Abstract
Haplotypes of the complement 4 (C4) and steroid 21-hydroxylase [21-OHase; steroid hydrogen-donor: oxygen oxidoreductase (21-hydroxylating), EC 1.14.99.10] repeated gene complex were studied in nine families with at least one member affected with a mild form of 21-OHase deficiency. DNA probes from different parts of the repeated C4/21-OHase unit were used to follow the segregation of hybridization patterns in the families. Ten structurally distinct haplotypes of the C4/21-OHase gene region were identified, and the encoded phenotype was assigned to 34 of the 36 C4/21-OHase haplotypes. Four structurally different haplotypes with three C4/21-OHase repeat units were found. Eight of the nine haplotypes found with triplications of the C4/21-OHase repeat unit encoded the mild form of 21-OHase deficiency, whereas one particular triplicated haplotype encoded a severe form of the disease. In one case the mild form of 21-OHase deficiency was encoded by a haplotype with a single C4/21-OHase repeat unit. Mild 21-OHase deficiency was predicted in a patient by the presence of a triplicated haplotype. The finding of deranged 21-OHase genes on all triplicated C4/21-OHase haplotypes indicate that most of these common haplotypes carry mutated 21-OHase genes, and thus may cause functional polymorphism of general importance in the population.
Full text
PDF
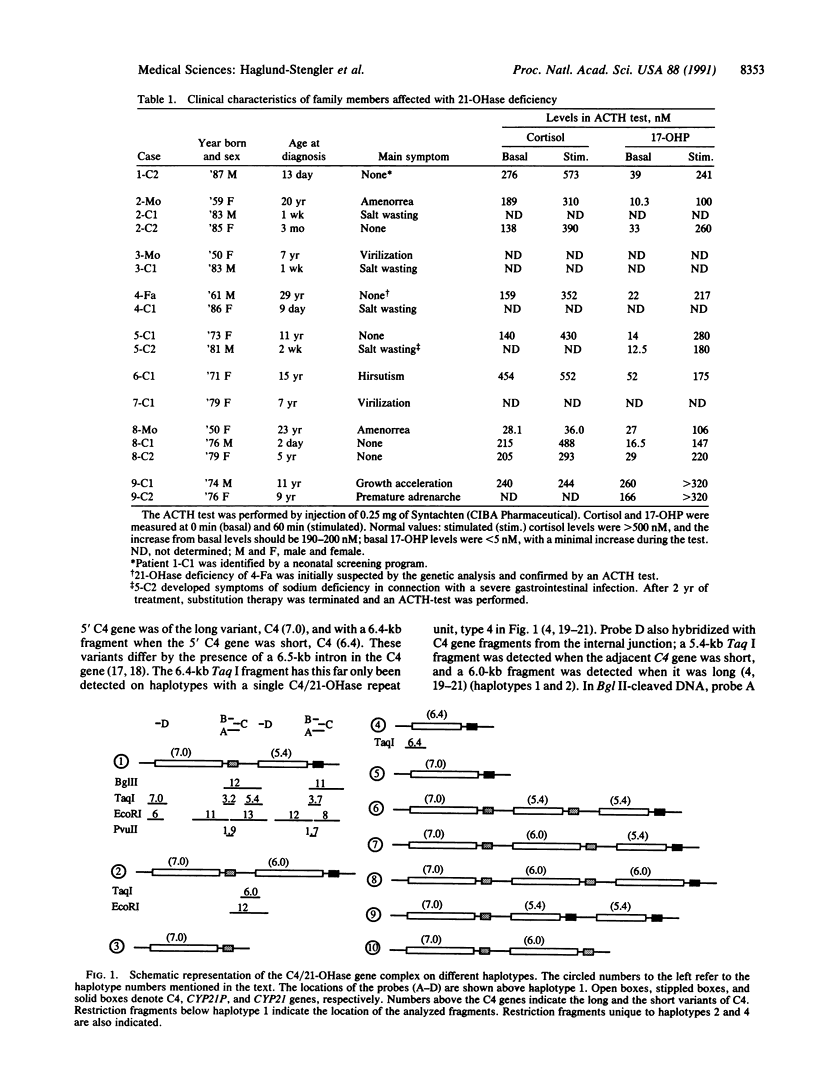
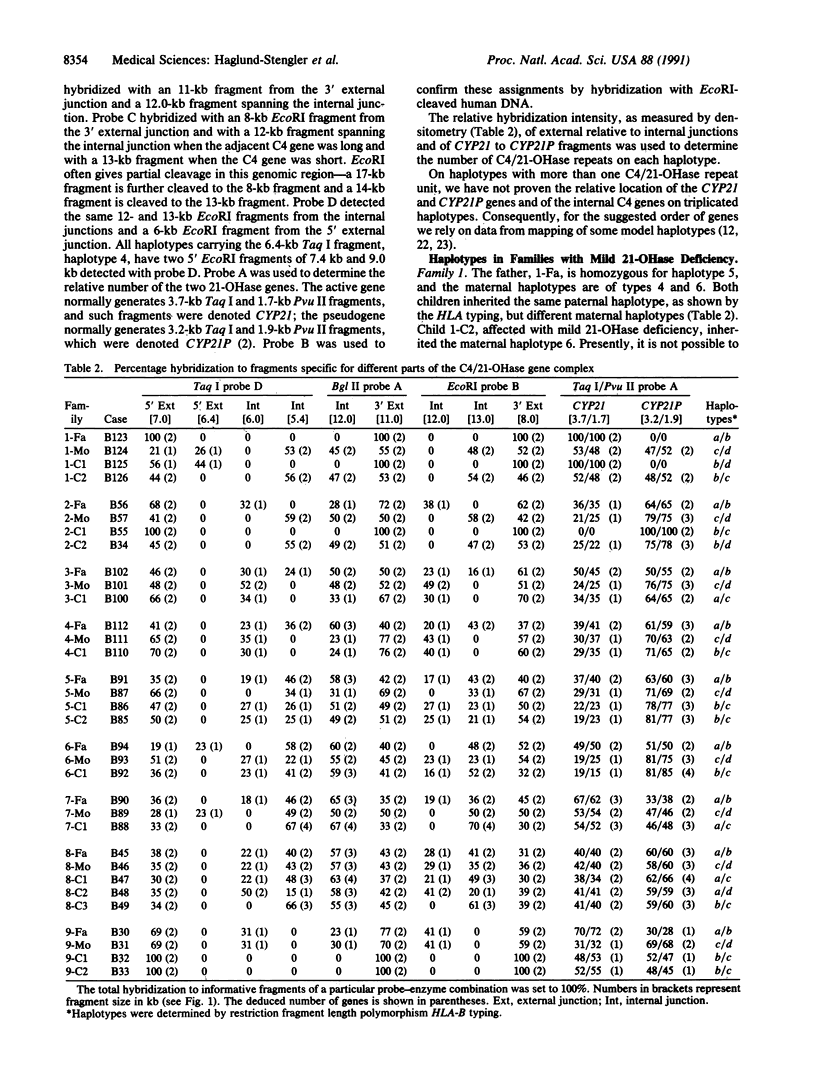


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Belt K. T., Palsdottir A., Yu Y. Molecular genetics of the fourth component of human complement and steroid 21-hydroxylase. Immunol Rev. 1985 Oct;87:39–60. doi: 10.1111/j.1600-065x.1985.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Porter R. R. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci U S A. 1985 Jan;82(2):521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Palsdottir A., Belt K. T., Porter R. R. Deletion of complement C4 and steroid 21-hydroxylase genes in the HLA class III region. EMBO J. 1985 Oct;4(10):2547–2552. doi: 10.1002/j.1460-2075.1985.tb03969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S., Sinnott P. J., Dyer P. A., Price D. A., Harris R., Strachan T. Pulsed field gel electrophoresis identifies a high degree of variability in the number of tandem 21-hydroxylase and complement C4 gene repeats in 21-hydroxylase deficiency haplotypes. EMBO J. 1989 May;8(5):1393–1402. doi: 10.1002/j.1460-2075.1989.tb03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham I., Sargent C. A., Dawkins R. L., Campbell R. D. Direct observation of the gene organization of the complement C4 and 21-hydroxylase loci by pulsed field gel electrophoresis. J Exp Med. 1989 May 1;169(5):1803–1818. doi: 10.1084/jem.169.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund-Stengler B., Ritzén E. M., Luthman H. 21-hydroxylase deficiency: disease-causing mutations categorized by densitometry of 21-hydroxylase-specific deoxyribonucleic acid fragments. J Clin Endocrinol Metab. 1990 Jan;70(1):43–48. doi: 10.1210/jcem-70-1-43. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Morel Y. The molecular genetics of 21-hydroxylase deficiency. Annu Rev Genet. 1989;23:371–393. doi: 10.1146/annurev.ge.23.120189.002103. [DOI] [PubMed] [Google Scholar]
- Mornet E., Couillin P., Kutten F., Raux M. C., White P. C., Cohen D., Boué A., Dausset J. Associations between restriction fragment length polymorphisms detected with a probe for human 21-hydroxylase (21-OH) and two clinical forms of 21-OH deficiency. Hum Genet. 1986 Dec;74(4):402–408. doi: 10.1007/BF00280494. [DOI] [PubMed] [Google Scholar]
- Olerup O., Luthman H., Ritzén E. M., Haglund-Stengler B. TaqI HLA-B and -DRB RFLP analysis can predict disease in siblings of affected children with 21-hydroxylase deficiency. Hum Genet. 1990 Oct;85(5):467–472. doi: 10.1007/BF00194218. [DOI] [PubMed] [Google Scholar]
- Palsdottir A., Fossdal R., Arnason A., Edwards J. H., Jensson O. Heterogeneity of human C4 gene size. A large intron (6.5 kb) is present in all C4A genes and some C4B genes. Immunogenetics. 1987;25(5):299–304. doi: 10.1007/BF00404422. [DOI] [PubMed] [Google Scholar]
- Partanen J., Kere J., Wessberg S., Koskimies S. Determination of deletion sizes in the MHC-linked complement C4 and steroid 21-hydroxylase genes by pulsed-field gel electrophoresis. Genomics. 1989 Aug;5(2):345–349. doi: 10.1016/0888-7543(89)90067-0. [DOI] [PubMed] [Google Scholar]
- Pollack M. S., Levine L. S., O'Neill G. J., Pang S., Lorenzen F., Kohn B., Rondanini G. F., Chiumello G., New M. I., Dupont B. HLA linkage and B14, DR1, BfS haplotype association with the genes for late onset and cryptic 21-hydroxylase deficiency. Am J Hum Genet. 1981 Jul;33(4):540–550. [PMC free article] [PubMed] [Google Scholar]
- Schneider P. M., Carroll M. C., Alper C. A., Rittner C., Whitehead A. S., Yunis E. J., Colten H. R. Polymorphism of the human complement C4 and steroid 21-hydroxylase genes. Restriction fragment length polymorphisms revealing structural deletions, homoduplications, and size variants. J Clin Invest. 1986 Sep;78(3):650–657. doi: 10.1172/JCI112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. L., Aston C. E., Morton N. E., Speiser P. W., New M. I. A segregation and linkage study of classical and nonclassical 21-hydroxylase deficiency. Am J Hum Genet. 1988 Jun;42(6):830–838. [PMC free article] [PubMed] [Google Scholar]
- Sinnott P., Collier S., Costigan C., Dyer P. A., Harris R., Strachan T. Genesis by meiotic unequal crossover of a de novo deletion that contributes to steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2107–2111. doi: 10.1073/pnas.87.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser P. W., Dupont B., Rubinstein P., Piazza A., Kastelan A., New M. I. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985 Jul;37(4):650–667. [PMC free article] [PubMed] [Google Scholar]
- Teisberg P., Jonassen R., Mevåg B., Gedde-Dahl T., Jr, Olaisen B. Restriction fragment length polymorphisms of the complement component C4 loci on chromosome 6: studies with emphasis on the determination of gene number. Ann Hum Genet. 1988 May;52(Pt 2):77–84. doi: 10.1111/j.1469-1809.1988.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Saueracker G., Kay P. H., Christiansen F. T., Anand R., Dawkins R. L. Extensive deletions and insertions in different MHC supratypes detected by pusled field gel electrophoresis. J Exp Med. 1988 Sep 1;168(3):933–940. doi: 10.1084/jem.168.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkmeister J. W., New M. I., Dupont B., White P. C. Frequent deletion and duplication of the steroid 21-hydroxylase genes. Am J Hum Genet. 1986 Oct;39(4):461–469. [PMC free article] [PubMed] [Google Scholar]
- White P. C., Grossberger D., Onufer B. J., Chaplin D. D., New M. I., Dupont B., Strominger J. L. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1089–1093. doi: 10.1073/pnas.82.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Congenital adrenal hyperplasia. (1). N Engl J Med. 1987 Jun 11;316(24):1519–1524. doi: 10.1056/NEJM198706113162406. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. Y., Belt K. T., Giles C. M., Campbell R. D., Porter R. R. Structural basis of the polymorphism of human complement components C4A and C4B: gene size, reactivity and antigenicity. EMBO J. 1986 Nov;5(11):2873–2881. doi: 10.1002/j.1460-2075.1986.tb04582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. Y., Campbell R. D. Definitive RFLPs to distinguish between the human complement C4A/C4B isotypes and the major Rodgers/Chido determinants: application to the study of C4 null alleles. Immunogenetics. 1987;25(6):383–390. doi: 10.1007/BF00396104. [DOI] [PubMed] [Google Scholar]



