Abstract
To assess the global and segmental left ventricular (LV) native T1 and extracellular volume fraction (ECV) in children and young adults with hypertrophic cardiomyopathy (HCM) compared to a control cohort. The study population included 21 HCM patients (mean 14.1 ± 4.6 years) and 21 controls (mean 15.7 ± 1.5 years). Native modified Look-Locker inversion recovery sequence was performed before and after contrast injection in 3 short axis planes. Global and segmental LV native T1 and ECV were quantified and compared between HCM patients and controls. Mean native T1 in HCM patients and controls was 1020.4 ± 41.2 and 965.6 ± 30.2 ms respectively (p < 0.0001). Hypertrophied myocardium had significantly higher native global T1 and global ECV compared to non-hypertrophied myocardium in HCM (p < 0.0001, = 0.14 and 0.048, = 0.01 respectively). In a subset of patients, ECV was higher in LV segments with LGE compared to no LGE (p < 0.0001). No significant correlation was identified between global native T1 and ECV and parameters of LV structure and function. Native T1 cut-off of 987 ms provided the highest sensitivity (95 %) and specificity (91 %) to separate HCM patients from controls. Global and segmental native T1 are elevated in HCM patients. LV segments with hypertrophy and/or LGE had higher ECV in a subset of HCM patients. LV native T1 and ECV do not correlate with parameters of LV structure and function. T1 in children and young adults may be used as a non-invasive tool to assess for HCM and related fibrosis.
Keywords: T1 mapping, Hypertrophic cardiomyopathy, Magnetic resonance imaging, Children
Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic disease with an incidence of 1:500 in the general population and is associated with ventricular hypertrophy and myocardial fibrosis [1]. Although the phenotypic presentation of HCM is uncommon in children, HCM accounts for 42 % cases of childhood cardiomyopathy [2]. Myocardial interstitial changes can be focal or global with potential for reversibility with treatment in early stages [3, 4]. The final common end point of irreversible fibrosis has been linked to increased risk of cardiac complications [5]. Cardiac MRI (CMR) can be used to non-invasively assess myocardial fibrosis using late gadolinium enhancement (LGE), however quantitative evaluation is limited [6]. T1 mapping is a potential tool for diagnosis of myocardial fibrosis, may be used to monitor therapy, and can serve as a surrogate endpoint in therapy trials [7].
Altered longitudinal relaxation time due to fibrosis can be noninvasively assessed by quantification of the myocardial native (non-contrast) T1, postcontrast myocardial T1, or calculating extracellular volume (ECV) fraction. Altered native T1 and postcontrast T1 reflects involvement of the myocyte and/or interstitium, while ECV represents the interstitial space [8]. Native T1 has proven to be of clinical relevance in adults with advantages of reduced scan time, cost effectiveness, zero risk of adverse effects from gadolinium-based contrast agents (GBCA), and faster postprocessing relative to calculation of ECV [9, 10]. Postcontrast T1 mapping however cannot be used in patients with contraindications to GBCA injection. There is limited literature on the role of native T1 mapping in children and young adults [11, 12].
The aim of this study was (a) to determine the reference values of LV native T1 in controls and compare to a group of children and young adults with HCM (b) to investigate the LV ECV in a subset of these HCM patients.
Materials and methods
This study was Health Insurance Portability and Accountability Act (HIPAA)-compliant and approved by our institutional review board (IRB). Informed written consent was obtained for performing the T1 modified Look-Locker (LL) inversion recovery (MOLLI) sequence per the IRB protocol.
Study population
Informed consent was obtained per IRB protocol. All children and young adults <25 years of age with HCM who consented to the T1 MOLLI during their clinically indicated cardiac MRI examination (CMR) between July 2013 and November 2015 were prospectively recruited in the study. The diagnosis of HCM was based on the demonstration of unexplained left ventricular (LV) hypertrophy in non-dilated ventricles in the absence of another cardiac or systemic disease contributing to the extent of hypertrophy. Trans-thoracic echocardiography is the initial modality of investigation in patients with suspected HCM. Practice guidelines for the diagnosis and treatment of HCM recommend exclusion of LV hypertrophy by infiltrative or storages diseases, metabolic disorders, and systemic/syndromic etiologies [13]. These secondary causes of myocardial hypertrophy were excluded clinically prior to labelling the diagnosis of HCM. Genetic mutation responsible for HCM cannot be identified in 30–40 % cases [2]. Genetic analysis was not routinely performed in our cohort to establish the diagnosis of HCM. We recruited controls who were undergoing a clinically indicated CMR examination, consented to the MOLLI sequence, and had normal cardiac function without any known cardiovascular disease, congenital or acquired by history, echocardiography, or MRI.
MR imaging
Clinical CMR protocol
All CMR studies were performed on a 1.5-T MRI scanner (Aera; Siemens AG Healthcare Sector, Erlangen, Germany). General anesthesia was utilized as clinically necessary per our clinical protocol. Balanced short axis steady-state free-precession (bSSFP) images covering the entire left ventricle (temporal resolution = 37.1 ms TR = 3.1 ms; TE = 1.3 ms; section thickness = 6 mm for young adults and 5 mm for children; field of view = 320mm × 240 mm for young adults and 280–300 mm × 188–225 mm for children) was performed to assess LV systolic function and morphology. Late gadolinium enhancement (LGE) images were acquired approximately 15 min following 0.2 mmol/kg gadopentetate dimeglumine administration (Magnevist; Bayer Healthcare, Wayne, NJ) in short axis, 2-chamber, and 4-chamber planes using both 2-dimensional single-shot and segmented inversion-recovery sequences. Scanning parameters used for single-shot sequence were: TR between 650 and 800 ms, TE 1.8 ms, trigger pulse 2–4 depending on HR, 2 for HR 60–80, 3 for 80+ and 4 for 100+, FOV: 260 mm × 75 %, matrix: 176 × 105, voxel: 1.5 × 1.6 × 6.0 mm and for segmented inversion-recovery sequence were: TR between 400 and 800 ms, TE 3.08 ms, trigger pulse 2–4 depending on HR, 2 for HR 60–80, 3 for 80+ and 4 for 100+, FOV: 300 × 90 %, matrix: 240 × 166, voxel: 1.2 × 1.3 × 6.0 mm.
MOLLI acquisition
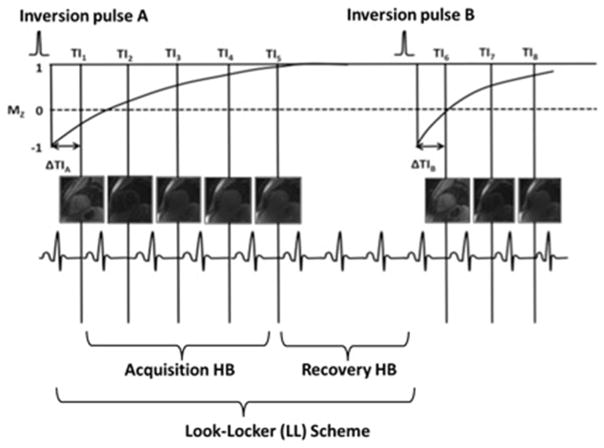
Myocardial T1 maps were obtained in the short-axis plane at the base, mid-chamber, and apex using a single-breath-hold, ECG-triggered, MOLLI sequence. MOLLI was acquired before and 12 min after contrast injection. The MOLLI sequence acquires single shot SSFP images at different inversion times that are all gated to the same cardiac phase (Fig. 1). At the end of each LL scheme, several dummy cardiac cycles are needed for full T1 signal recovery before the net inversion pulse is played out. We optimized our sequence parameters by adjusting the number of recovery heartbeat based on patient’s heart rate [14, 15], which allow for adequate T1 recovery between inversion pulses (Table 1). Spatial resolution was also optimized by reducing slice thickness and in-plane pixel size to compensate for partial volume averaging and loss of spatial resolution due to cardiac motion with high heart rates [14]. Other scanning parameters include: number of inversions = 2, MOLLI TI start = 129 ms, MOLLI T1 increment = 80 ms, number of acquisition heartbeats = 5, repetition time (TR) = 2.9 ms, bandwidth = 1050 Hz/pixel, and parallel imaging with a reduction factor of R = 2. Acquisition time will vary depending on heart rate of the patient, typically 11 s for each breath hold. We scanned at three short axis planes at base, mid-chamber, and apex, so overall duration was 3 × 11 s. Pixel based T1 value is calculated by a non-linear curve fitting using the three-parameter signal model [16],
Fig. 1.
Pre-contrast modified Look-Locker (LL) inversion recovery (MOLLI) pulse sequence scheme. In each LL scheme, images are read out after each inversion pulse during the following several heartbeats (acquisition heartbeats). Pauses (recovery heartbeats) in between the pulse experiments allow undisturbed T1 signal recovery. TI inversion time, ΔTI interval between the inversion pulse and first image acquisition
Table 1.
MOLLI pulse sequence parameters
| Heart rate | Recovery heart beats | Field of view (%) | Matrix (%) | Pixel size | Slice thickness |
|---|---|---|---|---|---|
| ≤70 | 5 | 360 × 85 | 256 × 66 | 2.1 × 1.4 | 8 |
| 71–80 | 6 | 360 × 85 | 256 × 66 | 2.1 × 1.4 | 8 |
| 81–90 | 7 | 320 × 85 | 240 × 75 | 1.8 × 1.3 | 8 |
| 91–100 | 8 | 320 × 85 | 240 × 75 | 1.8 × 1.3 | 8 |
| 101–110 | 9 | 280 × 85 | 224 × 75 | 1.7 × 1.25 | 6 |
| 111–125 | 10 | 280 × 85 | 224 × 75 | 1.7 × 1.25 | 6 |
| 126–140 | 11 | 280 × 85 | 224 × 75 | 1.7 × 1.25 | 6 |
MOLLI modified Look-Locker (LL) inversion recovery
| (1) |
where S(TI), signal intensity acquired at TI.
Image analysis
LV volumetric, functional analysis and LV mass calculation were derived using QMASS (Medis Medical Imaging Systems, Leiden, the Netherlands) by manual segmentation of the epicardial and endocardial borders by CR, attending radiologist with 15 years of experience in pediatric cardiovascular imaging. Heart rate, LV ejection fraction (LVEF), LV end-diastolic volume index (LVEDVi) and end-systolic volume index (LVESVi), and LV mass index (LV massi) were calculated. Segmental myocardial wall thickness was manually measured in end-diastole on basal, mid-chamber, and apical bSSFP images on PACS (Centricity, General Electric, Milwaukee, WI). LV segments with late gadolinium enhancement were qualitatively evaluated and compared with LV T1. E/A ratio derived from echocardiography within 2 weeks of CMR was used a marker of diastolic dysfunction.
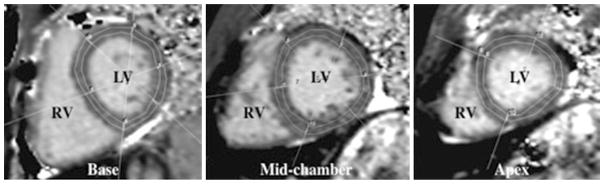
The scale on a color-coded image of the native T1 map can be used to visually determine T1 relaxation time. Manual segmentation of the LV mid-myocardium on short axis T1 maps at the LV basal, mid-chamber and apical slices was performed using a 16-segment AHA model (Fig. 2). Utmost care was taken to avoid contamination from blood pool or tissue beyond the myocardium. A single radiologist with 2 years of experience in cardiovascular imaging and blinded to results of other clinical data analyzed the MOLLI images on PACS. Segmentation was reviewed for consistency by another observer with 15 years of experience in pediatric cardiovascular imaging and adjustments were made accordingly. Mean T1 values for each segment were used for the data analysis. Segments in the T1 map with poor motion correction were excluded to avoid erroneous values. The mean T1 of the combined 16 LV segments was used to determine global T1.
Fig. 2.
Segmentation of left ventricular myocardium. T1 short axis maps of a 16-year-old male with history of loss of consciousness. Region of interests drawn in the left ventricle according to the AHA 16-segment model. RV right ventricle, LV left ventricle
ECV was calculated using pre- and post-contrast T1 values in those patients who received gadopentetate dimeglumine. CMR scans in patients who did not receive an extracellular contrast agent or in patients that did not have an hematocrit drawn within 7 days of the CMR study were excluded. The regions of interest for the blood pool were placed in the center of the LV to avoid any trabeculations. ECV was calculated using a correction for blood hematocrit.
| (2) |
Segmental myocardial hypertrophy was defined as a Z-score of >+2.00 for maximum end-diastolic myocardial wall thickness using EchoIMS (Merge Healthcare, Chicago, IL). LV myocardial segments with normal Z-score were considered non-hypertrophied. Segmental analyses of ECV in a small number of subset of HCM patients was done based on the presence or absence of hypertrophy and/or LGE.
Statistical analyses
Statistical analysis was performed using Microsoft Excel 2011 (Microsoft, Redmond, WA) and JMP® Pro11.0.0 (SAS Institute, Cary, NC). Quantitative data are presented as mean ± one standard deviation for normative data and median with inter-quartile range (IQR) for non-normative data. Comparison between two groups was performed using independent samples t test for parametric data and Mann–Whitney test for non-parametric data. Matched-pair t test was calculated to compare two sets of measurements. One-way analysis of variance (ANOVA) was performed for multiple comparisons of group means. Correlation between continuous variables was evaluated using the Pearson correlation (r) for parametric data and Spearman rank correlation (ρ) for nonparametric data. Receiver operating characteristic curve (ROC) analysis was performed to determine the cut-off LV native T1 to distinguish patients with HCM from controls. All patients with HCM were grouped as positive cases and controls were grouped as negative to determine a threshold for a LV native T1. To reduce the problem of multiple comparisons, the family wise error rate was maintained using Bonferroni correction. Statistical tests conducted were two-tailed. p < 0.05 was considered statistically significant.
Results
Population characteristics
T1 mapping was completed in 45 subjects with 3 patients excluded due to poor motion correction. Our final cohort included 42 subjects (30 males; mean age, 14.9 ± 3.5 years) with native T1 obtained during the clinical CMR examination. There were 21 subjects in the patient group (15 male) and 21 subjects in the control group (15 male). All HCM patients received extracellular gadolinium contrast followed by acquisition of delayed enhancement images. ECV was calculated in 9 HCM patients and 4 controls who had an hematocrit available within 7 days of the CMR study. Seven segments in the LV apex (4/336 in HCM patients and 3/336 in controls) were excluded due to poor motion correction. Seven subjects (5 HCM patients and 2 controls) were scanned under general anesthesia as per the clinical protocol. Characteristics of the HCM patients and controls are listed in Table 2. There was no significant difference between the age of HCM patients (mean age 14.1 ± 4.6 years; range 2–21 years) (p = 0.17) and the controls (mean 15.7 ± 1.5 years; range 12–18 years).
Table 2.
Population characteristics
| Variable | Control | HCM | p value |
|---|---|---|---|
| Number | 21 | 21 | N/A |
| Age (years) | 15.7 ± 1.5 | 14.1 ± 4.6 | 0.17 |
| Male [n (%)] | 15 (71.4) | 15 (71.4) | N/A |
| Weight (kg) | 64.9 ± 18.9 | 71.2 ± 35.1 | 0.49 |
| Height (cm) | 168.3 ± 13.2 | 159.9 ± 26.1 | 0.22 |
| Systolic BP (mmHg) | 118.5 ± 11.0 | 111.5 ± 11.3 | 0.06 |
| Diastolic BP (mmHg) | 68.3 ± 7.9 | 65.0 ± 8.8 | 0.17 |
Values are mean ± SD
HCM hypertrophic cardiomyopathy, BP blood pressure, N/A not applicable
LV myocardial native T1
Mean native T1 was higher in females compared to males in HCM group (1057.5 ± 47.8 vs. 1005.6 ± 28.0 ms, p = 0.03). However, no significant gender difference was identified in native T1 (967.2 ± 19.4 ms in females vs. 964.7 ± 16.4 ms in males, p = 0.40). The number of cohort is however very small to draw any conclusions.
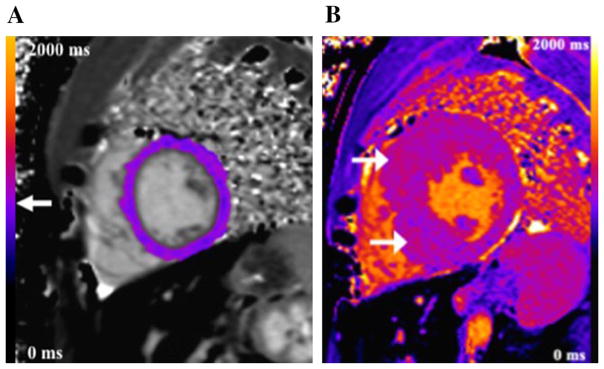
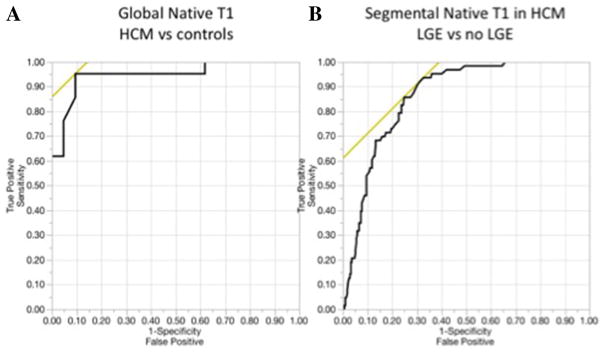
An example of a color-coded T1 map in a control (Fig. 3a) and a patient with hypertrophic cardiomyopathy (HCM) is shown (Fig. 3b). Twenty-one subjects with HCM had higher global mean LV native T1 (1020.4 ± 41.2 ms) compared to controls (965.6 ± 30.2 ms) (p < 0.001). ROC analysis showed a cut-off global LV native T1 of 987 ms yielding a sensitivity of 95 % and specificity of 91 % for distinguishing myocardium in HCM patients from controls with an area under the ROC of 0.95 (95 % confidence interval, 0.83–0.99) (Fig. 4a).
Fig. 3.
Native T1 maps. a Color-coded image of a native T1 map in the LV mid-chamber short axis in a 16-year-old male (control) who underwent CMR to evaluate coronary arteries that were not well seen on echocardiography. The scale can be used to visually determine T1 relaxation time (arrow). On quantitative analysis, native LV T1 was 972 ms. b Example of color-coded T1 map in a 16-year-old patient with hypertrophic cardiomyopathy (HCM). Maximum single wall thickness was 2.6 cm in the mid-chamber anterior septum. Color-coded T1 map in the LV mid-chamber short axis shows patchy areas of increased T1 relaxation (arrows), more marked in hypertrophic segments. Color scale ranges from 0 to 2000 ms
Fig. 4.
Receiver operating characteristic curves a ROC for determining a cut-off for the global LV native T1 value of controls vs. patients with HCM. A T1 cut-off value of 987 ms yielded sensitivity of 95 % and specificity of 91 % with an area under the ROC of 0.95 (95 % confidence interval, 0.83–0.99). b The cutoff LV native T1 of 1019 ms yielded a sensitivity of 94 % and specificity of 67 % to differentiate segments with and without LGE in patients with HCM and had an area under the ROC of 0.86 (95 % confidence interval, 0.82–0.90)
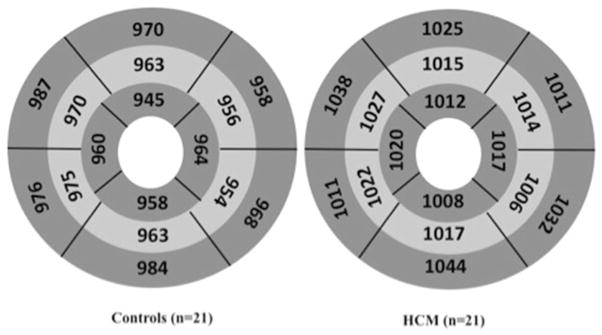
LV native T1 was significantly higher in patients compared to controls on a segmental comparison (AHA 16-segment model) with differences ranging from 3.5 to 6.8 % (Fig. 5). On segmental analysis of controls, the septum (segments 2, 3, 8, 9, and 14) had a significantly increased native T1 compared to the lateral wall (segments 5, 6, 11, 12, and 16) (973.3 ± 16.7 and 959.2 ± 22.8 ms respectively; p = 0.005). In patients with HCM, hypertrophied LV segments (115 segments with mean wall thickness, 17.1 mm ± 6.9) had significantly higher native T1 compared to non-hypertrophied segments (217 segments with mean wall thickness, 8.0 mm ± 2.2) (1043.0 ± 45.9 vs. 1009.5 ± 44.9 ms, p < 0.0001). Non-hypertrophied segments in HCM patients had an elevated native T1 when compared to LV segments of controls (333 segments) 965.6 ± 30.2 ms (range 944.0–995.0 ms) (p < 0.0001) (p < 0.0001 by ANOVA test). However, native T1 in HCM only had a very weak correlation with LV wall thickness (p = 0.01, ρ = 0.14). On excluding the septal segments (2, 3, 8, 9, and 14), mean native T1 of hypertrophied segments (1042.2 ± 44.3 ms) was higher compared to non-hypertrophied segments (993.8 ± 139.3 ms) in HCM patients (p < 0.0001).
Fig. 5.
Segmental comparison of LV native T1 values in controls and patients. Segmental comparison of LV native T1 in controls (n = 21) and HCM patients (n = 21) using the AHA 16 segment model. All data are listed as mean values and were significantly different on comparison by segments (p < 0.05) with percentage differences of 3.5–6.8 %
LV segments with LGE (63 segments) had higher LV native T1 compared to segments without LGE (269 segments) (1068.5 ± 36.2 vs. 1009.0 ± 48.9 ms, p < 0.001). The cutoff LV native T1 of 1019 ms yielded a sensitivity of 94 % and specificity of 67 % to differentiate segments with and without LGE in patients with HCM and had an area under the ROC of 0.86 (95 % confidence interval, 0.82–0.90) (Fig. 4b).
Extracellular volume fraction
On segmental analysis in HCM patients, ECV was higher in segments with LV hypertrophy (66 segments) (mean wall thickness, 19.4 mm ± 7.9) compared to non-hypertrophied segments (78 segments) (mean wall thickness, 8.2 mm ± 2.5) (25.3 ± 0.05 vs. 23.7 ± 0.04 %) (p = 0.048, ρ = 0.01). Mean ECV in segments with LGE (54 segments) was significantly elevated compared to segments with no LGE (90 segments) (27.0 ± 0.05 vs. 22.9 ± 0.04 %, p < 0.0001). Comparison of native T1 and ECV between the subgroups pssatient population is illustrated in Table 3.
Table 3.
Native T1 and ECV
| Patient subgroups | Native T1 (ms)/ECV (%) | p value |
|---|---|---|
| Global native T1 | ||
| Controls | 965.6 ± 30.2 ms | <0.0001 |
| HCM | 1020.4 ± 41.2 ms | |
| Segmental native T1 in HCM | ||
| Hypertrophied | 1043.0 ± 45.9 ms | <0.0001 |
| Non-hypertrophied | 1009.5 ± 44.9 ms | |
| Segmental native T1 in HCM | ||
| LGE | 1068.5 ± 36.2 ms | <0.001 |
| No LGE | 1009.0 ± 48.9 ms | |
| Segmental ECV in HCM | ||
| Hypertrophied | 25.3 ± 0.05 % | 0.048 |
| Non-hypertrophied | 23.7 ± 0.04 % | |
| Segmental ECV in HCM | ||
| LGE | 27.0 ± 0.05 % | <0.0001 |
| No LGE | 22.9 ± 0.04 % | |
Values are mean ± SD
HCM hypertrophic cardiomyopathy, LGE late gadolinium enhancement, ECV extracellular volume fraction
Native T1 and clinical variables
Descriptions of quantitative LV structure and function analysis are summarized in Table 4. With the exception of the LV mass i, the values were similar between HCM patients and controls. There was no significant correlation of global LV native T1 with parameters of LV structure and function derived from CMR and echocardiography, namely heart rate, LVEF, LVEDVi, LVESVi, and E/A ratio (ρ = 0.20, 0.05, −0.14, −0.09, and −0.21 respectively). In a subset of HCM patients, ECV did not significantly correlate with heart rate, LVEF, LVEDVi, LVESVi, and E/A ratio (ρ = 0.18, −0.23, 0.01, 0.11, and 0.09 respectively).
Table 4.
Left ventricular structure and functional analyses
| Parameter | Control | HCM | p value |
|---|---|---|---|
| Heart rate | 69.2 ± 12.3 | 75.9 ± 19.1 | 0.19 |
| LVEF (%) | 60.0 ± 5.6 | 63.3 ± 7.6 | 0.12 |
| LVEDVi (mL/m2) | 94.7 ± 14.1 | 89.1 ± 16.5 | 0.25 |
| LVESVi (mL/m2) | 37.0 ± 6.4 | 33.2 ± 11.6 | 0.19 |
| LV massi (g/m2) | 57.0 ± 14.0 | 93.6 ± 60.8 | 0.01 |
| E/A ratio | 2.2 ± 0.4 | 2.0 ± 0.7 | 0.11 |
Values are mean ± SD
HCM hypertrophic cardiomyopathy, LVEF left ventricular ejection fraction, LVEDVi left ventricular end diastolic volume index, LVESVi left ventricular end systolic volume index, E peak early diastolic mitral annulus velocity, A atrial contraction wave
Discussion
Our study shows elevated LV native T1 relaxation times in children with HCM compared to controls. Based on our findings, the mean LV native T1 in control children and young adults ranges from 944.0 to 995.0 ms. Mean LV native T1 value in our controls using the MOLLI sequence was similar to the LV native T1 in normal adult subjects (964.6 ± 35.3 ms) as reported by Kellman et al. using a similar T1 mapping sequence [17].
The higher native T1 in the interventricular septum compared to the lateral LV wall in our study has been described previously [18, 19]. This regional variation is not believed to represent true variability, but can be explained by the introduction of errors from increased sampling error in the lateral wall due to partial volume averaging of voxels at the myocardium-blood and myocardium-lung interface. The increased distance of the receiver coil elements from the lateral wall relative to the interventricular septum, leading to a differential signal gradient between the interventricular septum and the lateral wall could also contribute to this variability. Therefore, some authors suggest only inclusion of the interventricular septum for LV T1 region of interest analysis [20].
LV native T1 was significantly higher in children with HCM by global and segmental analyses and the non-hypertrophied segments in HCM had higher LV native T1 values compared to controls. These findings are compatible with the findings of studies in adults that demonstrate increased T1 relaxation times in both hypertrophied and remote non-hypertrophied myocardium [21, 22]. A study performed by Hinojar et al. showed similar findings with higher native T1 in the septum of adults with gene-positive/phenotype-negative HCM compared to a control population [23]. LV T1 had a very weak correlation with LV wall thickness and LV mass in our population, which suggests the need for a larger study with histopathology correlation to better understand the microscopic process leading to increase in T1 relaxation time of non-hypertrophied and hypertrophied myocardium in children.
In patients with HCM, areas of late gadolinium enhancement (LGE) are representative of replacement fibrosis secondary to microvascular ischemia [24]. Studies have shown that adults with LGE in HCM have higher risk of ventricular arrhythmias and sudden cardiac death [25]. In our study, LV native T1 in HCM was significantly higher in segments with LGE compared to segments with no LGE. Based on our findings, a cut-off LV native T1 of 1019 ms can be used to identify myocardial segments with LGE with high sensitivity. Statistical comparison of global ECV between the two groups was not possible due to the small number of controls. In this study, segments with LGE had a higher ECV with respect to segments without LGE in patients with HCM. Our findings were analogous to what has been described in adults with HCM having LGE [26].
The significance of T1 mapping to detect subclinical myocardial changes before the onset of systolic and diastolic dysfunction has been described in adults with heart failure [40]. Ellims et al. in their study in adults with 51 HCM patients have described a moderate correlation between post-contrast myocardial T1 and parameters of diastolic function (E/e′) (r = −0.48, p < 0.001) [27]. In our study, factors related to cardiac function including heart rate, LVEF, LVEDVi, LVESVi, and E/A ratio showed no significant association with global LV native T1 in HCM patients or ECV in a subset of HCM patients with available ECV. The differences in results between our study and theirs may be related to our smaller study population and could reflect greater sensitivity of E/e′ ratio for the detection of early changes in cardiac function [27].
Our study has several limitations. There is an overlap in T1 values between controls and patients that may limit the utility of LV native T1 mapping on an individual patient level. Our results are based on a small cohort and future studies should be conducted in a larger population to provide a guide for clinical applications of T1 mapping in children. We did not correlate LV native T1 or ECV with myocardial histopathology. There is no defined role of endomyocardial biopsy in diagnosis of patients with suspected HCM. However, histological validation of myocardial fibrosis by CMR in children and young adults with HCM should be ideally conducted prior to using it as a tool for risk stratification. The functional parameters used represent global LV function, which may not decline until later in the disease and can explain the lack of correlation with native T1. Also, we used only one parameter i.e. E/A as a representative of diastolic function. The hematocrit value was not available for all HCM patients limiting comprehensive evaluation of ECV and comparison with LV native T1. We acknowledge the small number of patients with ECV in our study. This study was primarily focused on native T1 and a dedicated study should be performed to determine the role of ECV in children and young adults with HCM.
Global and segmental LV native T1 is elevated in patients with HCM relative to controls. LV segments with hypertrophy and/or LGE had higher ECV. LV native T1 and ECV did not correlate with parameters of LV structure and function. Native T1 can be used to quantify myocardial fibrosis in pediatric patients without a risk of neuronal deposition of GBCA. HCM is a progressive disease and T1 mapping can be used to determine disease progression and timely intervention in children and young adults. Further work should be geared to determine whether T1 mapping can be used to guide cardiac risk stratification in children and young adults with HCM.
Acknowledgments
We thank Samantha Schoeneman, BA, Marci Messina, RT®(MR), and Brian Reilly, RT for their assistance with this project. Support by the the National Institutes of Health (NHLBI1R01 HL117888).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by an appropriate institutional review board.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287(10):1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Colan SD. Hypertrophic cardiomyopathy in childhood. Heart Fail Clin. 2010;6(4):433–444. doi: 10.1016/j.hfc.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115(7):888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 4.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83(6):1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 5.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51(14):1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 6.Hamlin SA, Henry TS, Little BP, Lerakis S, Stillman AE. Mapping the future of cardiac MR imaging: case-based review of T1 and T2 mapping techniques. Radiographics. 2014;34(6):1594–1611. doi: 10.1148/rg.346140030. [DOI] [PubMed] [Google Scholar]
- 7.Maestrini V, Treibel TA, White SK, Fontana M, Moon JC. T1 mapping for characterization of intracellular and extracellular myocardial diseases in heart failure. Curr Cardiovasc Imaging Rep. 2014;7:9287. doi: 10.1007/s12410-014-9287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB Society for Cardiovascular Magnetic Resonance I, Cardiovascular Magnetic Resonance Working Group of the European Society of C. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99(13):932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772–782. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 11.Hussain T, Dragulescu A, Benson L, Yoo SJ, Meng H, Windram J, Wong D, Greiser A, Friedberg M, Mertens L, Seed M, Redington A, Grosse-Wortmann L. Quantification and significance of diffuse myocardial fibrosis and diastolic dysfunction in childhood hypertrophic cardiomyopathy. Pediatr Cardiol. 2015;36(5):970–978. doi: 10.1007/s00246-015-1107-7. [DOI] [PubMed] [Google Scholar]
- 12.Kozak MF, Redington A, Yoo SJ, Seed M, Greiser A, Grosse-Wortmann L. Diffuse myocardial fibrosis following tetralogy of Fallot repair: a T1 mapping cardiac magnetic resonance study. Pediatr Radiol. 2014;44(4):403–409. doi: 10.1007/s00247-013-2840-9. [DOI] [PubMed] [Google Scholar]
- 13.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW American College of Cardiology Foundation/American Heart Association Task Force on Practice G. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58(25):e212–260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. doi: 10.1186/1532-429X-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67(6):1644–1655. doi: 10.1002/mrm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. doi: 10.1186/1532-429X-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers T, Dabir D, Mahmoud I, Voigt T, Schaeffter T, Nagel E, Puntmann VO. Standardization of T1 measurements with MOLLI in differentiation between health and disease– the ConSept study. J Cardiovasc Magn Reson. 2013;15:78. doi: 10.1186/1532-429X-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012;14:27. doi: 10.1186/1532-429X-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6(4):475–484. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, Watkins H, Neubauer S. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5(6):726–733. doi: 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]
- 22.Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, Gebker R, Doltra A, Kelle S, Khan S, Rogers T, Arroyo Ucar E, Cummins C, Carr-White G, Nagel E, Puntmann VO. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the international T1 multicenter cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2015;8(12) doi: 10.1161/CIRCIMAGING.115.003285. [DOI] [PubMed] [Google Scholar]
- 23.Cecchi F, Sgalambro A, Baldi M, Sotgia B, Antoniucci D, Camici PG, Sciagra R, Olivotto I. Microvascular dysfunction, myocardial ischemia, and progression to heart failure in patients with hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2(4):452–461. doi: 10.1007/s12265-009-9142-5. [DOI] [PubMed] [Google Scholar]
- 24.Leonardi S, Raineri C, De Ferrari GM, Ghio S, Scelsi L, Pasotti M, Tagliani M, Valentini A, Dore R, Raisaro A, Arbustini E. Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur Heart J. 2009;30(16):2003–2010. doi: 10.1093/eurheartj/ehp152. [DOI] [PubMed] [Google Scholar]
- 25.Brouwer WP, Baars EN, Germans T, de Boer K, Beek AM, van der Velden J, van Rossum AC, Hofman MB. In-vivo T1 cardiovascular magnetic resonance study of diffuse myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2014;16:28. doi: 10.1186/1532-429X-16-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohyama Y, Volpe GJ, Lima JA. Subclinical myocardial disease in heart failure detected by CMR. Curr Cardiovasc Imaging Rep. 2014;7:9269. doi: 10.1007/s12410-014-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellims AH, Iles LM, Ling LH, Hare JL, Kaye DM, Taylor AJ. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson. 2012;14:76. doi: 10.1186/1532-429X-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]