Abstract
Background
Temporary abdominal closure (TAC) following damage control surgery (DCS) for injured patients has been generalized to septic patients. However, direct comparisons between these populations are lacking. We hypothesized that patients with intra-abdominal sepsis would have different resuscitation requirements and lower primary fascial closure rates than trauma patients.
Study Design
We performed a three year retrospective cohort analysis of patients managed with TAC for trauma (n=77) or intra-abdominal sepsis (n=147). All patients received negative pressure wound therapy (NPWT) TAC with intention for planned relaparotomy and sequential abdominal closure attempts at 24-48 hour intervals.
Results
At presentation, trauma patients had higher rates of hypothermia (31% vs. 18%), severe acidosis (27% vs. 14%), and coagulopathy (68% vs. 48%), and septic patients had higher vasopressor infusion rates (46% vs. 27%). Forty-eight hours after presentation, septic patients had persistently higher vasopressor infusion rates (37% vs. 17%), and trauma patients had received more red blood cell transfusions (6.0 vs. 0.0 units), fresh frozen plasma (5.0 vs. 0.0 units), and crystalloid (8,290 vs. 7,159 ml). Among patients surviving to discharge, trauma patients had higher primary fascial closure (PFC) rates (90% vs. 76%). For trauma patients, independent predictors of failure to achieve PCF were ≥2.5 L NPWT output at 48 hours, ≥10 L crystalloid administration at 48 hours, and ≥10 U PRBC+FFP at 48 hours. For septic patients, relaparotomy within 48 hours predicted successful PFC; requirement for ≥3 diagnostic/therapeutic laparotomies predicted failure to achieve PFC.
Conclusions
Traumatic injury and intra-abdominal sepsis are associated with distinct pathophysiologic insults, resuscitation requirements, and outcomes. Failure to achieve primary fascial closure in trauma patients was attributable to the triad of hypothermia, acidosis, and coagulopathy; failure to achieve fascial closure in septic patients was dependent upon operative course. Indications and optimal techniques for TAC may differ between these populations.
Level of Evidence
level IV – therapeutic, and level III – prognostic
Keywords: damage control surgery, temporary abdominal closure, trauma, sepsis, primary fascial closure
Introduction
Temporary abdominal closure (TAC) gained notoriety with the advent of damage control surgery (DCS) and recognition that early physiologic resuscitation, rather than definitive surgical management, leads to improved outcomes after severe trauma and hemorrhagic shock. Stone et al. (1) described abbreviated laparotomy for a cohort of 31 trauma and emergency general surgery patients with major coagulopathy and exsanguinating hemorrhage in 1983. This approach involved repair of major vascular injuries, control of gastrointestinal and genitourinary injuries, packing the abdomen with laparotomy pads, and closing the fascia under tension with intention for interval laparotomy following resolution of coagulopathy (1). Since that time, DCS and TAC have become important strategies in managing injured patients with hemorrhagic shock, hypothermia, acidosis, coagulopathy, and massive bowel edema (2-6).
Evolving indications for TAC have recently been clarified by expert review of current evidence (7). These guidelines build on previous work emphasizing TAC as an important strategy for managing intra-abdominal sepsis in order to facilitate early diagnosis and treatment of residual infection, remove cytokine-rich peritoneal fluid, prevent abdominal compartment syndrome, and defer anastomosis until physiologic optimization (4, 6, 8, 9). In addition, the utilization of abbreviated laparotomy and TAC for septic patients to facilitate timely physiologic resuscitation in the ICU may mitigate their risk for the morbidity and mortality associated with acute kidney injury (AKI) (10). While TAC following DCS for hemorrhagic shock following trauma has been studied extensively, TAC of the septic abdomen has progressed at a more indolent pace.
Several authors have compared TAC outcomes across various etiologies (11-14), and guidelines from the World Society of Emergency Surgery have elucidated best practices in TAC management of intra-abdominal sepsis. A recent prospective observation study provided a detailed description of uninjured emergency general surgery TAC patients (15). However, direct comparisons between injured and septic patients managed with TAC are lacking. While both promote a robust pro-inflammatory innate immune response, injury and infection are different pathophysiologic insults treated with differing resuscitation strategies, and patients with intra-abdominal sepsis may be disproportionately affected by persistent inflammation and bowel edema. Therefore, better understanding of the essential differences between these patient populations may inform management decisions regarding resuscitation and operative strategy.
The purpose of this study was to compare TAC for traumatic abdominal injuries and intra-abdominal sepsis by identifying associations among pathophysiologic insult, resuscitation course, operative strategy, successful abdominal closure, and complications including intestinal fistula formation, fascial dehiscence, and mortality. We hypothesized that patients with traumatic injury and intra-abdominal sepsis would have different pathophysiologic characteristics and resuscitation requirements, and that primary fascial closure rates would be lower in septic patients.
Methods
We performed a retrospective analysis of patients managed with TAC for traumatic injury or intra-abdominal sepsis at our institution over a 49 month period ending June 2015. Institutional Review Board approval was obtained. Study subjects were identified by identifying patients who had an operation by a surgeon in the Division of Acute Care Surgery with CPT code modifier 58 (planned reoperation) or 78 (unplanned reoperation). Inclusion criteria were age ≥ 18, TAC for traumatic injury or intra-abdominal sepsis, and survival for ≥ 24 hours following presentation. Exclusion criteria were initial exploratory laparotomy at an outside facility, pre-existing intestinal fistula, and initial laparotomy performed to address secondary abdominal compartment syndrome. Patients who initially presented with traumatic injury and developed a secondary infection necessitating laparotomy and temporary abdominal closure were excluded. Patients with necrotizing pancreatitis were excluded due to significant differences in the pre and post-operative courses associated with this disease process.
During the study period there was a general consensus on open-abdomen management within the Acute Care Surgery group. However, specific operative techniques were not protocolized during the study period, and were ultimately at the discretion of the attending surgeon. All patients were managed with negative pressure wound therapy (NPWT) TAC with intention for planned relaparotomy and sequential fascial closure attempts at 24-48 hour intervals, favoring relaparotomy within 24 hours if possible (16). During the study period, our institution followed strict postoperative resuscitation protocols for both trauma and sepsis patients, as previously described (17-19). Sequential fascial closure was performed by placing successive simple interrupted or figure-of eight sutures at the cranial and caudal ends of the fasciotomy, advancing toward the center of the wound until further closure would result in fascial disruption or elevated airway pressures. NPWT included primarily the use of individualized vacuum pack (i.e. Barker technique (20), and occasional use of commercial (ABThera system, KCI, San Antonio, TX) vacuum assisted closure dressings. Primary fascial closure was performed by one of five methods: running slowly absorbable suture, running slowly absorbable suture with retention sutures, interrupted permanent or slowly absorbable suture, interrupted polyglactin suture, and interrupted polyglactin suture with retention sutures. Retention suture techniques included non-absorbable external retention sutures placed through the full thickness abdominal wall (penetrating both skin and peritoneum) and internal retention sutures placed within the abdominal wall (not penetrating skin or peritoneum) to evenly distribute tension on the fascia and reinforce the primary closure. Retention suture placement was performed only at the time of final primary fascial closure, and not during sequential fascial closure. If visceral edema and/or critical loss of abdominal domain precluded primary fascial closure during the index hospital admission, a planned ventral hernia was created with biologic or polyglactin mesh bridge placement, split thickness skin grafting, or a combination of the two. Clinical and laboratory data were recorded by physician-performed chart review. Baseline characteristics for septic patients were assessed at the time of consultation with the Acute Care Surgery service. For patients surviving to discharge, long term follow-up range was three months-three years depending upon clinic visit dates and availability of electronic medical record reports.
Hypothermia was defined as Tmin < 35.0°C. Severe acidosis was defined as pH < 7.20. Coagulopathy was defined as international normalized ratio (INR) > 1.5 (21) or coagulopathy on thromboelastograph (TEG) (rapid TEG with at least 2 of 4 conditions: activated clotting time (ACT) > 142 seconds (s), clot formation (K) time > 143 s, alpha angle < 64°, and maximum amplitude (MA) < 52 mm; or standard TEG with at least 2 of 4 conditions: reaction time > 600 s, K time > 180 s, alpha angle < 53°, and MA < 50 mm). Critical loss of abdominal domain was determined by individual surgeon clinical judgement that the abdominal cavity was unable to accommodate the abdominal contents within fascial boundaries. Acute respiratory distress syndrome (ARDS) was defined according to Berlin criteria (22). Acute kidney injury (AKI) was defined as a two-fold increase in serum creatinine (23). Multiple organ failure (MOF) was defined as dysfunction or failure of at least two organ systems such that homeostasis could not be maintained without intervention (24, 25).
Statistical analysis was performed using SPSS version 23 (IBM, Armonk, NY) to perform the Kruskal-Wallis test and Fisher’s Exact test to assess differences between injured and septic patients as appropriate. pH values were missing at random (MAR) for two patients (1%). Both TEG and INR were MAR for eight patients (4%). Analysis was performed on the available data without imputing missing data. Categorical variables were reported as n (%); continuous variables were reported as median [interquartile range]. Multiple logistic regression was performed to identify factors associated with planned ventral hernia formation, intestinal fistula formation, and ARDS. Independent variables were identified by significant correlation (r > 0.2) to the outcome variable, excluded for collinearity with other dependent variables, and entered into the regression equation. Independent predictors were reported as odds ratios (OR) with 95% confidence intervals. The strength of models predicting failure to achieve primary fascial closure for trauma and sepsis patients was assessed by calculating the area under the receiver operating characteristic curve (AUROC) with 95% confidence interval. The Hosmer and Lemeshow Test was performed to ensure that model significance was ≥ 0.05.
Results
Trauma cohort
Seventy-seven trauma patients were included (Table 1). Fifteen patients (19%) had penetrating injuries; median injury severity score was 32. Trauma patients had high rates of hypothermia (31%), severe acidosis (27%), and coagulopathy (68%) at presentation (Table 2). Forty-eight hours after admission, hypothermia and acidosis each affected 3% of all trauma patients, and the incidence of coagulopathy decreased to 31% (Table 3). Net fluid balance at 48 hours was 7.2 L positive. Total crystalloid administration was 8.3 L, and median PRBC and FFP administration was 6 units and 5 units, respectively. Among 58 patients surviving to discharge, primary fascial closure (PFC) was achieved in 52 (90%). Independent predictors of failure to achieve PFC included ≥2.5 L NPWT output at 48 hours, ≥10 L crystalloid administration at 48 hours, and ≥10 U PRBC+FFP at 48 hours.
Table 1.
Initial diagnosis for all patients.
| All patients n = 224 |
|
|---|---|
| Trauma | 77 (34%) |
| Blunt trauma | 62 (28%) |
| Penetrating trauma | 15 (7%) |
| Sepsis | 147 (66%) |
| Bowel ischemia | 74 (33%) |
| Hollow viscous perforation | 50 (22%) |
| Inflammation/infection without perforation | 20 (9%) |
| Anastomotic leak | 3 (1%) |
Table 2.
Baseline characteristics for trauma and sepsis patients (TEG: thromboelastograph, INR: international normalized ratio, data are depicted as median [interquartile range] or n (%),).
| At presentation | Trauma n = 77 |
Sepsis n = 147 |
p |
|---|---|---|---|
| Age (years) | 44 [30-56] | 61 [53-72] | <0.001 |
| Male | 51 (66%) | 75 (51%) | 0.029 |
| Injury Severity Score | 32 [23-44] | ||
| Hypothermia (Tmin < 35.0°C) | 24 (31%) | 27 (18%) | 0.030 |
| Severe acidosis (pH < 7.20) | 21 (27%) | 20 (14%) | 0.012 |
| Coagulopathy (per TEG or INR > 1.5) | 52 (68%) | 70 (48%) | 0.015 |
| Vasopressor infusion | 21 (27%) | 68 (46%) | 0.006 |
| Solid organ resection or repair | 43 (56%) | 3 (2%) | <0.001 |
| Bowel resection or repair | 24 (31%) | 116 (79%) | <0.001 |
Table 3.
Comparisons between trauma and sepsis patients at 48 hours (TEG: thromboelastograph, INR: international normalized ratio, data are depicted as n (%) or median [interquartile range]).
| At 48 hours | Trauma n = 77 |
Sepsis n = 147 |
p |
|---|---|---|---|
| Hypothermia (Tmin < 35.0°C) | 2 (3%) | 4 (3%) | 0.994 |
| Severe acidosis (pH < 7.20) | 2 (3%) | 0 (0%) | 0.107 |
| Coagulopathy (per TEG and/or INR > 1.5) | 24 (31%) | 61 (41%) | 0.349 |
| Vasopressor infusion | 13 (17%) | 54 (37%) | 0.003 |
| Net fluid balance (mL) | 7,203 [2,852-9,853] | 6,748 [4,640-10,121] | 0.777 |
| Total crystalloid administration (mL) | 8,290 [6,580-10,631] | 7,159 [5,364-9,428] | 0.001 |
| NPWT output (mL) | 1,702 [1,025-3,712] | 1,900 [1,082-3,200] | 0.811 |
| Red blood cell transfusions (units) | 6.0 [4.0-11.0] | 0.0 [0.0-3.0] | <0.001 |
| Plasma transfusions (units) | 5.0 [3.0-10.0] | 0.0 [0.0-2.0] | <0.001 |
| Underwent relaparotomy | 68 (88%) | 135 (92%) | 0.486 |
Sepsis cohort
One hundred forty-seven sepsis patients were included (Table 1). Sepsis patients were older than trauma patients (age 61 vs. 44 years) and included equal proportions of male and female subjects (Table 2). Rates of hypothermia, acidosis and coagulopathy on admission were each significantly lower in the sepsis cohort; vasopressor infusion requirements were higher (46% vs. 27%). Forty-eight hours after presentation, septic patients had persistently high rates of vasopressor requirement (37%) despite having net fluid balance 6.7 L positive. Septic patients also had higher incidence of AKI and MOF (Table 4). Among the 112 septic patients who survived to discharge, PFC was achieved in 85 (76%). Performance of relaparotomy within 48 hours increased the probability of PFC; performance of ≥3 diagnostic/therapeutic laparotomies was associated with failure to achieve PFC (Table 5). Post-discharge deaths rates were significantly higher in the sepsis cohort (21% vs. 9%, p = 0.047, follow-up range three months – three years).
Table 4.
Comparisons between trauma and sepsis patients at discharge (ICU: intensive care unit, data are depicted as n (%) or median [interquartile range]).
| At discharge | Trauma n = 77 |
Sepsis n = 147 |
p |
|---|---|---|---|
| Primary fascial closure (all patients) | 56 (73%) | 104 (71%) | 0.755 |
| Primary fascial closure (patients surviving to discharge) | 52 (90%) | 85 (76%) | 0.031 |
| Days to closure | 2.0 [1.0-3.0] | 2.0 [1.0-3.0] | 0.498 |
| Number of operations | 2.0 [2.0-4.0] | 2.0 [2.0-3.5] | 0.379 |
| Diagnostic/therapeutic laparotomies | 2.0 [2.0-3.0] | 2.0 [2.0-3.0] | 0.312 |
| Laparotomies for closure only | 0.0 [0.0-1.0] | 0.0 [0.0-1.0] | 0.726 |
| Days between operations | 1.0 [1.0-1.5 | 1.0 [1.0-1.5] | 0.921 |
| Fascial dehiscence after primary closure | 5 (9%) | 6 (6%) | 0.422 |
| Intestinal fistula prior to discharge | 1 (1%) | 5 (3%) | 0.355 |
| Acute respiratory distress syndrome | 14 (18%) | 22 (15%) | 0.452 |
| Acute kidney injury | 19 (25%) | 61 (41%) | 0.015 |
| Multiple organ failure | 16 (21%) | 59 (40%) | 0.006 |
| Days on mechanical ventilation | 10 [4-17] | 9 [4-18] | 0.676 |
| ICU length of stay (days) | 13 [5-23] | 14 [5-28] | 0.182 |
| ICU-free days | 4 [0-10] | 4 [1-10] | 0.723 |
| Hospital length of stay (days) | 19 [8-31] | 20 [10-39] | 0.723 |
| Mortality | 19 (25%) | 35 (24%) | 0.886 |
Table 5.
Multivariate models predicting failure to achieve primary fascial closure during admission (OR: odds ratio, CI: confidence interval, NPWT: negative pressure wound therapy, PRBC: packed red blood cell transfusion, FFP: fresh frozen plasma transfusion). Trauma model: AUROC (95% CI) = 0.864 (0.763-0.966); Hosmer and Lemeshow significance = 0.703. Sepsis model: AUROC (95% CI) = 0.718 (0.624-0.812); Hosmer and Lemeshow significance = 0.792.
| Population Factors |
OR | 95% CI | p |
|---|---|---|---|
| Trauma | |||
| ≥2.5 L NPWT output at 48 hours | 5.7 | 1.3-24.4 | 0.020 |
| ≥10 L crystalloid infusion at 48 hours | 5.1 | 1.1-23.6 | 0.036 |
| ≥10 U PRBC+FFP at 48 hours | 4.9 | 1.0-23.6 | 0.049 |
| Sepsis | |||
| Relaparotomy within 48 hours | 0.2 | 0.0-0.6 | 0.009 |
| ≥3 diagnostic/therapeutic laparotomies | 2.2 | 1.4-3.3 | <0.001 |
Total study population
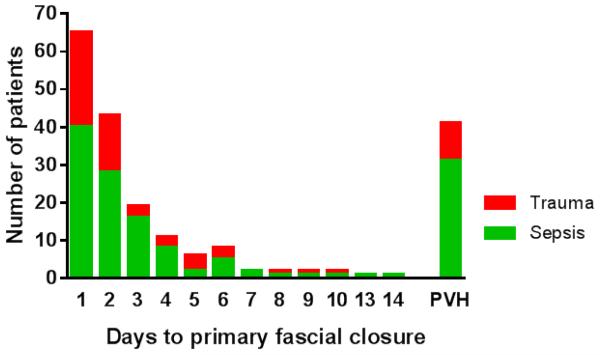
Ninety-five percent of all primary fascial closures occurred within one week of presentation (Figure 1). Incidence of fascial dehiscence was not significantly different among fascial closure techniques: running slowly absorbable suture (4/61), running slowly absorbable suture with retention sutures (3/49), interrupted permanent or slowly absorbable suture (1/24), interrupted polyglactin suture (0/11), and interrupted polyglactin suture with retention sutures (1/9). Among patients surviving to discharge, the long term incidence of intestinal fistula was 7% (11/170), and was significantly higher in planned ventral hernia patients compared to patients with primary fascial closure (Figure 2).
Figure 1.
Ninety-five percent of primary fascia closures occurred within one week of presentation (PVH: planned ventral hernia).
Figure 2.
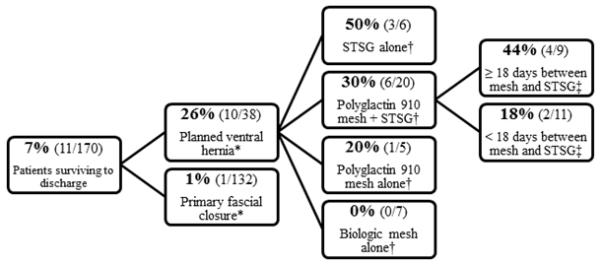
Incidence of intestinal fistula formation among patients surviving to discharge (STSG: split thickness skin graft, *p < 0.001, †p = 0.152, ‡p = 0.202).
Patients who had ≥18 days between polyglactin 910 mesh placement and interval STSG had higher incidence of intestinal fistula than patients with a < 18 day interval, though this association did not reach statistical significance. Factors associated with fistula formation included longer duration of TAC therapy (OR 1.6, 95% CI 1.0-2.6, p < 0.038) and planned ventral hernia formation (OR 117.7, 95% CI 4.1-3,354.5, p = 0.005) (model AUROC = 0.973 (95% CI 0.942-1.00), Hosmer and Lemeshow significance = 0.961). Additionally, patients whose primary fascial closure included retention suture placement had lower incidence of intestinal fistula formation than patients closed without retention sutures (2% (2/86) vs. 11% (9/84), p = 0.026). Both fistulas in the retention suture group occurred in patients who had external retention sutures (2/33); there were no fistulas among patients closed with internal retention sutures (0/53).
Only three of 38 (8%) patients discharged with a planned ventral hernia underwent interval abdominal wall reconstruction. One patient developed painful hernia incarceration, and was repaired. The other two abdominal wall reconstruction cases were performed in conjunction with enterocutaneous fistula takedown. All three reconstructions involved components separation with mesh reinforcement, and occurred at 676, 684, and 746 days after initial laparotomy. The other 35 patients discharged with a planned ventral hernia either were not suitable operative candidates, were offered reconstruction but deferred, or were lost to follow-up.
Discussion
Our results indicate that trauma patients and intra-abdominal sepsis patients have different physiologic characteristics, resuscitation requirements, and outcomes following TAC. Vasopressor requirements were persistently higher in septic patients despite roughly equal net fluid balance and greater crystalloid infusion volumes for injured patients, which may reflect differences in resuscitation strategies for hemorrhagic shock and distributive shock. Lower rates of primary fascial closure among septic patients may be related to their propensity for persistent inflammation and bowel edema and necessity for frequent washouts due to contamination. The observation that septic patients had lower fascial closure rates than trauma patients despite similar number of operations, days between operations, and days to closure may have been attributable to persistent massive visceral edema, prompting early planned ventral hernia formation rather than subjecting the patient to prolonged open abdomen therapy and multiple laparotomies, risking intestinal fistula formation (26). The non-significant trend toward increased incidence of intestinal fistula formation in patients with longer interval between polyglactin 910 mesh placement and STSG may be attributable to mesh erosion into the bowel. Increased mortality following discharge among septic patients may reflect the advanced age of this group as well as the propensity of the septic population to develop the persistent inflammatory, immunosuppressed, catabolic syndrome (PICS) described by Rosenthal and Moore (27), particularly in the context of higher rates of AKI and MOF.
A previous study found that trauma patients had higher rates of fascial closure, a trend toward lower incidence of enterocutaneous fistula (12% vs. 16%), and lower mortality (20% vs. 36%) compared to patients with abdominal sepsis or necrotizing pancreatitis (11). However, only 29% of patients in this study achieved primary fascial closure, which may be due to the inclusion of a substantial proportion of patients with necrotizing pancreatitis (11). Other authors have also concluded that achieving abdominal closure is more likely in non-septic abdomens than in patients with intra-abdominal sepsis when using NPWT plus sequential closure (13, 14), which is likely related to persistent inflammation and bowel edema among patients with intra-abdominal sepsis. A retrospective review of 103 patients managed with TAC at a level I trauma center found no differences in primary closure rates, fistula development, or mortality between injured and non-injured patients (12). However, infection and mesenteric ischemia only contributed to 39 out of 87 cases of TAC for non-trauma patients, limiting direct comparison between TAC for the septic abdomen and the traumatic abdomen (12). The potential benefits of a short duration between polyglactin mesh bridge placement and mesh excision and STSG placement have been previously reported by Jernigan et al. (28), who postulated that this strategy prevented mesh erosion into the bowel. In a review of 274 patients with giant abdominal wall defects, subjects who developed a fistula had an average 27 day interval between mesh placement and wound coverage compared to an 18 day interval in patients without fistula formation (p = 0.04) (28).
The major limitations of this study are its retrospective design, the fact that long term data were dependent upon clinic visit dates and availability of electronic medical record reports, and propensity to generate false positive results by making multiple comparisons. Despite these limitations, we sought to provide a direct comparison between injured and septic patients, as these two populations are often considered in isolation or bundled together. Better understanding of the differences between these cohorts may promote development of management strategies tailored more precisely to underlying pathophysiology. Currently, we are implementing a protocol with the goal of limiting crystalloid resuscitation volumes, increasing primary fascial closure rates, and decreasing intestinal fistula rates by employing strictly standardized TAC technique, direct peritoneal resuscitation, and 3% hypertonic saline intravenous resuscitation (29-32). Surgical techniques providing continuous fascial traction may also facilitate late primary fascial closures (33, 34), achieving the bimodal frequency distribution of TAC duration described in TAC literature (35). We also plan to use biologic mesh to bridge small fascial defects when skin flap coverage is feasible, due to findings that biologic meshes may have a lower propensity for infectious wound complications than synthetic meshes (36), and may often be salvaged when infection does occur, avoiding the necessity for mesh explantation (37).
Conclusions
Trauma and intra-abdominal sepsis patients exhibit distinct pathophysiologic insults and resuscitation requirements following damage control laparotomy. Injured patients are more likely to achieve primary fascial closure than septic patients when they are managed with the same planned relaparotomy and sequential fascial closure protocol. Failure to achieve primary fascial closure in trauma patients was attributable to the triad of hypothermia, acidosis, and coagulopathy persisting 48 hours after presentation, whereas failure to achieve fascial closure in septic patients was dependent upon operative course. Specific adjunctive therapies and techniques may be necessary to avoid planned ventral hernia formation and associated complications in patients with intraabdominal sepsis requiring damage control laparotomy.
Acknowledgement
The authors acknowledge Cindy Scalamonti, CSTR for her assistance in maintaining, accessing, and ensuring the quality of our institutional data registry.
The authors were supported in part by grants P30 AG028740 (SCB) awarded by the National Institute on Aging and by R01 GM105893-01A1 (AMM), P50 GM111152–01 (SCB, FAM, AMM) awarded by the National Institute of General Medical Sciences (NIGMS). TJL was supported by a post-graduate training grant (T32 GM-08721) in burns, trauma and perioperative injury by NIGMS.
Footnotes
Author Contributions
T.J.L., F.A.M., and S.C.B. contributed to literature review and study design. J.R.J., C.A.C., R.S.S., P.A.E., A.M.M., F.A.M., and S.C.B. contributed to planned relaparotomy protocol development. T.J.L. contributed to data acquisition. T.J.L., F.A.M., and S.C.B. contributed to data analysis. J.R.J., C.A.C., R.S.S., P.A.E., A.M.M., F.A.M., and S.C.B. made critical revisions.
The authors have no relevant conflicts of interest.
This work has not been presented at any meetings and has never been submitted elsewhere.
References
- 1.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197(5):532–5. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burch JM, Ortiz VB, Richardson RJ, Martin RR, Mattox KL, Jordan GL., Jr. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg. 1992;215(5):476–83. doi: 10.1097/00000658-199205000-00010. discussion 83-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotondo MF, Schwab CW, McGonigal MD, Phillips GR, 3rd, Fruchterman TM, Kauder DR, Latenser BA, Angood PA. 'Damage control': an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375–82. discussion 82-3. [PubMed] [Google Scholar]
- 4.Diaz JJ, Jr., Cullinane DC, Dutton WD, Jerome R, Bagdonas R, Bilaniuk JW, Collier BR, Como JJ, Cumming J, Griffen M, et al. The management of the open abdomen in trauma and emergency general surgery: part 1-damage control. J Trauma. 2010;68(6):1425–38. doi: 10.1097/TA.0b013e3181da0da5. [DOI] [PubMed] [Google Scholar]
- 5.Moore EE, Thomas G. Orr Memorial Lecture. Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am J Surg. 1996;172(5):405–10. doi: 10.1016/s0002-9610(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 6.Waibel BH, Rotondo MF. Damage control in trauma and abdominal sepsis. Crit Care Med. 2010;38(9 Suppl):S421–30. doi: 10.1097/CCM.0b013e3181ec5cbe. [DOI] [PubMed] [Google Scholar]
- 7.Roberts DJ, Bobrovitz N, Zygun DA, Ball CG, Kirkpatrick AW, Faris PD, Parry N, Nicol AJ, Navsaria PH, Moore EE, et al. Indications for use of thoracic, abdominal, pelvic, and vascular damage control interventions in trauma patients: A content analysis and expert appropriateness rating study. J Trauma Acute Care Surg. 2015;79(4):568–79. doi: 10.1097/TA.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 8.Perez D, Wildi S, Demartines N, Bramkamp M, Koehler C, Clavien PA. Prospective evaluation of vacuum-assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg. 2007;205(4):586–92. doi: 10.1016/j.jamcollsurg.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Sartelli M, Abu-Zidan FM, Ansaloni L, Bala M, Beltran MA, Biffl WL, Catena F, Chiara O, Coccolini F, Coimbra R, et al. The role of the open abdomen procedure in managing severe abdominal sepsis: WSES position paper. World J Emerg Surg. 2015;10:35. doi: 10.1186/s13017-015-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White LE, Hassoun HT, Bihorac A, Moore LJ, Sailors RM, McKinley BA, Valdivia A, Moore FA. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg. 2013;75(3):432–8. doi: 10.1097/TA.0b013e31829de6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuei BJ, Skinner JC, Bernard AC, Kearney PA, Boulanger BR. The open peritoneal cavity: etiology correlates with the likelihood of fascial closure. Am Surg. 2004;70(7):652–6. [PubMed] [Google Scholar]
- 12.Kritayakirana K, P MM, Brundage S, Purtill MA, Staudenmayer K, D AS. Outcomes and complications of open abdomen technique for managing non-trauma patients. J Emerg Trauma Shock. 2010;3(2):118–22. doi: 10.4103/0974-2700.62106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quyn AJ, Johnston C, Hall D, Chambers A, Arapova N, Ogston S, Amin AI. The open abdomen and temporary abdominal closure systems--historical evolution and systematic review. Colorectal Dis. 2012;14(8):e429–38. doi: 10.1111/j.1463-1318.2012.03045.x. [DOI] [PubMed] [Google Scholar]
- 14.Bruhin A, Ferreira F, Chariker M, Smith J, Runkel N. Systematic review and evidence based recommendations for the use of negative pressure wound therapy in the open abdomen. Int J Surg. 2014;12(10):1105–14. doi: 10.1016/j.ijsu.2014.08.396. [DOI] [PubMed] [Google Scholar]
- 15.Bruns BR, Ahmad SA, O'Meara L, Tesoriero R, Lauerman M, Klyushnenkova E, Kozar R, Scalea TM, Diaz JJ. Nontrauma open abdomens: A prospective observational study. J Trauma Acute Care Surg. 2016;80(4):631–6. doi: 10.1097/TA.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 16.Pommerening MJ, DuBose JJ, Zielinski MD, Phelan HA, Scalea TM, Inaba K, Velmahos GC, Whelan JF, Wade CE, Holcomb JB, et al. Time to first take-back operation predicts successful primary fascial closure in patients undergoing damage control laparotomy. Surgery. 2014;156(2):431–8. doi: 10.1016/j.surg.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Moore FA. The use of lactated ringer's in shock resuscitation: the good, the bad and the ugly. J Trauma. 2011;70(5 Suppl):S15–6. doi: 10.1097/TA.0b013e31821a4d6e. [DOI] [PubMed] [Google Scholar]
- 18.Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363(9425):1988–96. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- 19.Moore FA, McKinley BA, Moore EE, Nathens AB, West M, Shapiro MB, Bankey P, Freeman B, Harbrecht BG, Johnson JL, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61(1):82–9. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 20.Brock WB, Barker DE, Burns RP. Temporary closure of open abdominal wounds: the vacuum pack. Am Surg. 1995;61(1):30–5. [PubMed] [Google Scholar]
- 21.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 22.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 23.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative w Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75(2):257–77. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 25.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–74. [PubMed] [Google Scholar]
- 26.Dubose JJ, Scalea TM, Holcomb JB, Shrestha B, Okoye O, Inaba K, Bee TK, Fabian TC, Whelan J, Ivatury RR, et al. Open abdominal management after damage-control laparotomy for trauma: a prospective observational American Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg. 2013;74(1):113–20. doi: 10.1097/TA.0b013e31827891ce. discussion 1120-2. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal MD, Moore FA. Persistent inflammatory, immunosuppressed, catabolic syndrome (PICS): A new phenotype of multiple organ failure. J Adv Nutr Hum Metab. 2015;1(1) doi: 10.14800/janhm.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jernigan TW, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G, Bee TK. Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg. 2003;238(3):349–55. doi: 10.1097/01.sla.0000086544.42647.84. discussion 55-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JW, Garrison RN, Matheson PJ, Franklin GA, Harbrecht BG, Richardson JD. Direct peritoneal resuscitation accelerates primary abdominal wall closure after damage control surgery. J Am Coll Surg. 2010;210(5):658–64. 64–7. doi: 10.1016/j.jamcollsurg.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JW, Neal Garrison R, Matheson PJ, Harbrecht BG, Benns MV, Franklin GA, Miller KR, Bozeman MC, Richardson JD. Adjunctive treatment of abdominal catastrophes and sepsis with direct peritoneal resuscitation: indications for use in acute care surgery. J Trauma Acute Care Surg. 2014;77(3):393–8. doi: 10.1097/TA.0000000000000393. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 31.Harvin JA, Mims MM, Duchesne JC, Cox CS, Jr., Wade CE, Holcomb JB, Cotton BA. Chasing 100%: the use of hypertonic saline to improve early, primary fascial closure after damage control laparotomy. J Trauma Acute Care Surg. 2013;74(2):426–30. doi: 10.1097/TA.0b013e31827e2a96. discussion 31-2. [DOI] [PubMed] [Google Scholar]
- 32.Bradley MJ, Dubose JJ, Scalea TM, Holcomb JB, Shrestha B, Okoye O, Inaba K, Bee TK, Fabian TC, Whelan JF, et al. Independent predictors of enteric fistula and abdominal sepsis after damage control laparotomy: results from the prospective AAST Open Abdomen registry. JAMA Surg. 2013;148(10):947–54. doi: 10.1001/jamasurg.2013.2514. [DOI] [PubMed] [Google Scholar]
- 33.Cothren CC, Moore EE, Johnson JL, Moore JB, Burch JM. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg. 2006;192(2):238–42. doi: 10.1016/j.amjsurg.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Burlew CC, Moore EE, Biffl WL, Bensard DD, Johnson JL, Barnett CC. One hundred percent fascial approximation can be achieved in the postinjury open abdomen with a sequential closure protocol. J Trauma Acute Care Surg. 2012;72(1):235–41. doi: 10.1097/TA.0b013e318236b319. [DOI] [PubMed] [Google Scholar]
- 35.Regner JL, Kobayashi L, Coimbra R. Surgical strategies for management of the open abdomen. World J Surg. 2012;36(3):497–510. doi: 10.1007/s00268-011-1203-7. [DOI] [PubMed] [Google Scholar]
- 36.Darehzereshki A, Goldfarb M, Zehetner J, Moazzez A, Lipham JC, Mason RJ, Katkhouda N. Biologic versus nonbiologic mesh in ventral hernia repair: a systematic review and meta-analysis. World J Surg. 2014;38(1):40–50. doi: 10.1007/s00268-013-2232-1. [DOI] [PubMed] [Google Scholar]
- 37.Slater NJ, van der Kolk M, Hendriks T, van Goor H, Bleichrodt RP. Biologic grafts for ventral hernia repair: a systematic review. Am J Surg. 2013;205(2):220–30. doi: 10.1016/j.amjsurg.2012.05.028. [DOI] [PubMed] [Google Scholar]