Abstract
Rationale
Sympathetic nervous system control of inflammation plays a central role in hypertension. The gut receives significant sympathetic innervation, is densely populated with a diverse microbial ecosystem, and contains immune cells that greatly impact overall inflammatory homeostasis. Despite this uniqueness, little is known about the involvement of the gut in hypertension.
Objective
Test the hypothesis that increased sympathetic drive to the gut is associated with increased gut wall permeability, increased inflammatory status, and microbial dysbiosis and that these gut pathological changes are linked to hypertension.
Methods and Results
Gut epithelial integrity and wall pathology were examined in spontaneously hypertensive rat (SHR) and chronic Angiotensin II infusion rat models. The increase in blood pressure in SHR was associated with gut pathology that included increased intestinal permeability and decreased tight junction proteins. These changes in gut pathology in hypertension were associated with alterations in microbial communities relevant in blood pressure control. We also observed enhanced gut-neuronal communication in hypertension originating from paraventricular nucleus of the hypothalamus and presenting as increased sympathetic drive to the gut. Finally, angiotensin converting enzyme inhibition (captopril) normalized blood pressure and was associated with reversal of gut pathology.
Conclusions
A dysfunctional sympathetic-gut communication is associated with gut pathology, dysbiosis, and inflammation, and plays a key role in hypertension. Thus, targeting of gut microbiota by innovative probiotics, antibiotics, and fecal transplant, in combination with current pharmacotherapy, may be a novel strategy for hypertension treatment.
Keywords: Hypertension, microbiota, gut, inflammation, autonomic nervous system
Subject Terms: Hypertension, High Blood Pressure, Autonomic Nervous System, Basic Science Research, Inflammation
INTRODUCTION
The Centers for Disease Control estimates that hypertension contributes to about 1,000 American deaths each day1, 2. Hypertension is the most modifiable risk factor for cardiovascular disease (CVD) and stroke and also plays an important role in obesity, diabetes mellitus, and metabolic syndrome3. Despite advances in blood pressure (BP) control, approximately 15% of hypertensive patients remain resistant to available lifestyle modifications and pharmacotherapy4–8. Neurogenic components have been implicated as a cause of resistant hypertension, as evidenced by measurements of sympathetic outflow and norepinephrine overflow8, 9. Specifically, altered autonomic nervous system (ANS) activity and associated increases in peripheral and neuroinflammation have been implicated in the pathogenesis of resistant hypertension8, 10, 11. Our recent evidence, demonstrating increased peripheral and neuroinflammation in hypertension as a result of autonomic influences on bone marrow (BM) activity, further supports this concept12. However, the specific mechanism of autonomic dysregulation in hypertension pathophysiology remains elusive.
It has become evident in recent years that a symbiotic relationship exists between the gut and its microbiota for regulation of both local gut and systemic immunity13, 14. Imbalances in this host-gut microbiota eubiosis are potentially of major pathophysiological consequence to the host. For example, imbalances in this symbiotic relationship have been associated with diverse diseases, such as obesity, nervous system disorders, chronic kidney disease, inflammatory bowel disease (IBD), and CVD14, 15. However, the involvement of the host-gut relationship in hypertension has yet to be investigated in detail despite the fact that: (i) The gut is the most highly innervated peripheral organ, with significant numbers of the motor fibers to the gut identified as sympathetic nerves16. It is pertinent to note that elevated sympathetic nervous system activity is a hallmark of both animal and human hypertension8, 9; (ii) ANS influences gut permeability17, inflammatory state18, and microbial communities in IBD and other diseases19; (iii) gut microbial products, such as short chain fatty acids (SCFAs), lipopolysaccharides (LPS), and neurotransmitters influence both the immune and vascular systems20, 21; and (iv) studies from our group and others have demonstrated a link between gut dysbiosis and hypertension22, 23. This evidence, taken together, led us to the following hypothesis: increased sympathetic activity to the gut alters gut pathology in such a way that is conducive to increased gut permeability, altered inflammatory status, and microbial dysbiosis impacting BM production of proinflammatory cells. Thus, we propose that hypertension is, at least in part, a result of pathophysiological changes in the gut. The present study was designed to provide experimental evidence to support or refute this hypothesis.
METHODS
All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Full details of all experimental protocols are presented in the Methods section in the Online Supplemental Material.
RESULTS
Alterations in gut pathology in rat models of hypertension
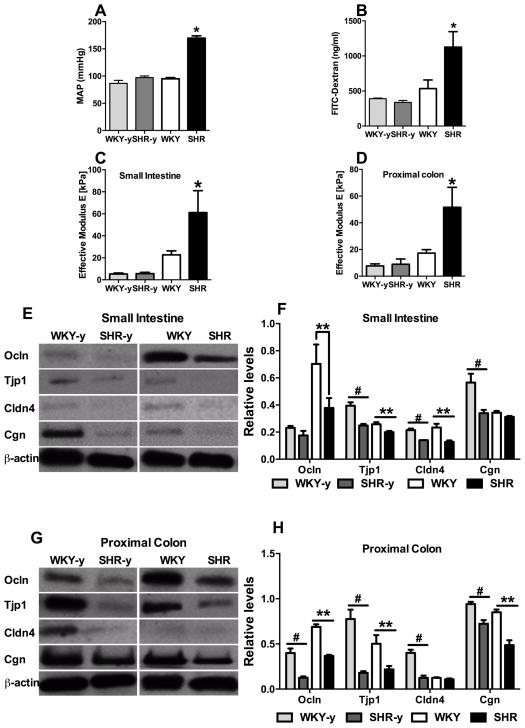
First, we investigated if the integrity of the gut epithelial barrier is altered in the spontaneously hypertensive rat (SHR) model using various techniques: (i) oral fluorescein isothiocyanate (FITC)-dextran for epithelial permeability, (ii) an innovative Multi-Scale Indention (MSI) System to measure gut wall stiffness24, 25, and (iii) Western blotting for tight junction proteins. Both young pre-hypertensive and adult SHR with established hypertension along with age-matched, normotensive Wistar Kyoto rats (WKY) were used to investigate gut associations with BP (Fig. 1A). We observed a 2.1-fold increase in FITC-dextran accumulation in adult SHR plasma (Figure 1B, p<0.05), whereas no difference was observed between young WKY and pre-hypertensive SHR. These data provide evidence to support the proposal that adult SHR with established hypertension have a leaky intestinal barrier. This enhanced permeability has been associated with an increase in tissue stiffness (effective modulus) 26. Accordingly, using the MSI, we observed a 2.7-fold and 3.0-fold (p<0.05) increase in stiffness in the small intestine and proximal colon, respectively, in the adult SHR as compared with age-matched WKY (Figure 1C–D). However, no change was observed between young WKY and pre-hypertensive SHR. Next, we compared tight junction proteins by Western blotting. Levels of occludin (Ocln), tight junction protein 1 (Tjp1), and claudin 4 (Cldn4) were decreased by 46.3% (p<0.05), 22.5% (p<0.05), and 45.4% (p<0.05), respectively in the small intestine of adult SHR compared to adult WKY (Figure 1E, F). Comparable decreases were observed in Ocln, Tjp1, and Cldn4 of the proximal colon in adult SHR (Figure 1G, H). Interestingly, the proximal colon from pre-hypertensive SHR also demonstrated significant decreases in all tight junction proteins measured (Ocln 68.6%, p<0.05; Tjp1 76.9%, p<0.05; Cldn4 69.0%, p<0.05; Cgn 23.1%, p<0.05) compared with age matched WKY controls (Figure 1G, H). Similarly, the small intestine of pre-hypertensive SHR showed a 24% decrease in Ocln (p<0.05), 36.9% in Tjp1 (p<0.05), 34.7% in Cldn4 (p<0.05), and 39.9% in Cgn (p<0.05) (Figure 1E, F). These observations support the suggestion that decreases in gut tight junction proteins may precede increases in gut permeability, stiffness and high BP.
Figure 1. Permeability and stiffness of small intestine and colon are increased in adult spontaneously hypertensive rat (SHR).
A, Mean arterial blood pressure (MAP) of young Wistar Kyoto rats WKY (WKY-y), young SHR (SHR-y), and adult WKY and SHR. B, Intestinal mucosa-to-blood permeability assessed by fluorescein isothiocyanate (FITC)-dextran 4kDa concentration in the plasma revealed increased FITC-dextran concentration in adult, but not young, SHR (n=4–7/group). C–D, Effective modulus measured by a Multi-Scale Indentation System is increased in both small intestine and proximal colon of WKY-y, SHR-y, and adult WKY, SHR (n=4–5/group). E–J, Western blotting for tight junction proteins in small intestine (E, G, I) and proximal colon (F, H, J) in WKY-y, SHR-y, and adult WKY, SHR. *p<0.05, SHR vs all other groups; # p<0.05, WKY-y vs SHR-y; **p<0.05, SHR vs WKY.
To investigate whether gut permeability is compromised in another model of hypertension, we evaluated the angiotensin II (Ang II)-infusion rat model of hypertension (Online Figure IA). An increase in plasma FITC-dextran (Online Figure IB) was observed in Ang II-infused rats that was comparable to adult SHR, suggesting enhanced gut permeability. However, mean effective modulus was not significantly changed in this model (Online Figure IC–D). Protein levels of tight junctional proteins were also reduced in both small intestine and proximal colon of Ang II-infused rats (Online Figure 1E–H). For example, Tjp1 by 48% (p<0.01) and 46% (p<0.05), and Cgn by 43% (p<0.01) and 49% (p<0.001) in small intestine and proximal colon, respectively. Ocln was decreased by 55% (p<0.01) only in the proximal colon. Although the differences between the SHR and Ang II-infusion models are evident, these observations suggest that both rat hypertension models exhibit an increase in gut permeability and decrease in tight junction proteins.
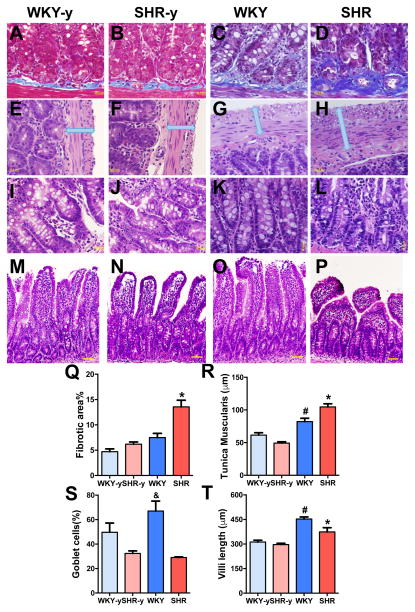
Next, we investigated whether the observed increased permeability is associated with alterations in gut pathology in the SHR. Figures 2A–P depict changes in the SHR small intestine compared with normotensive WKY rats, in both young and adult rats. We observed an 1.8-fold increase in fibrotic area in the adult SHR (Figure 2A–D, Q, p<0.05). An increase in the muscularis layer of the SHR small intestine was also observed (Figure 2E–H, R, p<0.05). We also observed a 57% decrease in numbers of goblet cells in adult SHR (Figure 2I–L, S). Figures 2M–P, T show that villi in the small intestine of adult SHR were much shorter and appeared stunted, compared with WKY. None of these pathological alterations were observed in young pre-hypertensive SHR as compared to age-matched WKY. The chronic Ang II-infusion rat model of hypertension was used to confirm these intestinal pathological changes. Similar to adult SHR, we observed increases in fibrotic area (Online Figure IIA–C), an increase in muscularis layer (Online Figure IID–F), no difference in villi length (Online Figure IIG–I), and a modest decrease in goblet cells (Online Figure IIJ–L).
Figure 2. Pathological changes were observed in the small intestine of adult spontaneously hypertensive rat (SHR).
A–D, Small intestine was stained with Masson’s trichrome to quantify the fibrosis in young and adult WKY and SHR (Q, n=4/group). E–H, Cross sections of the small intestine of WKY and SHR were also stained with hematoxylin-eosin to measure the thickness of tunica muscularis layer (R). I–L, The number of goblet cells per 100 epithelial cells was decreased in SHR (S). M–P, Villi lengths were shorter in SHR (T). *p< 0.05 SHR vs all other groups; #p< 0.05 WKY vs all other groups; &p<0.05 WKY vs SHR-y and SHR.
Effects of ACE inhibition (captopril) on gut pathology in the SHR
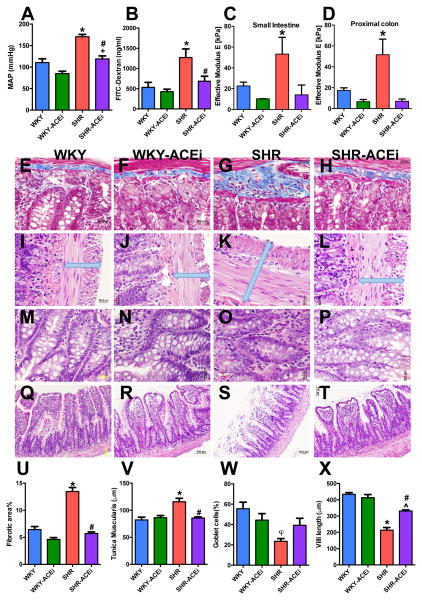
We investigated the effects of captopril, an effective antihypertensive medication in SHR27, to further explore the relationships between gut pathology and high BP. Adult WKY and SHR were treated with captopril for 4 weeks, which significantly reduced mean arterial pressure (MAP) in SHR (Figure 3A, 170 ± 5 mmHg water vs 119 ± 7 mmHg captopril, p<0.01), with only a slight reduction in MAP in WKY (110 ± 9 mmHg water vs 85 ± 5 mmHg captopril, p=0.071). ACE inhibition-related BP reduction was associated with a decrease in gut permeability in the SHR (Figure 3B, 1273 ± 212 ng/ml vs 685 ± 124 ng/ml, p<0.05), while no change was observed in WKY. Although effective modulus appears to be reduced in both the small intestine and proximal colon of captopril- treated SHR (Figure 3C–D), the values did not reach significance. We found a complete reversal in both fibrotic area (Figure 3E–H, U; p<0.001) and thickness of the muscularis layer (Figure 3I–L, V; p<0.01) with ACE-inhibition related decreased BP in SHR. Although this did not prevent the reduction in goblet cell numbers (Figures 3M–P, W), the villi length increased by 55% with ACE-inhibition reduced BP in treated SHRs (Figures 3Q–T, X; p<0.01). These data suggest that ACE inhibition-related decreased BP has beneficial effects on hypertension-linked gut pathology.
Figure 3. Antihypertensive treatment with ACEi has beneficial effects on gut pathology.
A, MAP of vehicle and captopril (ACEi) treated WKY and SHR (n=6–8/group). B, Intestinal mucosa-to-blood permeability assessed by FITC-dextran 4kDa concentration in the plasma revealed ACEi reverses increased permeability in the SHR. C–D, Mean effective modulus measured by a Multi-Scale Indentation System in both small intestine and proximal colon of vehicle and captopril treated WKY and SHR. E–H, Fibrotic area is decreased by ACEi in SHR (U). I–L, Cross sections of the small intestine of WKY and SHR shows protected tunica muscularis layer in ACEi treated SHR (V). M–P, The number of goblet cells per 100 epithelial cells was not improved by ACEi in SHR (W). Q–T, Villi lengths were improved by ACEi in SHR (X). *p<0.05 SHR vs all other groups; #p<0.05 SHR-ACEi vs SHR; +p<0.05 WKY-ACEi vs SHR-ACEi; φp<0.05 SHR vs WKY; ^p<0.05 SHR-ACEi vs WKY and WKY-ACEi.
Increase in gut inflammation in hypertension
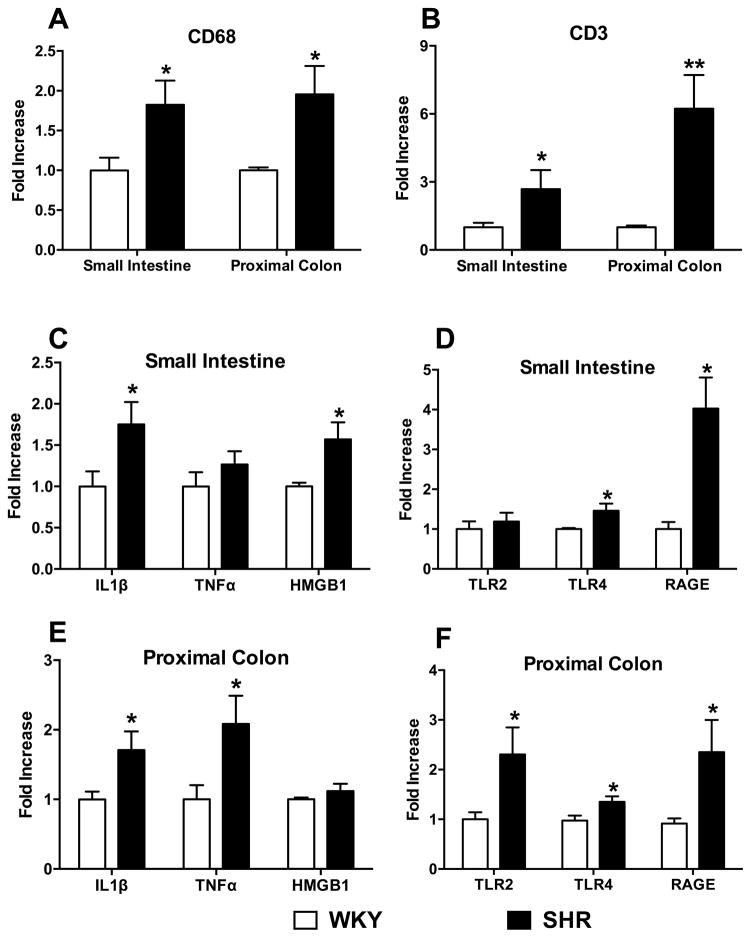
RT-PCR revealed that CD68 (Figure 4A) and CD3e (Figure 4B) gene expression, representative of macrophages and T-cells respectively, was elevated in both the small intestine (1.8 and 2.7-fold, respectively) and proximal colon (1.9 and 6.2-fold, respectively) in the SHR (p<0.05). An increase in inflammatory status was further confirmed by levels of cytokine mRNAs (Figure 4C–D). The mRNA levels of Interleukin 1β (IL-1β) and high mobility group box 1 (HMGB1) were 75% and 57% higher, respectively, in the SHR small intestine (p<0.05, Figure 4C). In the proximal colon, we observed a 70% and 108% increase in IL-1β and tumor necrosis factor α (TNFα) mRNA levels in the SHR (Figure 4D). No differences in HMGB1 levels were observed in the colon between the two strains. Next, we investigated the mRNA levels of three proposed receptors for HMGB128, 29: toll-like receptor 2 (TLR2), TLR4, and receptor for advanced glycation end-products (RAGE), in view of accumulating evidence that gut HMGB1 plays a critical role in systemic as well as gut inflammation30 (Figure 4E–F). Furthermore, these receptors are implicated in inflammation induced by bacterial products31. We observed increases in mRNAs for both TLR4 (46%) and RAGE (303%) in the small intestine (Figure 4E) and colon (35% and 135%, respectively, Figure 4F), while TLR2 was increased 130% only in the SHR colon (Figure 4F, p<0.05).
Figure 4. Inflammatory cells, cytokines, and their receptors are increased in the SHR small intestine and colon.
A–B, Small intestine and proximal colon of SHR show increased CD68 and CD3 mRNAs. C–D, Further mRNA revealed increased IL-1β, HMGB1, TLR4, and RAGE in the small intestine; and E–F, increased IL-1β, TNF-α, TLR2, TLR4, and RAGE in the proximal colon. *p<0.05, **p<0.01 vs WKY (n=5–7/group).
Our prior studies have demonstrated that hypertension impacts BM activity by influencing proliferation, mobilization, and differentiation of hematopoietic stem cells32. This cellular response contributes to increased peripheral and neuroinflammation in hypertension33. Thus, we sought to determine if altered BM cell activity contributes to increases in proinflammatory cells in the gut in hypertension using the chronic Ang II-infusion rat model. Enhanced green fluorescent protein Sprague Dawley (eGFP-SD) BM chimeric rats were generated and subjected to a 4-week Ang II-infusion (200ng/kg/min)33. This infusion resulted in a significant increase in MAP (saline 95 ± 2 mmHg vs. Ang II-infusion 150 ± 3 mmHg; p<0.001). The hypertensive response to Ang II-infusion in eGFP-chimeric rats was comparable to naïve SD rats33. We observed significant numbers of green fluorescent cells in the small intestine and colon of both the saline and Ang II-treated rats (Online Figure III). However, levels of these BM-derived eGFP+ cells were 2.2- and 1.3-fold higher in small intestine and colon, respectively, of Ang II-treated rats compared to saline. Double staining with inflammatory markers documented an increase in CD3+ T-cells, CD68+ macrophages, and Iba1+ macrophages in Ang II-treated rats (Online Figure IV). Quantification revealed increases of 103% in CD3+ T-cells, of 278% in CD68+ macrophages, and of 101% in Iba1+ macrophages in the small intestine of Ang II-treated rats. Similar increases were observed in the colon of the Ang II-treated animals. In contrast, no significant changes in number of cells double-stained for neurons (NeuN antibody) and smooth muscle cells (alpha smooth muscle actin antibody) were observed comparing the two groups (data not shown). Taken together, these data indicate that the increase in inflammatory cells in hypertension is, in part, a result of mobilization of BM cells to gut.
Gut perfusion is decreased in hypertension
We compared the blood perfusion of the gut in WKY and SHR using Laser Spackle Contrast Imaging33 and observed a significant decrease in mesenteric blood flow normalized to BP, in a section of small intestine in anesthetized adult WKY and SHR (Figure 5). This interesting observation is consistent with reports indicating that decreased gut blood flow is associated with proinflammatory gut pathophysiology34.
Figure 5. Blood perfusion to the gut is decreased in the SHR.
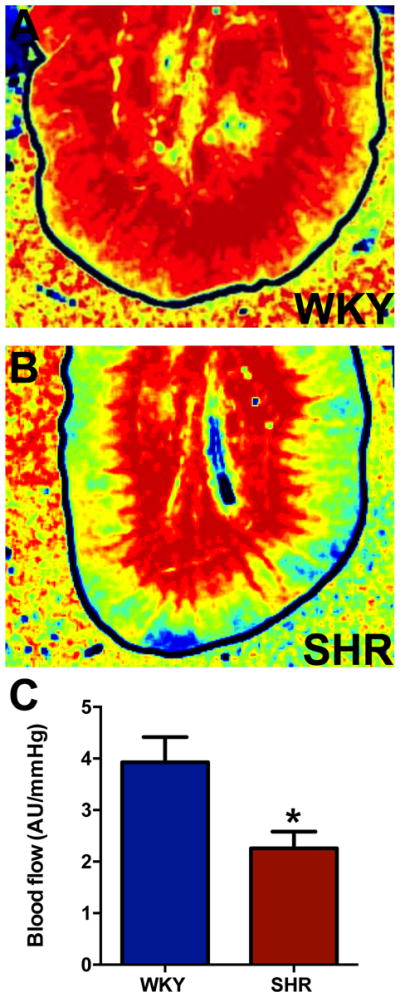
A, Representative blood flow intensity maps during the last minute of the three-minute sample period. Blood flow levels are indicated by the standard “rainbow color map”, i.e. high flow is red and low flow is blue. B, Average blood perfusion normalized to blood pressure (i.e. AU (Arbitrary perfusion units) per mmHg of systolic pressure) over the three-minute sample period is decreased between WKY and SHR. *p<0.05 vs WKY (n=3/group).
Altered microbial community in hypertensive rat gut
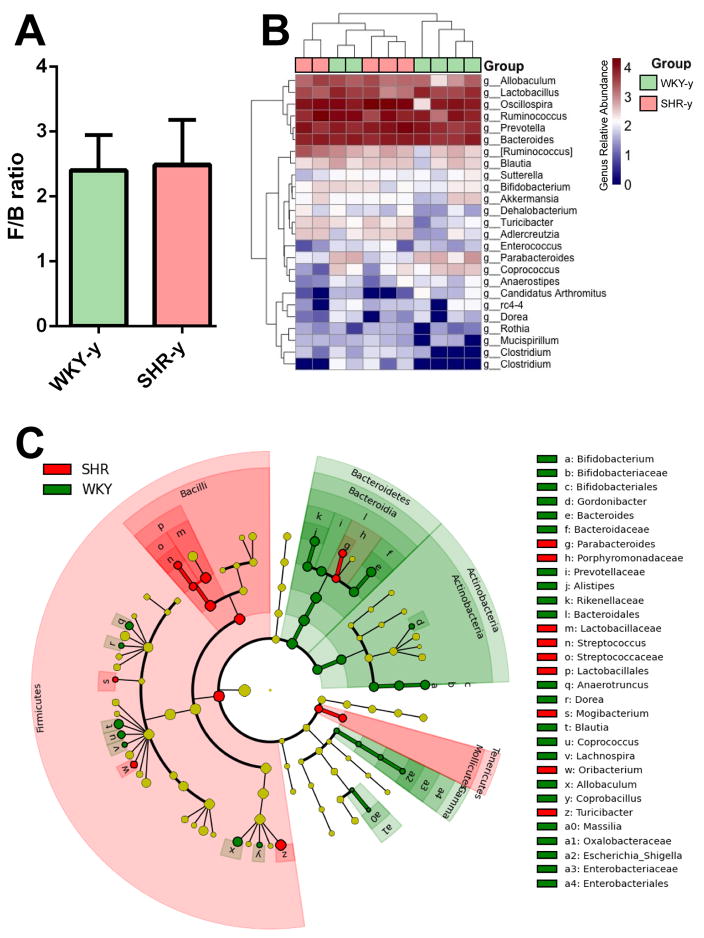
The above data indicate that hypertension is associated with increases in gut permeability and inflammatory status. These findings led us to investigate whether there are also differences in gut bacterial taxa between the normotensive WKY and SHR. Our previous data indicated a significant increase in the ratio of the Firmicutes to Bacteroidetes (F/B ratio, an indicator of gut microbial dysbiosis) phyla in the gut of SHR vs. WKY22. Here, we examined whether this shift in F/B ratio is associated with the SHR strain or the development of hypertension in the SHR strain. We found that the F/B ratio in pre-hypertensive SHR is not different from age-matched WKY (Figure 6A). This finding supports the suggestion that the BP increase in the SHR is closely associated with the development of gut dysbiosis. Of the total number of genera represented in the gut microbiota, members of 25 most abundantly represented genera were compared in the pre-hypertensive SHR and the age matched WKY using a heat map (Figure 6B), The heat map did not show any clustering between SHR and WKY indicating that the abundance levels of selected genera in pre-hypertensive SHR were not significantly different from the age matched WKY.
Figure 6. Gut microbial community is altered in hypertensive SHR.
A, Comparison of microbiota composition between young WKY and SHR. The Firmicutes/Bacteroidetes ratio (F/B ratio) was calculated as a biomarker of gut dysbiosis. B, Heat map indicating the genus-level changes in the young WKY and SHR groups. Each green or red box represent one animal (WKY-y green, SHR-y light red). The relative abundance of the bacteria in each genus is indicated by a gradient of color from blue (low abundance) to red (high abundance). C, Red circles indicate taxa enriched in the SHR, green circles indicate taxa enriched in WKY rats, while yellow circles indicate no difference between the groups. Each circle represents one bacterial taxa with circle size being proportional to abundance/effect size. Gamma on the bottom right in light green is an abbreviation for Gammaproteobacteria. (n=5–6/ group).
Fecal bacterial analysis was done to determine specific bacterial genera that may be associated with the observed changes in permeability and inflammation in the adult SHR. We observed a significant increase in gram-positive Streptococcus genus and Streptococcaceae family in the gut of the SHR, both belonging to the order of Lactobacillales (lactic acid-producing bacteria) and the phylum of Firmicutes (Figure 6C, in red). This was consistent with changes we had previously observed in the Ang II-infusion hypertensive rodent model22. Furthermore, we observed a significant decrease in the Bifidobacterium genus and the related Bifidobacteriaceae family of Actinobacteria phyla (Figure 6C, in green), a gram-positive bacterium with probiotic properties35, 36. We also observed a decrease in several bacterial genera of the Bacteroidetes phyla. These changes begin to pinpoint specific bacterial genera with implications in inflammation and hypertension.
Dysfunctional autonomic-gut communication in hypertension
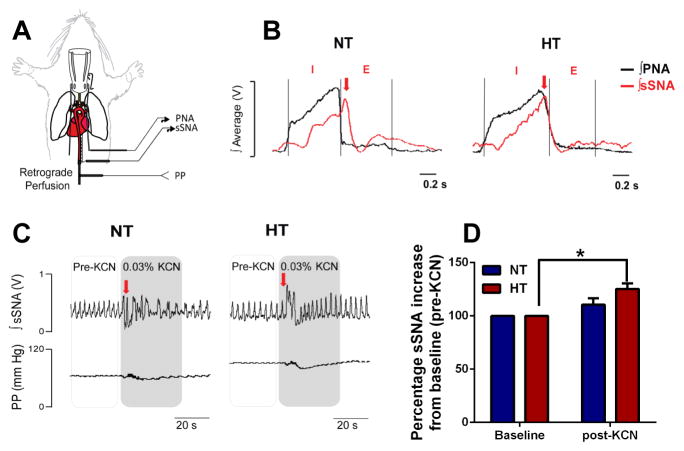
Activity of the splanchnic sympathetic nerve innervating gut in young 4 week-old normotensive (NT) rats and SHR was determined using a decerebrated artificially-perfused rat preparation (Figure 7A) as established previously37. This in situ preparation revealed classic phrenic nerve inspiratory (I) and expiratory (E) activity patterns (black trace), and robust splanchnic sympathetic nerve activity (sSNA; red trace) (Figure 7B) in both groups. We observed a shift in the peak of sSNA (red arrow) from the E phase in NT rats to the I phase in HT rats, typical of the respiratory-sympathetic uncoupling pattern in other neuronal beds of hypertensive rodent models12, 38–40. Furthermore, a bolus i.a. challenge with 0.1 ml of 0.03% KCN showed a significant increase in the immediate increase in sSNA bursting pattern in the HT rats only (by ~25% vs. pre-KCN baseline, Figure 7C–D), consistent with augmented chemoreflex sensitivity and typical of elevated sympathetic responses in hypertensive rodent models39. This response was consistent over several preparations.
Figure 7. Elevated splanchnic sympathetic nerve activity in the SHR.
A, In situ decerebrated artificially-perfused rat preparation (schematics on the left) reveals respiratory uncoupling of the B, sSNA (red trace) in the hypertensive (HT) SHR compared to normotensive (NT) control rats. Phrenic nerve activity (PNA, black trace) inspiratory (I) and expiratory (E) phases were used to determine respiratory uncoupling. Note the typical shift in the peak of sSNA burst from the E phase in the NT (left panel, red arrow) to the I phase of PNA in the HT (right panel, red arrow). C–B, Intra-arterial bolus KCN injection produced a ~25% higher sSNA burst from baseline (pre-KCN) in the HT compared to NT rats (n=5/group). *p<0.05 baseline HT vs post-KCN HT.
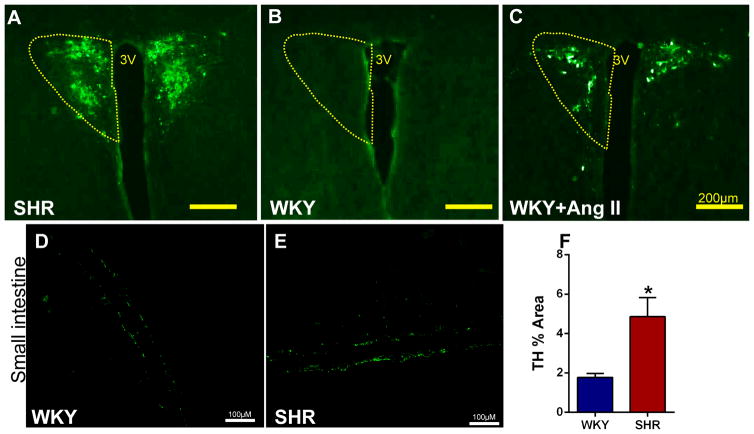
To confirm that the elevated sympathetic drive continues into established HTN, we performed pseudorabies virus expressing green fluorescent protein (PRV-GFP) retrograde labeling from the gut in adult SHR and Ang II-infused rats. Application of PRV-GFP to proximal colon and small intestine showed significant GFP fluorescent labeling in neurons of the paraventricular nucleus (PVN) of the hypothalamus in SHR and WKY rats infused with Ang II s.c. within 4 days (Figure 8A–C), but not in WKY controls within the same time period and following a similar experimental protocol. These responses were consistent across several animals. Finally, we observed an increase in density of tyrosine hydroxylase (TH) immunoreactivity (1.8 ± 0.2 WKY, 4.9 ± 0.9 SHR, p<0.05), and a doubling in the mean intensity of TH fluorescence in the small intestine of SHR (Figure 8D–F; p<0.05). These observations confirmed augmented PVN-gut connectivity in both adult SHR and Ang II-infusion hypertension models.
Figure 8. Higher PRV-GFP retrograde labeling from the small intestine to the PVN is associated with increased TH immunoreactivity in the small intestine of the SHR compared to WKY.
A–B, GFP staining reveals robust PRV retrograde labeling from small intestine to the PVN in the SHR compared to WKY. C, Retrograde labeling in WKY is enhanced following chronic Ang II-infusion. D–E, Representative images of tyrosine hydroxylase (TH) immunoreactivity in WKY and SHR small intestinal tissue. F, Quantification of TH staining revealed increased immunoreactivity in the small intestine of SHR (n=3/group). *p<0.05 SHR vs WKY.
DISCUSSION
Our study demonstrates important changes in the gut in two rodent models of hypertension, suggesting a gut link with hypertension. The major findings are: (i) increased permeability of gut epithelial barrier and inflammatory state is associated with BP elevation; (ii) enhanced sympathetic neuronal communication between the PVN and gut; and (iii) a shift in gut microbial genera is likely associated with the pathophysiological and immune status of the gut and high blood pressure. Together, these changes could be important in the initiation and establishment of hypertension.
Additional significance of our study is the association of gut pathology and microbial dysbiosis with hypertension, and data from pre-hypertensive SHR are relevant in support of this concept. They show that changes in sympathetic nerve activity and gut tight junction proteins precede changes in gut pathology, dysbiosis and hypertension. This view is further supported by ACE-inhibition induced BP lowering in the SHR, which reversed gut pathology. It is pertinent to note that sympathetic tone in SHR is also attenuated by ACE-inhibition41, 42. Therefore, one cannot deduce if changes in the gut pathology are blood pressure or sympathetic activity driven or dependent on both. Further experiments would be needed to resolve this. Our data suggest that pro-hypertensive signals are perceived in the PVN and transmitted via central neural pathways to increase sympathetic activity and norepinephrine (NE) release to the gut. This could lead to alterations of gut junction proteins resulting in permeability changes, dysbiosis, and pathology. Chronic elevation of TH in the gut of SHR, a key enzyme in NE generation, is consistent with this contention. In addition, gut dysbiosis and lactate producing bacterial populations are increased in hypertensive rats22, 43. These bacteria also generate a variety of neurotransmitters themselves (eg. serotonin44, dopamine45, and histamine46) that may potentially be involved in BP regulation. Furthermore, carbohydrates fermented by Streptococcus predominately yield lactic acid as a major end-product47. Lactic acid can potentially contribute to modulation of the immune system in both gut and circulation48, 49. Support for this view comes from our experimental findings that: (i) the lactic acid-producing bacterial population is increased in the SHR and Ang II-infusion rat hypertension model22, (ii) increased inflammation in the small intestine and colon of hypertensive animals was associated with both the increase in F/B ratio as well as hypertension development, and (iii) increased peripheral inflammation is associated with hypertension33.
The increase in gut inflammatory cells in hypertension observed in this study could be a result of activation of resident immune cells or their infiltration from the BM. Although the resident immune cells undergo regular turnover, our data indicate that increased recruitment of BM inflammatory cells into the gut plays an important role in hypertension-associated gut inflammation in addition to the resident immune cells. The mechanism of this increase remains to be elucidated, but it could be a result of stimulation and recruitment of inflammatory cells by a shift in bacterial release of SCFAs50, 51, and/or direct ANS-mediated regulation of BM inflammatory cell activity, as shown previously12. Interestingly, gut microbiota reportedly promote Ang II-related hypertension, as germ free mice are protected from Ang II-induced vascular dysfunction and hypertension52, an effect suggested to be mediated by a dampened immune response.
The brain can also influence intestinal homeostasis by gut motility and mucus secretion. Involvement of mucus in mucosal immune responses is well-recognized13. Our data demonstrate pathological changes in the gut of hypertensive animals. This includes increased permeability, flexibility and leakiness, fibrosis, and muscular tissues in the gut wall. In addition, stunted villi and decreased goblet cells were observed in both rat hypertension models. Goblet cells produce mucins, which protect the gut from pathogen invasion, thereby regulating gut immune responses13. A decrease in goblet cells in hypertensive animals could be associated with decreased mucin, contraction of mucin-degrading bacterial population, and potential expansion of other more harmful bacterial populations. Consequently, some commensal bacterial taxonomic branches are decreased in SHR and enriched in WKY rats. These include, Bacteriodetes-Alistipes, Bacteriodetes-Bacteroides, and Actinobacteria-Bifidobacterium branches that potentially impact gut immune status.
Recently, we advanced a brain-gut-BM axis hypothesis and proposed dysfunctional brain-gut interactions in hypertension7. Our current study, demonstrating an increased sympathetic drive associated with gut pathology and microbial dysbiosis, provides new evidence in support of that proposal. However, our findings generate questions for future investigation. For example: (i) does sympathetic activation precede or accompany changes in the gut? We favor the former scenario based on evidence that increases in sympathetic drive precede increases in BP in both animal models and human hypertension10, and our data presented here showing elevated sympathetic drive is present prior to pathological changes in the gut. (ii) Does microbial dysbiosis promote hypertension? Recent fecal transplantation studies suggest that this is highly likely52, 53. (iii) It is pertinent to recognize the well-established role of the afferent parasympathetic arm in the paradigm of gut-brain axis54, 55. Evidence indicates that microbiota communication with the brain involves afferent vagal signaling pathways56, and its activation modulates both neurochemical and behavioral effects. A recent report has described the role of the vagal anti-inflammatory pathway in modulation of intestinal muscularis layers and resident macrophages57. Considering that vagal activity is dampened in rodent models of hypertension10, it is possible that the shift in the balance of sympathetic and parasympathetic influences in the gut, rather than exaggerated sympathetic drive alone, contribute to hypertension-associated gut pathophysiology, dysbiosis, and immune system activation. Finally, therapeutic implications of this study for human hypertension must be investigated and clinical study (NCT 02188381) is underway.
Supplementary Material
Novelty and Significance.
What Is Known?
Increased sympathetic activity, inflammation and gut dysbiosis in hypertension.
Gut houses largest number of immune cells and trillions of microorganisms.
The central nervous system-immune system communication is altered in hypertension.
What New Information Does This Article Contribute?
Hypertension is associated with increased gut wall permeability, altered tight junction proteins and fibrosis, decrease in goblet cells and shorter villi, and microbial dysbiosis.
ACE inhibitor, captopril, which lowers blood pressure in spontaneously hypertensive rat, reverses gut pathology.
Sympathetic nerve activity and tight junction proteins, but not gut wall pathology and microbial composition, are altered in pre-hypertensive animals.
Abundant evidence reveals that both sympathetic nervous system and inflammation are key players in the development and establishment of hypertension. The gastrointestinal tract, which houses the largest number of immune cells in the body and contains trillions of microorganisms, is emerging as an important organ in the maintenance of normal body homeostasis. Despite this, the involvement of gut in blood pressure control and hypertension is not known. This coupled with recent evidence of gut microbial dysbiosis in hypertension led us to test the following hypothesis in this study: sympathetic nervous system impacts gut physiology to maintain blood pressure homeostasis; its increased activity is associated with increased gut wall permeability, pathology, and dysbiosis in hypertension. We demonstrate that gut wall was significantly thicker, more permeable, fibrotic, and had less numbers of goblet cells and stunted villi in hypertensive animals. These changes in the gut were associated with increase in the sympathetic nerve activity to the gut. ACE inhibitor treatment lowered blood pressure and attenuated gut pathology. Further investigation into the mechanism of this proposed dysfunctional sympathetic-gut communication is necessary to evaluate the potential of gut and microbiota as novel targets with the use of pro-and/or prebiotics as combination therapy for drug resistant hypertension.
Acknowledgments
SOURCES OF FUNDING
This study was supported by National Institutes of Health grant HL33610 (M.K.R.) and American Heart Association predoctoral fellowship 18590018 (M.M.S.).
Nonstandard Abbreviations and Acronyms
- Ang II
Angiotensin II
- ACE
Angiotensin converting enzyme
- ANS
Autonomic nervous system
- BP
Blood pressure
- BM
Bone marrow
- CVD
Cardiovascular disease
- IBD
Inflammatory bowel disease
- MAP
Mean arterial pressure
- MSI
Multi-Scale Indentation System
- NE
Norepinephrine
- PVN
Paraventricular nucleus of hypothalamus
- PRV
Pseudorabies virus
- SCFAs
Short chain fatty acids
- sSNA
Splanchnic sympathetic nerve activity
- SHR
Spontaneously hypertensive rat
- SD
Sprague Dawley
- TH
Tyrosine hydroxylase
- WKY
Wistar Kyoto
Footnotes
DISCLOSURE
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.CDC. High blood pressure facts. 2016 cdc.Gov.
- 3.Yoon SS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United states, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 4.Modolo R, de Faria AP, Sabbatini AR, Barbaro NR, Ritter AM, Moreno H. Refractory and resistant hypertension: Characteristics and differences observed in a specialized clinic. J Am Soc Hypertens. 2015;9:397–402. doi: 10.1016/j.jash.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Judd E, Calhoun DA. Apparent and true resistant hypertension: Definition, prevalence and outcomes. J Hum Hypertens. 2014;28:463–468. doi: 10.1038/jhh.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui M, Dudenbostel T, Calhoun DA. Resistant and refractory hypertension: Antihypertensive treatment resistance vs treatment failure. Can J Cardiol. 2016;32:603–606. doi: 10.1016/j.cjca.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow axis: Implications for hypertension and related therapeutics. Circ Res. 2016;118:1327–1336. doi: 10.1161/CIRCRESAHA.116.307709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dibona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114:1804–1814. doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- 11.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–138. discussion 138–140. [PMC free article] [PubMed] [Google Scholar]
- 12.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, Li W, Santisteban MM, Yuan W, Febo M, Mocco J, Feng Y, Scott E, Baekey DM, Raizada MK. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63:542–550. doi: 10.1161/HYPERTENSIONAHA.113.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 14.Alexander KL, Targan SR, Elson CO., 3rd Microbiota activation and regulation of innate and adaptive immunity. Immunol Rev. 2014;260:206–220. doi: 10.1111/imr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 16.Cervi AL, Lukewich MK, Lomax AE. Neural regulation of gastrointestinal inflammation: Role of the sympathetic nervous system. Auton Neurosci. 2014;182:83–88. doi: 10.1016/j.autneu.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Schaper J, Wagner A, Enigk F, Brell B, Mousa SA, Habazettl H, Schafer M. Regional sympathetic blockade attenuates activation of intestinal macrophages and reduces gut barrier failure. Anesthesiology. 2013;118:134–142. doi: 10.1097/ALN.0b013e3182784c93. [DOI] [PubMed] [Google Scholar]
- 18.Straub RH, Wiest R, Strauch UG, Harle P, Scholmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut. 2006;55:1640–1649. doi: 10.1136/gut.2006.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: Catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 20.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor gpr43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubiano A, Qi Y, Guzzo D, Rowe K, Pepine C, Simmons C. Stem cell therapy restores viscoelastic properties of myocardium in rat model of hypertension. J Mech Behav Biomed Mater. 2015;59:71–77. doi: 10.1016/j.jmbbm.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart DC, Rubiano A, Santisteban MM, Shenoy V, Qi Y, Pepine CJ, Raizada MK, Simmons CS. Hypertension-linked mechanical changes of rat gut. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canby CA, Palmer PJ, Wall KR, Tomanek RJ. Effects of captopril on left ventricular structure and function in shr with established hypertension. Basic Res Cardiol. 1989;84:306–318. doi: 10.1007/BF01907978. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of high mobility group box 1 (hmgb1) through toll-like receptor 4 in macrophages requires cd14. Mol Med. 2013;19:88–98. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. Hmgb1 signals through toll-like receptor (tlr) 4 and tlr2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 30.Palone F, Vitali R, Cucchiara S, Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A, Stronati L. Role of hmgb1 as a suitable biomarker of subclinical intestinal inflammation and mucosal healing in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1448–1457. doi: 10.1097/MIB.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 31.Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res. 2015;2015:489821. doi: 10.1155/2015/489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Zingler M, Harrison JK, Scott EW, Cogle CR, Luo D, Raizada MK. Angiotensin ii regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension. 2016;67:574–584. doi: 10.1161/HYPERTENSIONAHA.115.06474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:178–191. doi: 10.1161/CIRCRESAHA.117.305853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granger DN, Holm L, Kvietys P. The gastrointestinal circulation: Physiology and pathophysiology. Compr Physiol. 2015;5:1541–1583. doi: 10.1002/cphy.c150007. [DOI] [PubMed] [Google Scholar]
- 35.Sugahara H, Odamaki T, Fukuda S, Kato T, Xiao JZ, Abe F, Kikuchi J, Ohno H. Probiotic bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep. 2015;5:13548. doi: 10.1038/srep13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 37.Pickering AE, Paton JF. A decerebrate, artificially-perfused in situ preparation of rat: Utility for the study of autonomic and nociceptive processing. J Neurosci Methods. 2006;155:260–271. doi: 10.1016/j.jneumeth.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Zubcevic J, Potts JT. Role of gabaergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol. 2010;95:909–918. doi: 10.1113/expphysiol.2010.054007. [DOI] [PubMed] [Google Scholar]
- 39.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simms AE, Paton JF, Allen AM, Pickering AE. Is augmented central respiratory-sympathetic coupling involved in the generation of hypertension? Respir Physiol Neurobiol. 2010;174:89–97. doi: 10.1016/j.resp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda K, Tsuda S, Ura M, Shima H, Nishio I, Masuyama Y. Inhibitory actions of captopril on norepinephrine release from adrenergic nerve endings in spontaneously hypertensive rats. Jpn Heart J. 1988;29:475–483. doi: 10.1536/ihj.29.475. [DOI] [PubMed] [Google Scholar]
- 42.Richer C, Doussau MP, Giudicelli JF. Postsynaptic alpha adrenoceptors and attenuation of vascular sympathetic responses in shrs by captopril and enalapril. Eur Heart J. 1983;4(Suppl G):55–60. doi: 10.1093/eurheartj/4.suppl_g.55. [DOI] [PubMed] [Google Scholar]
- 43.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 44.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33:574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 45.Özogul F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen. International Journal of Food Science & Technology. 2011;46:478–484. [Google Scholar]
- 46.Rossi F, Gardini F, Rizzotti L, La Gioia F, Tabanelli G, Torriani S. Quantitative analysis of histidine decarboxylase gene (hdca) transcription and histamine production by streptococcus thermophilus pri60 under conditions relevant to cheese making. Appl Environ Microbiol. 2011;77:2817–2822. doi: 10.1128/AEM.02531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983;49:209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- 48.Tsai YT, Cheng PC, Pan TM. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl Microbiol Biotechnol. 2012;96:853–862. doi: 10.1007/s00253-012-4407-3. [DOI] [PubMed] [Google Scholar]
- 49.Schiffrin EJ, Brassart D, Servin AL, Rochat F, Donnet-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: Criteria for strain selection. Am J Clin Nutr. 1997;66:515s–520s. doi: 10.1093/ajcn/66.2.515S. [DOI] [PubMed] [Google Scholar]
- 50.Iraporda C, Errea A, Romanin DE, Cayet D, Pereyra E, Pignataro O, Sirard JC, Garrote GL, Abraham AG, Rumbo M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 2015;220:1161–1169. doi: 10.1016/j.imbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karbach SH, Schonfelder T, Brandao I, Wilms E, Hormann N, Jackel S, Schuler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schafer K, Munzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin ii-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM., Jr Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67:469–474. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: Paradigm shift in neuroscience. J Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forsythe P, Kunze WA. Voices from within: Gut microbes and the cns. Cell Mol Life Sci. 2013;70:55–69. doi: 10.1007/s00018-012-1028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P, Boeckxstaens GE. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.