Abstract
In addition to their carcinogenic activity, polycyclic aromatic hydrocarbons (PAHs) are suspected to be developmental neurotoxicants. We evaluated the effects of PAHs with two in vitro models that assess distinct “decision nodes” in neurodifferentiation: neuronotypic PC12 cells, which characterize the transition from cell replication to neurodifferentiation, neurite outgrowth and neurotransmitter specification; and embryonic neural stem cells (NSCs), which evaluate the origination of neurons and glia from precursors. We compared an environmentally-derived PAH mixture from a Superfund contamination site (Elizabeth River Sediment Extract, ERSE) to those of a single PAH, benzo[a]pyrene (BaP). In PC12 cells, BaP impaired the transition from cell replication to neurodifferentiation, resulting in higher numbers of cells, but with reduced cell size and deficits in all indices of neuronal features (neurite formation, development of dopamine and acetylcholine phenotypes). ERSE was far less effective, causing only modest changes in cell numbers and size and no impairment of neurite formation or neurotransmitter specification; in fact, ERSE evoked a slight increase in emergence of the acetylcholine phenotype. In the NSC model, this relationship was entirely reversed, with far greater sensitivity to ERSE than to BaP. Furthermore, ERSE, but not BaP, enhanced NSC differentiation into neurons, whereas both ERSE and BaP suppressed the glial phenotype. Our studies provide a cause-and-effect relationship for the observed association of developmental PAH exposure to behavioral deficits. Further, PAH sensitivity occurs over developmental stages corresponding to rudimentary brain formation through terminal neurodifferentiation, suggesting that vulnerability likely extends throughout fetal brain development and into early childhood.
Keywords: Benzo[a]pyrene, Neural stem cells, Neurodifferentiation, PC12 cells, Polycyclic aromatic hydrocarbons
1. INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) have long been studied for their carcinogenic properties but more recent work indicates that these agents are also capable of disrupting brain development. Prenatal PAH exposure in children is associated with increased risk of subsequent behavioral deficits (Perera et al. 2012). Similar outcomes are seen with animal models of exposure to specific PAHs such as benzo[a]pyrene (BaP), which impairs neurodifferentiation and evokes corresponding neurobehavioral impairment (Chen et al. 2012; Hood et al. 2000; Slotkin and Seidler 2009; Wormley et al. 2004). The animal studies thus provide a cause-and-effect relationship for the human epidemiological findings. However, a major difference between human and animal studies is that real-world PAH exposures comprise complex mixtures of compounds, whereas laboratory findings tend to focus on individual PAH components. It is certainly reasonable to expect that various PAHs will have different propensities to act as developmental neurotoxicants, or that a complex mixture could produce outcomes greater than those anticipated from the total PAH burden. Indeed, we recently showed that BaP interacts with other neurotoxicants in an in vitro model of neurodifferentiation (Slotkin et al. 2013). Likewise, tobacco smoke, a complex PAH-containing mixture, augments the adverse effects of nicotine directed toward neurodifferentiation and synaptic function (Slotkin et al. 2014, 2015). The current study focuses on providing a proof-of-principle that the effect of a complex PAH mixture on neurodifferentiation can be distinguished from those of a single model compound, BaP.
We chose an environmentally-relevant PAH mixture derived from the Elizabeth River in southeastern Virginia, designated as a Superfund contamination site by the U.S. Environmental Protection Agency. This estuary was the site of creosote-utilizing industries that resulted in heavy PAH contamination and associated damage to piscine populations. Elizabeth River Sediment Extract (ERSE) containing this complex PAH mixture, has been well-characterized chemically (Fang et al. 2014) and has been shown to evoke behavioral deficits and dysmorphology in Fundulus heteroclitus (killifish), a species native to the area (Brown et al. 2016). Behavioral deficits can emerge from a direct impact on neurodifferentiation or alternatively, from any number of indirect effects such as those on motor systems, or on other physiological events required to establish adult function. Accordingly, in the current study, we examined the effects of ERSE on neurodifferentiation in vitro, so as to determine whether it directly targets neuronal development, and also to evaluate how ERSE’s effects might differ from those of BaP. We chose cell systems that model two distinct “decision nodes” in neurodifferentiation, neuronotypic PC12 cells and rat embryonic neural stem cells (NSCs; PC12 cells are rat-derived, so the models are from the same species. PC12 cells are already committed to a neuronal phenotype and neurodifferentiation involves a number of key processes: changing from growth by cell replication to cell enlargement, extension of neuritic projections and selection of a neurotransmitter phenotype, dopamine or acetylcholine (Teng and Greene 1994). The PC12 model has been used in thousands of studies to evaluate many developmental neurotoxicants, including a full characterization of BaP (Slotkin et al. 2013, 2014; Slotkin and Seidler 2009). In contrast, the NSC model considers an earlier decision node, the point at which neural stem cells choose to become neurons or glia; for that purpose, we used NSCs derived from rat neuroepithelium on embryonic day 14, when phenotypic separation into neurons and glia is determined (Slotkin et al. 2016; Rodier 1988). We recently showed how diverse developmental neurotoxicants have the ability to divert neurodifferentiation toward or away from these two phenotypes (Slotkin et al. 2016).
2. MATERIALS & METHODS
2.1 ERSE preparation and analysis
ERSE was prepared from sediment samples obtained from the Atlantic Wood Industries Superfund site as described previously (Fang et al. 2014). Analysis of the PAH composition of ERSE was determined by gas chromatography/mass spectrometry (Fang et al. 2014) and is shown in Table 1. The total PAH concentration was 31 μM, so that equivalent PAH concentrations in our experiments can be readily calculated from the percentage of ERSE present in the cultures as indicated. The maximum ERSE studied was 10% addition to the culture medium by volume, corresponding to a final total PAH concentration of about 3 μM, thus encompassing the range of concentrations shown to disrupt neurobehavioral development and to elicit dysmorphogenesis in piscine models (Brown et al. 2016).
TABLE 1.
PAH COMPOSITION OF ELIZABETH RIVER SEDIMENT EXTRACT
| Compound | Molecular weight |
ppb* in ERSE |
μM in ERSE |
μM in medium at 1% ERSE |
μM in medium at 10% ERSE |
|---|---|---|---|---|---|
| Acenaphthene | 154 | 346 | 2.25 | 0.0225 | 0.225 |
| Acenaphthylene | 152 | 12.5 | 0.08 | 0.0008 | 0.008 |
| Anthracene | 178 | 109 | 0.61 | 0.0061 | 0.061 |
| 1,2-Benzanthracene | 228 | 99 | 0.43 | 0.0043 | 0.043 |
| Benzo(a)fluoranthene | 252 | 11.5 | 0.05 | 0.0005 | 0.005 |
| Benzo(b)chrysene | 278 | 4.6 | 0.02 | 0.0002 | 0.002 |
| Benzo(b)fluoranthene | 252 | 50 | 0.20 | 0.0020 | 0.020 |
| Benzo(k)fluoranthene | 252 | 33 | 0.13 | 0.0013 | 0.013 |
| 1,2-Benzofluorene | 216 | 56 | 0.26 | 0.0026 | 0.026 |
| 3,4-Benzofluorene | 216 | 13 | 0.06 | 0.0006 | 0.006 |
| Benzo(g,h,i)perylene | 276 | 17 | 0.06 | 0.0006 | 0.006 |
| Benzo(c)phenanthrene | 228 | 2.6 | 0.01 | 0.0001 | 0.001 |
| Benzo(a)pyrene | 252 | 51 | 0.20 | 0.0020 | 0.020 |
| Benzo(e)pyrene | 252 | 36 | 0.14 | 0.0014 | 0.014 |
| Carbazole | 167 | 232 | 1.39 | 0.0139 | 0.139 |
| Chrysene | 228 | 78 | 0.34 | 0.0034 | 0.034 |
| Dibenzo(a,h)anthracene | 278 | 3.5 | 0.01 | 0.0001 | 0.001 |
| Dibenzo(a,j)anthracene | 278 | 3.4 | 0.01 | 0.0001 | 0.001 |
| Dibenzo(a,l)pyrene | 302 | 5.7 | 0.02 | 0.0002 | 0.002 |
| Dibenzofuran | 168 | 173 | 1.03 | 0.0103 | 0.103 |
| Dibenzothiophene | 184 | 45 | 0.24 | 0.0024 | 0.024 |
| 2,6-Dimethylnaphthalene | 156 | 32 | 0.20 | 0.0020 | 0.020 |
| Fluoranthene | 202 | 506 | 2.51 | 0.0251 | 0.251 |
| Fluorene | 166 | 248 | 1.50 | 0.0150 | 0.150 |
| Indeno(1,2,3-c,d)pyrene | 276 | 4.4 | 0.02 | 0.0002 | 0.002 |
| 1-Methylnaphthalene | 142 | 155 | 1.09 | 0.0109 | 0.109 |
| 1-Methylphenanthrene | 191 | 55 | 0.29 | 0.0029 | 0.029 |
| 2-Methylphenanthrene | 191 | 49 | 0.26 | 0.0026 | 0.026 |
| Naphthalene | 128 | 1,591 | 12.4 | 0.1243 | 1.243 |
| Perylene | 252 | 11 | 0.04 | 0.0004 | 0.004 |
| Phenanthrene | 178 | 573 | 3.22 | 0.0322 | 0.322 |
| Picene | 278 | 18 | 0.06 | 0.0006 | 0.006 |
| Pyrene | 202 | 331 | 1.64 | 0.0164 | 0.164 |
| Retene | 234 | 55 | 0.23 | 0.0023 | 0.023 |
| Total PAH | 5045 | 31 | 0.31 | 3.1 |
parts per billion (ng/ml)
2.2 PC12 cell cultures and treatments
Because of the clonal instability of the PC12 cell line (Fujita et al. 1989), the experiments were performed on cells that had undergone fewer than five passages. As described previously (Qiao et al. 2003; Song et al. 1998), PC12 cells (American Type Culture Collection CRL-1721, obtained from the Duke Comprehensive Cancer Center, Durham, NC) were seeded onto poly-D-lysine-coated plates in RPMI-1640 medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% horse serum (Sigma), 5% fetal bovine serum (Sigma), and 50 μg/ml penicillin streptomycin (Invitrogen, Carlsbad, CA). Incubations were carried out with 5% CO2 at 37°C, standard conditions for PC12 cells. Twenty-four hours after plating, we initiated neurodifferentiation (Jameson et al. 2006b; Slotkin et al. 2007; Teng and Greene 1994) by changing the medium to include 50 ng/ml of 2.5 S murine nerve growth factor (Promega Corporation, Madison, WI). Test substances were added simultaneously so as to be present throughout neurodifferentiation: 10 μM BaP (Sigma) dissolved in dimethylsulfoxide (DMSO; Sigma), or ERSE concentrations ranging from 0.03% to 10% by volume. The medium was changed every 48 hr with the continued inclusion of nerve growth factor and test substances, and continued for 6 days. At the end of each study, the cultures were examined under a microscope to verify the outgrowth of neurites.
Control samples for the BaP studies contained the corresponding final concentration of DMSO vehicle (0.1%), which has no effect on PC12 cell viability or differentiation (Qiao et al. 2001, 2003; Song et al. 1998). Because the ERSE studies required dilution of the culture medium with up to 10% of water, we performed preliminary studies to evaluate the impact on cell number and differentiation. We found no effects of concentrations up to 1% and only a small effect (<5%) on any of the parameters with addition of 10% water. Accordingly, we ran separate water controls for the various concentrations of ERSE. To facilitate presentation, the values of the various experiments were normalized to a constant control value so they could be presented together; however, statistical evaluations were conducted only against the corresponding contemporaneous control with the same vehicle.
2.3 PC12 cell assays
Cells were harvested, washed, and the DNA and protein fractions were isolated and analyzed as described previously (Slotkin et al. 2007). Measurements of DNA, total protein and membrane protein were used as biomarkers for cell number, cell growth and neurite growth (Qiao et al. 2003; Song et al. 1998). Effects on cell number were determined by measuring DNA content, since each neuronotypic cell contains only a single, diploid nucleus (Winick and Noble 1965). DNA per cell is constant, so that cell growth entails an obligatory increase in total protein per cell (protein/DNA ratio) as well as in membrane protein per cell (membrane protein/DNA ratio). If cell growth represents simply an increase in the perikaryal area, then membrane protein decreases less than total protein because of the decline in the surface-to-volume ratio (volume increases with the cube of the perikaryal radius, whereas surface area increases with the square of the radius); however, when neurites are formed as a consequence of neurodifferentiation, this produces an increase in membrane protein larger than that predicted from this simple 2/3-power geometric relationship. Each of these biomarkers has been validated in prior studies by direct measurement of cell number (Powers et al. 2010; Roy et al. 2005), perikaryal area (Roy et al. 2005) and neurite formation (Das and Barone 1999; Howard et al. 2005; Song et al. 1998). To assess neurodifferentiation into dopamine and acetylcholine phenotypes, we assayed the enzymatic activities of tyrosine hydroxylase (TH) and choline acetyltransferase (ChAT), respectively, using established techniques (Jameson et al. 2006a, b).
2.4 NSC cultures and treatments
The techniques for NSC preparation, culturing and assays have all appeared previously (Slotkin et al. 2016). Primary neural stem cells (passage zero; MTI-GlobalStem, Gaithersburg, MD) were isolated from rat cortical neuroepithelium on embryonic day 14 and were frozen in DMEM/F-12 medium with N2 supplement (MTIGlobalStem) and 10% DMSO. Cells were thawed and plated at 35,000 cells/cm2 on 12 mm coverslips pre-coated with poly-L-ornithine, contained in 24-well culture plates. The culture medium consisted of DMEM/F-12, GlutaMAX™ with N2 Supplement, 20 ng/ml human fibroblast growth factor and 20 ng/ml epidermal growth factor (all from MTI-GlobalStem). Cultures were maintained in a humidified incubator at 37° C with 5% CO2. Twenty-four hours later, the medium was changed to initiate spontaneous differentiation by eliminating the two growth factors, with the addition of 200 μM ascorbic acid and the test compounds, BaP and ERSE. In this case, the final concentration of DMSO for BaP experiments was 0.05%, which we found in preliminary studies to have no effect on any of the NSC parameters. Ten percent ERSE, however, had noticeable effects and accordingly, we added sodium chloride to the ERSE so as to achieve isotonic conditions, at which point there was no longer any vehicle difference. Thus, controls for the ERSE experiments contained equivalent volumes of isotonic saline. As described above, the data for the two types of experiments were normalized to the same control so as to be presented together but statistical comparisons were made only to the control group with the corresponding vehicle. After 3 days, half the medium was replaced, including the indicated treatment agents, and the exposures were continued for another 3 days (6 day total exposure, parallel to the PC12 studies).
As with the PC12 cell studies, the NSC studies included a positive control, 10 μM dexamethasone (Slotkin et al. 2016). In every experiment, we verified the ability of dexamethasone to evoke significant reductions in cell numbers and specifically to impair emergence of the glial phenotype. The dexamethasone results are not shown here because they are not relevant to PAH effects other than to validate each NSC experiment, and because they replicated the previously published effects (Slotkin et al. 2016).
2.5 NSC assays
At the end of the exposure period, the medium was removed and the coverslips washed with Dulbecco's phosphate-buffered saline, fixed with 4% paraformaldehyde and washed three times with Dulbecco's phosphate-buffered saline containing additional Ca2+ and Mg2+. Cells were permeabilized for 30 min in phosphate buffered saline containing 0.2% Triton X-100, washed three times with phosphate buffered saline (without Triton), followed by a 30 min incubation in BlockAid™ solution. Cells expressing a neuronal or astroglial phenotype were identified by immunocytochemistry according to manufacturers’ instructions, using microtubule-associated protein 2 (MAP2) for neurons and glial fibrillary acidic protein (GFAP) for astroglia. After permeabilization, the coverslips were incubated for 1 hr at room temperature using rabbit anti-MAP2 (1:200) and rat anti-GFAP (1:20) in BlockAid™. Coverslips were rinsed four times with phosphate-buffered saline and then incubated for 1 hr at room temperature with the appropriate fluor-conjugated secondary antibodies (donkey anti-rabbit IgG Alexa Fluor 647 and goat anti-rat IgG Alexa Fluor 555) diluted 1:400 in BlockAid™. After an additional five rinses with phosphate buffered saline, coverslips were incubated for 5 min with 300 nM 4',6-diamidino-2-phenylindole (DAPI) nucleic acid stain to label individual cells. Coverslips were rinsed three times with phosphate buffered saline and mounted onto glass slides using ProLong Diamond Antifade mountant. All reagents for the NSC assays were obtained from MTIGlobalStem except for rabbit anti-MAP2 (EMD Millipore, Billerica MA) and antifade mountant (Thermo Fisher Scientific, Waltham, MA).
Images of 3 to 4 fields/slide (each field = 3.22 × 105 μm2) were captured using a Zeiss Axio Imager widefield fluorescence microscope with 200× magnification and quantified for total cells (DAPI-positive stain for nuclei); across the multiple fields in a given culture, thousands of cells were counted. Each cell was then examined to see if it expressed a neuronal phenotype (MAP2-positive) or a glial phenotype (GFAP-positive). A cell was counted as positive only when the stain for a given phenotype coincided with a DAPI-stained nucleus. Values were averaged across the fields to render a single value for each culture.
2.6 Data analysis
Each study was performed using multiple, separate batches of cells, with 3-6 independent cultures for each treatment in each batch. Each batch of cells comprised a separately prepared, frozen and thawed preparation. Cultures within each batch were considered independent samples because each represented a separate plating, on a separate culture dish (PC12 cells) or cover slip (NSCs), individually treated and cultured for the 6 day treatment period. Results are presented as mean ± SE, with treatment comparisons carried out by analysis of variance (ANOVA) followed by Fisher’s Protected Least Significant Difference Test for post-hoc comparisons of individual treatments. The initial comparisons included factors of treatment and cell batch, and in each case, we found that the treatment effects for each type of experiment were the same across the different batches of cells, although the absolute values differed from batch to batch. Accordingly, we normalized the results across batches prior to combining them for presentation, so that multiple treatments could be compared readily on the same graph; however statistical comparisons were carried out on the original data, retaining the batch factor, so that treatment groups would be compared only to their contemporaneous controls. Significance for all tests was assumed at p < 0.05 (two-tailed).
3. RESULTS
3.1 PC12 cells
Complete dose-response curves for BaP effects on neurodifferentiation in PC12 cells have been published previously (Slotkin and Seidler 2009), so for the current study, we included a single BaP concentration (10 μM) as a positive control for comparison with ERSE. BaP evoked a substantial increase in PC12 cell numbers after 6 days of exposure, as evidenced by higher DNA content (Figure 1A); ERSE also evoked a significant increase but the effect was more modest and was restricted to the highest concentration. The increase in cell numbers evoked by BaP was associated with smaller cells, as evidenced by a reduction in the total protein/DNA ratio (Figure 1B); again, the highest concentration of ERSE produced a statistically significant, but much smaller effect. BaP reduced the membrane protein/DNA ratio to the same extent that it did the total protein/DNA ratio (Figure 1C) and consequently, there was no change in the proportion of membrane protein relative to total protein (Figure 1D). ERSE was without significant effect on either of these parameters.
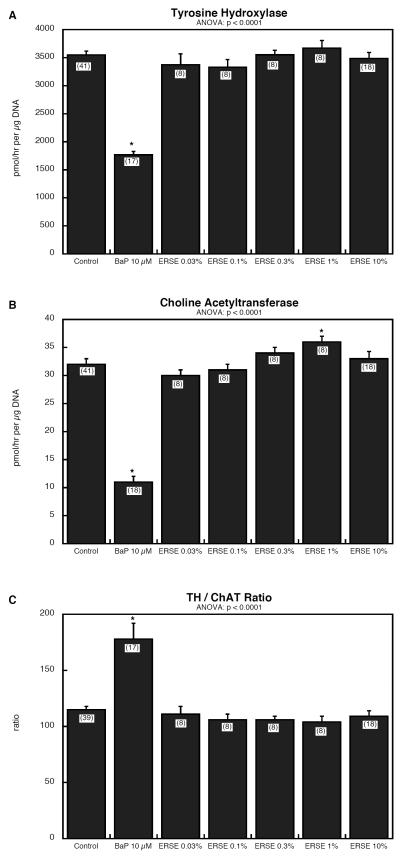
Figure 1.
Effects of BaP and ERSE on cell growth parameters in PC12 cells: (A) DNA content, (B) total protein/DNA ratio, (C) membrane protein/DNA ratio and (D) membrane protein/total protein ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel and asterisks denote groups that differ significantly from control values.
BaP had a profound effect on PC12 cell differentiation into neurotransmitter phenotypes, evoking robust reductions in both TH (dopamine phenotype, Figure 2A) and ChAT (acetylcholine phenotype, Figure 2B). Because the impairment of the cholinergic phenotype was greater, the TH/ChAT ratio increased (Figure 2C), connoting diversion of differentiation in favor of the dopamine phenotype. Neither of these effects was seen with ERSE, which had no significant effect on TH (Figure 2A) and actually evoked a slight increase in ChAT at 1% concentration (Figure 2B). The latter effect regressed when the concentration was raised to 10%. Because ERSE caused a nonsignificant increase in TH at the 1% concentration point as well, there was no significant change in the TH/ChAT ratio (Figure 2C).
Figure 2.
Effects of BaP and ERSE on markers for neurodifferentiation phenotypes in PC12 cells: (A) tyrosine hydroxylase activity (TH), (B) choline acetyltransferase activity (ChAT) and (C) the TH/ChAT ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel and asterisks denote groups that differ significantly from control values.
3.2 NSCs
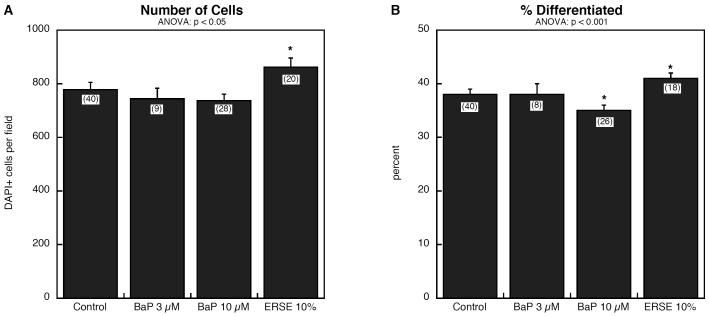
As there was no prior information about BaP effects on NSC differentiation, we evaluated two concentrations, one matching that of the PC12 experiments (10 μM) and another corresponding to the total PAH concentration (3 μM) achieved with 10% ERSE in the culture medium. BaP had no discernible effect on the number of cells after 6 days of exposure but in contrast, ERSE evoked a significant increase (Figure 3A). The two agents also differed in their impact on the proportion of cells showing differentiation (Figure 3B). Whereas ERSE enhanced overall differentiation, an equivalent concentration of BaP (3 μM) had no effect and a higher concentration (10 μM) actually reduced the proportion of differentiated cells.
Figure 3.
Effects of BaP and ERSE on cell number (A) and differentiation (B) in embryonic NSCs. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel and asterisks denote groups that differ significantly from control values.
Both BaP and ERSE impaired the emergence of the glial phenotype in NSCs (Figure 4A) but ERSE was more effective than an equivalent concentration (3 μM) of BaP, which only produced a nonsignificant decrease. The two agents differed in their impact on the neuronal phenotype (Figure 4B): BaP had little or no effect, whereas ERSE enhanced the emergence of neurons. Accordingly, both BaP and ERSE evoked a decrease in the glia/neuron ratio but the effects involved different events at the cellular level (Figure 4C). Again, for the glia/neuron ratio, ERSE was far more effective than was 3 μM BaP, the equivalent concentration to total PAHs achieved with 10% ERSE in the medium.
Figure 4.
Effects of BaP and ERSE on neurodifferentiation phenotypes in embryonic NSCs: (A) glia, (B) neurons and (C) glia/neuron ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel and asterisks denote groups that differ significantly from control values.
4. DISCUSSION
Results of this study indicate three main findings. First, PAHs exert direct effects on neurodifferentiation at specific decision nodes that specify development into neurons and glia, and later, that determine neurite formation and selection of neurotransmitter phenotype. Second, these effects differ between ERSE, a complex PAH mixture, and a single PAH component, BaP. Third, these differences are highly selective for the early vs. late node, with greater effects of ERSE on the neuron/glia decision and of BaP on the neurotransmitter decision. Most importantly though, our findings provide a biologic basis for the association of early-life PAH exposures with subsequent behavioral deficits in children and in piscine models (Brown et al. 2016; Perera et al. 2012).
In our earlier work with BaP in PC12 cells, we showed that, by itself, this PAH impairs the transition from cell replication to cell growth and neurodifferentiation, leading to higher numbers of cells, smaller cells, deficient neurite formation and impaired emergence of both the dopamine and acetylcholine phenotypes (Slotkin and Seidler 2009). These same effects were seen here. BaP increased DNA content (more cells) while reducing the total protein/DNA ratio (smaller cells). It also lowered membrane protein/DNA ratio to the same extent as total protein/DNA, so the membrane protein/total protein ratio was unchanged. As noted before (Slotkin and Seidler 2009), a cell that is simply smaller will have an increased membrane/total protein ratio because of its higher surface-to-volume ratio. Since BaP evoked equivalent changes in membrane and total protein (no change in the ratio), the smaller overall cell size reflected a parallel loss of membrane area, i.e. impairment of the neurite formation that is characteristic of neurodifferentiation. This was further confirmed by the adverse effects of BaP on emergence of both neurotransmitter phenotypes, with a greater effect on acetylcholine (ChAT activity) than dopamine (TH activity), leading to a rise in the TH/ChAT ratio.
For the markers of cell number and size, ERSE evoked in the same direction as BaP but the effect was much smaller; ERSE was devoid of discernible effects on membrane protein/DNA, thus indicating little or no impact on neurite formation. Some of the difference can be attributed to the higher concentration tested for BaP (10 μM) compared to the 10% ERSE mixture (total PAH 3 μM); however, our earlier work showed that 3 μM BaP still has robust effects on all four parameters, about one-half to one-third those of the higher concentration (Slotkin and Seidler 2009). Indeed, the measures of neurotransmitter phenotype provide definitive proof that the effects of ERSE are fundamentally different from those of BaP, since ERSE did not impair either TH or ChAT and in fact, actually evoked a significant increase in the latter parameter at 1% concentration in the medium (corresponding to 0.3 μM total PAH, well below the concentration of BaP). Accordingly, for this decision node, where cells are precommitted to a neuronal phenotype, and are destined to exit the mitotic cycle, form neurites and specify their neurotransmitter identity, ERSE and BaP diverge from each other not only in magnitude of effect (BaP >> ERSE) but even in the direction of effects.
NSCs test an earlier decision node, the point where neural precursors choose a neuronal or glial phenotype, and for this node, the relationships between ERSE and BaP were entirely reversed: ERSE had a much greater effect than BaP, even at equivalent total concentrations. ERSE exposure of NSCs evoked an increase in cell numbers, whereas BaP was without effect even at a 3-fold higher concentration. Even more notably, ERSE enhanced overall NSC differentiation whereas BaP reduced it. For BaP, then, the results for NSCs are in the same direction as for PC12 cells — impaired transition from cell replication to differentiation, resulting in more cells that are less differentiated — but with a clearly lower sensitivity. BaP thus targets the second decision node (modeled by PC12 cells) much more than the first (modeled by NSCs). For ERSE, we found the opposite, namely greater sensitivity for the early decision node. The differences between BaP and ERSE were even more stark considering the phenotypes resulting from NSC differentiation: whereas both BaP and ERSE impaired emergence of the glial phenotype, ERSE uniquely enhanced formation of neurons, resulting in a more profound drop in the glia/neuron ratio despite the fact that we tested a higher concentration of BaP. For NSCs, then, the PAH mixture was far more effective than could be accounted for by an equivalent, or even higher concentration of a single component, pointing toward contributions of multiple PAHs, and potential interactions among them. This conclusion is consonant with our earlier finding of interactions between BaP and other developmental neurotoxicants (Slotkin et al. 2013) or in a complex mixture of PAHs and other components in tobacco smoke (Slotkin et al. 2014).
Although our comparison used BaP as a model PAH, the ERSE mixture contains many other PAHs that are in higher concentration than BaP, most notably naphthalene, phenanthrene, fluoranthene and acenaphthene (Table 1). With regard to the aryl hydrocarbon receptor as one potential mechanism for our observed effects, BaP is orders of magnitude more potent than phenanthrene, naphthalene and many other of the higher-concentration ERSE components, but fluoranthene is more potent than BaP (Barron et al. 2004; Piskorska-Pliszczynska et al. 1986). The aryl hydrocarbon receptor contributes to adverse effects of PAHs on an earlier stage of mouse NSC development (proliferation and migration), but the effect may be species-specific, as it was not seen in human NSCs (Gassmann et al. 2010). Even then, in mouse NSCs, BaP was more effective than 3-methylcholanthrene despite the fact that the latter has ten-fold greater affinity for the receptor (Piskorska-Pliszczynska et al. 1986). It is thus difficult at this point to assign a specific mechanism or mode of action that explains all aspects of PAH effects on neurodifferentiation. However, our results do indicate that these mechanisms undergo a major transition as cells progress from the first decision node (formation of glia vs. neurons) to the later node (neurotransmitter phenotype, neurite development), with a corresponding shift in the relative effects of BaP and the PAH mixture. Accordingly, there is no single, generic effect of PAHs on neurodevelopment and the present results point to the likelihood that multiple mechanisms contribute to the adverse effects, rather than just those mediated by the aryl hydrocarbon receptor; these mechanisms can thus produce effects that are greater or less than those predicted from a single PAH component, depending on developmental stage. Finally, although the developmental toxicity and neurotoxicity of the ERSE mixture has been studied exclusively for its known, high contamination with PAHs, there are potentially other, unidentified contaminants that could contribute to adverse outcomes or that could interact with the PAHs. Unfortunately, this implies that different PAH mixtures, or PAH mixtures from different sources (with different secondary contaminants) may each have individual neurodevelopmental “fingerprints,” requiring empirical identification of adverse effects on a one-by-one basis.
5. CONCLUSIONS
In conclusion, our studies provide a cause-and-effect relationship for the observed association of developmental PAH exposure to behavioral deficits in children (Perera et al. 2012) or in animal models (Brown et al. 2016). Here, we demonstrated a direct effect on neurodifferentiation centered around two distinct nodes, one determining the emergence of neurons and glia from neural stem cells, and the other involving terminal differentiation stages associated with neurite formation and neurotransmitter specification. Equally important, we showed that a complex PAH mixture can produce effects that differ in direction and magnitude from those of a single PAH like BaP, pointing to interactions among the mixture components. In turn, this reinforces the need to consider environmentally relevant PAH mixtures, rather than just single compounds, in preclinical studies. Third, our results demonstrate how in vitro models can provide the missing link between exposure and outcomes seen in population studies, as well as identifying specific phases of vulnerability (decision nodes) of developing neural cells, and hence of brain assembly itself. Finally, the fact that adverse PAH effects on neurodifferentiation occur over an extended period, ranging from initial formation of neurons and glia through late stages of differentiation, neurite formation and neurotransmitter specification, means that the developing brain is likely to be sensitive to these environmental contaminants over a wide window of development, ranging from the rudiments of brain formation, through end stages of differentiation that occur late in gestation or in early childhood.
Acknowledgments
Funding: Supported by NIH ES010356.
Abbreviations
- ANOVA
analysis of variance
- BaP
benzo[a]pyrene
- ChAT
choline acetyltransferase
- DAPI
4',6-diamidino-2-phenylindole
- DMSO
dimethylsulfoxide
- ERSE
Elizabeth River sediment extract
- GFAP
glial fibrillary acidic protein
- MAP2
microtubule-associated protein 2
- NSC
neural stem cell
- PAH
polycyclic aromatic hydrocarbon
- TH
tyrosine hydroxylase
Footnotes
Conflict of interest statement: TAS received consultant income in the past three years from Acorda Therapeutics (Ardsley NY), Pardieck Law (Seymour, IN) and Walgreen Co. (Deerfield, IL)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barron MG, Heintz R, Rice SD. Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Marine Environ. Res. 2004;58:95–100. doi: 10.1016/j.marenvres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol. Teratol. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tang Y, Jiang X, Qi Y, Cheng S, Qiu C, Peng B, Tu B. Early postnatal benzo(a)pyrene exposure in Sprague-Dawley rats causes persistent neurobehavioral impairments that emerge postnatally and continue into adolescence and adulthood. Toxicol. Sci. 2012;125:248–261. doi: 10.1093/toxsci/kfr265. [DOI] [PubMed] [Google Scholar]
- Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol. Appl. Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- Fang M, Getzinger GJ, Cooper EM, Clark BW, Garner LVT, Di Giulio RT, Ferguson L, Stapleton HM. Effect-directed analysis of Elizabeth River porewater: developmental toxicity in zebrafish (Danio rerio) Env. Toxicol. Chem. 2014;33:2767–2774. doi: 10.1002/etc.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann K, Abel J, Bothe H, Haarmann-Stemmann T, Merk HF, Quasthoff KN, Rockel TD, Schreiber T, Fritsche E. Species-specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ. Health Perspect. 2010;118:1571–1577. doi: 10.1289/ehp.0901545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal. Toxicol. 2000;12:511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang DR. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol. Appl. Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Adverse neurodevelopmental effects of dexamethasone modeled in PC12 cells: identifying the critical stages and concentration thresholds for the targeting of cell acquisition, differentiation and viability. Neuropsychopharmacology. 2006a;31:1647–1658. doi: 10.1038/sj.npp.1300967. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ. Health Perspect. 2006b;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Camann D, Rauh V. Prenatal polyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6-7 years. Environ. Health Perspect. 2012;120:921–926. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J, Keys B, Safe S, Newman MS. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol. Lett. 1986;34:67–74. doi: 10.1016/0378-4274(86)90146-3. [DOI] [PubMed] [Google Scholar]
- Powers CM, Wrench N, Ryde IT, Smith AM, Seidler FJ, Slotkin TA. Silver impairs neurodevelopment: studies in PC12 cells. Environ. Health Perspect. 2010;118:73–79. doi: 10.1289/ehp.0901149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ. Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev. Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Rodier PM. Structural-functional relationships in experimentally induced brain damage. Prog. Brain Res. 1988;73:335–348. doi: 10.1016/S0079-6123(08)60514-2. [DOI] [PubMed] [Google Scholar]
- Roy TS, Sharma V, Seidler FJ, Slotkin TA. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Dev. Brain Res. 2005;155:71–80. doi: 10.1016/j.devbrainres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Adverse benzo[a]pyrene effects on neurodifferentiation are altered by other neurotoxicant coexposures: interactions with dexamethasone, chlorpyrifos, or nicotine in PC12 cells. Environ. Health Perspect. 2013;121:825–831. doi: 10.1289/ehp.1306528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Stadler A, Levin ED, Seidler FJ. Effects of tobacco smoke on PC12 cell neurodifferentiation are distinct from those of nicotine or benzo[a]pyrene. Neurotoxicol. Teratol. 2014;43:19–24. doi: 10.1016/j.ntt.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ. Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Benzo[a]pyrene impairs neurodifferentiation in PC12 cells. Brain Res. Bull. 2009;80:17–21. doi: 10.1016/j.brainresbull.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Card J, Levin ED, Seidler FJ. Diverse neurotoxicants target the differentiation of embryonic neural stem cells into neuronal and glial phenotypes. Toxicology. 2016;372:42–51. doi: 10.1016/j.tox.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Card J, Stadler A, Levin ED, Seidler FJ. Developmental neurotoxicity of tobacco smoke directed toward cholinergic and serotonergic systems: more than just nicotine. Toxicol. Sci. 2015;147:178–189. doi: 10.1093/toxsci/kfv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol. Appl. Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 218–224. [Google Scholar]
- Winick M, Noble A. Quantitative changes in DNA, RNA and protein during prenatal and postnatal growth in the rat. Dev. Biol. 1965;12:451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]
- Wormley D, Chirwa S, Harris E, Nayyar T, Wu J, Hood DB. Inhaled benzo(a)-pyrene impairs long-term potentiation in rat dentate gyrus: reduced capacity for long-term potentiation in the F1 generation. Cell. Mol. Biol. 2004;50:715–721. [PubMed] [Google Scholar]