Abstract
The relevance of the accessory vpr, vpu, and nef genes for human immunodeficiency virus type 1 (HIV-1) replication in human lymphoid tissue (HLT), the major site of viral replication in vivo, is largely unknown. Here, we show that an individual deletion of nef, vpr, or vpu significantly decreases HIV-1 replication and prevents CD4+ T-cell depletion in ex vivo HLT. However, only combined defects in all three accessory genes entirely disrupt the replicative capacity of HIV-1. Our results demonstrate that nef, vpr, and vpu are all essential for efficient viral spread in HLT, suggesting an important role in AIDS pathogenesis.
Human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) have several genes that are not absolutely required for viral spread in cell lines (7, 46) and are, therefore, called accessory genes. However, subsequent studies have indicated that these genes might play important roles in infected hosts in vivo, as well as in primary cells (reviewed in references 7 and 46). HIV-1 encodes Vif, Vpr, Vpu, and Nef. In contrast, HIV-2 and SIVmac do not encode Vpu but rather another late protein, Vpx (51). Vif suppresses the antiretroviral cellular enzyme APOBEC3G (36, 42, 48) and is essential for efficient viral replication in primary cells and in vivo (12). The in vivo importance and exact functions of the other accessory proteins are less clear, although it has been established that they modulate multiple host cell processes (reviewed in references 7 and 46). For example, Nef downregulates CD4 (2, 19, 37, 45), enhances virion infectivity (9, 37), alters T-cell activation (4, 13, 43), and interferes with major histocompatibility complex antigen presentation (26, 41, 50). Vpr is a virion-associated protein (10) which induces G2/M arrest (18, 40) and plays a role in the nuclear transport of the preintegration complex in nondividing cells (39). Vpr also enhances infection of macrophages (14), activates HIV transcription (1, 17), and induces apoptosis (38, 44, 47). Vpu promotes virion release (49, 54) by counteracting host restriction factors (30, 52), downregulates CD4 during the late stages of HIV-1 infection (34, 53), and inhibits NF-κB activation (3).
Studies using the SIVmac model have demonstrated that nef is important for efficient replication in vivo and for disease progression (31). The contribution of the other accessory genes to SIV or HIV-1 virulence is less clear. Deletion of vpr neither attenuates SIV replication nor prevents disease progression in infected monkeys (20). Results obtained with chimeric simian-human immunodeficiency viruses suggest that vpu might contribute to viral pathogenesis (35). The role of the accessory genes in HIV-1 pathogenesis is even less clear than their role in SIVmac pathogenesis. The importance of Nef in HIV infection of humans has been confirmed in several long-term nonprogressors (11, 32). Also, it has been suggested that sequence variations in Vpr and Vpu might be associated with nonprogressive HIV-1 infection (5, 44).
Understanding the role of the accessory HIV-1 proteins in AIDS in humans requires adequate experimental systems. In vivo, critical events in HIV disease occur in lymphoid tissues (15, 16). It has been shown that Nef, concordantly with its important role in vivo (11, 31, 32), enhances HIV-1 replication in human lymphoid tissue ex vivo (24). This system supports productive HIV-1 infection without exogenous stimulation (22, 23) and provides a useful model for studying the importance of the accessory genes for HIV-1 replication in infected human individuals. In the present study, we used this system to investigate the role of accessory genes by infecting blocks of human tonsillar tissue with HIV-1 mutants containing single or combined deletions of vpu, vpr, and nef and evaluated virus replication and CD4+-T-cell depletion.
HIV-1 NL4-3 vpr and vpu deletion mutants (21) were kindly provided by Ronald C. Desrosiers through the AIDS Research and Reference Reagent Program. Full-length proviral pBRNL4-3 variants with single and combined deletions of vpr, vpu, and nef were generated with standard cloning techniques. Briefly, to generate the vpr-defective forms, the gag-pol-vif-vpr region of p210-19 containing a deletion in vpr (21) was inserted into full-length nef-open and nef-defective HIV-1 NL4-3 proviral clones (8) by using the NarI and EcoRI sites located in the 5′ long terminal repeat and the vpr gene. Similarly, a vpu-deleted EcoRI-NheI restriction fragment derived from p210-13 (21) was cloned into the NL4-3 proviral constructs to obtain vpu-deleted HIV-1 mutants. Finally, vpr-deleted NarI-EcoRI restriction fragments were inserted into the vpu- and/or nef-defective proviral constructs to obtain HIV-1 variants with combined defects in the accessory genes.
Human tonsils removed during routine tonsillectomies were dissected, set up in culture at the air-liquid interface, and infected as described earlier (23). Briefly, for testing of each virus in tissue from one donor, 100 ng of p24 was applied to each of 27 tissue blocks. For each HIV-1 variant, the experiment was repeated n times (each time with tissues from a different donor). Viral production was evaluated by measurement of the p24 core antigen released into the pooled medium bathing all 27 blocks by HIV-1 p24 enzyme-linked immunosorbent assay (Coulter, Miami, Fla.). In agreement with earlier observations for various HIV-1 isolates (23, 29), p24 production was first noted on day 6 postinoculation, and virus replication increased until the end of the experiment on day 12 or 15 (Fig. 1A).
FIG. 1.
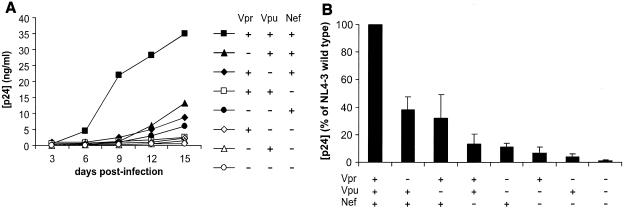
Replication of HIV-1 variants in human lymphoid tissue ex vivo. For each of the indicated HIV-1 variants, 27 tissue blocks were inoculated with 100 ng of p24 and medium was collected every 3 days. (A) Representative replication kinetics of wild-type NL4-3 and deletion mutants. (B) Average production of virus. Matched tissues from 13 donors were inoculated with the wild-type virus or with accessory gene-deleted mutants as indicated, and for each condition cumulative production of p24 by 27 tissue blocks over 15 days was measured. Presented are means ± standard errors of the means of these values expressed as percentages of those measured in cultures infected with the wild-type virus.
A single deletion of any of the accessory genes nef, vpr, and vpu decreased viral replication (Fig. 1A). On average, deletion of nef decreased the ability of HIV-1 NL4-3 to replicate in ex vivo-infected human lymphoid tissues to 13% ± 7% (n = 11, P = 0.001) of that of the wild-type parental virus and deletion of vpr and vpu decreased it to 38% ± 9% (n = 14, P = 0.003) and 32% ± 16% (n = 14, P = 0.002) of that of the wild-type virus, respectively (Fig. 1B). Combined deletion of vpr and vpu impaired HIV-1 replication more severely and reduced cumulative p24 production to 11% ± 3% (n = 12, P = 0.002) of that of the wild-type virus. Similarly, additional deletion of either vpr or vpu further attenuated replication of the nef-deleted HIV-1 variant (Fig. 1B). Thus, although vpr and vpu seem to be less critical than nef, both clearly contribute to efficient viral replication in ex vivo-infected human lymphoid tissue. Nevertheless, only the combined deletion of all three accessory genes completely disrupted HIV-1 replication (Fig. 1B).
We evaluated the number of productively infected CD4+ T cells by flow cytometry of cells mechanically isolated from control and infected-tissue blocks and stained for CD3, CD8, CD4, and p24 (23, 27). To account for CD4 downregulation by viral infection, productively infected CD4+ T cells were defined as CD3+ CD8− p24+. The numbers of HIV-1-infected cells in tissues infected with all tested mutants were significantly diminished, relative to those in tissues infected with wild-type virus (P < 0.008) (Fig. 2A). The progressive loss of CD4+ T lymphocytes is a major characteristic of HIV-1 infection and AIDS. We evaluated this loss in tissues infected by wild-type virus and accessory gene-deleted HIV-1 variants using flow cytometry. To normalize for differences in tissue block size and cellularity and to account for CD4 downregulation by viral infection, CD4+-T-cell depletion was expressed as the ratio of the number of CD8− T cells to the number of CD8+ T cells, the amount of which was not changed by HIV infection (23, 27). Consistent with findings in a previous study, wild-type NL4-3 HIV-1 depleted ex vivo-infected tissues of 40 to 50% of these cells within 12 days of infection. In contrast, the deletion mutants caused less CD4+-T-cell depletion (a maximum of about 15%) (Fig. 2B). There was a strong correlation (R2 = 0.88, P = 0.0006) between the number of productively infected CD4+ T cells and the level of their depletion (Fig. 2C). Although deletion of either of the accessory genes significantly decreased the efficiency of mutant replication, the number of infected cells, and therefore CD4+-T-cell depletion, from levels observed with the wild-type virus, donor-to-donor variability did not allow the ranking of different mutants in terms of these parameters.
FIG. 2.
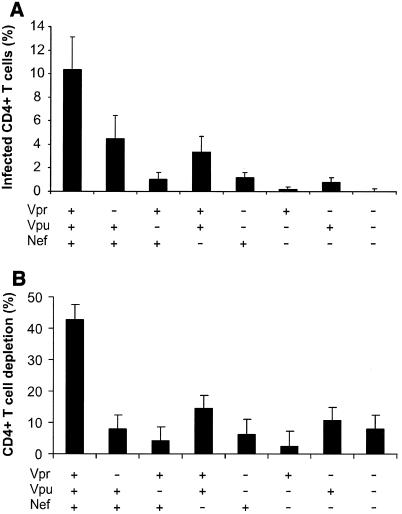
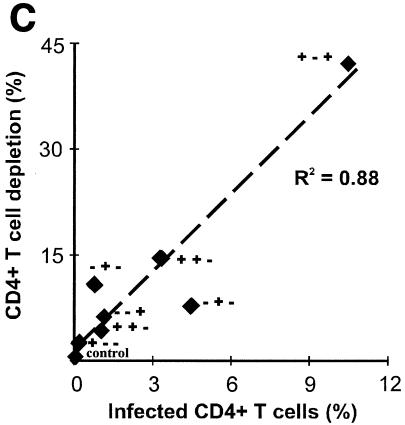
CD4+-T-cell depletion in human lymphoid tissue infected ex vivo with HIV-1 variants. (A) Percentages of infected cells; (B) loss of CD4+ T cells in human lymphoid tissue infected ex vivo with HIV-1. Productively infected CD4+ T cells were defined as CD3+ CD8− p24+, as described in the text. To evaluate CD4+-T-cell depletion, cells were mechanically isolated from control and infected matched tissues (27 pooled blocks for each variant) on day 12 postinfection, stained for CD3, CD4, CD8, and p24, and analyzed with flow cytometry. Depletion is expressed as 100% minus the percentage of CD4+ T cells that remained in the tissue after 12 days of infection, evaluated as described earlier (23, 29). Presented are average depletion values ± standard errors of the means for tissues from 4 to 12 donors. (C) Correlation between depletion and virus infection of CD4+ T cells in ex vivo-infected human lymphoid cultures. Accessory gene deletions are indicated in the following order: Vpr, Vpu, Nef.
We have previously shown that the loss of CD4+ T cells in ex vivo human lymphoid tissue results mainly from the death of HIV-1-infected cells (27, 28). Thus, although molecular mechanisms for low viral infectivity and virus production may be different for different mutants, they all result in a lower number of infected cells and hence less CD4+-T-cell depletion in infected tissues. However, more experiments need to be done to clarify whether the accessory genes might also play a direct role in cell killing, for example, through the proapoptotic activity reported for Vpr (38, 44, 47). We found that in 3 out of 13 experiments the vpr-deleted virus replicated with an efficiency similar to that of wild-type HIV-1 but caused no significant CD4+-T-cell depletion (1.3% ± 1.3% in tissues infected with vpr-deleted HIV-1 versus 36% ± 10% in tissues infected with wild-type virus). In any case, our data indicate that intact vpu, vpr, and nef genes are critical for the loss of CD4+ T lymphocytes, resulting in immunodeficiency associated with AIDS.
Many of the suggested mechanisms of facilitation of HIV-1 infection by accessory genes are related to their involvement in cell activation. For example, Vpr has been shown to potentiate Nef-induced activation of NFAT (33). We have previously shown, and we confirm in the present study (Fig. 3), that Nef enhances HIV-1 responsiveness to interleukin-2 (IL-2) in human lymphoid tissue ex vivo (24). It is unclear whether Vpu or Vpr might also facilitate cell activation by autocrine and paracrine cytokine production. Therefore, we tested in matched tissues from seven donors whether Vpu and Vpr change the sensitivity of the system to IL-2. Our results demonstrate that IL-2 stimulates the replication of both wild-type NL4-3 and its mutants (Fig. 3A). On average, however, exogenous IL-2 increased NL4-3 wild-type virus production about 13-fold, whereas production by the vpr and vpu deletion mutants was enhanced only 2.5- to 5.4-fold (Fig. 3B). Consequently, the difference between the replication rate of the wild-type HIV-1 and those of vpr- or vpu-deleted variants becomes more dramatic in IL-2-stimulated tissues: without IL-2, this difference was approximately two- to threefold, whereas in the presence of IL-2 it increased to five- to eightfold (Fig. 3B). Thus, accessory gene-deleted HIV-1 variants are less sensitive to stimulation by cytokines.
FIG. 3.
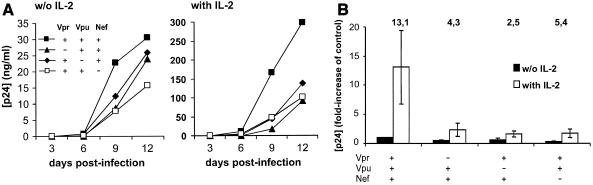
Effect of exogenous IL-2 on replication of HIV-1 variants in human lymphoid tissue ex vivo. Matched infected tissues were inoculated with HIV-1 variants and cultured without or with IL-2 (50 U/ml). For each condition and each donor tissue, 27 tissue blocks were inoculated. (A) Representative time course of p24 production in unstimulated and IL-2-stimulated tissues inoculated with wild-type virus or HIV-1 variants with accessory gene deletions. (B) Cumulative viral production over 12 days of infection by wild-type virus or HIV-1 variants with accessory gene deletions. Presented are means ± standard errors of the means of the fold increases of p24 production (relative to the replication of the wild-type virus in the absence of IL-2) in tissues from seven to nine donors inoculated ex vivo with the indicated HIV-1 variants. The numbers above each bar give the fold increase of virus production in the presence of IL-2 relative to production of the respective HIV-1 mutants in the absence of IL-2.
To investigate mechanisms by which accessory genes might affect viral spread in ex vivo-infected human lymphoid tissue, we measured the production of various cytokines known to affect HIV-1 replication. However, we did not detect any significant effect of nef, vpr, or vpu on the levels of macrophage inflammatory protein 1α (MIP-1α), MIP-1β, stromal cell-derived factor 1, RANTES, inducible protein 10, IL-1α, IL-1β, tumor necrosis factor alpha, IL-15, and IL-16 (data not shown). Thus, it remains to be clarified whether the described effects of mutant viruses occur at transcriptional or posttranscriptional levels. Previous data suggest, however, that accessory genes might contribute to efficient replication in human lymphoid tissue ex vivo by various mechanisms. We have demonstrated that the ability of Nef to enhance HIV-1 replication in ex vivo-infected human lymphoid tissue correlates with its functional activity in CD4 downmodulation (25). This Nef function might be critical for the production of fully infectious viral particles from CD4+ T cells (6). Vpu might enhance viral spread by both the same and different mechanisms because it downmodulates CD4 (34, 53) but it also increases the release of virus particles (49, 54). Finally, Vpr is known to play a role in virus transcription, cell proliferation, and apoptosis. Most likely, the multifunctional nef, vpu, and vpr genes enhance HIV-1 replication in ex vivo-infected human lymphoid tissue by several mechanisms. A major finding of our study is that the levels of viral replication and the numbers of infected cells are low in human lymphoid tissue infected with the vpu-, vpr-, or nef-defective viruses.
In conclusion, all three accessory genes, nef, vpr, and vpu, are important for efficient replication and CD4+-T-cell depletion in ex vivo-infected human lymphoid tissues. Some effects of these genes may be related to cell activation. Most importantly, our data suggest that, like Nef, Vpr and Vpu are relevant for efficient viral infection and for CD4 T-cell depletion in HIV-1-infected individuals.
Acknowledgments
We thank Thomas Mertens for constant support and Sofie Aftring and Nicola Bailer for excellent technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) and the Wilhelm-Sander-Stiftung.
REFERENCES
- 1.Agostini, I., J. M. Navarro, F. Rey, M. Bouhamdan, B. Spire, R. Vigne, and J. Sire. 1996. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J. Mol. Biol. 261:599-606. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 3.Akari, H., S. Bour, S. Kao, A. Adachi, and K. Strebel. 2001. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappa B-dependent expression of antiapoptotic factors. J. Exp. Med. 194:1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol. 71:6094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander, L., E. Weiskopf, T. C. Greenough, N. C. Gaddis, M. R. Auerbach, M. H. Malim, S. J. O'Brien, B. D. Walker, J. L. Sullivan, and R. C. Desrosiers. 2000. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J. Virol. 74:4361-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arganaraz, E. R., M. Schindler, F. Kirchhoff, M. J. Cortes, and J. Lama. 2003. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 278:33912-33919. [DOI] [PubMed] [Google Scholar]
- 7.Bour, S., and K. Strebel. 2000. HIV accessory proteins: multifunctional components of a complex system. Adv. Pharmacol. 48:75-120. [DOI] [PubMed] [Google Scholar]
- 8.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, E. A., G. Dehni, J. G. Sodroski, and W. A. Haseltine. 1990. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J. Virol. 64:3097-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein, D. A., M. P. Sherman, M. L. Penn, P. S. Chin, C. M. De Noronha, W. C. Greene, and M. A. Goldsmith. 2001. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J. Exp. Med. 194:1407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauci, A. S. 1996. Host factors and the pathogenesis of HIV-induced disease. Nature 384:529-534. [DOI] [PubMed] [Google Scholar]
- 16.Fauci, A. S. 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 262:1011-1018. [DOI] [PubMed] [Google Scholar]
- 17.Felzien, L. K., C. Woffendin, M. O. Hottiger, R. A. Subbramanian, E. A. Cohen, and G. J. Nabel. 1998. HIV transcriptional activation by the accessory protein, VPR, is mediated by the p300 co-activator. Proc. Natl. Acad. Sci. USA 95:5281-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher, T. M., III, B. Brichacek, N. Sharova, M. A. Newman, G. Stivahtis, P. M. Sharp, M. Emerman, B. H. Hahn, and M. Stevenson. 1996. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM). EMBO J. 15:6155-6165. [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs, J. S., D. A. Regier, and R. C. Desrosiers. 1994. Construction and in vitro properties of HIV-1 mutants with deletions in “nonessential” genes. AIDS Res. Hum. Retrovir. 10:343-350. [DOI] [PubMed] [Google Scholar]
- 22.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1:1320-1322. [DOI] [PubMed] [Google Scholar]
- 23.Glushakova, S., B. Baibakov, J. Zimmerberg, and L. Margolis. 1997. Experimental HIV infection of human lymphoid tissue: Correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histoculture inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res. Hum. Retrovir. 13:461-471. [DOI] [PubMed] [Google Scholar]
- 24.Glushakova, S., J.-C. Grivel, K. Suryanarayana, P. Meylan, J. D. Lifson, R. Desrosiers, and L. Margolis. 1999. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J. Virol. 73:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grivel, J.-C., A. Biancotto, Y. Ito, R. G. Lima, and L. B. Margolis. 2003. Bystander CD4+ T lymphocytes survive in HIV-infected human lymphoid tissue. AIDS Res. Hum. Retrovir. 19:211-216. [DOI] [PubMed] [Google Scholar]
- 28.Grivel, J.-C., N. Malkevitch, and L. Margolis. 2000. Human immunodeficiency virus type 1 induces apoptosis in CD4+ but not in CD8+ T cells in ex vivo-infected human lymphoid tissue. J. Virol. 74:8077-8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grivel, J.-C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:344-346. [DOI] [PubMed] [Google Scholar]
- 30.Hsu, K., J. Seharaseyon, P. Dong, S. Bour, and E. Marban. 2004. Mutual functional destruction of HIV-1 Vpu and host TASK-1 channel. Mol. Cell 14:259-267. [DOI] [PubMed] [Google Scholar]
- 31.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 32.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 33.Lahti, A. L., A. Manninen, and K. Saksela. 2003. Regulation of T cell activation by HIV-1 accessory proteins: Vpr acts via distinct mechanisms to cooperate with Nef in NFAT-directed gene expression and to promote transactivation by CREB. Virology 310:190-196. [DOI] [PubMed] [Google Scholar]
- 34.Levesque, K., Y. S. Zhao, and E. A. Cohen. 2003. Vpu exerts a positive effect on HIV-1 infectivity by down-modulating CD4 receptor molecules at the surface of HIV-1-producing cells. J. Biol. Chem. 278:28346-28353. [DOI] [PubMed] [Google Scholar]
- 35.Mackay, G. A., Y. Niu, Z. Q. Liu, S. Mukherjee, Z. Li, I. Adany, S. Buch, W. Zhuge, H. M. McClure, O. Narayan, and M. S. Smith. 2002. Presence of Intact vpu and nef genes in nonpathogenic SHIV is essential for acquisition of pathogenicity of this virus by serial passage in macaques. Virology 295:133-146. [DOI] [PubMed] [Google Scholar]
- 36.Marin, M., K. M. Rose, S. L. Kozak, and D. Kabat. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9:1398-1403. [DOI] [PubMed] [Google Scholar]
- 37.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel, C. A., M. Mukhtar, and R. J. Pomerantz. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J. Virol. 74:9717-9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 42.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9:1404-1407. [DOI] [PubMed] [Google Scholar]
- 43.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 44.Somasundaran, M., M. Sharkey, B. Brichacek, K. Luzuriaga, M. Emerman, J. L. Sullivan, and M. Stevenson. 2002. Evidence for a cytopathogenicity determinant in HIV-1 Vpr. Proc. Natl. Acad. Sci. USA 99:9503-9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steffens, C. M., and T. J. Hope. 2001. Recent advances in the understanding of HIV accessory protein function. AIDS 15(Suppl. 5):S21-S26. [DOI] [PubMed] [Google Scholar]
- 47.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Y. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stopak, K., C. de Noronha, W. Yonemoto, and W. C. Greene. 2003. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell 12:591-601. [DOI] [PubMed] [Google Scholar]
- 49.Strebel, K., T. Klimkait, F. Maldarelli, and M. A. Martin. 1989. Molecular and biochemical analyses of human immunodeficiency virus type 1 vpu protein. J. Virol. 63:3784-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tristem, M., C. Marshall, A. Karpas, J. Petrik, and F. Hill. 1990. Origin of vpx in lentiviruses. Nature 347:341-342. [DOI] [PubMed] [Google Scholar]
- 52.Varthakavi, V., R. M. Smith, S. P. Bour, K. Strebel, and P. Spearman. 2003. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. USA 100:15154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao, X. J., H. Göttlinger, W. A. Haseltine, and E. A. Cohen. 1992. Envelope glycoprotein and CD4 independence of vpu-facilitated human immunodeficiency virus type 1 capsid export. J. Virol. 66:5119-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]


