Abstract
Inflammatory bowel disease is believed to be caused by a combination of genetic and environmental stimuli such as our diet. Diets high in meat and fats and low in fruits and vegetables have been associated with new onset inflammatory bowel disease. This has triggered interest in using dietary modification as a treatment. The three principle models of dietary intervention are supplementation with selected dietary components, exclusion of selected dietary components, or use of dietary formulas in place of a normal diet. Despite the high level of interest in dietary interventions as a treatment for inflammatory bowel disease, few well designed clinical trials have been conducted to firmly establish the optimal diet to induce or maintain remission. This may be in part related to the challenges of conducting dietary intervention trials. This review examines these challenges and potential approaches to be used in dietary intervention trials.
Keywords: Crohn’s disease, Ulcerative colitis, Clinical trials, diet, nutrition
The inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC) are disorders of the intestinal mucosa, where environmental factors, intestinal microbiome, epithelial barrier, the gut-brain axis, innate and adaptive immune system are contributing to a complex pathophysiology against the backdrop of genetic dispositions. Current evidence suggests that environmental factors, including diet, may be important in the development and progression of IBD1. Environmental factors are thought to exert their effect through alterations in the composition of the gut microbiota, the mucosal immune system and epithelial barrier function, among other mechanisms. Given that diet is a modifiable environmental risk factor, it has become an attractive target for both prevention and treatment of IBD.
The incidence of IBD is highest in industrialized nations. In developing nations, where IBD was once rare, the incidence has increased as these nations have become more industrialized2. This has led to the hypothesis that westernization and particularly the Western diet may be a key trigger for IBD3. High dietary intake of total fats, polyunsaturated fatty acids, omega-6 fatty acids, and meat has been associated with an increased risk of both CD and ulcerative colitis UC4. Likewise, long-term intake of dietary fiber is associated with a decreased risk of developing CD5. These epidemiological associations have been strengthened by studies demonstrating that dietary milk fat and emulsifiers can exacerbate colitis in animal models of IBD6,7. A detailed review of diet as a risk factor for IBD is beyond the scope of this paper, but has been recently reviewed elsewhere4.
In addition to epidemiologic and basic science data, a major stimulus for the study of diet comes from patients with IBD who commonly identify foods that exacerbate symptoms8,9 and frequently ask their providers and community for dietary advice. Despite this, high-quality evidence for a direct effect of dietary modifications on reduction in symptoms and inflammation is limited. Several recent papers have reviewed the evidence supporting dietary interventions for IBD10,11 and as such these data will not be covered in detail here. Enteral nutritional therapy has been the most extensively studied dietary intervention and has repeatedly been demonstrated to produce improvements in clinical, biochemical, and mucosal healing outcomes in patients with CD. Recently, other diets for patients with IBD, predominantly exclusion diets such as the semi-vegetarian diet, the specific carbohydrate diet and the Crohn’s disease exclusion diet, have gained attention based mostly on small, non-randomized clinical trials12–14. For example, Chiba reported that remission was maintained in 94% of patients with CD who continued to follow a semi-vegetarian diet as compared to 33% of patients who returned to a regular diet12. Sigall-Boneh reported that 70% of patients achieved clinical remission and 70% had normalization of c reactive protein on the Crohn’s disease exclusion diet13. Other diets, such as the Paleolithic diet, are popular in the lay press, but have yet to be scientifically studied (Table 1).
Table 1.
Dietary interventions considered potentially beneficial for patients with inflammatory bowel disease
| Dietary intervention | Intervention type |
|---|---|
| Exclusive enteral nutrition with a defined formula40 | Modification of food + Exclusion |
| Partial enteral nutrition with a defined formula91 | Modification of food + Exclusion |
| Specific carbohydrate diet30,92,93 | Exclusion |
| Crohn’s disease exclusion diet13 | Exclusion (sometimes combined with Modification of food) |
| Semi-vegetarian diet12 | Exclusion |
| Low FODMAP diet94,95 | Exclusion |
| Anti-inflammatory diet31 | Exclusion |
| Mediterranean style diet71,96 | Exclusion |
| Gluten free97 | Exclusion |
| Additive free diet7,13,30,92,93 | Exclusion |
| High fiber21 | Supplementation |
| Omega-3 fatty acid supplements23,98 | Supplementation |
| Vitamin D supplements24 | Supplementation |
| Curcumin supplements27,28 | Supplementation |
The lack of high quality controlled trial data to guide dietary recommendations for patients with IBD is in stark contrast to the level of interest in the field. This is likely secondary to the unique challenges associated with conducting dietary clinical trials. Nonetheless, to definitively prove the efficacy of dietary interventions, such trials are essential. In this review, we discuss the unique issues that arise in designing, conducting and interpreting data from dietary trials involving patients with IBD.
Defining Diet
Dietary intake is fundamental to the growth, development and well-being of humans but remains highly challenging to categorize and measure due to the heterogeneity in food intake across cultures, age groups, and for a single individual over time. Diet can be represented as constituent chemicals, foods, food groups, or patterns of foods consumed (Figure 1)15. The chemical components that comprise foods include macronutrients, essential micronutrients, food additives, contaminants (agricultural chemical, microbial toxin, inorganic), chemicals formed during the cooking/processing of food, natural toxins, and other natural compounds15. Preparation, storage, and cooking of food may also alter nutritional value of foods16.
Figure 1.
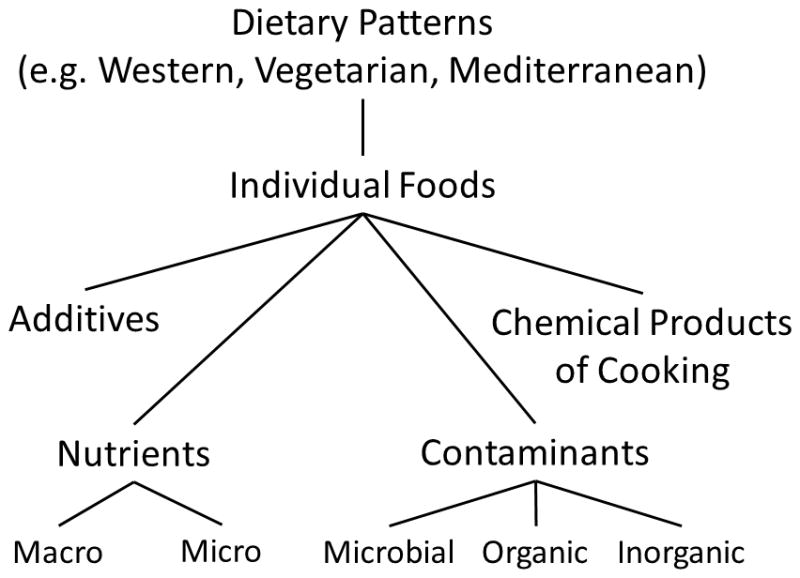
Categorization of diets
To define dietary needs, the Institute of Medicine has put forth Dietary Reference Intakes for macronutrients, fluids and electrolytes, vitamins, and minerals17. These values provide guidance on norms of intake and the physiological needs of individuals. In addition, food intake patterns, which take into consideration the varieties of foods eaten in conjunction, may be of importance in understanding the impact of dietary exposures on the pathophysiology of the human body18. The representation of diet as a dietary pattern is more challenging. For example, the western dietary pattern has been defined by the high intake of refined cereals and sugars, refined vegetable oils, fatty meats, dairy, and other “processed” foods19 while the Mediterranean diet has been characterized by fresh fruits and vegetables, legumes, lean meats (e.g. poultry) and fish, and use of olive oil as the principal fat for cooking20. Dietary patterns can also be defined by the exclusion of specific foods, such as vegan or gluten-free diets.
Because of the complexity of most natural foods and the large quantity of different combinations in which these foods can be consumed, dietary factors and their health effects may be difficult to isolate and specify. For example, investigations on the impact of the glycemic load on health outcomes typically necessitate comparing diets that differ in numerous, hard to standardize ways since the dietary glycemic load can be lowered by consuming more low-glycemic sugars such as fructose or lactose, by incorporating more high-fiber foods, or by lowering the overall carbohydrate content of the diet. Each of these interventions could result in different health effects. Similarly, exclusion of processed foods would likely reduce exposure to multiple factors, such as salts, sugars, and emulsifiers. Thus, when interpreting the results of a dietary intervention trial, one must consider the specific composition of the intervention diets and recognize that a biological effect could be attributed to a specific, isolated dietary factor of interest, associated dietary factors the investigators failed to standardize across intervention arms, or to a more global dietary change.
How to define intervention diets: Supplementation, Exclusion, and Replacement
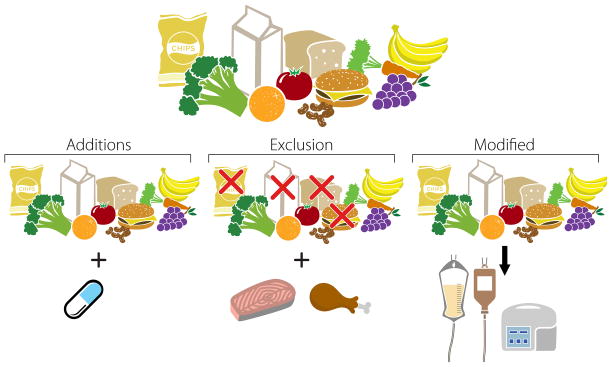
To date, the study of dietary interventions in patients with IBD has primarily concentrated on three approaches which can be used independently or in combination – supplementation with anti-inflammatory components, the exclusion/restriction of pro-inflammatory components, or replacement of the usual diet with nutritional formulas, the mechanism of which is not fully understood (Figure 2).
Figure 2.
Common designs of dietary interventions. Diet can be modified by adding ingredients or components in the form of whole foods or supplements. Exclusion diets focus on exclusion of selected components of the usual diet. However, to sustain adequate caloric intake the excluded nutrients must be replaced with another nutrient source. A third option is to replace usual diet with formula based diets delivered either parenterally or enterally.
Dietary supplementation
Dietary supplementation interventions presume that usual diets contain insufficient quantities of substances to meet the needs of patients with IBD. Supplementation can be in the form of a whole food, an ingredient, or a prepared dietary supplement (e.g. a capsule or liquid containing the additional dietary components). One should recognize that supplementation may result in reduction of consumption of another component of the diet, particularly if the supplementation has appreciable caloric content. In these cases, the investigators need to be aware that they are not assessing the impact of the supplemented food per se, but actually comparing the supplemented to the replaced food. Thus, as much thought needs to be given to which food(s) or dietary component(s) are replaced as to the choice of the specific supplement under investigation. Examples of dietary supplementation trials include randomized control trials of supplementation with prebiotics, i.e. dietary fiber,21 and probiotics22 in both CD and UC, omega-3 fatty acid supplementation in CD23, vitamin D supplementation in CD24, polyphenols found in green tea ((−)-epigallocatechin-3-gallate) for UC25, and curcumin for CD26 and UC27,28. Of these, dietary fiber, vitamin D, probiotics, green tea polyphenols and curcumin have some evidence of efficacy but have not been subject to large scale state of the art clinical trials, while omega-3 fatty acids showed no benefit in two large, well-designed placebo-controlled trials to prevent relapse of Crohn’s disease.
Exclusion diets
The underlying hypothesis for exclusion diets is that the patient’s usual diet may contain one or more ingredients that initiate or potentiate the inflammatory cascade. Examples include IgG4 guided exclusions29, semi-vegetarian diets12, the specific carbohydrate diet30 and similar diets described by Oledzki31 and Sigall-Boneh13. It is important to recognize the exclusion of a dietary component does not happen in isolation. Rather, exclusion diets could be better characterized as replacement diets, where one group of foods, ingredients, etc. is replaced by another (Figure 2). Furthermore, exclusion of select foods may also lead to exclusion of other dietary components because these items are generally consumed together (e.g., milk and breakfast cereal).
Following successful therapy with an exclusion diet, it may be possible to identify the key elements of the diet that were excluded by re-challenging patients in a systematic fashion. For example, Chiba reported that patients who resumed a regular diet after induction of remission with a semi-vegetarian diet were more likely to relapse than those who continued the semi-vegetarian diet12. However, the design did not isolate what aspect of the regular diet contributed to the higher relapse rate. In eosinophilic esophagitis, systematic re-challenge following treatment with an elemental diet or with a 6-item exclusion diet, allows for detection of the unique foods that drive the eosinophilic inflammation32. For such approaches, use of non-invasive biomarkers, such as calprotectin and c reactive protein, has great advantage over repeat cross sectional imaging or endoscopy, and may detect inflammation sooner than clinical symptoms33.
Dependent on the specific nature of the exclusion diet, prolonged periods of treatment with exclusion diets risk producing nutritional deficiency. Given the high prevalence of nutritional deficiencies among patients with IBD in general34, some trials may need to assess for nutritional deficiencies and replete those identified as part of the study protocol. As such, care must be taken when designing exclusion diets for long term use to assure adequately balanced nutrition. This can be partly overcome with nutritional supplements. Unfortunately, adherence to vitamin and supplements among patients with IBD is relatively poor, in part due to lack of understanding of the importance of the nutritional supplements35.
Many patients with IBD follow self-imposed restriction diets, avoiding foods that they believe worsen their symptoms8,36. Although we generally think of restriction diets as excluding selected food items from the diet, following a prescribed restriction diet may lead to more balanced nutrition for some patients.
Exclusive and Partial Enteral Nutrition
Enteral nutritional therapy, typically exclusive enteral nutrition (EEN) with elemental, semi-elemental and polymeric formula diets, is frequently used in the treatment of pediatric CD, particularly in Canada, Japan and Europe37–39. Of all dietary interventions, EEN is supported by the strongest clinical evidence. Commercially available formulae have proved efficacious in treating symptoms and intestinal inflammation in CD in addition to supporting nutritional needs40–42. However, the effectiveness is greatest when used as the exclusive source of nutrition41,43. The most common protocol involves the administration of a defined formula at 100% of caloric needs for 4–12 weeks in order to induce remission44. When compared to corticosteroids as treatment for children with CD for 10 weeks, EEN was significantly more effective than corticosteroids in healing the mucosa, as determined by both endoscopic as well as histologic criteria40.
EEN is an exclusion diet of sorts where the typical, whole foods diet is replaced by defined formulas. The mechanism of action is unknown, but may include reduction in luminal antigens and food exclusion, a direct anti-inflammatory effect of the formula, and/or changes in the gut microbiota or their metabolome45–48. The majority of formulas used for EEN are nutritionally complete, and if there are beneficial effects of the formula itself, such as more effective delivery of nutrients, then EEN could be both a dietary exclusion and supplementation approach.
Basic principles of clinical trials
The specific challenges associated with implementation of dietary intervention trials will be discussed in detail in subsequent sections of this review. Clinical trials of dietary interventions should follow the same principles of that of other interventions in IBD (Table 2). Careful consideration must be given to inclusion criteria, the choice of a comparator group, mode of treatment assignment, blinding, measuring adherence and statistical methods. Each is discussed below. Not addressed in this review is the selection of the outcomes of interest which will be specific to the study question. When studying a clinical outcome, such as remission, the principles do not differ from that of other clinical trials in IBD. Particularly in clinical trials where the treatment assignment is not masked, it is important to include objective measures of inflammation in addition to patient reported outcomes which can be influenced by factors other than bowel inflammation49.
Table 2.
Comparison of design features of dietary intervention and drug trials
| Dietary intervention trials | Drug trials | |
|---|---|---|
| Patient population | Often children | Typically adults first and children studied only after efficacy established in adults |
| Inclusion and exclusion criteria | Often exclude underweight or malnourished; May exclude those whose usual diet is similar to intervention diet | Nutrition or usual diet rarely part of inclusion criteria |
| Common outcomes | Disease activity, quality of life, anthropometric parameters | Disease activity, quality of life |
| Concomitant medications | Determined by study question | May exclude certain medications to avoid excessive immunosuppression |
| Intervention | Supplementation or exclusion of a dietary component | Addition or withdrawal of a medication |
| Comparator | Placebo if studying supplement; usual or standard diet if studying dietary pattern | Placebo or effective therapy |
| Randomization | Essential | Essential |
| Blinding | Sometimes impossible | Essential |
| Dose finding | Uncommon | Based on phase 1 and 2 trials |
| Sample size | Based on minimally important difference | Based on minimally important difference |
| Adherence measurement | 24 hour recalls, food frequency questionnaires, food checklists, and or measurement of biomarkers | Pill counts, electronic monitoring, direct observation therapy |
| Analytic approach | Intention to treat analysis, with or without additional per protocol analysis | Intention to treat analysis |
| Personnel | May include plant scientists, food scientists, registered dietitians, educational experts, behavior interventionists | May include experts in pharmacodynamics and pharmacokinetic measurements |
Inclusion criteria
The choice of whom to include in a dietary intervention trial is similar to that for drug intervention trials. The investigators must consider the hypothesized effect of the therapy on the disease process. For example, one may wish to exclude patients with a prior partial colonic resection if it is anticipated that the diet would be poorly tolerated in those with prior surgery. This may happen with therapies such as EEN that have high osmotic load. Similarly, some have proposed that isolated colonic Crohn’s disease responds less well to EEN than ileal disease50. There are limited data on existing dietary interventions for fistula although some promising results have been reported for EEN in patients with abdominal enterocutaneous fistula51. The fiber content of the diet should be considered when deciding whether to include patients with known or suspected stricturing disease. The impact of nutritional therapies on extraintestinal manifestations of IBD other than growth failure has not been well studied, but one could hypothesize that those manifestations that result from systemic inflammation may be less well controlled by dietary interventions than with systemic therapies. This is an important focus for future studies.
Whether a clinical trial should include patients with active disease or those in remission depends on the question to be answered. However, an important advantage to studying patients with active IBD is the knowledge that the patient’s usual diet was inadequate to provide control of the disease. It is potentially more challenging to show a benefit for maintenance of remission than for induction of a clinical response. In the latter design, the patients have already responded to another therapy; as such, the hypothesis being tested is that the dietary intervention acts synergistically with the therapy that induced remission to maintain remission.
Current nutritional status and other comorbidities may be an important inclusion criterion depending on the design of the trial. If a study diet is anticipated to result in weight loss, one may exclude underweight or undernourished patients. Metabolic disorders, such as diabetes mellitus, may also be important considerations depending on the composition of the study diets.
Finally, one important distinction of dietary intervention trials from drug trials is whether to consider the baseline diet as part of the inclusion criteria. For example, a study that wishes to test the effect of reducing consumption of a specific food group may want to only include patients who consume at least a minimum amount of this food group on a regular basis. Screening for such can be accomplished with food frequency questionnaires or other dietary measurement approaches. Exclusion of those whose usual diet is similar to the intervention may serve to increase statistical power.
Comparator groups
Choice of a comparator group for dietary intervention trials is more complicated than in drug trials. Replacing a food item with a well-defined alternative can increase the ability to detect a clinically meaningful difference and helps to define the study question. For example, a clinical trial could assign people to consume only red meat or chicken as their source of animal protein. This would help answer the question of whether outcomes are better when people consume red meat or chicken. However, this will not address the question of whether replacing red meat or chicken with fish would have the same effect.
The particular replacement dietary components may be more important for certain conditions than others. For example, gluten can be replaced with nearly anything and celiac disease will improve. However, exclusion of saturated fatty acids will only reduce LDL cholesterol if they are replaced with mono- or polyunsaturated fatty acids, not when replaced with carbohydrates.
The choice between a well-defined control diet and using the subject’s “usual diet” as the control should be driven by the underlying study question. Evaluating the subject’s usual diet allows one to more closely understand the impact the introduction of the study diet may have on the course of the disease. However, most people’s usual diet is quite variable and some participants may follow a diet that already approximates the study diet, thereby reducing the power to detect a difference between the treatment arms. In contrast, selecting a well-defined control diet is challenging. By manipulating the participant’s usual diet in any way, it is possible that any observed difference in outcomes in the clinical trial could be due to the effects of the study diet, the control diet, or both. As such, characterizing usual diet may provide insight into unintended effects of even the control diet.
Blinding
In drug development, the strongest evidence of efficacy comes from placebo-controlled RCTs. Placebo-controlled trials have been used for dietary intervention trials as well. In the simplest form, a dietary component can be encapsulated and provided in a pill form or within a solution52. In both cases, a placebo pill or solution can be used to achieve blinding of patients and investigators. However, this design may fail to reflect the impact of higher dietary consumption of a nutrient if the nutrient in the study product has different bioavailability than the same component included in food. Furthermore, nutrients are never consumed in isolation other than when provided as a supplement. Alternatively, investigators may provide the same food with and without a particular nutrient in a blinded fashion to demonstrate the efficacy of adding or removing the nutrient. Such designs have been used to assess the impact of fiber supplementation in a number of health conditions53–55. Such designs could be useful in patients with IBD to study the impact of selected vitamins, minerals, or additives, such as emulsifiers.
Creation of a placebo or control is more complicated if the study product has substantial caloric content. In such cases, consumption of the study product would be expected to reduce intake of other food. One approach is to provide comparable calories in the placebo. Alternatives are to have minimal caloric content in the placebo or not provide any control product. The choice between these strategies depends on the question to be answered. If the goal is to assess the impact of increasing consumption of a specific nutrient, providing a placebo with comparable calories will minimize the risk of contamination of the intervention by greater consumption of other foods that contain the same ingredient in the control group. In contrast, if the objective of the study is to assess the clinical impact of providing a nutritional product on disease course, the ideal control product may be one with minimal calories or not providing any control product, thereby reflecting the impact that the product would have when used in clinical practice.
The examples provided thus far have focused on dietary supplementation. Blinding is more difficult in exclusion diets since the relevant dietary component may be acquired from a number of different foods. Similarly, when the intervention is built around the entire diet, blinding is almost always infeasible. Thus, many trials of dietary interventions are open label or at most single blind (i.e. the evaluator is blinded but the patient is aware of the treatment) and the comparator group consumes a “control diet” or “their usual diet”. Because the participants in such studies are aware of the treatment, there is a greater risk for bias. In particular, there is the risk that participants assigned to one diet will also implement all or some aspects of the other diet on their own. This is sometimes referred to as contamination, opting-in or opting-out, and can be a particular problem if one of the study diets is “the participant’s usual diet.” Imagine a trial where patients are randomly assigned to either a high red meat diet which resembles the participant’s usual diet or a low red meat diet. If the participants are aware of the composition of the two diets, they will immediately know the hypothesis under study and may reduce red meat consumption in hopes of achieving a benefit.
One approach to avoiding this problem is to limit the participant’s knowledge to only the treatment arm to which they are assigned. While this has the advantage of reducing the risk of contamination, it creates additional organizational and perhaps analytic challenges56. A complimentary approach is to provide participants with their food. This is much more expensive, but in theory should reduce the risk of contamination and differential participation rates.
Randomization
Randomization is the cornerstone of clinical trials. Random assignment of treatment serves to avoid bias that can result when the treating clinician recommends one treatment over another based on a characteristic of the patient. Furthermore, when a trial is large enough, randomization serves to balance both the known and unknown potential confounders. Trials of dietary interventions should use randomization to achieve such balance and avoid selection bias in the same way as drug trials have. Stratified randomization can help assure balance on important covariates and may be particularly helpful when sample sizes are relatively small.
Randomization can be employed in designs other than the traditional parallel group clinical trial. Historically, crossover designs have been used to study chronic diseases. In a crossover design, participants are treated sequentially with both interventions, typically with a washout period between the treatment periods. The order of treatment is determined by random assignment. The main advantage of the design is increased statistical power as each participant serves as their own control. Key challenges of crossover designs are the potential for carryover effects from the first treatment period and the impact of missing data when participants are lost to follow-up. A variant of crossover trials is the N-of-1 trial design where an individual participant is randomly assigned to treatment periods with the different therapies. After several treatment periods, the data are used to assess whether the disease was better controlled with one of the two treatments. The result can be used to select a long term treatment for the individual patient. Repeating this experiment with multiple participants allows for pooling of the data to draw more generalizable conclusions. Although appealing in concept and reminiscent of advice often given to patients with IBD to try different dietary modifications to see what helps, the design has not been widely employed in a formal research setting. A 2011 systematic review identified only 108 N-of-1 trials published over a 25 year period, none of which were dietary interventions57.
Measuring dietary intake and adherence to the study diet
Just as adherence to medications can influence the effectiveness, adherence to therapeutic diets may be just as important. A recent study demonstrated that the effectiveness of EEN for Crohn’s disease was influenced by the proportion of calories consumed as regular table food relative to the formula43. Palatability, tolerability, satiety, ease of preparation, availability of ingredients or prepared foods, cost, and cultural, religious, and social acceptability may all influence adherence to a dietary intervention. One approach to maximize adherence is to include a run in period where participants sample the study diets prior to randomization. However, to avoid having a clinically important effect on the disease course, such run in periods need to be short.
Measuring dietary intake and adherence can be accomplished with simple checklists that are kept by the participants to report their adherence to the specific intervention, ideally daily. Other possibilities include 24-hour dietary recalls, food diaries, food frequency questionnaires (FFQs), evaluation by a registered dietitian, or directly measuring the food not consumed in controlled studies that provide some, most, or all of the food (analogous to pill counts for drug studies). The FFQ method is a validated approach to capture dietary exposure data over time58,59. The FFQ method emphasizes longer-term dietary exposures, and as such respondents must estimate past exposures. The FFQ questions need to be framed to encompass the time that the participant is in the clinical trial if the goal is to measure adherence to the study diets. FFQ are considered relatively poor instruments to assess compliance with a dietary intervention, as it would be relatively easy for participants to manipulate their responses to the standardized questions to appear more compliant than they were.
Dietary recall and food records can provide greater dietary detail than the FFQ method. The 24-hour dietary recall and food record methods capture food intake on one or more specified days. With 24-hour dietary recall, study participants are prompted by a trained interviewer to recall foods consumed over the previous 24 hours while with the food record method, food intake is logged real-time in a diary. Unlike the estimations used in answering a FFQ, these two methods capture actual daily intake including details on quantity, preparation, and other specifics. Both are open-ended, i.e. participants list their actual foods consumed, rather than respond to questions about consumption in broad food categories as in the FFQ. Whereas food records can introduce bias by impacting an individual’s food consumption, unannounced 24-hour dietary recalls are a more objective measure. The 24-hour dietary recall approach is much more expensive and is less feasible for very large studies with repeated measures over the course of time. An automated, web-based version of dietary recall with direct-entry of data by study participants has been developed by the National Cancer Institute and may become a viable, lower-cost alternative to traditional dietary recall in the future60,61. The use of 24-hour recalls introduces the possibility of sampling bias pending the frequency of specific dietary exposures.
Each of these methods is subject to bias from errors in the assessment of diet62. An alternative is to measure adherence through the use of biomarkers, if such markers exist23. An example for an established biomarker commonly used in research is the plasma phospholipid concentration of the fatty acids C15:0, C17:0, and C16:1 n-7 trans, all of which are biomarkers for dairy fat consumption63. More complex biomarkers are currently in development, some of which are based on the plasma and/or urinary metabolome64. Newer technologies have the potential to improve precision while reducing cost and participant burden. Mobile phone food records using photograph-based assessments and the usage of sensor technology to estimate portion sizes are in active development and hold promise for the future of dietary assessment65. As novel tools are developed for measuring human physiology, the opportunities for improved monitoring of adherence should expand66
In most clinical trials of medications, the study drugs are provided to the participant without cost. This model has been used on occasion in dietary intervention trials20. Implementation is easier when the dietary intervention is limited, such as addition of a special beverage, bread, etc. More complex dietary interventions may require delivery of complete sets of ingredients or prepared meals. Such designs are becoming increasingly feasible with the many vendors providing this service to the general public. For example, an ongoing trial of the specific carbohydrate diet has employed this strategy (https://clinicaltrials.gov/ct2/show/NCT02610101?term=suskind&rank=8). However, the cost may be substantial and the sustainability of such a model is less clear. Specifically, if a dietary intervention is efficacious when the key components are provided to participants at no cost, the effectiveness when implemented outside of a clinical trial may be substantially less, particularly if the diet is difficult to follow. One approach to this is to provide the diet only during the first part of the trial and to test the sustainability of the diet in the second half of the trial as is planned in a forthcoming trial comparing the Mediterranean style diet the specific carbohydrate diet (http://www.pcori.org/research-results/2016/comparative-effectiveness-specific-carbohydrate-and-mediterranean-diets-induce). Thus, dietary intervention trials commonly test both the biological effect of a diet and the ability and willingness of participants to consume this diet.
Dose finding in dietary intervention trials
An important aspect of drug development is identifying the minimum effective dose. For some dietary interventions, the same principle may apply. The principles of pharmacokinetics and pharmacodynamics can be used to predict the minimum effective dose of a drug. For example, dosing of vedolizumab was demonstrated to nearly fully occupy the α4β7 receptor at doses used in clinical trials67,68. For dietary interventions, particularly exclusion diets, the question may be how much of a substance is too much or too little. For dietary interventions that provide additional amounts of a specific nutrient, it is intuitive to consider measuring the concentration of the nutrient in the plasma or urine. Unfortunately, circulating concentrations of most micronutrients are not representative of even recent intake, as they are homeostatically regulated or because their storage in tissues or secretion are important additional determinants of plasma concentrations69.
Ideally, the same principles should apply as with drug development. If the target pathway is known, dose finding studies to assess the amount of a nutrient required to influence the pathway can be used to optimize the intervention before testing in a large scale clinical trial. When possible, early phase trials should assess the impact of differing amounts of the dietary component on tolerability and biomarkers relevant to the study question. Nutrigenomics is an evolving discipline in which the expression of genes is measured in response to a dietary intervention, and may be helpful in IBD in order to understand on how an altered nutritional exposure could regulate gene expression and ultimately phenotype70,71.
Statistical considerations
An extensive overview of the statistical approaches to clinical trials is beyond the scope of this manuscript. Here, we highlight several key concepts that should be considered in the design and implementation of dietary intervention trials. A more extensive discussion of this topic, specifically as it applies to dietary intervention studies is available from several recently published methods-focused manuscripts72–75. Sample size calculations for dietary intervention trials should follow the same principles as for other trials. One of the most pervasive issues that plagues nutrition clinical trials are underpowered studies. Not only are they often false negative, as would be expected, but they are more likely to result in ‘significant’ findings that are purely due to chance because the study was concluded on a ‘random high’ or because of residual confounding despite randomization. Even more problematic are trials that end up being underpowered due to high attrition rates, especially if these were unevenly divided between the control and the intervention arm. This leads to biased estimations of the treatment effect. For a recent discussion of these and other issues see Yelland et al76. Even if a best effort attempt at determining a realistic sample size are made, calculations are still often based on a too optimistic assessment of the likely effect size77. This state of affairs underscores the need for more multi-center trials with better statistical and organizational support.
Drawing upon the importance of randomization to balance confounders between the treatment groups, the primary analysis for most trials should employ the principle of intention to treat, whereby all participants are analyzed according to their assigned diet. Additional per protocol analyses can be conducted that provide additional information on the potential effectiveness of an intervention if followed as described in the protocol. However, per protocol analyses do not fully take advantage of the benefits of randomization and therefore it is important to reassess for potential imbalance of confounder variables and adjust for these as needed.
Per protocol analyses often use a single predefined definition of adherence to determine who is included in the analysis. However, adherence is difficult to measure in dietary trials and the level of adherence that is necessary to achieve the outcome is rarely known at the time the trial is designed. Sensitivity analyses examining the effectiveness of the dietary intervention across a range of levels of adherence can generate important hypotheses. For example, in celiac disease, complete exclusion of gluten is necessary to effectively treat the disease. In contrast, reduction of intake of foods with a high glycemic index will improve glucose control in diabetes, even if adherence is not 100%.
Dietary quality control and reproducibility
The source of food and how it is prepared adds to the heterogeneity of dietary interventions. At present, food product labeling largely does not distinguish between different plant varieties, or cultivars, despite substantial differences in nutrient profiles. For example, purple potatoes may have a vastly different nutrient profile when compared to white potatoes78,79. Similarly, sourdough bread may differ in its immunogenicity from yeast bread80, or a cheese may have different health effects dependent on whether it was made from raw or pasteurized milk, or from cows that were grain- or pasture-fed81. Importantly, none of these issues would be apparent from a food label or nutrient database. Understanding the impact and role of “processing” foods is important in dietary trials and collaboration with food scientists may be a valuable opportunity for clinicians.
Quality control in dietary intervention trials can be optimized by directly providing foods to study participants. This approach eliminates the variability of available foods and allows investigators to ascertain dietary exposure details. However, the cost of this approach may be prohibitive for longer or larger trials, and differential food preparation may still introduce variability.
Though it has been suggested that single-center studies may lack the rigor of multi-center studies, single-center studies often observe larger treatment effects. This may be related to stricter treatment protocols, reduced variability in food sources, or more homogenous participant populations82,83. In contrast, multinational trials may introduce cultural variability in food preparation and consumption that may be challenging to capture and result in disparate study results. Studies that house participants and provide and measure all dietary consumption allow for the greatest control of dietary exposures. Such designs may be used for early phase studies to assess tolerability of the dietary intervention and the impact of short term exposures on biomarkers. However, the duration of time required to measure clinical outcomes generally make this study design impractical for large scale IBD trials.
Personnel
In addition to the traditional personnel supporting clinical trials, rigorous dietary studies may require additional expertise and oversight. Scientists and physicians may develop the clinical questions and provide expertise in study design, but the involvement of other experts can provide essential insight. Food scientists study the physical, chemical, and biochemical properties of food and the principles of food processing84. Food scientists can provide guidance on safe production, preservation, and packaging of foods. Similarly, registered dietitians can help assure that study diets meet patients’ nutritional requirements and also guide patients on the practical aspects of consuming specialized diets.
Education is arguably one of the most important aspects of a clinical trial involving a diet. Diets are inherently varied with essentially unlimited food choices available to consumers and this represents a challenge and leaves room for error. To ensure consistency, appropriate education of patients, family members, and the clinical or research team is critical. Registered dietitians can guide counseling and support of subjects and also direct the research team on creating an appropriate study diet.
Most studies of diet for the treatment of disease have relied on education in the form of careful dietary counseling by a physician, registered dietitian or certified nutrition specialists. This is time consuming and generally requires additional personnel. However, dietary instructions must be adequately conveyed to subjects and also to parents or other family members in studies involving pediatric subjects. The frequency of dietary counseling sessions is an important consideration. The most common approach has been a more thorough initial session with distribution of detailed reference guides with subsequent follow-up sessions so that the study team can assess compliance, answer questions, and determine additional educational needs13. Although it requires further resources, to improve compliance, it may be helpful to have a mechanism in place for questions to be answered by a study team member in real time. Consideration should be given to the amount of oversight and attention given to the respective study arms, as differential guidance may bias study results.
Regulatory considerations
When does a dietary intervention become a drug, a food for special medical purposes (also known as a medical food), or a nutritional supplement? Although a full discussion is beyond the scope of this review, investigators should recognize that under certain circumstances a dietary intervention could be considered as a drug, a medical food, or a supplement. Exclusion diets do not meet any of these definitions. However, dietary interventions that supplement or replace the usual diet could potentially meet the definition of a supplement, medical food or a drug. In the latter cases additional regulations would apply to the conduct of clinical trials and marketing of the product.
Unique considerations for dietary intervention trials in children
The dietary requirements of children are unique in that nutrition must support normal growth and development85. As children progress from infancy to childhood and adolescence, physiology and nutritional requirements change vastly. The design of clinical trials that will include children must consider the nutritional requirements for normal growth and development. The duration of the trial may be an important consideration. For example, 4 to 12 weeks of a restriction diet is unlikely to lead to significant nutritional deficiencies whereas this could be a problem in longer maintenance trials.
The differential role of food chemical component exposures at different stages of growth and development requires further study, in particular the role of dietary exposures in the developing immune system. A recent randomized trial following infants through childhood demonstrated the protective effect of early exposure to peanut protein on the future development of peanut allergies86. This study demonstrates the importance of timing of dietary exposure on the development of an aberrant immunological response. As such, the analytic plan of dietary intervention trials in children may want to include subgroup analyses stratified by age.
Self-selected dietary interventions have lower compliance than prepared meals and motivated parents can partner to encourage increased compliance in their children87. Trials of EEN for CD in adults have demonstrated poor efficacy and adherence88. In contrast, pediatric studies have repeatedly demonstrated the efficacy of EEN at inducing remission of active CD89. The difference in outcomes between children and adults may in part be due to stricter adherence enforced by parents in pediatric studies. Alternatively, it is possible that dietary interventions may be more effective in new onset disease.
Children with IBD often require lifelong immunosuppressive therapy, and the concern for possible adverse events such as infection and cancer often plays a role for families to seek out alternative therapies, including diet. Experimental dietary trials in children are important to develop improved therapies for disease in children, and this potential benefit must be balanced with ethical implications of studying an investigational therapy in a minor who enters into a trial with consent obtained by proxy from a parent or guardian. The child’s assent should be obtained if they are able to comprehend the relevant issues around their participation in a trial90.
Generalizability
The goal of all clinical trials is to generate new knowledge that can be applied broadly to patients who are representative of those included in the study. To date, many dietary intervention studies for patients with IBD have been conducted in a single center. The logistics of designing and implementing such trials make international studies very challenging.
Certain food products may not be available in all regions. It may be difficult to find specialized food products or adequate variety in smaller, rural markets. Additionally, similar food products in different regions or in different countries may not contain identical ingredients. Of course, cultural differences in food ingredients and food preparation must also be considered.
The majority of the exclusion diets that have been proposed for the treatment of IBD require significant restrictions and preparing food at home becomes essential. Use of more convenient, packaged foods becomes challenging and in most cases, is not allowed. Preparing food primarily or exclusively at home can be expensive and time consuming and may limit the generalizability of the dietary interventions. Utilization of pre-packaged fresh or frozen meals delivered to subjects at regular intervals is an approach that will ensure uniformity and may also increase compliance. Moreover, such approaches may be generalizable more broadly with increased availability of food delivery services. Additionally, whether to provide the food to the subject alone or to the entire family will need to be considered and this may be especially relevant in pediatric trials.
Conclusions
CD and UC are chronic debilitating diseases for which there is no medical cure. Our currently available therapies target the downstream inflammatory process rather than the underlying stimulus of the disease, are not completely effective and are associated with substantial risks and side effects. Dietary patterns are associated with incidence of IBD and diet is the most readily modifiable of the likely environmental triggers, thereby representing an ideal therapeutic target. Formula-based diets have demonstrated efficacy in CD; however, these are generally impractical for long term management of CD and do not appear to be effective for UC. Furthermore, the mechanism of action of these diets is unclear. It is unknown whether the efficacy is driven by exclusion of select dietary components, increased delivery and/or absorption of other dietary components, both of these, or some other mechanism. The available data on less extreme restriction diets and some novel dietary supplements suggests potential therapeutic benefit, but further research is needed to define the optimal diet for patients with IBD. Conducting such research is challenging, but not beyond the reach of the IBD community. Nonetheless, it will likely require creating new models of research teams and adapting the commonly used study designs to address special challenges that are unique to dietary interventions. Ultimately, identification of dietary interventions that can improve the course of IBD could have immediate and long lasting impact on the management of these diseases.
Acknowledgments
This work was supported in part by National Institutes of Health grant K24-DK078228.
The authors acknowledge Nestle Health Science who provided support for initial literature searches. No writing assistance was provided and all views expressed in this manuscript are those of the authors. The authors jointly drafted and edited the manuscript.
Footnotes
Potential Conflicts of Interest: Dr. Lewis has served as a consultant for and received research funding from Nestle Health Science. Dr. Kratz has received honoraria and compensation for travel as well as a research grant from dairy-related organizations. Dr. Reinisch has served as a consultant for Nestle Health Science. Drs. Gottlieb, Lee, and Albenberg report no potential conflicts of interest.
References
- 1.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel disease. Gastroenterology & hepatology. 2013 Jun;9(6):367–374. [PMC free article] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 3.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011 Apr;106(4):563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 4.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011 Apr;106(4):563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013 Nov;145(5):970–977. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012 Jul 5;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015 Mar 5;519(7541):92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sandler RS, Lewis JD. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Digestive diseases and sciences. 2013 May;58(5):1322–1328. doi: 10.1007/s10620-012-2373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limdi JK, Aggarwal D, McLaughlin JT. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflammatory bowel diseases. 2016 Jan;22(1):164–170. doi: 10.1097/MIB.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 10.Lee D, Albenberg L, Compher C, Baldassano R, Piccoli D, Lewis JD, Wu GD. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology. 2015 May;148(6):1087–1106. doi: 10.1053/j.gastro.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penagini F, Dilillo D, Borsani B, Cococcioni L, Galli E, Bedogni G, Zuin G, Zuccotti GV. Nutrition in Pediatric Inflammatory Bowel Disease: From Etiology to Treatment. A Systematic Review. Nutrients. 2016;8(6) doi: 10.3390/nu8060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba M, Abe T, Tsuda H, Sugawara T, Tsuda S, Tozawa H, Fujiwara K, Imai H. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010 May 28;16(20):2484–2495. doi: 10.3748/wjg.v16.i20.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigall-Boneh R, Pfeffer-Gik T, Segal I, Zangen T, Boaz M, Levine A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis. 2014 Aug;20(8):1353–1360. doi: 10.1097/MIB.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 14.Obih C, Wahbeh G, Lee D, Braly K, Giefer M, Shaffer ML, Nielson H, Suskind DL. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2015 Nov 30; doi: 10.1016/j.nut.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Willett W. Nutritional epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 16.Hoffman R, Gerber M. Food Processing and the Mediterranean Diet. Nutrients. 2015 Sep 17;7(9):7925–7964. doi: 10.3390/nu7095371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs DR, Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003 Sep;78(3 Suppl):508S–513S. doi: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 19.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005 Feb;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 20.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA. Primary prevention of cardiovascular disease with a Mediterranean diet. The New England journal of medicine. 2013 Apr 4;368(14):1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 21.Wedlake L, Slack N, Andreyev HJ, Whelan K. Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis. 2014 Mar;20(3):576–586. doi: 10.1097/01.MIB.0000437984.92565.31. [DOI] [PubMed] [Google Scholar]
- 22.Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clinical and experimental gastroenterology. 2014;7:473–487. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feagan BG, Sandborn WJ, Mittmann U, Bar-Meir S, D’Haens G, Bradette M, Cohen A, Dallaire C, Ponich TP, McDonald JW, Hebuterne X, Pare P, Klvana P, Niv Y, Ardizzone S, Alexeeva O, Rostom A, Kiudelis G, Spleiss J, Gilgen D, Vandervoort MK, Wong CJ, Zou GY, Donner A, Rutgeerts P. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA. 2008 Apr 9;299(14):1690–1697. doi: 10.1001/jama.299.14.1690. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010 Aug;32(3):377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 25.Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (−)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis. 2013 Aug;19(9):1904–1912. doi: 10.1097/MIB.0b013e31828f5198. [DOI] [PubMed] [Google Scholar]
- 26.Suskind DL, Wahbeh G, Burpee T, Cohen M, Christie D, Weber W. Tolerability of curcumin in pediatric inflammatory bowel disease: a forced-dose titration study. Journal of pediatric gastroenterology and nutrition. 2013 Mar;56(3):277–279. doi: 10.1097/MPG.0b013e318276977d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, Ching JY, Cheong PK, Avidan B, Gamus D, Kaimakliotis I, Eliakim R, Ng SC, Ben-Horin S. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2015 Aug;13(8):1444–1449. e1441. doi: 10.1016/j.cgh.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, Yamada M, Iwaoka Y, Kanke K, Hiraishi H, Hirayama K, Arai H, Yoshii S, Uchijima M, Nagata T, Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2006 Dec;4(12):1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Rajendran N, Kumar D. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: a pilot study. Colorectal Dis. 2011 Sep;13(9):1009–1013. doi: 10.1111/j.1463-1318.2010.02373.x. [DOI] [PubMed] [Google Scholar]
- 30.Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, Eshee L, Mason D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. Journal of pediatric gastroenterology and nutrition. 2014 Oct;59(4):516–521. doi: 10.1097/MPG.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 31.Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutrition journal. 2014;13:5. doi: 10.1186/1475-2891-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kavitt RT, Hirano I, Vaezi MF. Diagnosis and Treatment of Eosinophilic Esophagitis in Adults. The American journal of medicine. 2016 Sep;129(9):924–934. doi: 10.1016/j.amjmed.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011 May;140(6):1817–1826. e1812. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang C, Ross V, Mahadevan U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm Bowel Dis. 2012 Apr 5; doi: 10.1002/ibd.22906. [DOI] [PubMed] [Google Scholar]
- 35.Greenley RN, Stephens KA, Nguyen EU, Kunz JH, Janas L, Goday P, Schurman JV. Vitamin and mineral supplement adherence in pediatric inflammatory bowel disease. Journal of pediatric psychology. 2013 Sep;38(8):883–892. doi: 10.1093/jpepsy/jst037. [DOI] [PubMed] [Google Scholar]
- 36.Vagianos K, Clara I, Carr R, Graff LA, Walker JR, Targownik LE, Lix LM, Rogala L, Miller N, Bernstein CN. What Are Adults With Inflammatory Bowel Disease (IBD) Eating? A Closer Look at the Dietary Habits of a Population-Based Canadian IBD Cohort. JPEN J Parenter Enteral Nutr. 2016 Mar;40(3):405–411. doi: 10.1177/0148607114549254. [DOI] [PubMed] [Google Scholar]
- 37.Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schutz T, van Gemert W, van Gossum A, Valentini L, Lubke H, Bischoff S, Engelmann N, Thul P. ESPEN Guidelines on Enteral Nutrition: Gastroenterology. Clin Nutr. 2006 Apr;25(2):260–274. doi: 10.1016/j.clnu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Sandhu BK, Fell JM, Beattie RM, Mitton SG, Wilson DC, Jenkins H. Guidelines for the Management of Inflammatory Bowel Disease in Children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010 Jan 13; doi: 10.1097/MPG.0b013e3181c92c53. [DOI] [PubMed] [Google Scholar]
- 39.Caprilli R, Gassull MA, Escher JC, Moser G, Munkholm P, Forbes A, Hommes DW, Lochs H, Angelucci E, Cocco A, Vucelic B, Hildebrand H, Kolacek S, Riis L, Lukas M, de Franchis R, Hamilton M, Jantschek G, Michetti P, O’Morain C, Anwar MM, Freitas JL, Mouzas IA, Baert F, Mitchell R, Hawkey CJ. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006 Mar;55( Suppl 1):i36–58. doi: 10.1136/gut.2005.081950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, Russo PM, Cucchiara S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2006 Jun;4(6):744–753. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. Journal of gastroenterology. 2014 Apr;49(4):638–645. doi: 10.1007/s00535-013-0815-0. [DOI] [PubMed] [Google Scholar]
- 42.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database of Systematic Reviews. 2007:1. doi: 10.1002/14651858.CD000542.pub2. update of Cochrane Database Syst Rev. 2001;(3):CD000542. [DOI] [PubMed] [Google Scholar]
- 43.Lee D, Baldassano RN, Otley AR, Albenberg L, Griffiths AM, Compher C, Chen EZ, Li H, Gilroy E, Nessel L, Grant A, Chehoud C, Bushman FD, Wu GD, Lewis JD. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm Bowel Dis. 2015 Aug;21(8):1786–1793. doi: 10.1097/MIB.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 44.Day AS, Whitten KE, Lemberg DA, Clarkson C, Vitug-Sales M, Jackson R, Bohane TD. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol. 2006 Oct;21(10):1609–1614. doi: 10.1111/j.1440-1746.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- 45.Gassull MA. Review article: the role of nutrition in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004 Oct;20( Suppl 4):79–83. doi: 10.1111/j.1365-2036.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 46.Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, Morelli L. Enteral nutrition and microflora in pediatric Crohn’s disease. JPEN J Parenter Enteral Nutr. 2005 Jul-Aug;29(4 Suppl):S173–175. doi: 10.1177/01486071050290S4S173. discussion S175–178, S184-178. [DOI] [PubMed] [Google Scholar]
- 47.van den Bogaerde J, Kamm MA, Knight SC. Immune sensitization to food, yeast and bacteria in Crohn’s disease. Aliment Pharmacol Ther. 2001 Oct;15(10):1647–1653. doi: 10.1046/j.1365-2036.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- 48.Van Den Bogaerde J, Cahill J, Emmanuel AV, Vaizey CJ, Talbot IC, Knight SC, Kamm MA. Gut mucosal response to food antigens in Crohn’s disease. Aliment Pharmacol Ther. 2002 Nov;16(11):1903–1915. doi: 10.1046/j.1365-2036.2002.01360.x. [DOI] [PubMed] [Google Scholar]
- 49.Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D’Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV, Jr, Marteau P, Munkholm P, Murdoch TB, Ordas I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O’Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015 Sep;110(9):1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 50.Afzal NA, Davies S, Paintin M, Arnaud-Battandier F, Walker-Smith JA, Murch S, Heuschkel R, Fell J. Colonic Crohn’s disease in children does not respond well to treatment with enteral nutrition if the ileum is not involved. Digestive diseases and sciences. 2005 Aug;50(8):1471–1475. doi: 10.1007/s10620-005-2864-6. [DOI] [PubMed] [Google Scholar]
- 51.Yan D, Ren J, Wang G, Liu S, Li J. Predictors of response to enteral nutrition in abdominal enterocutaneous fistula patients with Crohn’s disease. European journal of clinical nutrition. 2014 Aug;68(8):959–963. doi: 10.1038/ejcn.2014.31. [DOI] [PubMed] [Google Scholar]
- 52.Di Sabatino A, Volta U, Salvatore C, Biancheri P, Caio G, De Giorgio R, Di Stefano M, Corazza GR. Small Amounts of Gluten in Subjects With Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2015 Sep;13(9):1604–1612. e1603. doi: 10.1016/j.cgh.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, He J, Wildman RP, Reynolds K, Streiffer RH, Whelton PK. A randomized controlled trial of dietary fiber intake on serum lipids. European journal of clinical nutrition. 2006 Jan;60(1):62–68. doi: 10.1038/sj.ejcn.1602268. [DOI] [PubMed] [Google Scholar]
- 54.Bliss DZ, Savik K, Jung HJ, Whitebird R, Lowry A, Sheng X. Dietary fiber supplementation for fecal incontinence: a randomized clinical trial. Research in nursing & health. 2014 Oct;37(5):367–378. doi: 10.1002/nur.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soltoft J, Krag B, Gudmand-Hoyer E, Kristensen E, Wulff HR. A double-blind trial of the effect of wheat bran on symptoms of irritable bowel syndrome. Lancet. 1976 Feb 7;1(7954):270–272. doi: 10.1016/s0140-6736(76)91402-1. [DOI] [PubMed] [Google Scholar]
- 56.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, Loewenstein G, Brennan TA, Asch DA, Volpp KG. Randomized trial of four financial-incentive programs for smoking cessation. The New England journal of medicine. 2015 May 28;372(22):2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabler NB, Duan N, Vohra S, Kravitz RL. N-of-1 trials in the medical literature: a systematic review. Medical care. 2011 Aug;49(8):761–768. doi: 10.1097/MLR.0b013e318215d90d. [DOI] [PubMed] [Google Scholar]
- 58.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. American journal of epidemiology. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 59.Johnson RK. Dietary intake--how do we measure what people are really eating? Obesity research. 2002 Nov;10( Suppl 1):63S–68S. doi: 10.1038/oby.2002.192. [DOI] [PubMed] [Google Scholar]
- 60.Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S, Potischman N. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. Journal of the Academy of Nutrition and Dietetics. 2012 Aug;112(8):1134–1137. doi: 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson FE, Dixit-Joshi S, Potischman N, Dodd KW, Kirkpatrick SI, Kushi LH, Alexander GL, Coleman LA, Zimmerman TP, Sundaram ME, Clancy HA, Groesbeck M, Douglass D, George SM, Schap TE, Subar AF. Comparison of Interviewer-Administered and Automated Self-Administered 24-Hour Dietary Recalls in 3 Diverse Integrated Health Systems. American journal of epidemiology. 2015 Jun 15;181(12):970–978. doi: 10.1093/aje/kwu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E, Davy BM. Dietary biomarkers: advances, limitations and future directions. Nutrition journal. 2012;11:109. doi: 10.1186/1475-2891-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001 Mar;131(3):828–833. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 64.Boeing H. Nutritional epidemiology: New perspectives for understanding the diet-disease relationship? European journal of clinical nutrition. 2013 May;67(5):424–429. doi: 10.1038/ejcn.2013.47. [DOI] [PubMed] [Google Scholar]
- 65.Stumbo PJ. New technology in dietary assessment: a review of digital methods in improving food record accuracy. The Proceedings of the Nutrition Society. 2013 Feb;72(1):70–76. doi: 10.1017/S0029665112002911. [DOI] [PubMed] [Google Scholar]
- 66.Kalantar-Zadeh K, Yao CK, Berean KJ, Ha N, Ou JZ, Ward SA, Pillai N, Hill J, Cottrell JJ, Dunshea FR, McSweeney C, Muir JG, Gibson PR. Intestinal Gas Capsules: A Proof-of-Concept Demonstration. Gastroenterology. 2016 Jan;150(1):37–39. doi: 10.1053/j.gastro.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 67.Rosario M, Dirks NL, Gastonguay MR, Fasanmade AA, Wyant T, Parikh A, Sandborn WJ, Feagan BG, Reinisch W, Fox I. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015 Jul;42(2):188–202. doi: 10.1111/apt.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyant T, Estevam J, Yang L, Rosario M. Development and validation of receptor occupancy pharmacodynamic assays used in the clinical development of the monoclonal antibody vedolizumab. Cytometry Part B, Clinical cytometry. 2015 Apr 23; doi: 10.1002/cyto.b.21236. [DOI] [PubMed] [Google Scholar]
- 69.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr. 2003 Mar;133( Suppl 3):875S–880S. doi: 10.1093/jn/133.3.875S. [DOI] [PubMed] [Google Scholar]
- 70.Laursen L. Interdisciplinary research: Big science at the table. Nature. 2010 Dec 23;468(7327):S2–4. doi: 10.1038/468S2a. [DOI] [PubMed] [Google Scholar]
- 71.Marlow G, Ellett S, Ferguson IR, Zhu S, Karunasinghe N, Jesuthasan AC, Han DY, Fraser AG, Ferguson LR. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Human genomics. 2013;7:24. doi: 10.1186/1479-7364-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bland JM, Altman DG. Best (but oft forgotten) practices: testing for treatment effects in randomized trials by separate analyses of changes from baseline in each group is a misleading approach. Am J Clin Nutr. 2015 Nov;102(5):991–994. doi: 10.3945/ajcn.115.119768. [DOI] [PubMed] [Google Scholar]
- 73.Brown AW, Li P, Bohan Brown MM, Kaiser KA, Keith SW, Oakes JM, Allison DB. Best (but oft-forgotten) practices: designing, analyzing, and reporting cluster randomized controlled trials. Am J Clin Nutr. 2015 Aug;102(2):241–248. doi: 10.3945/ajcn.114.105072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Souza RJ, Eisen RB, Perera S, Bantoto B, Bawor M, Dennis BB, Samaan Z, Thabane L. Best (but oft-forgotten) practices: sensitivity analyses in randomized controlled trials. Am J Clin Nutr. 2016 Jan;103(1):5–17. doi: 10.3945/ajcn.115.121848. [DOI] [PubMed] [Google Scholar]
- 75.Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr. 2015 Oct;102(4):721–728. doi: 10.3945/ajcn.115.113548. [DOI] [PubMed] [Google Scholar]
- 76.Yelland LN, Makrides M, McPhee AJ, Quinlivan J, Gibson RA. Importance of adequate sample sizes in fatty acid intervention trials. Prostaglandins, leukotrienes, and essential fatty acids. 2016 Apr;107:8–11. doi: 10.1016/j.plefa.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Summers MJ, Chapple LS, McClave SA, Deane AM. Event-rate and delta inflation when evaluating mortality as a primary outcome from randomized controlled trials of nutritional interventions during critical illness: a systematic review. Am J Clin Nutr. 2016 Mar 9; doi: 10.3945/ajcn.115.122200. [DOI] [PubMed] [Google Scholar]
- 78.Charepalli V, Reddivari L, Radhakrishnan S, Vadde R, Agarwal R, Vanamala JK. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J Nutr Biochem. 2015 Aug 14; doi: 10.1016/j.jnutbio.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Lin-Wang K, Deng C, Warran B, Wang L, Yu B, Yang H, Wang J, Espley RV, Zhang J, Wang D, Allan AC. Comparative Transcriptome Analysis of White and Purple Potato to Identify Genes Involved in Anthocyanin Biosynthesis. PLoS One. 2015;10(6):e0129148. doi: 10.1371/journal.pone.0129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M. How the sourdough may affect the functional features of leavened baked goods. Food microbiology. 2014 Feb;37:30–40. doi: 10.1016/j.fm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 81.Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. European journal of nutrition. 2013 Feb;52(1):1–24. doi: 10.1007/s00394-012-0418-1. [DOI] [PubMed] [Google Scholar]
- 82.Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Critical care medicine. 2009 Dec;37(12):3114–3119. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- 83.Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Annals of internal medicine. 2011 Jul 5;155(1):39–51. doi: 10.7326/0003-4819-155-1-201107050-00006. [DOI] [PubMed] [Google Scholar]
- 84.Potter NN, Hotchkiss JH. Food Science. 5. Springer US; 1995. [Google Scholar]
- 85.Peckos PS. Nutrition during growth and development. Child development. 1957 Sep;28(3):273–285. doi: 10.1111/j.1467-8624.1957.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 86.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G, Team LS. Randomized trial of peanut consumption in infants at risk for peanut allergy. The New England journal of medicine. 2015 Feb 26;372(9):803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Metz JA, Kris-Etherton PM, Morris CD, Mustad VA, Stern JS, Oparil S, Chait A, Haynes RB, Resnick LM, Clark S, Hatton DC, McMahon M, Holcomb S, Snyder GW, Pi-Sunyer FX, McCarron DA. Dietary compliance and cardiovascular risk reduction with a prepared meal plan compared with a self-selected diet. Am J Clin Nutr. 1997 Aug;66(2):373–385. doi: 10.1093/ajcn/66.2.373. [DOI] [PubMed] [Google Scholar]
- 88.Wall CL, Day AS, Gearry RB. Use of exclusive enteral nutrition in adults with Crohn’s disease: a review. World J Gastroenterol. 2013 Nov 21;19(43):7652–7660. doi: 10.3748/wjg.v19.i43.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Day AS, Lopez RN. Exclusive enteral nutrition in children with Crohn’s disease. World J Gastroenterol. 2015 Jun 14;21(22):6809–6816. doi: 10.3748/wjg.v21.i22.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004 Aug-Sep;364(9436):803–811. doi: 10.1016/S0140-6736(04)16942-0. [DOI] [PubMed] [Google Scholar]
- 91.Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, Takahashi H, Kinouchi Y, Hiwatashi N, Funayama Y, Sasaki I, Tsuji I, Shimosegawa T. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment Pharmacol Ther. 2006 Nov 1;24(9):1333–1340. doi: 10.1111/j.1365-2036.2006.03120.x. [DOI] [PubMed] [Google Scholar]
- 92.Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. Journal of pediatric gastroenterology and nutrition. 2014 Jan;58(1):87–91. doi: 10.1097/MPG.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 93.Obih C, Wahbeh G, Lee D, Braly K, Giefer M, Shaffer ML, Nielson H, Suskind DL. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2016 Apr;32(4):418–425. doi: 10.1016/j.nut.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 94.Prince AC, Myers CE, Joyce T, Irving P, Lomer M, Whelan K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016 May;22(5):1129–1136. doi: 10.1097/MIB.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 95.Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis. 2009 Feb;3(1):8–14. doi: 10.1016/j.crohns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Ananthakrishnan AN, Khalili H, Song M, Higuchi LM, Richter JM, Nimptsch K, Wu K, Chan AT. High School Diet and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2015 Oct;21(10):2311–2319. doi: 10.1097/MIB.0000000000000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herfarth HH, Martin CF, Sandler RS, Kappelman MD, Long MD. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2014 Jul;20(7):1194–1197. doi: 10.1097/MIB.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, Willett WC, Richter JM, Chan AT. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2014 May;63(5):776–784. doi: 10.1136/gutjnl-2013-305304. [DOI] [PMC free article] [PubMed] [Google Scholar]