Abstract
Rationale
The vascular adventitia is a complex layer of the vessel wall consisting of vasa vasorum microvessels, nerves, fibroblasts, immune cells, and resident progenitor cells. Adventitial progenitors express the stem cell markers, Sca1 and CD34 (AdvSca1 cells), have the potential to differentiate in vitro into multiple lineages, and potentially contribute to intimal lesions in vivo.
Objective
While emerging data support the existence of AdvSca1 cells, the goal of this study was to determine their origin, degree of multipotency and/or heterogeneity, and contribution to vessel remodeling.
Methods and Results
Using two in vivo fate-mapping approaches combined with a smooth muscle cell (SMC) epigenetic lineage mark, we report that a subpopulation of AdvSca1 cells is generated in situ from differentiated SMCs. Our data establish that the vascular adventitia contains phenotypically distinct subpopulations of progenitor cells expressing SMC, myeloid, and hematopoietic progenitor-like properties and that differentiated SMCs are a source to varying degrees of each subpopulation. SMC-derived AdvSca1 cells exhibit a multipotent phenotype capable of differentiating in vivo into mature SMCs, resident macrophages, and endothelial-like cells. Following vascular injury, SMC-derived AdvSca1 cells expand in number and are major contributors to adventitial remodeling. Induction of the transcription factor Klf4 in differentiated SMCs is essential for SMC reprogramming in vivo while in vitro approaches demonstrate that Klf4 is essential for maintenance of the AdvSca1 progenitor phenotype.
Conclusions
We propose that generation of resident vascular progenitor cells from differentiated SMCs is a normal physiological process that contributes to the vascular stem cell pool and plays important roles in arterial homeostasis and disease.
Keywords: Smooth muscle cells, Kruppel-like factor 4, reprogramming, vascular biology, progenitor cell, adventitia, vascular remodeling
Subject Terms: Cellular Reprogramming, Vascular Biology, Stem Cells, Smooth Muscle Proliferation and Differentiation
INTRODUCTION
The static arrangement of concentric layers of intima, media, and adventitia found in most blood vessels fails to capture the dynamics of cell movement between the individual layers. Most studies that address this question have focused on migration of smooth muscle cells (SMCs) into the intimal layer, where cells from the circulation and from the vessel wall interact during progression of atherosclerotic plaques1. Little, if any, attention has been paid to the possibility that SMCs may also move in the opposite direction, into the adventitia. In part, this is due to the prevailing notion that the adventitia is an inert bystander tissue whose function is to provide structural support for the artery wall in the form of collagen-rich matrix produced by adventitial fibroblasts. However, this notion is challenged by recent studies showing that the adventitia actually contains a highly dynamic population of leukocytes, microvessels, adipocytes and resident progenitor cells that collectively both maintain the artery wall and respond robustly to many kinds of vascular injury2–5. In some cases, the adventitia is even the main site of pathological change such as in the formation of tertiary lymphoid organs in diseased arteries, fibrotic changes that reduce compliance and promote renal damage in hypertension, and as a focus for inflammatory cell accumulation in development of medial dissections and aneurysms6–9. In smaller vessels, the adventitia and associated perivascular cells are important signaling niches for resident progenitor cells in skeletal muscle10, kidney11, and bone marrow hematopoietic stem cells12. The perivascular niche is also a residence site for dormant cancer cells with metastatic potential13. Thus, factors governing the cell composition of the adventitia are important to identify for the health and maintenance of blood vessels and the tissues and stem cell populations they support.
In recent years, reports demonstrating the existence of resident cardiovascular progenitor cells have had profound effects on our understanding of cardiovascular biology and tissue regeneration14–23. Emerging data suggest that several distinct progenitor populations with the capacity to differentiate into endothelial cells, smooth muscle cells (SMCs), fibroblasts, and macrophages reside in a specialized niche in the adventitia at the media-adventitia border. Hu, et.al.2 described a population of vascular progenitor cells in the aortic root adventitia of ApoE−/− mice that express the progenitor markers Sca1 and CD34 and differentiate to vascular SMCs in vitro (AdvSca1 progenitors). Our group showed similar cells cluster in an adventitial domain of sonic hedgehog (Shh) signaling, and while SMC marker negative, were found to express transcription factors known to activate SMC markers (e.g. serum response factor [SRF] and myocardin)4. They also express high levels of SRF co-repressors (eg. KLF4) suggesting AdvSca1(+) progenitors are specified for the SMC fate, but transcriptional repression maintains their progenitor phenotype. AdvSca1 progenitors were shown to have the potential to self renew or to differentiate in vitro into SMCs, endothelial cells, osteoblasts, chondrocytes, or adipocytes4, 24. These cells are not unique to murine vessels as adventitial-derived CD34(+) progenitor cells have also been isolated from human vessels14, 25. In addition, an intriguing finding was the existence of local, resident AdvSca1 myeloid progenitors with hematopoietic potential that reside in a similar adventitial niche5, 26. The presence of resident vascular progenitor cells has significant implications for their potential therapeutic use in the treatment of vascular diseases and regenerative medicine. While accumulating evidence supports their existence, a number of important questions remain unanswered. Several groups2, 5, 24 demonstrated that these cells do not originate from bone marrow and our previous findings4 demonstrated that adventitial progenitors do not arise from cardiac neural crest. Therefore, the origin of AdvSca1 progenitor cells remains unclear. In addition, the degree of heterogeneity of AdvSca1 progenitors and the mechanism underlying maintenance of the AdvSca1 progenitor cell phenotype are also unclear.
Vascular SMCs are specialized cells that express high levels of SMC-specific proteins, such as smooth muscle myosin heavy chain (Myh11/SMMHC) and smooth muscle-α-actin (Acta2/αSMC)27. Under pathological conditions, such as atherosclerosis and restenosis, however SMCs are capable of undergoing phenotypic and functional changes resulting in a proliferative, inflammatory phenotype characterized by decreased expression of SMC contractile proteins and increased production of pro-inflammatory cytokines. As a result, mature SMCs are major contributors to pathological neointima formation28–32. Our previous report using a highly specific SMC fate-mapping approach in the setting of restenosis demonstrated that the majority of proliferating intimal cells derive from mature SMCs33. Remarkably, we also consistently detected reporter-positive, but SMC marker-negative SMC-derived cells in the arterial adventitia, suggesting that mature SMCs contribute to both intimal and adventitial remodeling. In addition, recent reports demonstrated that a high percentage of SMCs in atherosclerotic lesions lack detectable expression of conventional SMC markers, but exhibit a macrophage-like phenotype31,34, 35. These findings suggest that SMCs exhibit an even greater degree of plasticity than previously recognized. In light of our findings that SMCs contribute to adventitial remodeling and since the origin of AdvSca1 progenitor cells remains unknown, we sought to determine if mature SMCs contribute to the vascular progenitor pool. Using fate mapping and lineage tracing approaches, in this report we demonstrate that a distinct subpopulation of AdvSca1 progenitors derive from differentiated SMCs that undergo a reprogramming-like process in situ to generate multipotent progenitor cells. In addition, we show here that the pluripotency-associated transcription factor, Klf4, regulates the generation of SMC-derived AdvSca1 cells and is essential for the maintenance of the AdvSca1 progenitor cell phenotype.
METHODS
Mice
Myh11-CreERT2 transgenic mice and Rosa26-LacZ or Rosa26-YFP reporter mice were bred to generate tamoxifen-inducible SMC-specific β-galactosidase- or YFP-expressing mice (Myh11-CreERT-βGal/YFP). To activate Cre, adult male mice received 1-mg IP tamoxifen injections for 5 consecutive days 8 weeks before they were sacrificed. Timed pregnant females (Rosa26-YFP females bred to male Myh11-CreERT-Rosa26-YFP mice) received 1-mg IP tamoxifen injections at e15 and e16 to label developing embryos. SM22α-Cre transgenic mice were bred to Rosa26-YFP to generate SMC-specific YFP-expressing mice (SM22α-Cre-YFP). For in vivo Matrigel™ plug assays, SMC-derived AdvSca1 cells were isolated as described below, resuspended in 700 μl Matrigel™ plus 100ng/ml VEGF and 100ng/ml FGF2, and injected subcutaneously into wild type C57BL/6 recipients. Plugs were harvested 14 days post-injection, fixed in 4% buffered PFA, and embedded in OCT for immunofluorescent staining. To induce vascular injury, mice were subjected to wire-induced femoral artery injury or carotid artery ligation injury, as previously demonstrated33, 36. Four weeks after femoral artery injury or three days following carotid artery ligation injury, uninjured and injured arteries were harvested, perfusion fixed in 4% buffered PFA, and embedded in OCT for immunofluorescent staining. SM22α-CreKI transgenic mice (Taglntm2(cre)Yec; JAX stock 006878) were bred to Klf4 floxed mice and Rosa26-YFP mice to generate SMC-specific YFP-expressing Klf4-deficient mice (SM22α-CreKI-YFP KO). This alternative SMC Cre driver, in which Cre recombinase is activated very late in development compared to the traditional SM22α-Cre mouse line (TagIn-Cre; JAX stock 004746), was used to generate SMC-specific Klf4 KO mice as use of TagIn-Cre mice resulted in embryonic lethality. Mice were maintained in the Center for Comparative Medicine, and procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver.
Cell analysis
Isolated aortic arch plus left and right carotid arteries, descending aortas, and left and right femoral arteries were digested to single cells by digestion at 37°C for 1hr in collagenase buffer. For flow sorting, single cell suspensions were incubated with a rat anti-mouse monoclonal APC-Sca1 antibody (eBiosciences); live cells were sorted on a MoFlo high-speed cell sorter based on Sca1-APC and endogenous YFP expression. For FACS analysis, single cell suspensions were stained with various combinations of rat anti-mouse monoclonal antibodies (PerCP-Cy5.5-Sca1, PE-Cy7-CD45, APC/e780-Ly6C, PE-Cy7-CD115, APC-CD140a, APC-CD140b, PE-CD31; all antibodies were from eBiosciences). Flow cytometry was performed on a Galios cytometer (Becton Dickenson). Data were analyzed using Kaluza Software (Beckman Coulter). To quantitate AdvSca1 populations in WT versus SMC-specific Klf4 KO mice, vessels from 8–10-day old mice were individually digested to single cell suspensions, labeled with anti-APC-Sca1 antibodies, and analyzed for endogenous YFP and Sca1-APC expression. To analyze the effect of Klf4 on maintenance of the AdvSca1 progenitor cell phenotype, AdvSca1 cells were sorted based on Sca1 expression, transfected with non-targeting or Klf4-targeting siGENOME SMARTpool siRNAs (100 nm; Thermo Scientific) or transduced with an empty vector adenovirus or an adenovirus expressing Klf4 (100 MOI; Vector Bioloabs, Malvern, PA).
Immunofluorescence, Chromatin Immunoprecipitation, and Quantitative RT-PCR
OCT-embedded tissues or fixed cells were permeabilized with MeOH followed by 0.05% Tween-20 in PBS, blocked in 3% horse serum, and sequentially incubated with specific primary and secondary antibodies. Antibodies used include monoclonal rat anti-mouse Sca1 (1:100; BD Pharmingen), monoclonal rat anti-mouse CD34 (1:50; Abcam), FITC-conjugated polyclonal goat anti-GFP (1:200; Abcam), Cy3-conjugated monoclonal anti-smooth muscle alpha actin (αSMA; 1:2000; Sigma), monoclonal rat anti-mouse CD45 (1:100; BD Pharmingen), polyclonal rabbit anti-Klf4 (1:100; Abcam), monoclonal rat anti-mouse F4/80 (1:50; Abcam), polyclonal rabbit anti- smooth muscle alpha actin (αSMA; 1:200; Abcam), and polyclonal rabbit anti-SMMHC (1:100; Biomedical Technologies, Inc.). Sections/cells were imaged using a laser-scanning confocal microscope (LSM 780 spectral, Carl Zeiss, Thornwood, NY). Tissues were stained for LacZ activity using a kit from GTS, Inc. according to the protocols provided. To quantitate cell populations in uninjured compared to injured carotid arteries, total numbers of Sca1 or CD45(−) YFP(+), Sca1 or CD45(+) YFP(+), and Sca1 or CD45(+) YFP(−) adventitial cells were counted in three high-powered fields per vessel from n=3 uninjured and injured vessels by two independent investigators. Chromatin Immunoprecipitation (ChIP) was performed on flow-isolated cell populations as previously described37. Antibodies for ChiP include anti-H3K4Me2, anti-H4-Ac (Millipore), and anti-SRF (Santa Cruz Biotechnologies). DNA was purified with QIAquick PCR purification kits (Qiagen). Real-time PCR with Power SYBR Green mastermix (Life Technologies) and primers designed to flank CaRG elements in the Acta2 and Myh11 was used to analyze chromatin immunoprecipitation (primer sequences are available in the online Supplement). Data from a minimum of 3 independent experiments were normalized to YFP+ SMCs. Quantitative RT-PCR (qPCR) was used with total RNA isolated from flow-isolated cell populations as previously described33. Primer sequences are available in the online Supplement. β-actin was used for normalization. To compare among individual experiments, data was normalized to YFP+ SMCs for SMC genes (αSMA, SMMHC, SM22α, and myocardin) or AdvSca1-MA cells for progenitor cell genes (Klf4, CD34, VEGF, SDF-1α, CD31, Flk1, Flt1).
RESULTS
Genetic fate-mapping reveals adventitial SMC-derived progenitor cells
We previously used tamoxifen (tmx)-inducible Myh11CreERT2 transgenic mice crossed with floxed-stop ROSA reporter mice (Myh11-CreER-βGal/YFP) to fate map mature SMCs in response to vascular injury33. Tmx given to adult mice before vascular injury genetically, permanently, and efficiently marked only Myh11/SMMHC-expressing SMCs through Cre-mediated reporter knock-in. Since tmx was given before injury and then stopped, SMCs expressing Myh11/SMMHC and their progeny were the only reporter-positive cells throughout the experimental time period allowing tracking of mature SMCs in response to vascular injury even if levels of Myh11/SMMHC (or other SMC markers) were no longer detectable; any non-SMC that potentially gained expression of Myh11/SMMHC after injury would not be labeled as tmx was not in the system to activate Cre. Using this approach, we found that the majority of intimal Acta2/αSMA-expressing cells originate from mature SMCs (33 and Supplemental Figure I), establishing that neointimal SMCs derive from mature differentiated SMCs. However, similar to a recent report in the setting of atherosclerosis33, we also detected populations of YFP(+) SMC-derived intimal cells that expressed no detectable levels of SM markers (asterisks in Supplemental Figure 1) suggesting that the contribution of SMCs to intimal hyperplasia is underestimated if solely using expression of conventional SMC marker proteins to identify intimal SMCs. Mice not treated with tamoxifen exhibited no genetic labeling of SMCs or adventitial cells (Supplemental Figure II). In addition to intimal SMCs, we made the entirely unanticipated observation that over time mature SMCs migrate to the adventitia and reside in a niche at the adventitia-media border (33 and Figure 1A). Adventitial SMC-derived cells have lost SMC marker expression (Figure 1B&F, Acta2/αSMA shown), but gained expression of the stem cell markers, Sca1 and CD34 (Figure 1C,D,E,G) suggesting that differentiated SMCs generate AdvSca1 cells to contribute to the resident vascular progenitor cell pool.
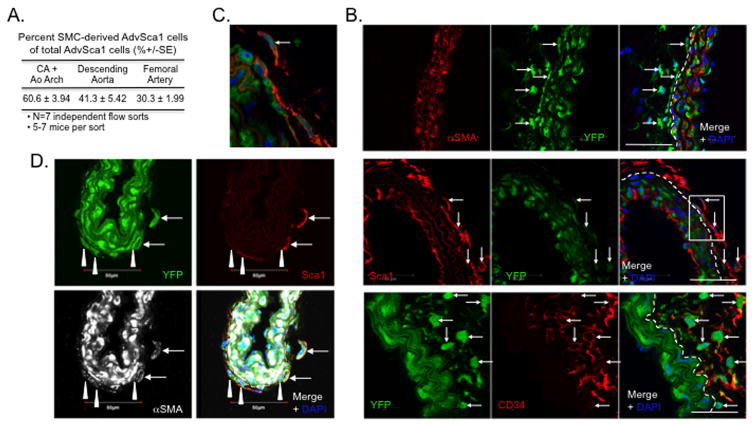
Figure 1. SMC-derived adventitial cells express stem cell markers.
Two-month old Myh11-CreERT-βGal or -YFP mice received I mg IP tamoxifen injections once a day for five consecutive days. Aortae, carotid arteries plus aortic arch, and femoral arteries were harvested 8-weeks post-tamoxifen. (A). Aortas were whole mount stained for X-Gal; histological section from a representative aorta showing βGalactosidase activity (blue reaction color). Arrows = X-Gal-positive SMC-derived adventitial cell. Arrowheads = external elastic lamina. Dashed line represents media-adventitia boundary. (B–E). Carotid or femoral arteries were immunofluorescently stained for αSMA (red) and YFP (green; B), Sca1 (red) and YFP (green; C&D), or CD34 (red) and YFP (green; E). Representative carotid arteries are shown in (B,C,&E); representative femoral artery shown in (D). Arrow = SMC-derived adventitial cells. A = adventitia; M = media. Dashed lines represent media-adventitia boundary. (F&G). Higher magnification images of inset boxes in (B&C). Scale bars=50μm. A minimum of 8 mice were analyzed.
Our previous report demonstrated that AdvSca1 progenitors appear in the adventitia of the ascending aorta and pulmonary trunk by e18.5, a developmental time point after the arterial media has acquired its complement of SMCs and has organized structured elastic lamellae4. Based on this, we hypothesized that SMC-derived AdvSca1 progenitors are established during the late embryonic-to-early postnatal period. To test this hypothesis, tmx was administered to timed-pregnant females at e15 and e16 to obtain restricted labeling of Myh11-expressing SMCs in embryos between e15 and e17, when all vascular beds exhibit strong Myh11/SMMHC expression and before the appearance of AdvSca1 cells. Pups were allowed to develop to 30 dpp, then carotid arteries plus aortic arch, descending aortas, and femoral arteries were harvested, digested into single cells, immuno-labeled for Sca1, and flow sorted based on Sca1 and YFP expression (gating strategy shown in Supplemental Figure III). Three distinct cell populations were retrieved from each vessel: (A) Sca1(−)YFP(+): mature SMCs; (B) Sca1(+)YFP(−): non-SMC-derived AdvSca1(+) cells (hereafter referred to as AdvSca1-MA); (C) Sca1(+)YFP(+): SMC-derived AdvSca1(+) cells (hereafter referred to as AdvSca1-SM) (Supplemental Figure IVA&B). Using this approach, we found approximately 8% of the total Sca1(+) cell population were YFP-positive SMC-derived cells (Supplemental Figure IVB). In contrast to blood vessels, AdvSca1-SM cells were not detected in peripheral blood mononuclear cells (not shown). This is consistent with our previous finding of a lack of labeled cells in bone marrow33 and a previous report using a comparable SMC-specific labeling approach in which labeled cells were not detected in bone marrow cells or the circulation34. Others have used a similar in utero tamoxifen pulse to fate-map yolk sac-derived myeloid progenitors38 and hemogenic endothelial cells39, albeit tamoxifen was administered at much earlier developmental time points (e. g. e8.5). In contrast to these studies upon further examination in our system, while 5-d tmx treatment of adult mice resulted in highly efficient labeling of medial SMCs33, we were unable to achieve similar labeling delivering tmx in utero at these later time points (2 injections failed to efficiently label outer medial SMCs [Supplemental Figure IVC] and >2 in utero injections consistently resulted in premature termination of pregnancy) thus precluding the ability to accurately quantitate the percentage of AdvSca1 progenitors that originate from SMCs due to lack of genetic labeling. Nonetheless, using a highly selective fate-mapping approach, our data demonstrate that a population of resident AdvSca1 cells derives from mature SMCs.
In order to accurately quantitate the percentage of AdvSca1 cells that originate from SMCs, constitutive, SMC-specific SM22α-Cre transgenic mice were bred to ROSA26-YFP mice (SM22α-Cre-YFP) to label differentiating SMCs at the time in vascular development that SM22α is induced. We previously showed that AdvSca1 cells do not express SM22α4. Using this approach, we consistently found that a large percentage of total Sca1(+) cells in the carotid artery plus aortic arch, descending aorta, and femoral artery originate from SMCs (Figure 2A and Supplemental Figure V). By immunofluorescent staining, αSMA(−), but YFP(+)Sca1(+)CD34(+) cells were detected in the adventitia of 30d SM22α-Cre-YFP mice (Figure 2B&C). Consistent with in vivo staining, freshly isolated AdvSca1-SM cells from 30d SM22α-Cre-YFP mice express high levels of progenitor cell markers, including Sca1, CD34, and the pluripotency-associated transcription factor, Klf4, but very dim to undetectable levels of αSMA (Supplemental Figure VI). To further strengthen that SMCs migrate into the adventitia and to provide evidence of the late developmental timing of SMC reprogramming, we analyzed arteries from 5-day old SM22α-Cre-YFP mice and detected Sca1(+)YFP(+) adventitial cells still expressing residual SMC markers (Figure 2D). Specific chromatin modifications occur selectively in SMC gene loci of SM markers (eg. Acta2/αSMA, Myh11/SMMHC) and are associated with specification of the SMC lineage during development40, 41. Unlike most which are lost from SMC genes in response to SMC phenotypic switching (eg. H4Ac), one chromatin mark in particular, H3K4 dimethylation (H3K4Me2), is retained and serves as a highly specific SMC lineage marker in both mouse and human tissues40, 42. As a secondary approach to demonstrate SMC origin of YFP(+)Sca1(+) AdvSca1 cells, chromatin immunoprecipitation (ChIP) was conducted for expression of the H3K4Me2 mark in the Myh11 and Acta2 loci of isolated YFP(+)Sca1(+) compared to YFP(−)Sca1(+) cells. Similar to SMC-rich intact aortic media (not shown), H3K4Me2 was detected on the Myh11/SMMHC and Acta2/αSMA promoters of isolated YFP(+) mature SMCs and YFP(+)Sca1(+) AdvSca1-SM cells, but not YFP(−)Sca1(+) AdvSca1-MA cells (Supplemental Figure VIIA). In contrast, compared to mature YFP(+) SMCs, expression of the SMC differentiation-associated H4Ac chromatin mark40 was undetectable in YFP(+)Sca1(+) AdvSca1-SM cells (Supplemental Figure VIIB left; Myh11 shown). Binding of the transcription factor, SRF, to CArG boxes of SM gene promoters is essential for promoting the SMC differentiation program40. Loss of the SMC differentiation H4Ac chromatin mark was associated with undetectable SRF binding to SM gene promoters in YFP(+)Sca1(+) AdvSca1-SM cells (Supplemental Figure VIIB right; Myh11 shown). Collectively, using two independent genetic fate-mapping systems combined with a SMC lineage histone mark, we established that differentiated SMCs generate in situ a subpopulation of AdvSca1 progenitor cells.
Figure 2. Percentage of total AdvSca1 cells originating from differentiated SMCs.
(A). Carotid arteries (CA) plus aortic (Ao) arch, descending aorta, and femoral arteries were harvested from two-month old SM22α-Cre-YFP mice. Arteries from 5–7 mice were pooled, digested into single cell suspensions, labeled with an APC-conjugated anti-Sca1 antibody, and flow sorted based on endogenous YFP and Sca1 expression. The percent±SE SMC-derived YFP(+)Sca1(+) (quadrant B; Supplemental Figure 5) of total Sca1(+) cells (quadrants A + B; Supplemental Figure 5) from 7 independent sorts is shown in the table. (B). Representative immunofluorescent stains of carotid artery sections from two-month old SM22α-Cre-YFP mice for αSMA (red) and YFP (green; top panels), Sca1 (red) and YFP (green; middle panels), or CD34 (red) and YFP (green; bottom panels). Arrows = YFP(+) adventitial cells. Dashed lines represent media-adventitia boundary. A minimum of 8 mice were analyzed. Scale bars=50μm. (C). Higher magnification image of inset box in (B, middle panel). (D). Representative immunofluorescent stain of a carotid artery section from a five day-old SM22α-Cre-YFP mouse for YFP (green), Sca1 (red), and αSMA (white). Arrows = SMC-derived AdvSca1+ cells expressing residual levels of αSMA; Arrowheads = SMC-derived adventitial cells expressing residual levels of αSMA and low/absent levels of Sca1. Dashed line represent media-adventitia boundary. Scale bars=50μm.
Differentiated SMCs generate phenotypically distinct subpopulations of AdvSca1 cells
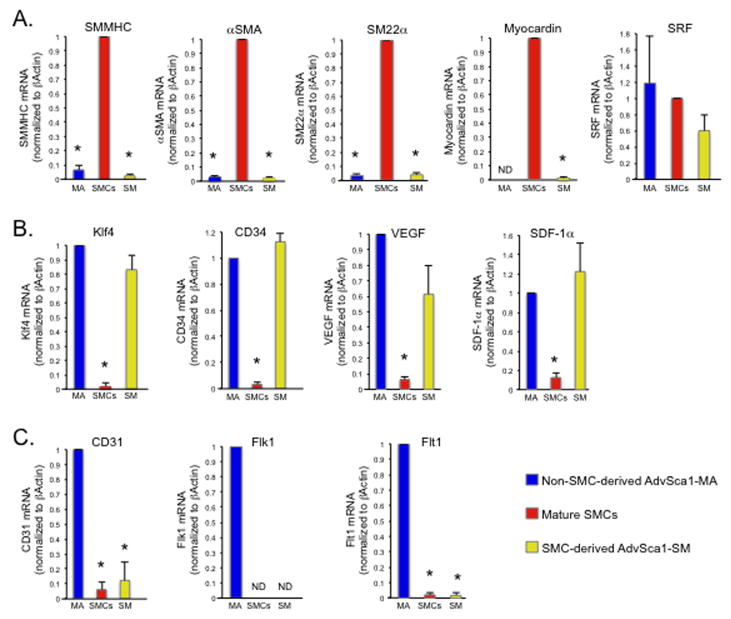
We used quantitative RT-PCR (qPCR) to further characterize individual cell populations. Mature SMCs, AdvSca1-SM progenitors, and AdvSca1-MA progenitors were isolated by flow sorting as described above. Compared to mature YFP(+)Sca1(−) SMCs, expression of the SMC-specific mRNAs, Myh11/SMMHC, Acta2/αSMA, TagIn/SM22α, and Mycdn/myocardin was undetectable in both AdvSca1-SM and AdvSca1-MA progenitors (Figure 3A). No difference in expression of SRF was detected among the cell populations (Figure 3A). However, combined with loss of SRF binding to SM gene promoters by ChIP analysis (Supplemental Figure 7), these results suggest that the SMC differentiation program is repressed in AdvSca1-SM cells. In contrast to SM-specific genes, Klf4, the progenitor cell marker CD34, and progenitor cell-associated cytokines, VEGF and SDF-1α, were highly expressed in both populations of AdvSca1 cells compared to mature SMCs (Figure 3B). Finally, CD31, Flk-1, and Flt-1, commonly associated with endothelial/monocyte progenitor cells, were selectively expressed by AdvSca1-MA, but not AdvSca1-SM progenitor cells (Figure 3C) suggesting two distinct populations of AdvSca1 progenitor cells reside in the vessel wall.
Figure 3. Progenitor cell marker expression in AdvSca1 cells.
Total RNA was isolated from cell populations from pooled, digested arteries from SM22α-Cre-YFP mice and analyzed by qPCR for the indicated mRNAs. Shown are fold changes in mRNA copy number±SE from an N=3 independent experiments using arteries from 10 pooled mice per experiment; *P<0.05. β-actin was used for normalization. (A). SMC markers. To compare among individual experiments, data was normalized to YFP(+) SMCs. (B). Progenitor cell markers expressed by both Sca1+ populations. To compare among individual experiments, data was normalized to AdvSca1-MA cells. (C). mRNAs selectively expressed in Sca1(+)YFP(−) cells. To compare among individual experiments, data was normalized to AdvSca1-MA cells. Shown for all panels are data obtained from cell populations isolated from pooled carotid arteries plus aortic arch. ND = not detectable.
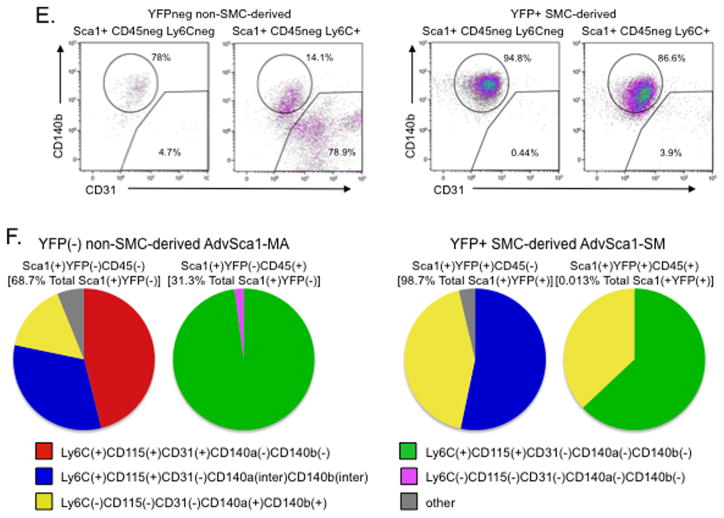
We used flow cytometry to further immunophenotypically characterize these subpopulations. Two recent reports identified a population of non-hematopoietic-derived resident adventitial Sca1(+)CD45(+) macrophage progenitor cells5,26. Gated YFP(+) and YFP(−) cells (gating strategy shown in Supplemental Figure VIIIA) were analyzed for expression of CD45 and the common monocyte progenitor marker Ly6C to determine if CD45(+) macrophage progenitors reside selectively in the YFP(−) population. Whereas a much higher percentage of CD45(+) cells was identified in the YFP(−) cell fraction, the YFP(+) cell fraction was found to contain a small percentage of CD45(+) cells (Figure 4A&F; Supplemental Table I). CD45(−) and CD45(+) cells from YFP(−) and YFP(+) populations were analyzed for Sca1 expression. Distinct populations of Sca1(+)CD45(−) and Sca1(+)CD45(+) were identified within both SMC-derived YFP(+) and non-SMC-derived YFP(−) cells (Figure 4B; Supplemental Table I). While the majority of Sca1(+)CD45(−) and Sca1(+)CD45(+) AdvSca1-MA progenitors also expressed Ly6C, only approximately half of Sca1(+)CD45(−) (54.6%) and Sca1(+)CD45(+) (56.1%) AdvSca1-SM progenitors expressed Ly6C (Figure 4C&F; Supplemental Table I). Ly6C(+) cells within the YFP(+)Sca1(+) and YFP(−)Sca1(+) populations co-expressed CD115, also a common monocyte progenitor marker (Figure 4D circled populations). Expression of the platelet derived growth factor receptors PDGF-Rβ/CD140b and PDGF-Rα/CD140a has been implicated in regulating a variety of progenitor cells and, in particular, specification of a SMC fate. CD31 is a cell surface marker expressed by a variety of cells, including mature endothelial cells and endothelial and myeloid progenitor cells. Compared to AdvSca1-MA cells, the vast majority of both CD45(−)Ly6C(+) and CD45(−)Ly6C(−) subpopulations of AdvSca1-SM cells expressed CD140b and CD140a (Figure 4E&F; Supplemental Table I; CD140b shown), but not CD31. In contrast, CD45(−)Ly6C(+) and CD45(−)Ly6C(−) subpopulations of AdvSca1-MA cells expressed either CD140b/CD140a or CD31; expression appeared to be mutually exclusive (Figure 4E&F; Supplemental Table I; CD140b shown). All non-SMC Sca1(+)CD45(+) cells were negative for CD140b, CD140a, and CD31 (Figure 4F; Supplemental Table I) whereas SMC-derived Sca1(+)CD45(+) cells either expressed Ly6C and CD115 or CD140b and CD140a; none expressed CD31 (Figure 4F; Supplemental Table I). Finally, the remaining YFP(+)Sca1(−)CD45(−) population (Supplemental Figure VIIIB circled population in left panel) did not express the myeloid markers, Ly6C, CD115, or CD31 (Supplemental Figure VIIIB middle and right panels; CD115 not shown) and only expressed low levels of CD140b and CD140a (Supplemental Figure VIIIB right two panels; Supplemental Figure VIIIC) verifying this population as mature SMCs.
Figure 4. FACS profiling reveals distinct subpopulations of AdvSca1 cells.
Single cell suspensions were obtained from digested arteries from SM22α-Cre-YFP mice. (A). Representative flow cytometry plots showing expression of CD45 and Ly6C in YFP(−) and YFP(+) cells. (B). Representative flow cytometry plots showing expression of Sca1 in CD45(−) and CD45(+) cells from YFP(−) and YFP(+) cell populations. (C). Representative flow cytometry plots showing expression of Ly6C in Sca1(+)CD45(−) and Sca1(+)CD45(+) cells from YFP(−) and YFP(+) cell populations. (D). Representative flow cytometry plots showing expression of Ly6C and CD115 in YFP(−) and YFP(+) cells. (E). Representative flow cytometry plots for expression of CD140b and CD31 in Sca1(+)CD45(−)Ly6C(−) and Sca1(+)CD45(−)Ly6C(+) cells from YFP(−) and YFP(+) cell populations. (F). Pie charts illustrating the various subpopulations of Sca1(+) progenitor cells within Sca1(+)YFP(−) and Sca1(+)YFP(+) groups. Shown for all are data obtained from cell populations isolated from carotid arteries plus aortic arch; N=4 independent analyses using pooled arteries from 5 mice.
To determine if AdvSca1-SM cells possess multipotency potential capable of differentiating into other cell lineages, in vivo Matrigel™ plug angiogenesis assays were performed. AdvSca1-SM cells were recovered from SM22α-Cre-YFP mice, resuspended in Matrigel™ plus growth factors, subcutaneously injected into syngeneic wild type mice (WT) mice, and plugs examined 14 days post-implantation for SMC-, macrophage-, and endothelial cell-specific markers (Myh11/SMMHC, F4/80, and von Willebrand factor [vWF], respectively). Analysis of YFP(+) cells demonstrated that AdvSca1-SM cells contribute to perivascular cells of functioning neovessels within Matrigel™ plugs that connected to the systemic circulation (Supplemental Figure IX). Many of these perivascular cells co-expressed SMMHC, a marker of differentiated SMCs (Figure 5A), demonstrating that AdvSca1-SM cells serve as SMC progenitor cells. In addition, we observed YFP(+)F4/80(+) cells largely in regions of the plug with recruited host-derived inflammatory cells demonstrating that AdvSca1-SM cells differentiate to macrophages (Figure 5B). Moreover, while the majority of endothelial cells in neovessels were host-derived (e.g. YFP−)(Supplemental Figure XA), YFP(+)vWF(+) endothelial-like cells were observed (Figure 5C and Supplemental Figure XB) supporting a role for AdvSca1-SM cells as endothelial cell precursors. Finally, undifferentiated YFP(+) cells still expressing Sca1 were observed surrounding newly developing vessels (Supplemental Figure XC). In vitro differentiation assays demonstrated the ability of SMC-derived AdvSca1 cells to also differentiate into adipocytes and chondrocytes (Supplemental Figure XI) thereby supporting the concept that AdvSca1-SM cells exhibit a multipotent progenitor cell phenotype. Collectively, these data suggest that differentiated SMCs can generate distinct subpopulations of multipotent progenitor cells that reside in the vascular adventitia.
Figure 5. AdvSca1-SM cells exhibit multipotency potential.
AdvSca1-SM cells were isolated from SM22α-Cre-YFP mice by flow sorting, embedded in Matrigel™, and subcutaneously implanted into syngeneic WT mice. Matrigel™ plugs were harvested 14 days post-implantation for immunofluorescence analysis. (A). Representative stains of neovessels within plugs for YFP (green) and the SMC-specific marker, SMMHC (red). (B). Representative stains for YFP (green) and the macrophage-specific marker, F4/80 (red). (C). Representative stain of an endothelial cord for YFP (green) and the endothelial cell-specific marker, von Willebrand Factor (vWF)(red). Arrows = AdvSca1-SM-derived endothelial cells. Scale bars=50μm. N=4 independent analyses.
AdvSca1-SM cells expand in number in response to vascular injury
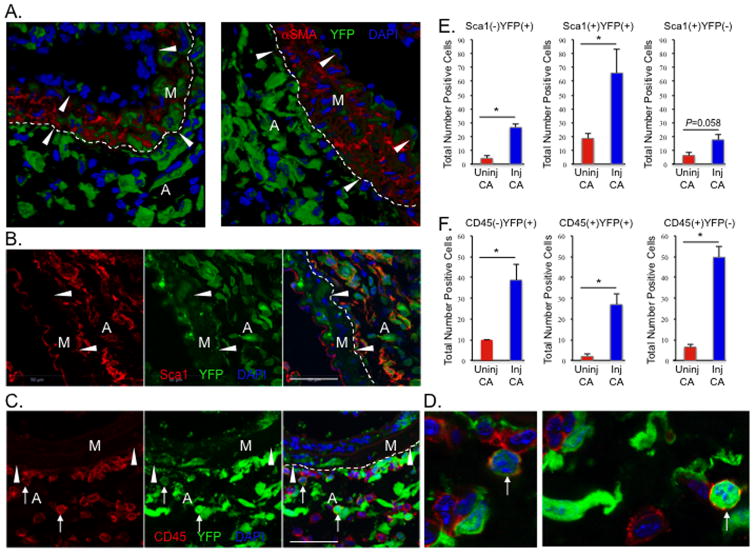
While the predominant focus of the vascular response to injury has been on neointima formation and vessel occlusion, previous reports have also demonstrated early activation and expansion of adventitial cells following injury43–46. To determine if AdvSca1-SM cells contribute to adventitial cell expansion early after vascular injury, carotid artery ligation injuries were performed on 2-mo old SM22α-Cre-YFP mice. Compared to uninjured contralateral controls (Supplemental Figure XIIA&B), there was a large increase in the numbers of YFP+αSMA− adventitial cells 3 days post injury (Figure 6A and Supplemental Figure XIIC). While this consisted of increases in both Sca1+YFP+ and Sca1−YFP+ adventitial cells, the majority of YFP+ adventitial cells co-expressed Sca1 (Figure 6B,E). Similarly, there were increased numbers of CD45+YFP+ and CD45−YFP+ adventitial cells in response to injury. Although rare in uninjured vessels, SMC-derived CD45+ adventitial cells increased in response to injury; the majority of CD45+ adventitial cells, however were YFP− non-SMC-derived (Figure 6C,D,F). These data are consistent with the concept that resident vascular progenitor cells, and in particular SMC-derived AdvSca1 cells, are activated early and are the dominant source of adventitial remodeling in response to vascular injury.
Figure 6. Injury-induced expansion of AdvSca1-SM cells.
Two-month old SM22α-Cre-YFP mice were subjected to carotid artery ligation injury and injured left and uninjured right arteries were harvested 3-days post injury for immunofluorescence analysis. (A). Representative stains of injured vessels for αSMA (red) and YFP (green) from 2 independent mice showing expansion of SMC-derived adventitial cells. (B). Representative stain of an injured vessel for Sca1 (red) and YFP (green). (C). Representative stain of an injured vessel for CD45 (red) and YFP (green). Arrows = YFP(+)CD45(+) adventitial cells. M = arterial media; A = arterial adventitia; arrowheads = internal (A) and external elastic laminae. (D). Higher magnification images of arrows in panel (C). For A–C, dashed lines represent media-adventitia boundary. (E&F). Total numbers of adventitial Sca1(−)YFP(+), Sca1(+)YFP(+), or Sca1(+)YFP(−) cells (panel E) or CD45(−)YFP(+), CD45(+)YFP(+), or CD45(+)YFP(−) cells (panel F) in uninjured compared to injured arteries were quantitated as described in Material and Methods. Total numbers±SE are recorded in the graphs. N=3 independent mice; *P<0.05. Scale bars=50μm.
SMC generation of AdvSca1-SM cells is dependent on induction of Klf4
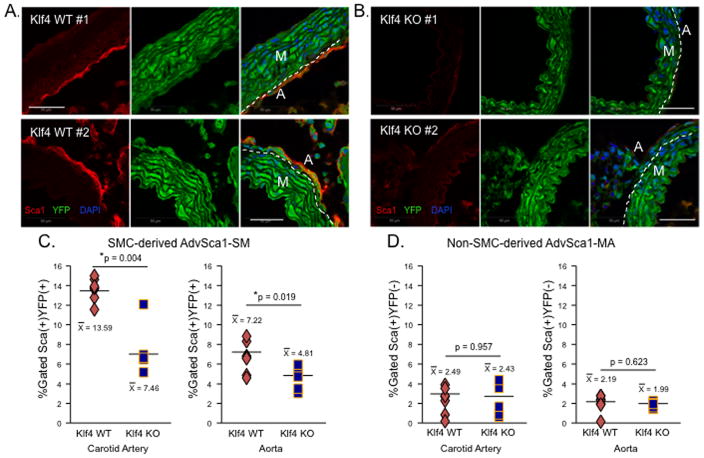
While many somatic cells, including SMCs, have been successfully reprogrammed in vitro to iPS cells using exogenous approaches47, endogenous mechanisms directing reprogramming are largely undefined. Klf4, a Kruppel-like transcription factor member, is not expressed in differentiated SMCs (Figure 3), but its induction contributes to SMC phenotypic switching48. Klf4 has been shown to be one of four genes necessary for in vitro iPS cell generation, underscoring its importance in maintenance of a progenitor cell phenotype49. Since Klf4 was highly expressed by AdvSca1-SM cells (Figure 3 and Supplemental Figure 6), we examined its role in SMC generation of AdvSca1-SM cells. To determine if induction of Klf4 in differentiated SMCs was required in vivo, aortas from 8–10-day old WT and SMC-specific Klf4-deficient mice (SM22α-CreKI-YFP Klf4 SM-KO) were examined for the presence of AdvSca1 cells. We found abundant numbers of adventitial Sca1+ cells in WT mice (Figure 7A). In contrast, adventitial Sca1+ cells were barely detectable in aortae from SM22α-CreKI-YFP Klf4 SM-KO mice (Figure 7B). FACS analysis was used to quantitate AdvSca1-SM and AdvSca1-MA cells in WT and Klf4 SM-KO mice. No difference in the numbers of AdvSca1-MA cells was observed, as anticipated since Klf4 was not deleted from non-SMC-derived cells (Figure 7D). Compared to WT mice, however, decreased numbers of AdvSca1-SM cells was confirmed in carotid arteries plus aortic arch and descending aortae of Klf4 SM-KO mice (Figure 7C) supporting the concept that Klf4 induction is necessary for SMCs to generate progenitor cells. Unfortunately, due to premature death of SM22α-CreKI-YFP Klf4 SM-KO mice by 4 wks of age (our unpublished observations and 50), we were unable to demonstrate the biological importance of Klf4 in the expansion of AdvSca1-SM cells after vascular injury. To test if Klf4 is sufficient to promote a progenitor cell phenotype as defined by expression of progenitor cell markers and repression of SM genes, cultured SMCs were transduced with adenoviruses expressing GFP (control) or Klf4-GFP. By FACS analysis, we observed approximately 50% transduction efficiency (Supplemental Figure XIIIA). Compared to GFP-transduced SMCs, Klf4-transduced SMCs expressed increased levels of cell surface Sca1 and CD34 (Supplemental Figure XIIIB&C). This was associated with downregulation of SMC-specific markers at the level of mRNA and protein (Supplemental Figure XIIIC&D).
Figure 7. SMC reprogramming is dependent on Klf4 induction.
Aorta and carotid arteries plus aortic arch were harvested from 8–10-day old SM22α-CreKI-YFP WT (A) or SM22α-CreKI-YFP Klf4 KO (B) mice. Representative immunofluorescent stains of aortic sections for Sca1 (red) and YFP (green); aortae from two independent WT and KO mice shown; N=6 per genotype total. Scale bars=50μm. Dashed lines represent media-adventitia boundary. (C&D). FACS analysis was used to quantitate SMC-derived AdvSca1-SM (C) or non-SMC-derived AdvSca1-MA (D) cells in WT versus Klf4 KO mice. Each symbol represents data from an individual mouse. N=7 WT and N=5 KO mice.
We previously demonstrated that Klf4 is downregulated as AdvSca1 cells differentiate in culture and acquire SMC markers4. Based on its known function as a co-repressor of SRF-dependent SMC gene transcription and as a regulator of self-renewal in embryonic stem cells, we sought to determine if Klf4 is critical for maintenance of a progenitor cell phenotype. We used in vitro approaches to silence Klf4 using control non-targeting or Klf4-specific siRNAs or overexpress Klf4 using adenoviruses expressing empty vector (control) or wild type Klf4. Compared to control siRNA, Klf4 knockdown decreased Sca1 mRNA expression (Supplemental Figure XIVA) and reduced the number of Sca1-positive cells, as evaluated by immunofluorescence staining (Figure 8A&B left). This was associated with a trend toward an increased fraction of cells exhibiting αSMA-positive contractile filaments (Figure 8A&B right) suggesting that loss of Klf4 accelerates differentiation of AdvSca1 cells toward a SMC fate. In contrast to Klf4 silencing, adenoviral-mediated over-expression of Klf4 resulted in increased Sca1 expression and decreased αSMA expression (Supplemental Figure XIVB). This was associated with increased numbers of Sca1-positive cells (Figure 8C&D left) and blocked the differentiation of AdvSca1 cells to SMCs, as measured by decreased fraction of cells exhibiting αSMA-positive contractile filaments (Figure 8C&D right). Collectively, these data are consistent with an essential role for Klf4 in SMC reprogramming, similar to a recent report31, and in the maintenance of the AdvSca1 progenitor phenotype.
Figure 8. Klf4 maintains the progenitor phenotype.
(A&B). Isolated AdvSca1 cells from WT mice were cultured for 24 h, transfected with siRNAs targeting GFP (control) or KLF4, and evaluated 72 hrs after siRNA transfection. (A) Representative images from treated cultures with immunostaining for αSMA (green), Sca1 (red), and DAPI (blue). Sca1-positive and αSMA-positive cells from each treatment group were counted and normalized to total cell numbers. N=3 independent experiments; *P<0.05. (C&D). Isolated AdvSca1 cells from WT mice were transduced with an empty adenovirus (control) or a Klf4-expressing adenovirus then cultured for 7 days. (C) Representative images from treated cultures with immunostaining for αSMA (green), Sca1 (red), and DAPI (blue). (D) Sca1-positive and αSMA-positive were counted and normalized to total cell numbers. N=3 independent experiments; *P<0.05.
DISCUSSION
In previous experiments to determine the fate of medial SMCs in injured arteries, we made the entirely unexpected observation that some mature SMCs migrate from the media into the adventitia, and become SMC marker negative33. In this report, using two independent SMC genetic fate-mapping systems combined with a SMC lineage-specific chromatin mark, we demonstrate that SMCs moving into the adventitia gain expression of progenitor cell markers (Sca1, CD34, Klf4) and become residents within the adventitial progenitor niche that we and others have described2, 4, 5. We propose that this phenomenon is a type of in situ SMC reprogramming with the evidence to support this as follows: (1) no detectable SMC differentiation marker expression by AdvSca1-SM cells, (2) no detectable binding of SRF to CArG elements in SMC marker gene promoter regions in these cells, (3) loss of the H4Ac differentiation-associated mark on SMC promoter CArG elements by AdvSca1-SM cells, but (4) retention of the H3K4Me2 SMC lineage mark, (5) gain of expression of functional progenitor marker genes by AdvSca1-SM cells, and (6) gain of multipotential fate capabilities as defined by differentiation in vivo into SMCs, macrophages, and endothelial cells. Further study showed that SMC-derived progenitor cells reside in the adventitia of aortic arch and carotid arteries, descending aorta, and femoral arteries, each from distinct embryonic lineages, in numbers representing approximately 60%, 40%, and 30%, respectively, of the total adventitial Sca1+ progenitor cell population of these vessels. Taken together, our data suggest the novel possibility that during vascular development differentiated SMCs migrate from the outer media into the inner adventitia where they become reprogrammed to a progenitor-like state. Since formation of AdvSca1-SM cells was observed in adult mice after treatment with tamoxifen, this would also suggest SMC reprogramming is a continual process likely serving as a mechanism to replace resident vascular progenitor cells. This activity would be similar, in principle, to the reversion of mature differentiated cells to fate-restricted progenitor cells in regeneration of tracheal cell types following lung injury51 and stomach crypt cells in response to stem cell ablation52. An important difference and the novel aspect of our findings, however, is that our data support the concept that formation of AdvSca1-SM cells from mature SMCs is a normal physiological process, in contrast to these previous studies in which differentiated cells revert to progenitor cells only in the setting of experimental tissue injury or stem cell ablation. We are aware that Myh11/SMMHC can sometimes be expressed by microvascular pericytes and that some myeloid and myofibroblastic cells express Acta2/αSMA and Tagln/SM22α (but not Myh11). At this time, we cannot rule out the possibility that some of the AdvSca1-SM cells we identify here may have an origin from adventitial cells. However, vasa vasorum microvessels are sparse in normal murine adventitia and a substantial origin of AdvSca1-SM cells from these alternate sources seems unlikely.
While remodeling of the adventitia has been known to occur following many forms of vascular injury, it is widely assumed that medial SMCs do not directly participate in this response. This is likely due to the vast majority of studies in the literature that identify SMCs in injured arteries by their expression of conventional SM marker proteins (eg. Acta2/αSMA, Myh11/SMMHC). By those criteria, intimal SMCs, many plaque macrophages31,34, and AdvSca1-SM cells, which lose expression of these markers, would not be recognized as SMC-derived. Recent advances in SMC fate-mapping systems that permanently mark mature SMCs has allowed reliable identification of these cells as SMC-derived even if they lose all characteristics of SMCs. Using these systems, our data (Supplemental Figure I) and recent reports by others31, 34 have conclusively demonstrated that the percentage of SMCs contributing to intimal lesion formation and atherosclerosis is much greater than previously estimated from expression of traditional SMC markers. While the mechanisms regulating SMC migration into the adventitia remain unclear, it is well known that breaks in the internal elastic lamina facilitate migration of SMCs into the intima. We assert that it would not be completely unexpected that breaks in the external elastic lamina, as observed during vascular development and following injury to the vessel wall, would result in a similar movement of SMCs into the adventitia. Our ongoing studies are addressing this possibility in more detail.
From their earliest description, it was evident that the AdvSca1-positive cell population in the aortic adventitia was heterogeneous. For example, Hu et. al.2 reported that Sca1-positive cells isolated from the aortic root adventitia of adult ApoE−/− mice contained ~60% fibroblastic cells, ~20% epithelioid cells, and occasional adipocyte-like cells. Passman et. al. 4, Rev. in 16 found that about 50% of freshly isolated aortic AdvSca1 cells from wild type C57Bl/6 mice lost Sca1 expression and differentiated into SMC marker-positive cells over 8 days ex vivo, while ~25% proliferated as Sca1-positive cells without SMC differentiation, and the remaining 25% lost expression of Sca1 but did not acquire detectable levels of SMC marker expression. Psaltis et. al.5 found that aortic cells isolated from wild type C57Bl/6 mice exhibited hematopoietic colony forming activity predominantly for progenitors expressing a macrophage fate. Further study showed that co-expression of Sca1 and CD45 in the adventitia marked the CFU-M progenitor cells and these Sca1+/CD45+ cells comprised about 36% of the total aortic Sca1-positive cell population21. The remaining Sca1+/CD45− cell population was shown in previous studies to possess SMC differentiation potential without hematopoietic colony forming activity2, 4. Our results reported in the present study confirm and extend this prior work supporting the concept that the aortic adventitia contains at least two types of AdvSca1 progenitor cells. Based on expression of cell surface markers, our data suggest that SMC-derived AdvSca1 cells express predominantly a myogenic progenitor phenotype (AdvSca1-SM) whereas non-SMC-derived AdvSca1 cells express predominantly a macrophage progenitor cell phenotype (AdvSca1-MA). However, myogenic and macrophage progenitor cell phenotypes were observed within subpopulations of both the AdvSca1-SM and AdvSca1-MA populations supporting the likelihood of multipotent fate decisions. Indeed, our in vivo Matrigel™ plug assays and in vitro differentiation assays demonstrated the ability of AdvSca1-SM cells to differentiate into SMCs, macrophages, endothelial-like cells, adipocytes, and chondrocytes further supporting the multipotency of these cells.
Induction of pluripotent stem cells from somatic cells can be accomplished through forced overexpression of the transcription factors, Oct-4, Sox2, Klf4, and c-Myc49. Our current and previous4 results together with our unpublished RNA-Seq data demonstrate high expression levels of Klf4 and Myc/c-Myc, but not POU5F1/Oct4 or Sox2, in both populations of AdvSca1 cells further suggesting that these cells possess a fate-restricted multipotent, but not pluripotent phenotype. This would be similar to the detection of fate-restricted progenitor cells during regeneration of axolotl limb53, zebrafish fin54, mouse digit tip55, 56, and both zebrafish57 and neonatal mouse58 hearts after apex amputation injury. Further, our finding that the AdvSca1-SM subset arises predominantly from pre-existing SMCs offers an explanation for the previously puzzling observation that despite being SMC marker-negative, AdvSca1 cells express a transcription factor profile typically found in mature SMCs4. Our findings suggest very little contribution of AdvSca1-SM cells to the previously identified CFU-M subset5, 26. However, our findings suggest that AdvSca1-SM CD45+ cells contribute to the adventitial myeloid cell population, which is consistent with differentiation of AdvSca1-SM cells into macrophages as observed in in vivo Matrigel™ plug assays, and to expansion of adventitial CD45+ cells following vascular injury.
The adventitia is a complex layer of the vessel wall than previously thought that responds rapidly and robustly to many forms of arterial injuryRev. in 17. Previous studies point to the adventitial fibroblast as the main responders to vessel injury. For instance, Shi, et. al.43 and Scott, et. al.44 reported that adventitial cells, referred to as fibroblasts, proliferated earlier and to a greater extent than medial SMCs in response to balloon arterial injury. Our data demonstrate that AdvSca1-SM, in particular, are robustly activated and expand considerably in numbers early after arterial injury. Mobilization of AdvSca1-SM cells might participate in medial repair by differentiating into medial SMCs, and/or in neointimal formation. We propose that AdvSca1-SM progenitor cells may also play major roles during injury-mediated adventitial remodeling. In support of this model, and in agreement with our data, a recent report demonstrates that AdvSca1 cells are major sources of angiotensin II-induced adventitial fibrosis leading to artery wall stiffening and hypertension59. Similarly, using the mdx mouse model of Duchenne muscular dystrophy, Ieronimakis et. al.60 found that the major collagen-producing cell type associated with late onset cardiac fibrosis was the coronary AdvSca1 cell. Ongoing studies using fate-mapping approaches to selectively label SMC-derived AdvSca1-SM cells are addressing these possibilities.
The pluripotency-associated transcription factor, Klf4, is also well known to regulate SMC phenotypic changes and multiple lines of evidence support an important role for Klf4 in vascular disease progression31, 48,. Using in vivo and in vitro approaches, our studies establish that SMC generation of AdvSca1-SM cells is dependent on induction of Klf4. In vivo targeted deletion of the Klf4 gene in SMCs resulted in selective loss of AdvSca1-SM cells, but not AdvSca1-MA cells suggesting that failure to induce Klf4 prevents AdvSca1-SM cell generation from SMCs and establishment of this subpopulation of resident AdvSca1 cells. In support of these in vivo findings, overexpression of Klf4 in cultured SMCs promoted a progenitor cell phenotype as defined by loss of SMC differentiation markers and gain of progenitor cell markers. Unfortunately, early postnatal death of SMC-specific Klf4 SM-KO mice precluded our ability to define the biological importance of Klf4 on expansion of AdvSca1-SM cells in response to vascular injury. We recognize that, compared to WT mice, AdvSca1-SM cell numbers decreased in SMC-specific Klf4 KO mice by only 50%. This is likely due to the late developmental activation of Cre recombinase and thus SMC deletion of Klf4 in SM22α-CreKI compared to SM22α-Cre mice61. If migration into the adventitia and reprogramming occurred prior to Cre activation and inactivation of KLF4 in a subset of SMCs, the potential for Klf4 to be induced and promote AdvSca1-SM cell generation in this subset would remain. Finally, loss- and gain-of-function studies demonstrated that Klf4 is critical for the maintenance of the AdvSca1 progenitor cell phenotype. Collectively, our data demonstrate that Klf4 is a critical regulator of AdvSca1-SM cell generation and maintenance of the resident vascular progenitor cell pool.
In summary, our findings suggest that the local environment of the inner adventitia directs or stabilizes a Klf4-dependent SMC reprogramming-like process to maintain a vascular progenitor cell pool in the artery wall. Depending on environmental cues, SMC-derived progenitor cells may express cell fates resembling tissue resident macrophages, mural cells, endothelial-like cells, adipocytes, and osteoblasts. Going forward, the identification of factors in addition to Klf4 that regulate SMC-to-progenitor cell transitions in vivo will broaden our understanding of the multiple roles SMCs play in vascular homeostasis and disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Multipotent vascular progenitor cells expressing stem cell markers Sca1 and CD34 (AdvSca1 cells) reside in a unique niche in the inner adventitia of several vascular beds.
Using bone marrow transplant and adoptive transfer approaches, it has been shown that AdvSca1 cells do not originate from bone marrow or circulating cells.
AdvSca1 progenitor cells appear late during vascular development after the arterial media is fully formed, vascular smooth muscle cells (SMCs) have acquired a differentiated phenotype, and layering of the vessel wall has stopped.
What New Information Does This Article Contribute?
Differentiated SMCs in the outer media migrate into the inner adventitia and lose expression of SMC markers, gain expression of progenitor cell markers, and contribute to a subpopulation of AdvSca1 progenitor cells.
SMC-derived AdvSca1 cells can differentiate to mural cells, macrophage-like cells, and endothelial-like cells within Matrigel™ implants in vivo. They can also give rise to adipocytes and chondrocytes under defined in vitro conditions at lower frequencies.
Formation and maintenance of AdvSca1 cells from SMCs is dependent on induction of the pluripotency-associated transcription factor, Kruppel-like factor 4 (KLF4).
The vascular adventitia is a complex layer of the vessel wall containing populations of leukocytes, microvessels, and resident progenitor cells that collectively maintain the artery wall and respond robustly to arterial injury. Factors governing the cell composition of the adventitia are expected to contribute to the health and maintenance of blood vessels and the tissues and stem cell populations they support. Identification and characterization of resident vascular progenitor cells might have significant therapeutic implications in vascular diseases and regenerative medicine. We show that differentiated SMCs migrate from the arterial media into the adventitia and revert to multipotent vascular progenitor cells through a physiological reprogramming-like process. Depending on environmental cues, SMC-derived progenitor cells may adopt cell fates resembling tissue resident macrophages, mural cells, endothelial cells, adipocytes, and osteoblasts. SMC reprogramming is dependent on induction of the transcription factor, KLF4. Moving forward, identification of additional factors regulating the SMC-to-progenitor cell transition in vivo is expected to broaden our understanding of the multiple roles SMCs play in vascular homeostasis and disease. Manipulations of these cells in situ might have therapeutic applications in settings such as atherosclerosis/restenosis, aneurysm, ischemia, and tumor angiogenesis.
Acknowledgments
We thank Radu Moldovan and Greg Glazner of the UCD Advanced Microscopy Core Facility for assistance with confocal microscopy and Karen Helm and staff of the UC Cancer Center Flow Cytometry Core Facility.
SOURCES OF FUNDING
This work was funded by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health to MCMW-E (R21 HL114126, R01 HL121877, and R01 HL123650) and MWM (R01 HL123650 and R01 HL121877), and from the American Heart Association to JNR and MWM (AHA 0715320U). The University of Colorado Cancer Center Flow Cytometry Core Facility is funded through a support grant from the National Cancer Institute (P30 CA046934). Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advanced Light Microscopy Core supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082.
Nonstandard Abbreviations and Acronyms
- SMCs
smooth muscle cells
- AdvSca1
adventitial sca1-positive progenitor cells
- Sca1
stem cell antigen-1
- Shh
sonic hedgehog
- SRF
serum response factor
- Mhy11/SMMHC
smooth muscle myosin heavy chain
- Acta2/αSMA
smooth muscle-α-actin
- Tmx
tamoxifen
- Dpp
days post-partum
- AdvSca1-MA
non-SMC-derived AdvSca1(+) cells
- AdvSca1-SM
SMC-derived AdvSca1(+) cells
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative polymerase chain reaction
- iPS
induced pluripotent stem cell
- Klf4
kruppel-like factor 4
- Cre
Cre recombinase
- WT
wild type
- KO
knockout
- FACS
fluorescence-activated cell sorting
- YFP
yellow fluorescent protein
Footnotes
DISCLOSURES
The authors declare no competing financial interests.
References
- 1.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–82. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, Mueske CS, Gulati R, Sandhu GS, Simari RD. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabner R, Lötzer K, Döpping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJ. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–48. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–51. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poduri A, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Fibroblast angiotensin II type 1a receptors contribute to angiotensin II-induced medial hyperplasia in the ascending aorta. Arterioscler Thromb Vasc Biol. 2015;35:1995–2002. doi: 10.1161/ATVBAHA.115.305995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 11.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacic JC, Boehm M. Resident vascular progenitor cells: an emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res. 2009;2:2–15. doi: 10.1016/j.scr.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31:1523–1529. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- 19.Plass CA, Sabdyusheva-Litschauer I, Bernhart A, Samaha E, Petnehazy O, Szentirmai E, Petrasi Z, Lamin V, Pavo N, Nyolczas N, Jakab A, Murlasits Z, Bergler-Klein J, Maurer G, Gyongyosi M. Time course of endothelium-dependent and -independent coronary vasomotor response to coronary balloons and stents. Comparison of plain and drug-eluting balloons and stents. JACC Cardiovasc Interv. 2012;5:741–751. doi: 10.1016/j.jcin.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Orlandi A, Bennett M. Progenitor cell-derived smooth muscle cells in vascular disease. Biochem Pharmacol. 2010;79:1706–1713. doi: 10.1016/j.bcp.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Psaltis PJ, Harbuzariu A, Delacroix S, Holroyd EW, Simari RD. Resident vascular progenitor cells--diverse origins, phenotype, and function. J Cardiovasc Transl Res. 2011;4:161–176. doi: 10.1007/s12265-010-9248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Izpisua JC. Mending a faltering heart. Circ Res. 2016;118:344–351. doi: 10.1161/CIRCRESAHA.115.306820. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Wong MM, Campagnolo P, Simpson R, Winkler B, Margariti A, Hu Y, Xu Q. Adventitial stem cells in vein grafts display multilineage potential that contributes to neointimal formation. Arterioscler Thromb Vasc Biol. 2013;33:1844–1851. doi: 10.1161/ATVBAHA.113.300902. [DOI] [PubMed] [Google Scholar]
- 25.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 26.Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, Gulati R, Simari RD. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364–75. doi: 10.1161/CIRCRESAHA.115.303299. [DOI] [PubMed] [Google Scholar]
- 27.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 28.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 29.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 30.Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–37. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackers-Johnson M, Talasila A, Sage AP, Long X, Bot I, Morrell NW, Bennett MR, Miano JM, Sinha S. Myocardin regulates vascular smooth muscle cell inflammatory activation and disease. Arterioscler Thromb Vasc Biol. 2015;35:817–828. doi: 10.1161/ATVBAHA.114.305218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S, Weiser-Evans MCM. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–1308. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–7. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 35.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–46. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furgeson SB, Simpson PA, Park I, Vanputten V, Horita H, Kontos CD, Nemenoff RA, Weiser-Evans MC. Inactivation of the tumour suppressor PTEN, in smooth muscle promotes a pro-inflammatory phenotype and enhances neointima formation. Cardiovasc Res. 2010;86:274–82. doi: 10.1093/cvr/cvp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5′ CArG degeneracy in smooth muscle alpha-actin is required for injury-induced gene suppression in vivo. J Clin Invest. 2005;115:418–27. doi: 10.1172/JCI22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–51. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–36. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonals OG, Wanhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG boc chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 42.Gomez D, Shankman LS, Nguyen AT, Owens GK. Detection of histone modifications at specific gene loci in single cells in histological sections. Nat Methods. 2013;10:171–7. doi: 10.1038/nmeth.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation. 1996;93:340–348. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]
- 44.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 45.Ryan ST, Koteliansky VE, Gotwals PJ, Lindner V. Transforming growth factor-beta-dependent events in vascular remodeling following arterial injury. J Vasc Res. 2003;40:37–46. doi: 10.1159/000068937. [DOI] [PubMed] [Google Scholar]
- 46.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from inncent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida Y, Yamanaka S. Recent Stem Cell Advances: Induced Pluripotent Stem Cells for Disease Modeling and Stem Cell–Based Regeneration. Circulation. 2010;122:80–87. doi: 10.1161/CIRCULATIONAHA.109.881433. [DOI] [PubMed] [Google Scholar]
- 48.Salmon M, Gomez D, Greene E, Shankman L, Owens GK. Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22α promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ Res. 2012;111:685–96. doi: 10.1161/CIRCRESAHA.112.269811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida T, Gan Q, Franke AS, Ho R, Zhang J, Chen YE, Hayashi M, Majesky MW, Somlyo AV, Owens GK. Smooth and cardiac muscle-selective knock-out of Kruppel-like factor 4 causes postnatal death and growth retardation. J Biol Chem. 2010;285:21175–84. doi: 10.1074/jbc.M110.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–23. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–68. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–5. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 54.Tu S, Johnson SL. Fate restriction in the growing and regenerating zebrafish fin. Dev Cell. 2011;20:725–32. doi: 10.1016/j.devcel.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–13. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci U S A. 2011;108:20609–14. doi: 10.1073/pnas.1118017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–5. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, Montaniel KR, Saleh MA, Xiao L, Chen W, Owens GK, Humphrey JD, Majesky MW, Paik DT, Hatzopoulos AK, Madhur MS, Harrison DG. Origin of Matrix-Producing Cells That Contribute to Aortic Fibrosis in Hypertension. Hypertension. 2016;67:461–8. doi: 10.1161/HYPERTENSIONAHA.115.06123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ieronimakis N, Hays AL, Janebodin K, Mahoney WM, Jr, Duffield JS, Majesky MW, Reyes M. Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFβ1 signaling in the mdx mouse model of Duchenne muscular dystrophy. J Mol Cell Cardiol. 2013;63:122–34. doi: 10.1016/j.yjmcc.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 2006;26:e23–4. doi: 10.1161/01.ATV.0000202661.61837.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.