continuous variable. In a sensitivity analysis, they were modeled as dichotomous scores with values below 50 (mean) indicating poor physical or mental quality of life. Pre-specified stratified analysis was carried out by type of IBD, and by age of onset among the older IBD group. The influence of disease activity on HRQoL was examined by calculating the Pearson correlation co-efficient between the SCCAI or HBI and SIBDQ in the two population groups. To examine the effect of IBD, the SF-12 PCS and MCS scores in the IBD cohort were compared to a group of healthy controls recruited for the registry. The study was approved by the Institutional Review Board of Partners Healthcare.
RESULTS
Study population
Our study included 1,607 patients with IBD who completed both quality of life questionnaires. Among these, 186 patients (99 CD, 87 UC) were older than age 60 years (mean age 68 years; range 61 – 94 years) while the remaining 1421 younger IBD patients (801 CD, 620 UC) had a mean age of 36 years (range 18 – 60 years) (Table 1). The older IBD patients had a greater age at diagnosis (49 vs. 26), longer disease duration (19 vs. 10 years) and were more likely to be male and former smokers (p ≤ 0.001 for all). Older CD patients were less likely to have penetrating disease behavior and perianal involvement and more likely to have isolated colonic disease (30% vs. 24%). No differences were noted in UC extent between the two groups. As has been noted before, older IBD patients were less likely to be ever users of immunomodulators (51% vs. 62%, p < 0.001) or anti-tumor necrosis factor α (anti-TNF) biologics (29% vs. 49%, p=0.027). At enrollment, there was no difference in disease activity between the older and younger IBD cohorts with similar HBI (3.7 vs. 3.3, p=0.62) and SCCAI scores (2.6 vs. 2.4, p=0.55). There was also no difference in the C-reactive protein (9.1 vs, 7.9 mg/L), hemoglobin (13.6g/dL vs. 13.7g/dL), and ESR (18.2mm/hr vs. 22.5mm/hr) between the two groups.
Table 1.
Characteristics of the study population
| Older IBD cohort (n=186) | Younger IBD cohort (n=1421) | p value | |
|---|---|---|---|
| Mean age at enrollment (in years) (SD) | 67.7 (5.8) | 36.1 (11.2) | |
| Mean age at diagnosis (in years) (SD) | 48.5 (18.4) | 26.4 (10.8) | < 0.001 |
| Mean disease duration (in years) (SD) | 19.2 (17.0) | 9.7 (8.2) | < 0.001 |
| Sex (% female) | 42 | 55 | 0.001 |
| Ever smoker, % | 60 | 30 | < 0.001 |
| IBD type, % | 0.417 | ||
| CD | 53 | 56 | |
| UC | 47 | 44 | |
| CD location, % | 0.036 | ||
| Terminal ileum | 26 | 25 | |
| Isolated colonic | 30 | 24 | |
| Ileocolonic | 42 | 50 | |
| Upper gastrointestinal | 2 | 0 | |
| CD phenotype, % | 0.088 | ||
| Inflammatory | 52 | 49 | |
| Stricturing | 28 | 21 | |
| Penetrating | 20 | 30 | |
| Perianal CD, % | 18 | 26 | 0.096 |
| UC extent, % | 0.406 | ||
| Proctosigmoiditis | 13 | 14 | |
| Left-sided colitis | 38 | 31 | |
| Pancolitis | 49 | 56 | |
| Prior IBD surgery, % | 34 | 32 | 0.525 |
| Steroid use, % | 0.077 | ||
| Current | 15 | 12 | |
| Past | 61 | 69 | |
| Never | 24 | 19 | |
| 5-aminosalicylates use, % | 0.277 | ||
| Current | 22 | 20 | |
| Past | 62 | 67 | |
| Never | 16 | 13 | |
| Immunomodulators use, % | 0.027 | ||
| Current | 22 | 25 | |
| Past | 30 | 36 | |
| Never | 49 | 38 | |
| Anti-TNF biologics, % | < 0.001 | ||
| Current | 10 | 24 | |
| Past | 20 | 25 | |
| Never | 71 | 51 | |
| Harvey Bradshaw index | 3.7 (3.9) | 3.3 (3.8) | 0.623 |
| SCCAI | 2.6 (2.7) | 2.4 (2.8) | 0.553 |
| CRP, mg/L | 7.9 (15.9) | 9.1 (16.4) | 0.536 |
| HGB, gm/dL | 13.7 (3.5) | 13.6 (2.5) | 0.690 |
| ESR, mm/hr | 22.5 (20.4) | 18.2 (19.5) | 0.084 |
SD – standard deviation; IBD – inflammatory bowel disease; CD – Crohn’s disease; UC – ulcerative colitis; SCCAI – simple clinical colitis activity index; CRP – c-reactive protein; HGB – hemoglobin; ESR – erythrocyte sedimentation rate
One-third of the patients in the older IBD cohort were diagnosed after the age of 60 years (34%) with a mean age of diagnosis of 67 years and a disease duration of 4 years (newly diagnosed older IBD cohort). The remaining were diagnosed at a mean age of 39 years and had disease for duration of 27 years (established older IBD cohort).
Comparison of IBD-specific and general HRQoL between elderly and younger patients
Among all included patients, the older IBD cohort had a modestly greater SIBDQ score when compared to younger IBD patients (56.2±10.4 vs. 54.4±11.3, p=0.04). Older patients were also more likely to have higher MCS scores on the SF-12 questionnaire (51.2±9.4 vs. 48.1±9.8, p < 0.001) but a lower PCS score (46.6±10.3 vs. 48.6±9.6, p=0.010) (Figure 1). These findings remained significant on multivariable analysis (Table 2). Adjusting for potential confounders, older IBD patients had a two-point higher SIBDQ compared to younger IBD patients (regression co-efficient 2.34, 95% confidence interval (CI) 0.82 – 3.87). The older cohort also had higher adjusted MCS scores (3.78, 95% CI 2.26 – 5.30) and lower PCS scores (−1.80, 95% CI −3.21 to −0.38) with all comparisons achieving independent statistical significance. We then modeled PCS and MCS scores as dichotomous outcomes, defining a score less than 50 to indicate poor quality of life. On multivariable analysis, older patients were more likely to have poor physical quality of life (Odds ratio (OR) 1.49, 95% CI 1.02 – 2.17) but significantly less likely to have a mental quality of life score below the mean (OR 0.48, 96% CI 0.33 – 0.69).
Figure 1. Comparison of quality of life in older and younger patients with inflammatory bowel diseases.
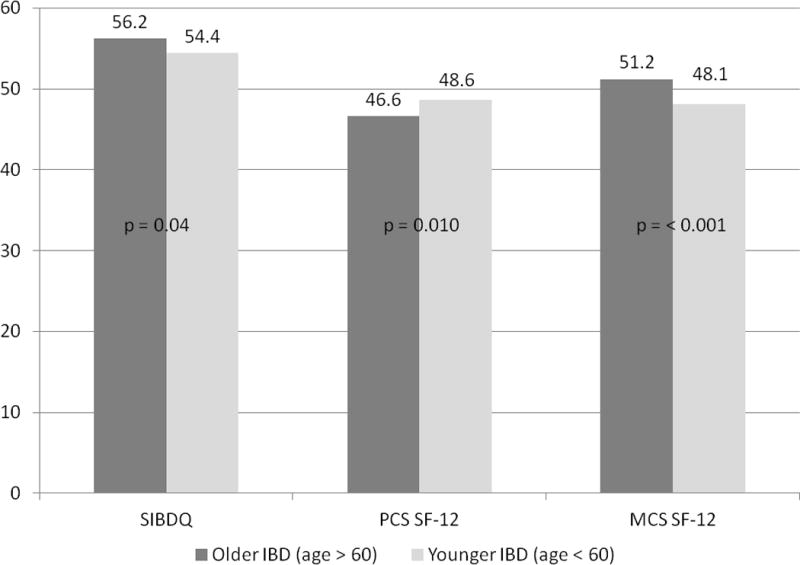
SIBDQ – short inflammatory bowel disease questionnaire; SF-12 short form health survey; PCS – physical composite scale score; MCS – mental composite scale score
Table 2.
Multivariable analysis of predictors of disease-related and general quality of life in patients with inflammatory bowel diseases
| Coefficient | 95% confidence interval | |
|---|---|---|
| SIBDQ | ||
| Age > 60 years (vs. younger IBD) | 2.34 | 0.82 – 3.87 |
| Male gender (vs female) | 2.33 | 1.38 – 3.27 |
| UC (vs CD) | 1.79 | 0.82 – 2.75 |
| Active disease† | −12.11 | −13.14 – −11.1 |
| Ever steroid use | −1.80 | −2.65 – −0.94 |
| Ever anti-TNF use | −0.80 | −1.40 – −0.21 |
| Ever smoker | −2.10 | −3.11 – −1.08 |
| SF-12 PCS | ||
| Age > 60 years (vs. younger IBD) | −1.80 | −3.21 – −0.38 |
| Male gender (vs female) | 1.45 | 0.59 – 2.32 |
| UC (vs CD) | 2.60 | 1.72 – 3.50 |
| Active disease† | −8.49 | −9.43 – −7.54 |
| Ever steroid use | −2.20 | −2.99 – −1.41 |
| Ever anti-TNF use | −0.90 | −1.45 – −0.35 |
| Ever smoker | −1.48 | −2.42 – −0.55 |
| SF-12 MCS | ||
| Age > 60 years (vs. younger IBD) | 3.78 | 2.26 – 5.30 |
| Male | 1.44 | 0.50 – 2.38 |
| Active disease† | −6.09 | −7.11 – −5.07 |
| Ever steroid use | −0.94 | −1.79 – −0.10 |
| Ever smoker | −1.82 | −2.84 – −0.81 |
Active disease was defined as an SCCAI > 2 in UC and HBI > 4 in CD
UC – ulcerative colitis; CD – Crohn’s disease; SIBDQ – short inflammatory bowel disease questionnaire; SF-12 short form health survey; PCS – physical composite scale score; MCS – mental composite scale score
Supplemental table 1 presents the results stratifying by type of IBD. No statistical difference in the SIBDQ was noted between older and younger patients among those with CD or UC. However, a significantly greater MCS and lower PCS remained among older CD patients compared to younger CD patients, and similarly with UC. Compared to healthy controls aged 50 and higher, older IBD patients had significantly lower SF-12 PCS (47.2 vs 53.3, p=0.025) and SF-12 MCS scores (49.2 vs55.1, p=0.027). Younger IBD patients also had a lower SF-12 PCS and MCS scores than healthy controls in the same age-group.
Comparison of newly diagnosed and established older IBD patients
There was no difference in the SIBDQ score between older IBD patients with established disease or those with a recent diagnosis (57.8 vs. 56.5, p=0.56). As well, there was no difference in the PCS or MCS components of the SF-12 except in older UC patients where those with a recent diagnosis had higher MCS scores than those with prolonged duration of disease (Supplemental Table 2). These findings remained on adjustment for other covariates.
Factors associated with quality of life
On multivariable analysis, male gender (co-efficient 2.33, 95% CI 1.38 – 3.27) and a diagnosis of UC (1.79, 95% CI 0.82 – 2.75) were associated with higher SIBDQ scores than women and those with CD respectively. Disease activity at enrollment was the strongest inverse predictor of SIBDQ with each 1 point increase in the HBI or SCCAI scores being associated with a 12-point lower SIBDQ score (−12.11, 95% CI −13.14 to −11.10). In addition, use of steroids (−1.80, 95% CI −2.65 to −0.94), anti-TNF biologics (−0.80, 95% CI −1.40 to −0.21), and ever smoking (−2.10, 95% CI −3.11 to −1.08) were independently associated with lower SIBDQ scores. In contrast, disease duration, location, extent, and phenotype did not predict HRQoL.
Given the strong impact of disease activity on SIBDQ, we examined if this correlation was stronger in older compared to younger patients under the hypothesis that impaired mobility and lower functional reserve in the elderly may lead to them experiencing worse quality of life for a given disease activity. However, we found a statistically similar correlation between disease activity and SIBDQ (rho = −0.54 vs. −0.65), PCS (rho= −0.43 vs. −0.54), and MCS scores (rho= −0.54 vs. −0.40) in older and younger IBD patients respectively, indicating a similar impact of disease activity on HRQoL regardless of age.
DISCUSSION
The older patient represents a subgroup that is particularly vulnerable to the impact of chronic disease. As many as one in five patients with IBD are older than age 60 years and this population is only expected to increase14, 16, 18, 32, 33. While a growing body of literature has studied the older IBD patient, the focus has been almost exclusively on differences in disease phenotype or in outcomes such as use of medications, hospitalizations, and surgery10–18, 32, 34. It is well recognized that an individual’s perception of impact of disease on quality of life is an important consequence of IBD. To our knowledge, few prior studies have examined IBD-specific and general quality of life in older patients with IBD.
One may hypothesize that older patients would be particularly vulnerable to some of the symptoms of IBD such as diarrhea and incontinence that may impair functional independence. However, an important and reassuring finding from our study was that this did not appear to be so with the SIBDQ actually being modestly higher in older IBD patients compared with younger ones. That this finding persisted on adjusting for disease phenotype and activity suggests that a more aggressive course in younger patients with newer onset disease is unlikely to explain this difference. We also found no difference in the quality of life between older patients with prolonged disease duration and newly diagnosed elderly IBD patients. This lack of effect of disease duration was also demonstrated previously in a study where the mean IBDQ scores for CD patients of more than 20 years duration was not significantly different from those newly diagnosed35. Importantly, in our study, the contribution of disease activity to the SIBDQ was similar between older and younger patients, suggesting that similar improvements in disease-specific HRQoL may be obtainable through mitigation of disease activity in both groups. Comparative studies on which is the most effective and safe way to achieve this endpoint, be it medical or surgical treatment, are essential as achievement of remission, rather than the modality (medical or surgical) to achieve it is an important determinant of HRQoL36.
Older patients demonstrated a significantly lower physical component score of the SF-12. In contrast, the mental component score of the SF-12 was better in older patients compared to younger individuals. This interesting dichotomy between the direction of effect of age on physical and mental quality have been reported for other chronic illnesses. In a large study of chronically ill individuals in Australia, compared with younger patients, older patients had a 6 point higher MCS score but an 8-point lower PCS score37. A few previous studies have found a similar association between age and quality of life in IBD patients38. In a cross-sectional study of Israeli adult IBD patients, Slonim-Nevo et al. reported SIBDQ and SF-36 MCS scores were positively associated with age, while SF-36 PCS scores were negatively associated with age39. One explanation for the reduction in the PCS-12 score compared to the other scales used in the elderly is that a greater comorbidity burden in older patients may result in impairment of QOL that is not related to their underlying IBD but a reflection of the burden of illness. In addition, factors such as restricted mobility, depression, unemployment, widowed status, and reduction of functional independence may contribute to a lower perceived physical quality of life in older patients40, 41. This finding emphasizes that in delivering comprehensive care to the older IBD patient, in addition to control of their IBD, it is essential for focus to be on these other factors that affect physical quality of life in these patients. The intriguingly higher MCS may relate to superior adaptation, resilience, and coping in older patients compared to younger individuals42, 43.
As reported by other studies, disease activity was the strongest determinant of health-related quality of life in our cohort7, 35, 38, 44, 45. As well, consistent with prior studies, CD patients had lower quality of life scores than those with UC7, 38, 44. In addition to disease activity, we identified associations between poor HRQoL and the female gender, steroid and anti-TNF use, and tobacco history, while prior IBD-related surgery, disease duration, and 5-aminosalicylates use did not modified HRQoL measures. The lack of effect of prior surgical treatment is also consistent with Casellas et al. who showed than an improvement in IBDQ was achieved with attainment of remission through either medical or surgical interventions36, 46. While medical therapy has been consistently shown to improve HRQoL in IBD47, the evidence is not as robust for steroids where, as demonstrated by Blondel-Kucharski et al., general HRQoL measured by SF-36 were worse for patients on steroids, regardless of dose or disease activity45.
We readily acknowledge several limitations of our study. First, being based at a referral center, it may be susceptible to bias introduced by the greater severity of disease in our population. Second, while representing one of the largest cohorts to our knowledge to examine disease-specific and overall HRQoL in IBD patients, there may be lower statistical power to determine effect in specific subgroups. Third, we assessed disease activity and QOL at a single time point which may not reflect longitudinal evolution of impact of disease. Fourth, we did not systematically screen for depression or evaluate physical functioning, frailty, and social support which may all influence general health-related quality of life.
In conclusion, assessment of disease-specific and general health-related quality of life is an important part of IBD care. We provide reassurance that the older IBD patient does not have a significantly lower IBD-specific quality of life but that such patients do have a lower general quality of life physical score suggesting the impact of factors beyond IBD severity. Thus, comprehensive care of the older IBD patient must include not only mitigation of disease activity but assessment of factors contributing to impairment of physical quality of life and institution of appropriate interventions to improve patient outcomes.
Supplementary Material
Acknowledgments
Source of funding: This work is supported by the National Institutes of Health (NIH) (P30 DK043351) to the Center for Study of Inflammatory Bowel Diseases. Ananthakrishnan is supported in part by a grant from the National Institutes of Health (K23 DK097142).
Footnotes
Conflicts of Interest: Ananthakrishnan has served on scientific advisory boards for Abbvie, Takeda, and Merck.
Authorship statement:
Velonias: study design, data collection, analysis and interpretation of data, drafting of the manuscript
Conway, Andrews, Khalili, Garber, Yajnik: study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content
Ananthakrishnan: study design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54 e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Loftus EV, Jr, Ng SC, et al. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622–9. doi: 10.1136/gutjnl-2011-301397. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Ng SC, Lakatos PL, et al. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001–10. doi: 10.1097/MIB.0b013e318281f3bb. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 5.Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–49. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 6.Alrubaiy L, Rikaby I, Dodds P, et al. Systematic review of health-related quality of life measures for inflammatory bowel disease. J Crohns Colitis. 2015;9:284–92. doi: 10.1093/ecco-jcc/jjv002. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RD. The quality of life in patients with Crohn’s disease. Aliment Pharmacol Ther. 2002;16:1603–9. doi: 10.1046/j.1365-2036.2002.01323.x. [DOI] [PubMed] [Google Scholar]
- 8.Kemp K, Griffiths J, Lovell K. Understanding the health and social care needs of people living with IBD: a meta-synthesis of the evidence. World J Gastroenterol. 2012;18:6240–9. doi: 10.3748/wjg.v18.i43.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananthakrishnan AN, Binion DG. Treatment of ulcerative colitis in the elderly. Dig Dis. 2009;27:327–34. doi: 10.1159/000228569. [DOI] [PubMed] [Google Scholar]
- 10.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis. 2009;15:182–9. doi: 10.1002/ibd.20628. [DOI] [PubMed] [Google Scholar]
- 11.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease A population-based cohort study. Gut. 2014;63:423–432. doi: 10.1136/gutjnl-2012-303864. [DOI] [PubMed] [Google Scholar]
- 12.Fabricius PJ, Gyde SN, Shouler P. Crohn’s disease in the elderly. Gut. 1985;26:461–465. doi: 10.1136/gut.26.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Saverymuttu SH, Keshavarzian A, et al. Is the pattern of inflammatory bowel disease different in the elderly? Age and Ageing. 1985;14:366–370. doi: 10.1093/ageing/14.6.366. [DOI] [PubMed] [Google Scholar]
- 14.Ha CY, Katz S. Clinical implications of ageing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2014;11:128–38. doi: 10.1038/nrgastro.2013.241. [DOI] [PubMed] [Google Scholar]
- 15.Jason H, Linda F, Akbar W. Characteristics and behavior of elderly-onset inflammatory bowel disease: A multi-center U.S. Study. Inflammatory Bowel Diseases. 2014;20:S40–S41. doi: 10.1097/MIB.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 16.Ananthakrishnan AN, Shi HY, Tang W, et al. Systematic Review and Meta-analysis: Phenotype and Clinical Outcomes of Older-onset Inflammatory Bowel Disease. J Crohns Colitis. 2016 doi: 10.1093/ecco-jcc/jjw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadilla J, Ferreiro R, Ollero V, et al. Clinical characteristics of inflammatory bowel disease in elderly patients. Journal of Crohn’s and Colitis. 2013;7:S276. [Google Scholar]
- 18.del Val JH. Old-age inflammatory bowel disease onset: a different problem? World J Gastroenterol. 2011;17:2734–9. doi: 10.3748/wjg.v17.i22.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Cheon JH, Moon CM, et al. Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older? Digestion. 2010;81:237–43. doi: 10.1159/000253850. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto S, Miyatani H, Yoshida Y. Ulcerative colitis: comparison between elderly and young adult patients and between elderly patients with late-onset and long-standing disease. Dig Dis Sci. 2013;58:1306–12. doi: 10.1007/s10620-012-2517-5. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen GC, Sheng L, Benchimol EI. Health Care Utilization in Elderly Onset Inflammatory Bowel Disease: A Population-based Study. Inflammatory Bowel Diseases. 2015;21:777–782. doi: 10.1097/MIB.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 22.Ananthakrishnan AN, Huang H, Nguyen DD, et al. Differential effect of genetic burden on disease phenotypes in Crohn’s disease and ulcerative colitis: analysis of a North American cohort. Am J Gastroenterol. 2014;109:395–400. doi: 10.1038/ajg.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:309–15. doi: 10.1002/ibd.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oxford EC, Nguyen DD, Sauk J, et al. Impact of coexistent celiac disease on phenotype and natural history of inflammatory bowel diseases. Am J Gastroenterol. 2013;108:1123–9. doi: 10.1038/ajg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taleban S, Stewart KO, Li DK, et al. Clinical Activity and Quality of Life Indices Are Valid Across Ulcerative Colitis But Not Crohn’s Disease Phenotypes. Dig Dis Sci. 2016;61:2627–35. doi: 10.1007/s10620-016-4180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 28.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–8. [PubMed] [Google Scholar]
- 30.Assessment OoPH. Health Status in Utah: The Medical Outcomes Study SF-12 (2001 Utah Health Status Survey Report) Salt Lake City, UT: Utah Department of Health; 2004. [Google Scholar]
- 31.Ganz ML, Sugarman R, Wang R, et al. The Economic and Health-related Impact of Crohn’s Disease in the United States: Evidence from a Nationally Representative Survey. Inflamm Bowel Dis. 2016;22:1032–41. doi: 10.1097/MIB.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 32.Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–77. doi: 10.1111/apt.12616. [DOI] [PubMed] [Google Scholar]
- 33.Katz S, Pardi DS. Inflammatory bowel disease of the elderly: frequently asked questions (FAQs) Am J Gastroenterol. 2011;106:1889–97. doi: 10.1038/ajg.2011.271. [DOI] [PubMed] [Google Scholar]
- 34.Heresbach D, Alexandre JL, Bretagne JF, et al. Crohn’s disease in the over-60 age group: A population based study. European Journal of Gastroenterology and Hepatology. 2004;16:657–664. doi: 10.1097/01.meg.0000108337.41221.08. [DOI] [PubMed] [Google Scholar]
- 35.Canavan C, Abrams KR, Hawthorne B, et al. Long-term prognosis in Crohn’s disease: factors that affect quality of life. Aliment Pharmacol Ther. 2006;23:377–85. doi: 10.1111/j.1365-2036.2006.02753.x. [DOI] [PubMed] [Google Scholar]
- 36.Casellas F, Lopez-Vivancos J, Badia X, et al. Impact of surgery for Crohn’s disease on health-related quality of life. Am J Gastroenterol. 2000;95:177–82. doi: 10.1111/j.1572-0241.2000.01681.x. [DOI] [PubMed] [Google Scholar]
- 37.Jayasinghe UW, Proudfoot J, Barton CA, et al. Quality of life of Australian chronically-ill adults: patient and practice characteristics matter. Health Qual Life Outcomes. 2009;7:50. doi: 10.1186/1477-7525-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin GP, Hungin AP, Chinn DJ, et al. Quality of life in patients with established inflammatory bowel disease: a UK general practice survey. Aliment Pharmacol Ther. 2004;19:529–35. doi: 10.1111/j.1365-2036.2004.1873.x. [DOI] [PubMed] [Google Scholar]
- 39.Slonim-Nevo V, Sarid O, Friger M, et al. Effect of psychosocial stressors on patients with Crohn’s disease: threatening life experiences and family relations. Eur J Gastroenterol Hepatol. 2016;28:1073–81. doi: 10.1097/MEG.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 40.Netuveli G, Blane D. Quality of life in older ages. Br Med Bull. 2008;85:113–26. doi: 10.1093/bmb/ldn003. [DOI] [PubMed] [Google Scholar]
- 41.Webb E, Blane D, McMunn A, et al. Proximal predictors of change in quality of life at older ages. J Epidemiol Community Health. 2011;65:542–7. doi: 10.1136/jech.2009.101758. [DOI] [PubMed] [Google Scholar]
- 42.Hildon Z, Montgomery SM, Blane D, et al. Examining resilience of quality of life in the face of health-related and psychosocial adversity at older ages: what is “right” about the way we age? Gerontologist. 2010;50:36–47. doi: 10.1093/geront/gnp067. [DOI] [PubMed] [Google Scholar]
- 43.Netuveli G, Wiggins RD, Montgomery SM, et al. Mental health and resilience at older ages: bouncing back after adversity in the British Household Panel Survey. J Epidemiol Community Health. 2008;62:987–91. doi: 10.1136/jech.2007.069138. [DOI] [PubMed] [Google Scholar]
- 44.Casellas F, Arenas JI, Baudet JS, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis. 2005;11:488–96. doi: 10.1097/01.mib.0000159661.55028.56. [DOI] [PubMed] [Google Scholar]
- 45.Blondel-Kucharski F, Chircop C, Marquis P, et al. Health-related quality of life in Crohn’s disease: a prospective longitudinal study in 231 patients. Am J Gastroenterol. 2001;96:2915–20. doi: 10.1111/j.1572-0241.2001.4681_b.x. [DOI] [PubMed] [Google Scholar]
- 46.Thaler K, Dinnewitzer A, Oberwalder M, et al. Assessment of long-term quality of life after laparoscopic and open surgery for Crohn’s disease. Colorectal Dis. 2005;7:375–81. doi: 10.1111/j.1463-1318.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 47.Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101–9. doi: 10.2147/ceg.s4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


