Abstract
Schwann cells and oligodendrocytes are the myelinating cells of the peripheral and central nervous system, respectively. Despite having different myelin components and different transcription factors driving their terminal differentiation there are shared molecular mechanisms between the two. Sox10 is one common transcription factor required for several steps in development of myelinating glia. However, other factors are divergent as Schwann cells need the transcription factor Egr2/Krox20 and oligodendrocytes require Myrf. Likewise, some signaling pathways, like the Erk1/2 kinases, are necessary in both cell types for proper myelination. Nonetheless, the molecular mechanisms that control this shared signaling pathway in myelinating cells remain only partially characterized. The hypothesis of this study is that signaling pathways that are similarly regulated in both Schwann cells and oligodendrocytes play central roles in coordinating the differentiation of myelinating glia. To address this hypothesis, we have used genome-wide binding data to identify a relatively small set of genes that are similarly regulated by Sox10 in myelinating glia. We chose one such gene encoding Dual specificity phosphatase 15 (Dusp15) for further analysis in Schwann cell signaling. RNA interference and gene deletion by genome editing in cultured RT4 and primary Schwann cells showed Dusp15 is necessary for full activation of Erk1/2 phosphorylation. In addition, we show that Dusp15 represses expression of several myelin genes, including myelin basic protein. The data shown here support a mechanism by which Egr2 activates myelin genes, but also induces a negative feedback loop through Dusp15 in order to limit overexpression of myelin genes.
Keywords: Schwann, Dusp15, Sox10, MEK-Erk, myelin
Graphical Abstract
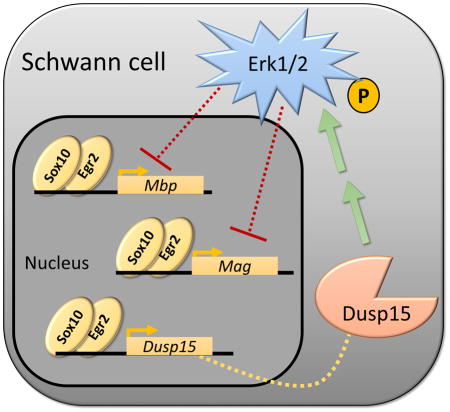
Erk signaling in Schwann cells is essential, but its regulation is still poorly understood. We describe Dual Specificity Phosphatase 15 (Dusp15) as a novel positive regulator of Erk1/2 phosphorylation which leads to the transcriptional repression of some myelin genes. In addition, Dusp15 is a target of the transcription factors Sox10 and Egr2. These findings point to another layer of regulation over myelin genes that Sox10 and Egr2 activate but also repress through the activation of Dusp15.
INTRODUCTION
Myelin is formed by myelinating glia called Schwann cells and oligodendrocytes in the peripheral and central nervous systems, respectively. Myelin is required for proper nerve transmission, and myelin formation is dependent on stage-specific actions of signaling pathways, many of which impinge on transcription factors regulating genes required for myelination of axons (Pereira et al. 2012, Salzer 2012, Grigoryan & Birchmeier 2015, Meijer & Svaren 2013, Mitew et al. 2013). Given the similar physiological roles of Schwann cells and oligodendrocytes, it is nonetheless clear that myelin constituents and gene regulatory networks diverge significantly between the two cell types. For example, principal myelin components include Myelin protein zero (Mpz) in Schwann cells of the peripheral nervous system, whereas Proteolipid protein 1 (Plp1) predominates in oligodendrocytes of the central nervous system. Indeed, even the developmental origins of these two cell types are distinct, as Schwann cells and oligodendrocytes arise from neural crest and neural tube, respectively (Stolt & Wegner 2015). Although some signaling pathways appear to be conserved in both cell types, there are significant differences in the physiological roles of neuregulin and PI3 kinase signaling (Noseda et al. 2016, Brinkmann et al. 2008).
The transcription factors that drive myelination are also quite divergent in Schwann cells versus oligodendrocytes. Although a number of transcription factors have been characterized in myelinating glia, only Sox10, YY1, and Zeb2 are required for myelination in both cell types (Britsch et al. 2001, Stolt et al. 2002, He et al. 2007, He et al. 2010, Weng et al. 2012, Quintes et al. 2016, Wu et al. 2016). However, we recently reported a comparative analysis of Sox10 binding patterns in peripheral nerve and spinal cord, where we found that only a minority of binding sites are conserved between the tissues (Lopez-Anido et al. 2015). Sites unique to each tissue are co-localized with binding sites of transcription factors that are important for development of each cell type, indicating that Sox10 binding specificity is strongly influenced by cell type-specific factors (Emery 2013, Weider et al. 2013, Lopez-Anido et al. 2015).
Despite major differences between Schwann cells and oligodendrocytes, there is a core of myelin genes that are expressed in both cell types (e.g. Mbp, Mag, Plp1, Gjb1/Cx32, Cnp), even though Sox10 and cell type-specific regulators may utilize distinct binding sites in each cell type for these shared genes (Lopez-Anido et al. 2015). Recent studies have identified a key role for Myelin regulatory factor (Myrf), a transcription factor that is induced in myelinating oligodendrocytes and is required for terminal differentiation of oligodendrocytes (Hornig et al. 2013, Bujalka et al. 2013, Emery et al. 2009, Koenning et al. 2012). It has been suggested that Myrf plays an analogous role in oligodendrocytes to that of the Early growth response 2 (Egr2/Krox20) transcription factor (Emery 2013), which is induced in myelinating Schwann cells and is required for myelination (Topilko et al. 1994, Le et al. 2005a). Interestingly, both Egr2 and Myrf are regulated by Sox10 in Schwann cells and oligodendrocytes, respectively (Reiprich et al. 2010, Hornig et al. 2013, Ghislain & Charnay 2006).
Analogous to the core myelin genes expressed between oligodendrocytes and Schwann cells, the MEK-Erk signaling pathway promotes myelination in both myelinating cell types. For example, in vivo studies have shown hypermyelination of axons in both the central and peripheral nervous system when the MEK-Erk pathway is constitutively activated (Ishii et al. 2013, Ishii et al. 2016, Jeffries et al. 2016). We propose that identifying shared target genes in both Schwann cells and oligodendrocytes will shed light on potentially shared regulators of signaling mechanisms in myelinating glia. To examine the role of one factor that is coordinately regulated in both Schwann cells and oligodendrocytes, we identified Dusp15, a member of the Dual specificity phosphatase (DUSP) family that appeared to be strongly regulated by Sox10 in both cell types. Interestingly, Dusp15 is also targeted by Egr2 and Myrf in Schwann cells and oligodendrocytes, respectively. The following experiments test the role of Dusp15 in regulation of Schwann cell signaling and gene expression.
METHODS
Bioinformatics Analysis
Global binding profiles and enrichment at select loci were obtained from previously published ChIP-Seq data (Lopez-Anido et al. 2015, Srinivasan et al. 2012, Bujalka et al. 2013, Yu et al. 2013). To focus on high confidence gene targets of Egr2 and Myrf, binding sites were assigned to the nearest TSS within 50kb using the annotatePeaks program in Homer (Heinz et al. 2010). Comparison of gene targets identified targets that contain either Egr2 or Myrf near the TSS versus those that contain both Egr2 and Myrf nearby. 2653 and 707 genes contain either Egr2 or Myrf binding sites, respectively, and 304 genes contain both Egr2 and Myrf sites nearby. To perform gene ontology (GO) analyses we used David (Database for Annotation, Visualization, and Integrated Discovery) (Huang et al. 2009). We performed GO terms clustering using the highest stringency and also identified Kegg pathways with a p-value < 0.05.
Out of 304 genes with Egr2/Myrf nearby, 102 genes (147 gene probe sets) are expressed in sciatic nerve as determined from a previous microarray study (Le et al. 2005a, Le et al. 2005b). Using a stringent cut off, expressed genes in sciatic nerve were defined as those with detection values greater than 2000. Egr2-dependent genes were identified by comparing expression in control nerves versus Egr2-deficient nerves from mice with a hypomorphic allele of Egr2 (Le et al. 2005a, Le et al. 2005b).
Cell culture and transfection
S16 (Toda et al. 1994) and RT4-D6P2T (Hai et al. 2002) (referred to as RT4, obtained from ATCC) rat Schwann cells were grown in supplemented DMEM (Corning cellgro, 10-017-CV) with penicillin, streptomycin and 5% bovine growth serum (Hyclone, SH30541.03HI). Primary rat Schwann cells were grown in DMEM media, supplemented with 0.2% of bovine pituitary extract (Sigma) and 2uM forskolin (Sigma) (Fambrough et al. 1999).
Cells were transfected with Sox10 siRNA (Ambion, 4390771) Dusp15 siRNA (IDT, RNC.RNAI.N001108598.12.2) or control siRNA (IDT or Ambion-NC1) using Lipofectamine 3000 (L3000015). Luciferase assays were performed with the RT4 Schwann cell line, constructs were also transiently transfected with Lipofectamine 3000 and harvested for analysis 48hrs post-transfection. The reporter construct contained the following coordinates from the rat chromosome 13 (rn5), Pro_Dusp15 chr3:154,831,512-154,831,734, cloned upstream of the pGL4 luciferase reporter containing the minimal E1B TATA promoter. To test if Sox10 is required for activity, reporters were transfected together with Sox10 and control siRNA. The Dusp15-KO cell line was transfected with 500ng of pcDNA3-MmDusp15-T7c (Muth et al. 2016).
Genome editing was performed by cloning guide RNA’s to remove the following sequence: (Rn5 chr3:154,823,256-154,831,564). Sequences for guide RNA were: CCGCCACGGCCCACGGGGGA and TGTGATTCCCGCGGCGATCG. The pX330 plasmid (Cong et al. 2013), purchased from Addgene (Fahmy & Khachigian 2004), was used to transfect the Cas9 system and clone each guide RNA.
Experimental animals and Nerve injury surgery
All animal experiments were performed according to protocols approved by the University of Wisconsin Graduate School. C57BL/6J mice were ordered from Jackson Laboratories and mice were selected at random. Sciatic nerves of 2-month old mice, 4 males and 3 females for 1 day experiment and 3 female mice for 4 day experiment, were transected following the surgery protocol described previously (Hung et al. 2015). As a control, the contralateral limb also received a sham operation consisting of only a skin incision. The nerve tissue distal to the transection and contralateral (sham) nerves were harvested and RNA was isolated, using the RNeasy Lipid Tissue Mini Kit (Qiagen Cat. 74804). Samples were coded prior to analysis and sham/injury samples were grouped to calculate changes in gene expression. The number of mice for these experiments was calculated in order to observe >2-fold changes in gene expression. Injury-induced gene expression changes were consistent with published gene expression profiles (Arthur-Farraj et al. 2012, Kim et al. 2012, Barrette et al. 2010).
Western Blot
RT4 Schwann cells were lysed in lysis buffer (150mM NaCl, 10% glycerol, 50nM Tris pH 8.0, 1% SDS 1% Triton and 1:100 protease inhibitor Sigma-Aldrich P8340) and heated for 5 minutes at 95°C before electrophoresis in an 10% SDS-PAGE (MIDSCI BCG01012). Total and phosphorylated Erk1/2 was measured using Erk1/2 (Cell Signal, #4695P) and P-Erk1/2 (Cell Signal, #4370P). Membranes were scanned and quantitated with the Odyssey Infrared Imager (LI-COR Biosciences).
RT-qPCR
RNA was isolated from cultured Schwann cells 48hrs after transfection using Tri Reagent (Ambion). RNA was converted to cDNA using the MMLV reverse transcriptase (Invitrogen). All cDNAs were analyzed from three independent experiments by RT-qPCR using Power SYBR Green Master Mix (Thermo Fischer Scientific) on the ViiA7 system (Applied Biosystems). Relative expression was calculated using Comparative Ct method (Livak & Schmittgen 2001). Primers used are listed in Table 1.
Table 1.
| Gene Name | Forward | Reverse |
|---|---|---|
| Dusp15 | AGCCACTTAACGAGCCCCTTT | TCGGCTGAGCTGCAGTGTTTA |
| Sox10 | CGAATTGGGCAAGGTCAAGA | CACCGGGAACTTGTCATCGT |
| Egr2 | GCACTCTGTGGCCCTAGAACA | GGCTGAGATGGCTCGAGAAA |
| Ccl2 | TGCATCCACTCTCTTTTCCA | CATTGAAAGTGTTGAACCAGGA |
| Vegfc | CGCTGTGTCCCATCATATTG | CTGTCTGGTCACTGGCAGAA |
| Mag | GGCTGAGTACGCAGAAATCC | AGGGGAGAGGGAGCTGTAAC |
| Mbp | CAGGATTCGGGAAGGCTGAG | GAGGAAGAGACAGCCGCTCTG |
| Gjb1 | CTCTGCACTGTGGATGGAGA | TTCAGAGAGAGAGGCCTTGG |
| Pmp22 | CAGATCCCTCCCTCCCATTC | TGTCCCCGCACTTTGGTTAT |
| Srebp-1c | GGAGCCATGGATTGCACATT | TCAAATAGGCCAGGGAAGTCA |
| Mpz | CCTGGAGGTGACGGTCACTT | CTGCAGTCAAATCCCCCAGTA |
Statistics
P-values were obtained from the Student’s two-tailed t-test.
RESULTS
Egr2 and Myrf have common target genes in Schwann cells and oligodendrocytes
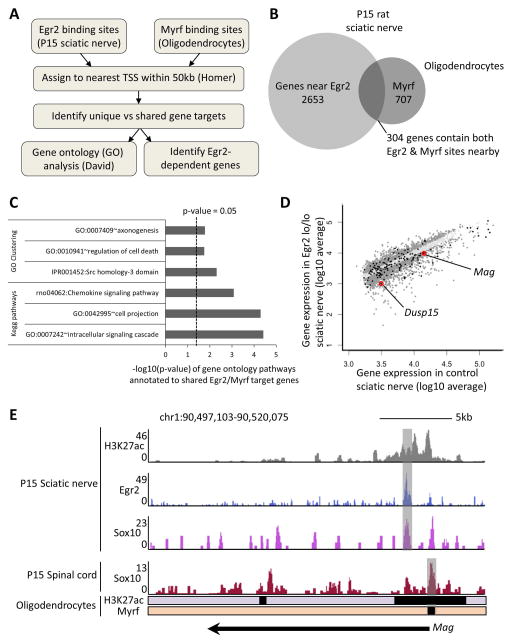
While Egr2 and Myrf are required for terminal differentiation in Schwann cells and oligodendrocytes, respectively, it remains unclear if they fulfill similar roles by targeting unique or shared gene targets. Therefore, we annotated Egr2 and Myrf binding sites to nearby genes and performed a comparative analysis to identify unique and shared gene targets (Figure 1A). To focus on high confidence gene targets of Egr2 (7179 total) and Myrf (2049) (Lopez-Anido et al. 2015, Srinivasan et al. 2012, Bujalka et al. 2013), binding sites were assigned to the nearest transcription start site (TSS) within 50kb. A comparison of gene targets revealed 2653 and 707 genes that contain either Egr2 or Myrf binding sites, respectively, and 304 genes that contain both Egr2 and Myrf sites nearby (Figure 1B and Supplementary Table 1). It is intriguing that a relatively small group of shared genes have nearby Egr2/Myrf sites because it suggests that select genes exhibit converged Egr2/Myrf regulation. To better understand the function of these shared genes we performed gene ontology (GO) and Kegg pathway analysis using David (Database for Annotation, Visualization, and Integrated Discovery) (Huang et al. 2009). Our analysis revealed enrichment in genes associated with axon guidance (e.g. Gas7), regulation of cell death (e.g. Ptprf, Foxo3), cell projection (e.g. Clic4, Ptprf, Gsn, Mbp), and intracellular signaling cascades (e.g. Gsn, Rab5b, Rtkn, Rhog) (Figure 1C and Supplementary Table 2). Interestingly, two genes producing proteins involved in axoglial contacts in CNS and PNS node formation, contactin 2/Tag1 (Traka et al. 2002) and neurofascin, were in this group. Neurofascin is present in both axons and Schwann cells/oligodendrocytes, although the NF155 isoform is specific to myelinating glia (Roche et al. 2014).
Figure 1. Egr2 and Myrf have common target genes in myelinating glia.
(A) Schematic depicts bioinformatics approach to compare Egr2 and Myrf binding sites in Schwann cells versus oligodendrocytes, respectively. Egr2 binding sites in Schwann cell-rich P15 rat sciatic nerve and Myrf sites in cultured oligodendrocytes were recently published (Lopez-Anido et al. 2015, Srinivasan et al. 2012, Bujalka et al. 2013). (B) Overlap analysis of genes with Egr2 and Myrf binding sites nearby identifies 304 genes that may be shared targets in Schwann cells and oligodendrocytes. (C) Gene ontology (GO) terms clustering and Kegg pathway analysis on Egr2/Myrf shared target genes reveals enrichment in signaling pathways and other processes. (D) Scatter plot represents gene expression for a given gene (represented by dots) in control versus Egr2-deficient sciatic nerve. Gene expression values were obtained from a previous microarray study on mice with a hypomorphic allele of Egr2 (Le et al. 2005a, Le et al. 2005b). Dark grey dots are either upregulated in Egr2-deficient nerve by >1.25-fold or downregulated by <0.8-fold. The black dots represent genes that are expressed in control nerve and also have an Egr2/Myrf binding event nearby, and these include (highlighted in red) the well-studied myelin gene Myelin-associated glycoprotein (Mag) as well as Dual specificity phosphatase 15 (Dusp15), a gene with an unknown role in peripheral nerve myelination. (E) ChIP-Seq analysis profiles depict genomic regions enriched with transcriptional regulators and enhancers in P15 rat sciatic nerve and spinal cord. Included are binding profiles of Sox10 and Egr2 (Lopez-Anido et al. 2015, Srinivasan et al. 2012). Profiles of H3K27ac and the oligodendrocyte-specific regulator Myrf in cultured oligodendrocytes were also obtained from published datasets (Yu et al. 2013, Bujalka et al. 2013, Lopez-Anido et al. 2015). Egr2- versus Myrf-enriched enhancers specific to Schwann cells versus oligodendrocytes are indicated by grey boxes.
To test if genes with nearby Egr2/Myrf sites are regulated by Egr2 in Schwann cells, we analyzed a previously published microarray on Egr2-deficient nerve from mice with a hypomorphic allele of Egr2 (Le et al. 2005a, Le et al. 2005b). 44 genes (e.g. Clic4, Abca2, Mag, Mbp, Fa2h, Syt11, Thra, Psmf1, Dusp15, Ptprf, Klf9, Nfix, Gsn) were downregulated <0.8-fold and 24 genes (e.g. Map4k4, Sox10, P2rx4, Fem1b, Stard13, Ninj1, Foxo3) were upregulated >1.25-fold in Egr2-deficient nerves (Figure 1D and Supplementary Table 3). One gene identified in the analysis was Mag (Myelin-associated glycoprotein), a critical myelin gene (Carenini et al. 1997, Yin et al. 1998) and a known Egr2 target (Jang et al. 2006). ChIP-Seq binding profiles indicate that while Mag is a shared target gene of Egr2/Myrf, Egr2 binds an intronic element and Myrf binds just upstream of the transcription start site (Figure 1E). Accordingly, our previous analysis on Sox10 binding events in Schwann cells and oligodendrocytes indicated that most sites are unique to one cell type or the other, and even shared genes have CNS- and PNS-specific enhancers (Lopez-Anido et al. 2015). Most of these genes have not yet been characterized in either oligodendrocyte or Schwann cell function, but the apparent parallel regulation by Sox10 and Egr2/Myrf may help identify critical genes required for myelination in both types of myelinating glia.
Sox10 and Egr2 control Dusp15 transcription
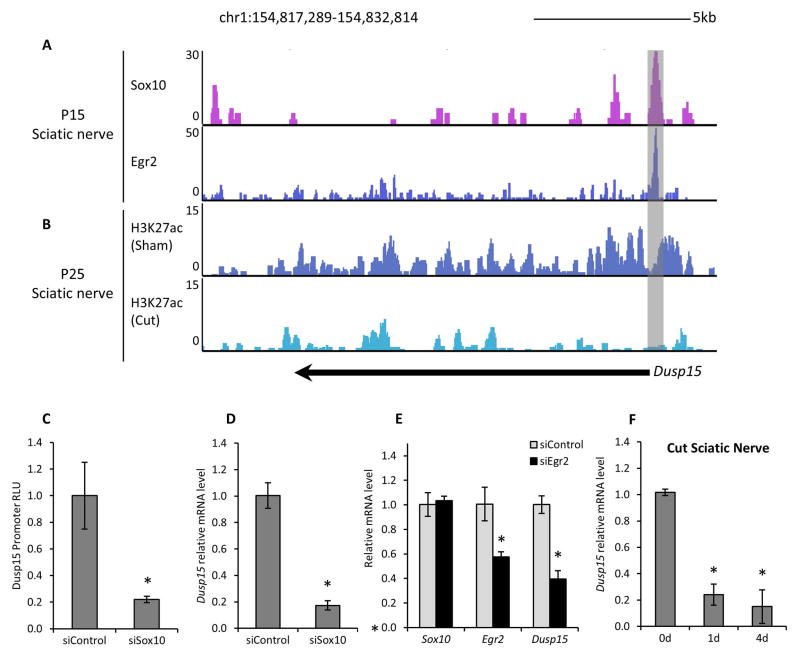
To determine the role of a gene that has not been characterized in Schwann cells, we turned to the Egr2-dependent gene Dual specificity phosphatase 15 (Dusp15), a gene with nearby Egr2/Myrf sites that is also reduced ~70% in Egr2-deficient nerve relative to wild type (Le et al. 2005a, Le et al. 2005b) (Figure 1D). Previous studies found that Dusp15 expression is induced upon differentiation of oligodendrocytes in culture (Schmidt et al. 2012) and PNS development (Patzig et al. 2011). A more recent study, in primary oligodendrocytes, showed how Sox10 and Myrf control Dusp15 transcriptional levels by binding to its promoter region and also observed an increase in Dusp15 mRNA in spinal cord after birth (Muth et al. 2016). Also, our analysis using Sox10 siRNA treatment in the S16 Schwann cell line identified Dusp15 as a potential Sox10 target gene (Srinivasan et al. 2012). To test if Dusp15 is a direct target of Sox10, we assayed ChIP-Seq datasets on Sox10 binding in P15 rat sciatic nerve. Sox10 ChIP-Seq in sciatic nerve (Srinivasan et al. 2012, Lopez-Anido et al. 2015) revealed a Sox10 binding site in the proximal promoter region (Figure 2A). ChIP-Seq in spinal cord (not shown), where Sox10 is only expressed in the oligodendrocyte lineage, also revealed Sox10 binding in the promoter region of Dusp15 (Lopez-Anido et al. 2015). Sox10 binding coincided with local enrichment with histone acetylation (histone H3K27 acetylation), which marks actively engaged enhancers (Figure 2B).
Figure 2. Sox10 and Egr2 regulate Dusp15 transcription.
(A) ChIP-Seq analysis of P15 sciatic nerve reveals Sox10 and Egr2 enrichment at the Dusp15 gene, with a grey box highlighting the promoter region. (B) ChIP-Seq analysis on P25 rat sham sciatic nerve show the H3K27ac-marked enhancer (grey box), which is diminished in cut nerve (3 d post-injury). (C) Luciferase assays were performed in S16 Schwann cells with constructs containing the promoter region of Dusp15 (Pro_Dusp15) and treated with Sox10 siRNA. Relative light units (RLU) are shown relative to siControl treatment after normalizing to β-galactosidase activity from a co-transfected CMV-lacZ plasmid. Quantitative RT-PCR was used to determine relative mRNA expression levels in (D) S16 rat Schwann cells treated with siRNA against Sox10. (E) S16 Schwann cells were treated with siRNA against Egr2 and quantitative RT-PCR was used to measure mRNA levels of Sox10, Egr2 and Dusp15. (F) Dusp15 levels were measured in uncut (sham) or cut sciatic nerve (1 and 4 d post-injury) using quantitative RT-PCR. 18S housekeeping gene was used to normalize all quantitative RT-PCR assays and error bars indicate the standard deviation of three biological replicates (n=7 for 1 d post-injury and 3 for 4 d post-injury). (*P<0.05)
To confirm direct Sox10 binding at the promoter, we performed sequence analysis to identify the Sox10 binding motif and also tested if enhancer activity was Sox10-dependent. We found that the promoter region is GC-rich, but contains two potential binding motifs for Sox10 (GACAAAGcccTAGTGTG, GACAACGgcaGCTTGTT) that conform to the previously published consensus motif. The two motifs appear to be inverted dimeric sites that have been shown to bind Sox10 cooperatively (Peirano & Wegner 2000) and are conserved in mammalian species. To examine enhancer activity, the promoter region surrounding the Sox10 binding sites were cloned into a luciferase reporter vector (termed Pro_Dusp15 reporter), and transfected into the RT4 Schwann cell line. We found that level of luciferase activity from the Pro_Dusp15 reporter was significantly reduced when Sox10 siRNA was cotransfected (Figure 2C). Mutation of the first Sox10 motif listed above was shown to reduce Sox10 responsiveness of the Dusp15 promoter in N2A transfection assays (Muth et al. 2016).
In addition, we tested if Dusp15 expression was Sox10-dependent. We measured Dusp15 relative expression levels by qRT-PCR after siRNA-mediated depletion of Sox10 in RT4 cells (Figure 2D) and found a 5-fold reduction in Dusp15 levels by Sox10 siRNA. Finally, we tested if Dusp15 levels were also downregulated in mouse sciatic nerves lacking Sox10. We employed P16 sciatic nerve from a conditional knockout of Sox10 in which the floxed allele was mated to the Dhh::cre line to delete Sox10 selectively from Schwann cells (Finzsch et al. 2010). Previous analysis of this mouse revealed that Sox10 plays an important role in mature stages of Schwann cell development, and also showed strong reduction in Sox10 target genes (Mbp, Mpz, Dhh, S100B, Erbb3, and Egr2). Quantitative RT-PCR of these nerve samples (n=2) confirmed that Sox10 is necessary for Dusp15 expression in vivo (data not shown).
Our in vivo ChIP-Seq data also showed a pronounced Egr2 binding site in P15 rat sciatic nerve (Srinivasan et al. 2012) (Figure 2A). The region contained known, consensus Egr2 binding motifs (Swirnoff & Milbrandt 1995), which are also conserved in mammalian species (e.g. ACGGGGGAG, GCGTGGGAC, GTGGGGGCG, CTGGGGGCA). As noted above (Le et al. 2005a), Egr2-deficient nerve exhibits lower Dusp15 expression levels than wild type. We also found that siRNA-mediated depletion of Egr2 in S16 cells reduced Dusp15 levels ~50% (Figure 2F), while Sox10 expression levels remained unchanged. Our findings indicate that Dusp15 is regulated by two of the major regulators of Schwann cell development, Sox10 and Egr2, as has been shown for many other myelin-associated genes (Jones et al. 2007, Jones et al. 2011, Svaren & Meijer 2008, Jang & Svaren 2009, Srinivasan et al. 2012, Bondurand et al. 2001, Jones et al. 2012).
Dusp15 is regulated by nerve injury
Given the dependence of Dusp15 expression on Egr2, we hypothesized that Dusp15 expression would decline after peripheral nerve injury, since Egr2 and many of its target genes are downregulated in the demyelination phase after nerve injury (Le et al. 2005a, Nagarajan et al. 2002, Topilko et al. 1997, Zorick et al. 1996). To test this we performed a nerve transection injury, where we observed a decline in Dusp15 expression 1 and 4 days after injury (Figure 2G). In addition, ChIP-Seq data for H3K27ac (Hung et al. 2015) around the Sox10 and Egr2 binding region upstream of Dusp15 revealed a dramatic reduction in the enhancer mark in injury samples (3d post-transection) when compared with control (Figure 1B). Our results are consistent with microarray analyses of gene expression changes after nerve injury, which also show a similar decrease in Dusp15 expression after injury (Arthur-Farraj et al. 2012, Kim et al. 2012, Barrette et al. 2010). Thus, in addition to being induced over myelination, Dusp15 is also downregulated along with myelin genes after nerve injury.
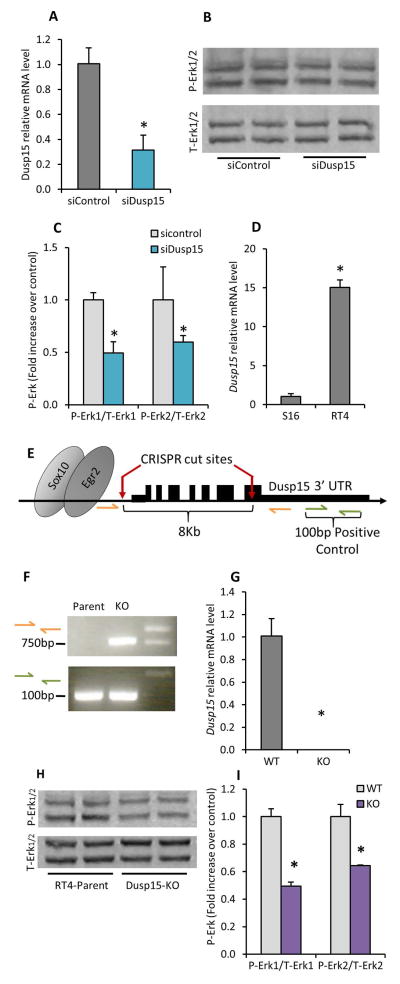
Dusp15 increases Erk1/2 phosphorylation
Dusp15 is a member of the dual specificity phosphatase family and peptide array experiments identified potential substrates with important signaling molecules, such as ErbB3 and p38 (Schmidt et al. 2012). This suggested the hypothesis that Dusp15 is an important regulator of signaling molecules in Schwann cells. Dusp15 mRNA levels were repressed in the RT4 Schwann cell line using siRNA and phosphorylated levels of ErbB3 and p38 were measured by Western blot. While Dusp15 was repressed 4-fold (Figure 3A), phosphorylated levels of ErbB3 and p38 remained unchanged (data not shown). Because some DUSP family proteins are also known to interact with Erk and Akt signaling pathways, we hypothesized that Dusp15 may regulate other signaling molecules important for Schwann cell myelination. Instead of an expected increase in Erk1/2 phosphorylation levels, the opposite was observed as phosphorylation was reduced in Dusp15-repressed samples (Figure 3B and 3C). These results show that Dusp15 positively regulates the Erk1/2 signaling pathway, probably through intermediary factors.
Figure 3. Dusp15 controls Erk1/2 phosphorylation levels.
(A) Dusp15 levels were measured using quantitative RT-PCR in RT4 Schwann cells treated with siRNA against Dusp15. (B) Western Blot analysis of Erk1/2 phosphorylation was performed and (C) quantified in RT4 Schwann cells treated with siRNA against Dusp15. (D) Dusp15 mRNA levels were compared between S16 and RT4 Schwann cells using quantitative RT-PCR. (E) Diagram depicting guide RNA (red) target sites for CRISPR/Cas9 deletion of the Dusp15 gene. (F) Genotyping of Cas9 treated cells was determined by PCR using primers (orange pair) flanking the deleted genomic and the uncut 3′UTR region in both lines (green). (G) Dusp15 mRNA levels in WT and KO cell lines were determined by quantitative RT-PCR. (H) Erk1/2 phosphorylation was measured in WT versus KO lines and compared to total levels of Erk1/2 by western blot analysis. (I) Band signals obtained from western blot analysis were normalized to Total-Erk (T-Erk) levels. All experiments represent average values of biological triplicates. (*P<0.05)
Creation of a Dusp15 Knockout in the RT4 Schwann cell line
One limitation of siRNA studies is that only partial depletion of the target gene is normally achieved. Therefore, to further investigate the function of Dusp15, the gene was deleted in RT4 Schwann cells using the new genome engineering technique, CRISPR-Cas9. The RT4 Schwann cell line was chosen due to the high levels of Dusp15 that it expresses when compared to other known Schwann cell lines like S16; we found that RT4 cells express 15-fold more Dusp15 than the S16 cell line (Figure 3D). Guide RNAs (gRNA) were designed to flank the coding sequence of Dusp15, spanning from the transcription start site to the 7th exon and leaving the 3′UTR intact (Figure 3E). Two consecutive rounds of transfection yielded a clone that lacked the Dusp15 coding region. To assay the absence of Dusp15 in the identified clonal population, genomic PCR was performed using primers that spanned the 3′UTR still present in the parent line (wildtype) and KO line and another primer set that flanked the 8 kb region between the two guide RNA targets. The wildtype line did not produce a band while the KO line amplified a 750bp band showing the absence of the Dusp15 gene (Figure 3F). Furthermore, mRNA levels of Dusp15 were not detected by quantitative RT-PCR (Figure 3G). Our CRISPR-Cas9 deletion line provided another tool to test the role of Dusp15. We measured Erk1/2 phosphorylation in the deletion line, and observed a downregulation in the deletion line relative to the parental RT4 cell line, as was seen with Dusp15 siRNA (Figure 3H and 3I). Although Dusp15 does not seem to regulate ErbB3 phosphorylation (data not shown), it does appear to be required for the full activation and phosphorylation of Erk1/2.
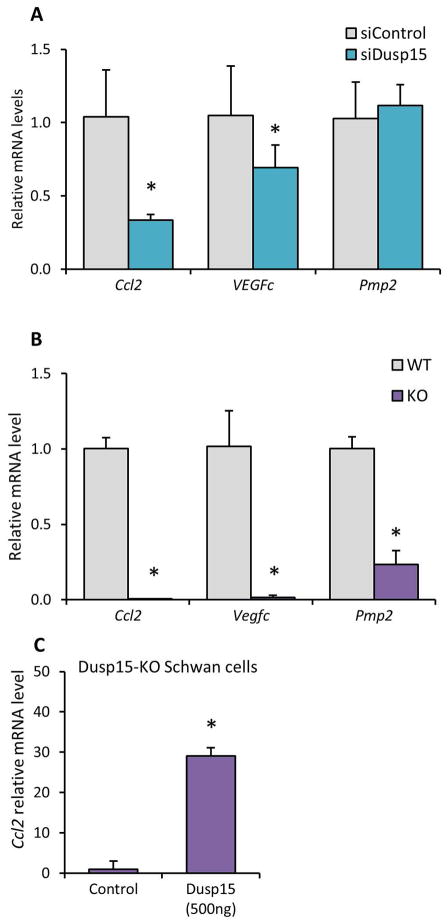
Regulation of Erk1/2 target genes in Schwann cells
Erk1/2 signaling plays an important role in promoting Schwann cell development, particularly during embryogenesis, but has also been implicated in the injury response of Schwann cells (Harrisingh et al. 2004, Napoli et al. 2012, Newbern & Birchmeier 2010, Ishii et al. 2013, Sheean et al. 2014, Ishii et al. 2016). Previous studies identified several Erk1/2 target genes during Schwann cell injury (Napoli et al. 2012). Therefore, we hypothesized that reduced levels of Erk1/2 phosphorylation were correlated with targets of the MEK-Erk pathway in Schwann cells: Ccl2/MCP-1, Vegfc (Napoli et al. 2012, Fischer et al. 2008) and Pmp2 (Sheean et al. 2014). Quantitative RT-PCR revealed that Ccl2 and Vegfc were significantly reduced by Dusp15 siRNA, while Pmp2 was unchanged (Figure 4A). In addition, the same mRNA levels were also measured in the Dusp15 knockout line, which showed a more striking decrease of the Erk1/2 regulated genes (Figure 4B). Finally, we tested if changes in Ccl2 mRNA levels could be rescued by ectopic expression of Dusp15, and found that transient transfection with a Dusp15 expression plasmid in the knockout line led to partial restoration of the expression levels (Figure 4C). Our results indicate that Dusp15 functions upstream of Erk1/2 activity.
Figure 4. Dusp15 is necessary for Erk activity.
Quantitative RT-PCR was used to determine Ccl2, Vegfc and Pmp2 mRNA levels in (A) RT4 Schwann cells treated with siRNA against Dusp15 and (B) Dusp15-KO-RT4 Schwann cell line. (C) Ccl2 mRNA levels were measured in Dusp15-KO Schwann cells transfected with an expression plasmid for Dusp15 using quantitative RT-PCR. (*P<0.05)
Dusp15 represses expression of myelin genes
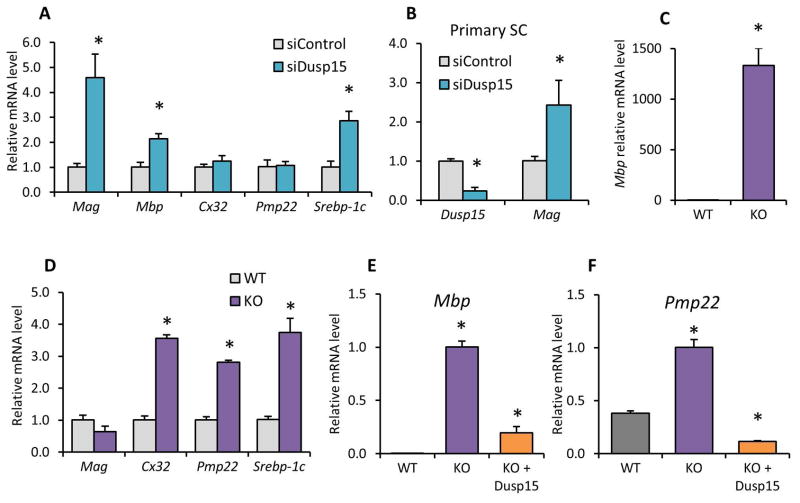
As we observed that Dusp15 loss leads to reduction of Erk1/2 phosphorylation, we also examined some Erk1/2-dependent myelin genes that have been characterized previously (Sheean et al. 2014, Napoli et al. 2012). Different myelin genes display different levels of Erk-dependence in various contexts, but we initially examined Mbp and Mag because these myelin genes were induced in Erk1/2-deficient Schwann cells (Newbern et al. 2011). In RT4 Schwann cells treated with siRNA against Dusp15 we measured mRNA levels of Mbp and other myelin genes (Mag, Mpz, Gjb1/Cx32, Pmp22) and also an important regulator of lipid synthesis that is developmentally regulated in Schwann cells, SREBP-1c (LeBlanc et al. 2005, de Preux et al. 2007, Verheijen et al. 2003, Verheijen et al. 2009, Norrmén et al. 2014). Consistent with previous results (Newbern et al. 2011), some of the myelin genes were induced, mainly, Mag, Mbp, and SREBP-1c, while others remained unchanged (Figure 5A). We decided to further test the Dusp15 effects in primary Schwann cells (Primary SC). When Dusp15 was repressed using siRNA we measured an increase in Mag mRNA mimicking the results found in RT4 Schwann cells (Figure 5B). Somewhat surprisingly, Mbp expression was dramatically induced in the Dusp15 knockout line compared with parental RT4 cells (Figure 5C). As it turns out, RT4 cells express relatively high levels of some myelin genes, but Mbp is virtually undetectable (Hai et al. 2002). Therefore, induction from the extremely low level of Mbp expression is quite dramatic.
Figure 5. Dusp15 represses myelin gene expression.
Mag, Mbp, Cx32, Pmp22 and Srebp-1c mRNA levels were measured by quantitative RT-PCR in (A) RT4 Schwann cells, Dusp15 and Mag in (B) Primary Schwann cells treated with siRNA against Dusp15 and in (C and D) Dusp15-KO Schwann cell line compared to parent RT4 Schwann cells. (E) Mbp and (F) Pmp22 mRNA levels were measured in Dusp15-KO Schwann cells treated with ectopic expression levels of Dusp15. All experiments represent biological triplicates, and expression levels were normalized to 18S rRNA. (*P<0.05)
The knockout line was also used to evaluate expression of the other myelin-associated genes by quantitative RT-PCR. In contrast to the siRNA experiments, Mag, was slightly reduced, but Cx32/Gjb1, Pmp22 and SREBP-1c were induced (Figure 5D). Finally, it was important to show if the induction seen in the myelin genes was due to the lack of Dusp15 rather than an off-target effect. Restoring Dusp15 expression in the knockout line by transfection of an overexpression plasmid brought Mbp and Pmp22 back to levels approximately equal to the expression in the parental RT4 line (designated WT in Figure 5E and 5F).
The Erk pathway represses myelin genes
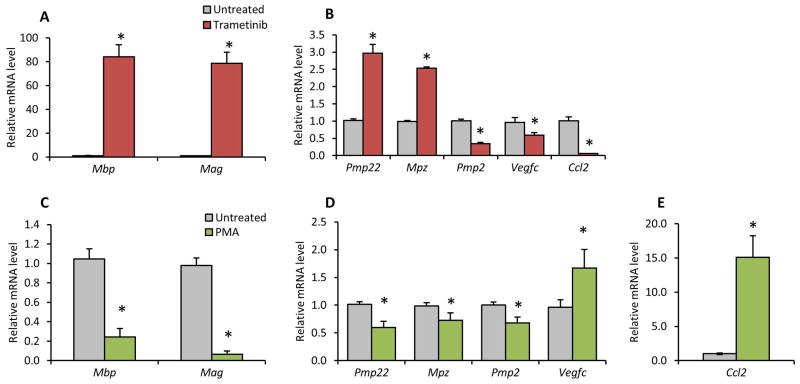
Since Dusp15 reduction leads to decreased Erk1/2 phosphorylation, we tested if myelin gene expression changes would also be observed with more direct manipulation of Erk1/2 phosphorylation. The parental RT4 Schwann cell line was treated with Trametinib, a MEK inhibitor, and quantitative RT-PCR indicated that Mbp and Mag were induced 80-fold each (Figure 6A) while other myelin genes (Pmp22, Mp11 and Mpz) were modestly induced (Figure 6B). Repression of Erk signaling was correlated with decreased expression of Erk-regulated target genes Vegfc and Ccl2, and the myelin gene Pmp2 (Figure 6B). We next performed complementary experiments to activate Erk signaling by the phorbol ester PMA, which targets PKC upstream of Erk. Quantitative RT-PCR showed that Erk1/2 activation repressed most of the genes that were activated by Dusp15 depletion. Mbp and Mag were reduced 80% (Figure 6C) and other myelin gene mRNA levels (Pmp22, Cx32 and Mpz) were modestly reduced (Figure 6D). Erk activated genes were also induced with PMA (Vegfc and Ccl2), while Pmp2 was not induced (Figure 6D and 6E). Overall, our results indicate that Erk activity downstream of Dusp15 represses myelin gene expression.
Figure 6. Erk activity represses myelin gene expression.
Mbp, Mag, Pmp22, Mpz, Pmp2, Vegfc and Ccl2 mRNA levels were measured in RT4 Schwann cells treated with (A and B) Trametinib 18nM, or with (C, D and F) PMA using quantitative RT-PCR. Samples were normalized to the level of 18S rRNA. Error bars represent the standard deviation from biological triplicates.
Erk signaling represses myelin gene expression in primary Schwann cells
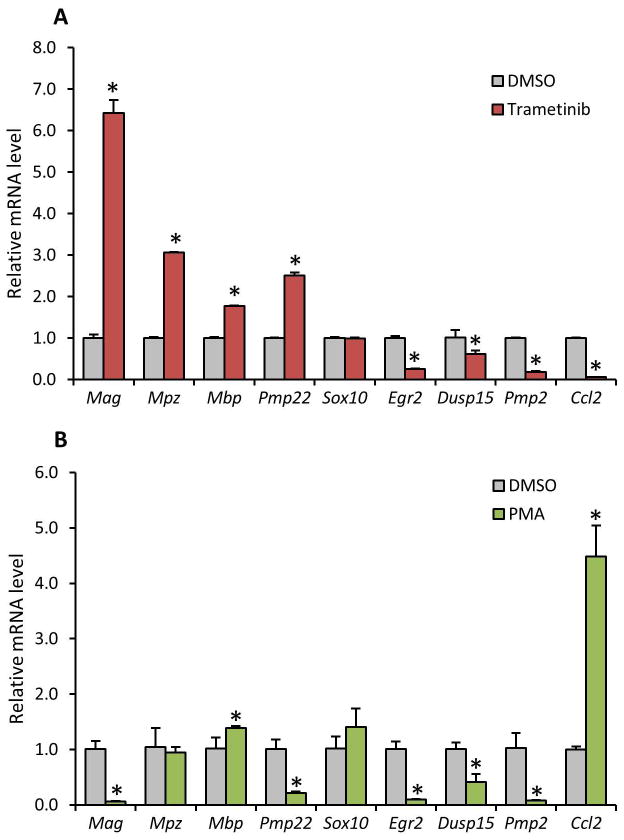
The experiments above were performed in the RT4 Schwann cell line, but we wanted to know if the effects of Erk signaling on myelin gene expression were present in primary Schwann cells. Primary rat Schwann cells were treated with Trametinib and several myelin genes, (Mag, Mpz, Mbp and Pmp22) were all induced as we had seen before (Figure 7A). The activation of Erk, through PKC using PMA, resulted in an almost mirror image effect on some of the genes, specifically Mag, Pmp22 and Ccl2 (Figure 7B). The gain-of-function experiment using PMA resulted in the expected results as we observed Mag and Pmp22 induced, while confirming an induction in Erk activity through increased Ccl2 mRNA levels. An interesting effect observed with Trametinib was the reduction of Egr2 mRNA levels and the induction of the myelin genes. This was also observed in E12.5 DRG that lacked Erk1/2 expression (Newbern et al. 2011).
Figure 7. Downstream Erk effects is conserved in Primary Schwann cells.
Mag, Mpz, Mbp, Pmp22, Sox10, Egr2, Dusp15, Pmp2 and Ccl2 were measured in Primary Schwann cells when these were treated with Trametinib (A) and PMA (B) as before. Genes were measured using quantitative RT-PCR and normalized to 18S rRNA. Error bars represent the standard deviation from biological triplicates. (*P<0.05)
DISCUSSION
Although oligodendrocytes and Schwann cells perform similar functions in the central and peripheral nervous system, respectively, the molecular pathways of these two cell types are significantly divergent. To elucidate potentially common pathways we looked at genes associated with binding of Sox10, a transcription factor expressed in both cells. ChIP-Seq analysis from Sox10 genomic occupancy in sciatic nerve and spinal cord tissue (Schwann cells and oligodendrocytes, respectively) identified a core of common genes (Lopez-Anido et al. 2015). Here, we found that a relatively small subset of these genes are associated with Egr2 binding in peripheral nerve and Myrf in oligodendrocytes (Srinivasan et al. 2012, Bujalka et al. 2013). Of these ~300 genes, relatively few have been characterized previously.
To begin to identity novel regulators of myelination, we focused on Dusp15, a member of the dual specificity phosphatase family, since it had potential to be an important regulator of intracellular signaling pathways. ChIP-Seq revealed that the Dusp15 promoter is bound by Sox10 in both sciatic nerve and spinal cord tissue (Lopez-Anido et al. 2015). Luciferase activity of the promoter showed that Sox10 directly activates Dusp15 and Sox10 cKO nerve further confirmed Dusp15 as a target of Sox10 in vivo. Egr2 also appears to control Dusp15 expression levels based on our ChIP-Seq analysis (Srinivasan et al. 2012), siRNA effects on Dusp15 mRNA expression levels, and microarray analysis from Egr2-deficient mice (Le et al. 2005a). Finally, developmental gene expression analysis (Patzig et al. 2011, Verdier et al. 2012) revealed that Dusp15 is induced throughout Schwann cell differentiation and exhibits peak expression at P15. Dusp15 induction through differentiation can be explained by parallel activation of Egr2 during myelination (Topilko et al. 1994, Murphy et al. 1996). Interestingly, the promoter region is also a binding site for Myrf and Sox10 in oligodendrocytes and lacZ reporter controlled by the Dusp15 promoter showed X-gal staining colocalizing with Sox10 positive cells in spinal cord and with Sox10/Egr2 expressing cells in peripheral nerve (Muth et al. 2016). The fact that Dusp15 is a common target of Egr2 and Myrf transcription factors suggested that it may have an important common role in both Schwann cells and oligodendrocytes.
While the direct targets of Dusp15 phosphatase activity are not resolved in this work, we show evidence for its regulation of the Erk1/2 pathway in Schwann cells. Positive regulation over the Erk pathway was unexpected since Dusp15 has been shown to have phosphatase activity (Schmidt et al. 2012, Alonso et al. 2004). Therefore, it is likely that Dusp15 targets an inhibitor of Erk1/2. We observed that Dusp15 is necessary for the full phosphorylation of Erk1/2, and depletion of Dusp15 led to reduction in previously described Erk1/2 target genes in Schwann cells, Ccl2 and Vegfc (Napoli et al. 2012, Fischer et al. 2008). Interestingly, loss of Dusp15 not only reduced Erk1/2 signaling but also activated several myelin genes, mainly Mbp, Mag (in siRNA experiments) and Pmp22. We confirmed our results by using genome editing to remove the Dusp15 open reading frame in the RT4 Schwann cell line and rescuing the effects described above by ectopic expression of Dusp15. While both siRNA and CRISPR technologies have limitations in terms of either a) partial depletion of target and b) potential compensatory/off-target changes in the selection of clones with the desired modification, the use of both technologies provide complementary benefits in analysis of Dusp15 function.
Dusp15-mediated repression of myelin genes and activation of the Erk pathway prompted us to test if the reduction of Erk1/2 phosphorylation leads to increased myelin gene expression. Indeed, we observed that inhibiting the Mek/Erk pathway also induced some myelin genes. Conversely, modulating the Erk pathway through PKC activation led to repression of Mbp, Mag, and Pmp22. Performing similar experiments in primary Schwann cells revealed the same trends in myelin gene expression as observed in RT4 Schwann cells. These effects are consistent with past findings of a Schwann cell-specific knockout of Erk1/2 (Newbern et al. 2011) which showed increased Mbp and Mag levels in E12.5 DRG samples even when Egr2 levels were repressed.
The role of Erk1/2 signaling in Schwann cell differentiation has been the subject of several studies (reviewed in Grigoryan & Birchmeier 2015, Newbern 2015). Erk1/2 signaling is activated after nerve injury and plays a role in demyelinating processes triggered by injury and other pathological conditions (Harrisingh et al. 2004, Napoli et al. 2012). Indeed, increased Erk1/2 signaling was also demonstrated in rodent models of the CMT1A neuropathy (Fledrich et al. 2014). On the other hand, embryonic Erk1/2 signaling is required for myelination and for activity of the pro-myelinating YY1 transcription factor (Newbern et al. 2011, He et al. 2010). Enhanced expression of either Erk or Mek1 signaling in Schwann cells has also been shown to increase myelin thickness (Ishii et al. 2013, Sheean et al. 2014, Ishii et al. 2016). Moreover, expression of a constitutively active Mek1 allele in Schwann cells can rescue defects observed with knockout of either the Shp2 or Erbb3 gene (Sheean et al. 2014). It should be noted that Erk1/2 activation is high in the early postnatal myelination period in mouse up to at least P15, but then subsides by P30 until it is re-activated upon nerve injury (Sheean et al. 2014, Harrisingh et al. 2004).
Given the many potential targets of Erk1/2 activity, it is possible that the effect of Erk activation is dependent upon the degree of activation and the stage of Schwann cell development (Ishii et al. 2016). Therefore, it is important to identify potential regulators of Erk1/2 activation that may give rise to context-specific aspects of Schwann cell differentiation. An interesting parallel of Dusp15 is the Shp2 tyrosine phosphatase, which is required for full Erk1/2 activation (Grossmann et al. 2009), and it seems possible that Dusp15 may play a partially overlapping role with this protein. However, it is notable that Shp2 deficiency results in decreased Egr2 activation, which is not observed upon depletion of Dusp15 in our studies. Interestingly, Erk1/2 activation has been studied extensively in oligodendrocyte development, where it promotes differentiation and increased myelin thickness (reviewed in Gaesser & Fyffe-Maricich 2016). Therefore, Erk1/2 activation is important in both the development of oligodendrocytes and Schwann cells leading us to suggest that Dusp15 may have similar roles in both myelinating cells.
Based on our analysis and the developmental regulation of Dusp15, it is notable that the increase in Dusp15 up to P15 parallels a similar increase in Erk1/2 phosphorylation (Sheean et al. 2014). However, Dusp15 mRNA continues to be expressed in mature nerve even when Erk1/2 signaling decreases. It is possible that Dusp15 may limit full activation of certain myelin genes, such as Mbp and Mag, during the myelination stage in Schwann cell development through the activation of Erk signaling. It is noted that a preliminary analysis of Dusp15 in oligodendrocytes, showed a similar repression of some myelin genes (Schmidt et al. 2012). Ultimately we predict that myelin gene repression is not the only function by Dusp15 in Schwann cells and a full analysis of the Dusp15 molecule in vivo will require loss-of-function studies in vivo.
The actual targets of Dusp15 are yet to be determined, but since it is myristoylated (Alonso et al. 2004), a posttranslational modification that directs the protein to the cytoplasmic membrane, it is likely that its possible targets may rest in the cytoplasmic side of the cell membrane. However, a reliable antibody has not been identified for Dusp15 to confirm the predicted localization. Although we were unable to validate some putative targets of Dusp15 activity (Schmidt et al. 2012), some potential targets are Fyn and other Src family kinases (Wu et al. 2007), Erbin (Tao et al. 2009, Liang et al. 2012), which have been shown to be necessary for Erk phosphorylation and Schwann cell myelination (Hossain et al. 2010).
Supplementary Material
Acknowledgments
We thank members of our lab and Michael Wegner (FAU, Erlangen, Germany) for helpful comments on the manuscript. This work was supported by grants from National Institutes of Health: NS075269 and NS083841 to JS, HD03352 P30 core grant. JRM was supported by the National Science Foundation Graduate Research Fellowship Program DGE-1256259 and the UW-Madison Advanced Opportunity Fellowship (SciMed GRS).
Abbreviations
- Dusp15
Dual specificity phosphatase 15
- Egr2
Early growth response 2
- Mbp
Myelin basic protein
- Myrf
Myelin regulatory factor
- Plp1
Proteolipid protein 1
- YY1
Ying-Yang 1
- ChIP-seq
Chromatin Immunoprecipitation-Sequencing
- TSS
Transcription start site
- Mag
Myelin associated glycoprotein
- PNS
Peripheral nervous system
- siRNA
short interfering RNA
- Dhh
Dessert hedgehog
- Mpz
Myelin protein zero
- Erk
Extracellular signal-regulated kinases
- CRISPR
clustered regularly interspaced short palindromic repeats
- CCL2
chemokine C-C motif ligand 2
- Pmp22
Peripheral myelin protein 22
- Cx32
Connexin 32
- MEK
MAPK/ERK kinase
- PMA
phorbol-12-myristate-13-acetate
- UTR
untranslated region
- Kegg
Kyoto Encyclopedia of Genes and Genomes
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors have no conflict of interest to declare.
References
- Alonso A, Narisawa S, Bogetz J, et al. VHY, a novel myristoylated testis-restricted dual specificity protein phosphatase related to VHX. J Biol Chem. 2004;279:32586–32591. doi: 10.1074/jbc.M403442200. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Calvo E, Vallières N, Lacroix S. Transcriptional profiling of the injured sciatic nerve of mice carrying the Wld(S) mutant gene: identification of genes involved in neuroprotection, neuroinflammation, and nerve regeneration. Brain Behav Immun. 2010;24:1254–1267. doi: 10.1016/j.bbi.2010.07.249. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Girard M, Pingault V, Lemort N, Dubourg O, Goossens M. Human Connexin 32, a gap junction protein altered in the X-linked form of Charcot-Marie-Tooth disease, is directly regulated by the transcription factor SOX10. Hum Mol Genet. 2001;10:2783–2795. doi: 10.1093/hmg/10.24.2783. [DOI] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalka H, Koenning M, Jackson S, et al. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 2013;11:e1001625. doi: 10.1371/journal.pbio.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carenini S, Montag D, Cremer H, Schachner M, Martini R. Absence of the myelin-associated glycoprotein (MAG) and the neural cell adhesion molecule (N-CAM) interferes with the maintenance, but not with the formation of peripheral myelin. Cell Tissue Res. 1997;287:3–9. doi: 10.1007/s004410050727. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Preux AS, Goosen K, Zhang W, et al. SREBP-1c expression in Schwann cells is affected by diabetes and nutritional status. Mol Cell Neurosci. 2007;35:525–534. doi: 10.1016/j.mcn.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Emery B. Playing the field: Sox10 recruits different partners to drive central and peripheral myelination. PLoS Genet. 2013;9:e1003918. doi: 10.1371/journal.pgen.1003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy RG, Khachigian LM. Locked nucleic acid modified DNA enzymes targeting early growth response-1 inhibit human vascular smooth muscle cell growth. Nucleic Acids Res. 2004;32:2281–2285. doi: 10.1093/nar/gkh543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D, McClure K, Kazlauskas A, Lander ES. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell. 1999;97:727–741. doi: 10.1016/s0092-8674(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm E, Bösl M, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Weishaupt A, Troppmair J, Martini R. Increase of MCP-1 (CCL2) in myelin mutant Schwann cells is mediated by MEK-ERK signaling pathway. Glia. 2008;56:836–843. doi: 10.1002/glia.20657. [DOI] [PubMed] [Google Scholar]
- Fledrich R, Stassart RM, Klink A, et al. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med. 2014;20:1055–1061. doi: 10.1038/nm.3664. [DOI] [PubMed] [Google Scholar]
- Gaesser JM, Fyffe-Maricich SL. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Charnay P. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 2006;7:52–58. doi: 10.1038/sj.embor.7400573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Birchmeier W. Molecular signaling mechanisms of axon-glia communication in the peripheral nervous system. Bioessays. 2015;37:502–513. doi: 10.1002/bies.201400172. [DOI] [PubMed] [Google Scholar]
- Grossmann KS, Wende H, Paul FE, et al. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci U S A. 2009;106:16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai M, Muja N, DeVries GH, Quarles RH, Patel PI. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J Neurosci Res. 2002;69:497–508. doi: 10.1002/jnr.10327. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. Embo J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kim JY, Dupree J, Tewari A, Melendez-Vasquez C, Svaren J, Casaccia P. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci. 2010;13:1472–1480. doi: 10.1038/nn.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig J, Fröb F, Vogl MR, Hermans-Borgmeyer I, Tamm ER, Wegner M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013;9:e1003907. doi: 10.1371/journal.pgen.1003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain S, Fragoso G, Mushynski WE, Almazan G. Regulation of peripheral myelination by Src-like kinases. Exp Neurol. 2010;226:47–57. doi: 10.1016/j.expneurol.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Huang dW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hung HA, Sun G, Keles S, Svaren J. Dynamic Regulation of Schwann Cell Enhancers after Peripheral Nerve Injury. J Biol Chem. 2015;290:6937–6950. doi: 10.1074/jbc.M114.622878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Dupree JL, Bansal R. Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS. J Neurosci. 2016;36:6471–6487. doi: 10.1523/JNEUROSCI.0299-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J. In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. Journal of Neurochemistry. 2006;98:1678–1687. doi: 10.1111/j.1471-4159.2006.04069.x. [DOI] [PubMed] [Google Scholar]
- Jang SW, Svaren J. Induction of myelin protein zero by early growth response 2 through upstream and intragenic elements. J Biol Chem. 2009;284:20111–20120. doi: 10.1074/jbc.M109.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, Fyffe-Maricich SL. ERK1/2 Activation in Preexisting Oligodendrocytes of Adult Mice Drives New Myelin Synthesis and Enhanced CNS Function. J Neurosci. 2016;36:9186–9200. doi: 10.1523/JNEUROSCI.1444-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Brewer MH, Srinivasan R, Krueger C, Sun G, Charney KN, Keles S, Antonellis A, Svaren J. Distal enhancers upstream of the Charcot-Marie-Tooth type 1A disease gene PMP22. Human Molecular Genetics. 2012;21:1581–1591. doi: 10.1093/hmg/ddr595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Jang SW, Mager GM, Chang LW, Srinivasan R, Gokey NG, Ward RM, Nagarajan R, Svaren J. Interactions of Sox10 and Egr2 in myelin gene regulation. Neuron Glia Biology. 2007;3:377–387. doi: 10.1017/S1740925X08000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Lopez-Anido C, Srinivasan R, Krueger C, Chang LW, Nagarajan R, Svaren J. Regulation of the PMP22 Gene through an Intronic Enhancer. J Neuroscience. 2011;31:4242–4250. doi: 10.1523/JNEUROSCI.5893-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Remacle AG, Chernov AV, et al. The MMP-9/TIMP-1 axis controls the status of differentiation and function of myelin-forming Schwann cells in nerve regeneration. PLoS One. 2012;7:e33664. doi: 10.1371/journal.pone.0033664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, Emery B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J Neurosci. 2012;32:12528–12542. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2005a;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Svaren J, LaPash C, Araki T, Schmidt RE, Milbrandt J. Nab proteins are essential for peripheral nervous system myelination. Nat Neurosci. 2005b;8:932–940. doi: 10.1038/nn1490. [DOI] [PubMed] [Google Scholar]
- LeBlanc SE, Srinivasan R, Ferri C, Mager GM, Gillian-Daniel AL, Wrabetz L, Svaren J. Regulation of cholesterol/lipid biosynthetic genes by Egr2/Krox20 during peripheral nerve myelination. J Neurochem. 2005;93:737–748. doi: 10.1111/j.1471-4159.2005.03056.x. [DOI] [PubMed] [Google Scholar]
- Liang C, Tao Y, Shen C, Tan Z, Xiong WC, Mei L. Erbin is required for myelination in regenerated axons after injury. J Neurosci. 2012;32:15169–15180. doi: 10.1523/JNEUROSCI.2466-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Anido C, Sun G, Koenning M, Srinivasan R, Hung HA, Emery B, Keles S, Svaren J. Differential Sox10 genomic occupancy in myelinating glia. Glia. 2015;63:1897–1914. doi: 10.1002/glia.22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D, Svaren J. Specification of Macroglia by Transcription Factors: Schwann Cells. In: Alvarez-Buylla A, Rowitch D, editors. Patterning and Cell Type Specification in the Developing CNS and PNS. Vol. 1. Elsevier; 2013. pp. 759–770. [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Murphy P, Topilko P, Schneider-Maunoury S, Seitanidou T, Baron-Van Evercooren A, Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122:2847–2857. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- Muth KN, Piefke S, Weider M, Sock E, Hermans-Borgmeyer I, Wegner M, Küspert M. The Dual-specificity phosphatase Dusp15 is regulated by Sox10 and Myrf in Myelinating Oligodendrocytes. Glia. 2016;64:2120–2132. doi: 10.1002/glia.23044. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Le N, Mahoney H, Araki T, Milbrandt J. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proc Natl Acad Sci U S A. 2002;99:8998–9003. doi: 10.1073/pnas.132080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21:922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM. Molecular control of the neural crest and peripheral nervous system development. Curr Top Dev Biol. 2015;111:201–231. doi: 10.1016/bs.ctdb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmén C, Figlia G, Lebrun-Julien F, et al. mTORC1 controls PNS myelination along the mTORC1-RXRγ-SREBP-lipid biosynthesis axis in Schwann cells. Cell Rep. 2014;9:646–660. doi: 10.1016/j.celrep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Noseda R, Guerrero-Valero M, Alberizzi V, et al. Kif13b Regulates PNS and CNS Myelination through the Dlg1 Scaffold. PLoS Biol. 2016;14:e1002440. doi: 10.1371/journal.pbio.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzig J, Jahn O, Tenzer S, et al. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J Neurosci. 2011;31:16369–16386. doi: 10.1523/JNEUROSCI.4016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano RI, Wegner M. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28:3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35:123–134. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Quintes S, Brinkmann BG, Ebert M, et al. Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat Neurosci. 2016;19:1050–1059. doi: 10.1038/nn.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiprich S, Kriesch J, Schreiner S, Wegner M. Activation of Krox20 gene expression by Sox10 in myelinating Schwann cells. J Neurochem. 2010;112:744–754. doi: 10.1111/j.1471-4159.2009.06498.x. [DOI] [PubMed] [Google Scholar]
- Roche SL, Sherman DL, Dissanayake K, et al. Loss of glial neurofascin155 delays developmental synapse elimination at the neuromuscular junction. J Neurosci. 2014;34:12904–12918. doi: 10.1523/JNEUROSCI.1725-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Axonal regulation of Schwann cell ensheathment and myelination. J Peripher Nerv Syst. 2012;17(Suppl 3):14–19. doi: 10.1111/j.1529-8027.2012.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F, van den Eijnden M, Pescini Gobert R, et al. Identification of VHY/Dusp15 as a regulator of oligodendrocyte differentiation through a systematic genomics approach. PLoS One. 2012;7:e40457. doi: 10.1371/journal.pone.0040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheean ME, McShane E, Cheret C, et al. Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev. 2014;28:290–303. doi: 10.1101/gad.230045.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Sun G, Keles S, Jones EA, Jang SW, Krueger C, Moran JJ, Svaren J. Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve. Nucleic Acids Res. 2012;40:6449–6460. doi: 10.1093/nar/gks313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Wegner M. Schwann cells and their transcriptional network: Evolution of key regulators of peripheral myelination. Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirnoff AH, Milbrandt J. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Mol Cell Biol. 1995;15:2275–2287. doi: 10.1128/mcb.15.4.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Dai P, Liu Y, Marchetto S, Xiong WC, Borg JP, Mei L. Erbin regulates NRG1 signaling and myelination. Proc Natl Acad Sci U S A. 2009;106:9477–9482. doi: 10.1073/pnas.0901844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K, Small JA, Goda S, Quarles RH. Biochemical and cellular properties of three immortalized Schwann cell lines expressing different levels of the myelin-associated glycoprotein. J Neurochem. 1994;63:1646–1657. doi: 10.1046/j.1471-4159.1994.63051646.x. [DOI] [PubMed] [Google Scholar]
- Topilko P, Levi G, Merlo G, Mantero S, Desmarquet C, Mancardi G, Charnay P. Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J Neurosci Res. 1997;50:702–712. doi: 10.1002/(SICI)1097-4547(19971201)50:5<702::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier V, Csárdi G, de Preux-Charles AS, Médard JJ, Smit AB, Verheijen MH, Bergmann S, Chrast R. Aging of myelinating glial cells predominantly affects lipid metabolism and immune response pathways. Glia. 2012;60:751–760. doi: 10.1002/glia.22305. [DOI] [PubMed] [Google Scholar]
- Verheijen MH, Camargo N, Verdier V, et al. SCAP is required for timely and proper myelin membrane synthesis. Proc Natl Acad Sci U S A. 2009;106:21383–21388. doi: 10.1073/pnas.0905633106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen MH, Chrast R, Burrola P, Lemke G. Local regulation of fat metabolism in peripheral nerves. Genes Dev. 2003;17:2450–2464. doi: 10.1101/gad.1116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weider M, Reiprich S, Wegner M. Sox appeal - Sox10 attracts epigenetic and transcriptional regulators in myelinating glia. Biol Chem. 2013;394:1583–1593. doi: 10.1515/hsz-2013-0146. [DOI] [PubMed] [Google Scholar]
- Weng Q, Chen Y, Wang H, et al. Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron. 2012;73:713–728. doi: 10.1016/j.neuron.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Ma MH, Brown KR, Geisler M, Li L, Tzeng E, Jia CY, Jurisica I, Li SS. Systematic identification of SH3 domain-mediated human protein-protein interactions by peptide array target screening. Proteomics. 2007;7:1775–1785. doi: 10.1002/pmic.200601006. [DOI] [PubMed] [Google Scholar]
- Wu LM, Wang J, Conidi A, et al. Zeb2 recruits HDAC-NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat Neurosci. 2016;19:1060–1072. doi: 10.1038/nn.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, Li C, Roder J, Trapp BD. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci. 1998;18:1953–1962. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Y, Kim B, et al. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell. 2013;152:248–261. doi: 10.1016/j.cell.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The Transcription Factors SCIP and Krox-20 Mark Distinct Stages and Cell Fates in Schwann Cell Differentiation. Mol Cell Neurosci. 1996;8:129–145. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.