Abstract
The ability to modulate the future liver remnant (FLR) is a key component of modern oncologic hepatobiliary surgery practice and has extended surgical candidacy for patients who may have been previously thought unable to survive liver resection. Multiple techniques have been developed to augment the FLR including portal vein embolization (PVE), associating liver partition and portal vein ligation (ALPPS), and the recently reported transhepatic liver venous deprivation (LVD). PVE is a well-established means to improve the safety of liver resection by redirecting blood flow to the FLR in an effort to selectively hypertrophy and ultimately improve functional reserve of the FLR. This article discusses the current practice of PVE with focus on summarizing the large number of published reports from which outcomes based practices have been developed. Both technical aspects of PVE including volumetry, approaches, and embolization agents; and clinical aspects of PVE including data supporting indications, and its role in conjunction with chemotherapy and transarterial embolization will be highlighted. PVE remains an important aspect of oncologic care; in large part due to the substantial foundation of information available demonstrating its clear clinical benefit for hepatic resection candidates with small anticipated FLRs.
Keywords: Portal vein, embolization, future liver remnant, hypertrophy, liver regeneration, liver cancer, hepatocellular carcinoma, liver metastases
Introduction
Extensive liver resections are increasingly being performed for the treatment of both primary and metastatic liver tumors. The safety of major liver resection is contingent upon the anticipated remaining liver after resection, commonly described as the future liver remnant (FLR)1,2. The FLR is a critical determinant associated with the risk of perioperative liver failure and death3. The ability to modulate the FLR is a key component of modern oncologic hepatobiliary surgery practice and has extended surgical candidacy for patients who may have been previously thought unable to survive liver resection. Multiple techniques have been developed to augment the FLR including portal vein embolization (PVE), associating liver partition and portal vein ligation (ALPPS), and the recently reported transhepatic liver venous deprivation (LVD)4-6.
PVE serves as a well-established means to improve the safety of liver resection by redirecting blood flow to the FLR in an effort to hypertrophy and ultimately improve functional reserve of the spared liver7-10. In appropriately selected patients, PVE can reduce perioperative morbidity and allow for safe, potentially curative hepatectomy for patients previously considered ineligible for resection based on anticipated small remnant livers8,9,11-18. For this patient subset, PVE is now utilized as the standard of care at many comprehensive hepatobiliary centers prior to major hepatectomy. This review will summarize the essential role of PVE as an adjunct to major hepatectomy, with focus on mechanisms, technique, and clinical outcomes.
Mechanisms of liver regeneration
The liver’s unique ability to regenerate following injury or resection has been studied and documented for centuries. The liver’s capacity to regenerate can be referenced as early as 750–700 B.C. in Hesiod’s Theogony19. In the setting of either liver injury or resection, massive hepatocyte proliferation can occur resulting in recovery of functional liver mass within 2 weeks after loss of upwards of two-thirds of the liver20. This regenerative response is typically mediated by the proliferation of surviving hepatocytes within the acinar architecture of the remnant liver. This response results in hypertrophy of the remnant liver rather than restoration of the resected lobes20.
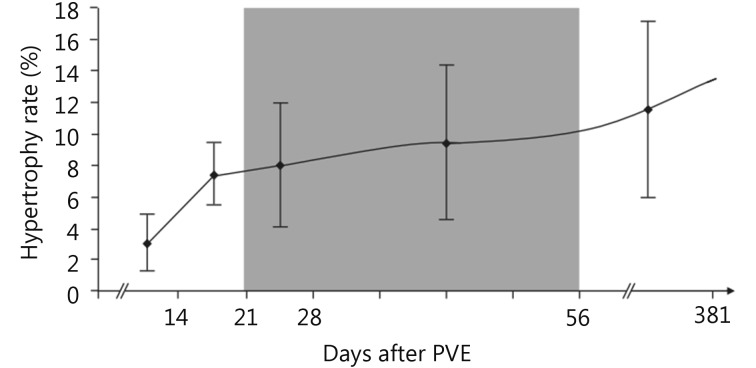
Regeneration of the liver is dependent on both the stimulus of injury and the condition of the liver parenchyma. Hepatocyte proliferation is proportional to the severity of the liver injury/resection; minor injuries (< 10% parenchymal involvement) induce only localized mitotic reactions while major injuries (> 50% parenchymal involvement) result in multiple mitotic waves throughout the entire liver19. Regeneration rates are dependent on the time from injury, with greatest rate of regeneration after PVE typically occurring within the first two weeks (Figure 1)21.
1.
Degree of hypertrophy of the sFLR over time after PVE with kinetics of FLR growth, plotted as median degree of hypertrophy after PVE (with interquartile ranges). The shaded zone, days 22-56 after PVE, represents the “plateau” period during which the degree of hypertrophy did not change significantly between measurement points. Used with permission from Ref.21.
Most information about the molecular and cellular events during liver regeneration comes from studies of partial hepatectomy in animal models19. Growth-factor stimulation in response to injury induces the production of cytokines and activates immediate response genes that signal hepatocytes for cell cycle progression and regeneration. Hepatocyte growth factor (HGF) is the most potent mitogen; other mitogens include transforming growth factor-α and epidermal growth factor. Insulin is synergistic with HGF and slower regeneration rates are seen in patients with diabetes22,23. Extra-hepatic factors are transported primarily from the gut via the portal vein and not the hepatic artery24-27.
PVE redirects portal flow to the intended FLR in an attempt to initiate hypertrophy of the non-embolized segments. Portal blood flow to the non-embolized hepatic segments measured by Doppler sonography increases significantly and then falls to near-baseline values after 11 days. The resultant hypertrophy rate correlates with the portal flow rate28,29. The predominant mechanism of cell death after PVE is cell-mediated apoptosis rather than necrosis observed after transarterial embolization (TAE)30. Direct correlation is observed clinically: compared to TAE, PVE is typically not associated with post-embolization syndrome, as characterized by nausea, fever, and pain.
Techniques for liver regeneration
As early as 1990, Makuuchi et al.15 first reported on the utility of PVE in promoting future liver remnant hypertrophy prior to hepatic resection in 14 patients with hilar cholangio-carcinoma. Since that time, PVE has continued to gain traction as a well-established technique to hypertrophy the FLR. Although this review will focus on the topic of PVE, alternative techniques have recently been proposed to hypertrophy the FLR, including ALPPS and transhepatic LVD4-6.
ALPPS is a surgical technique of parenchymal dissection of the liver in combination with ligation of the right portal vein and portal branches to segment 4 staged prior to resection of the diseased liver. Schnitzbauer et al.31 first reported on the outcomes of ALPPS for patients with bilobar disease burden which would previously have been considered ineligible for surgery. These patients were made eligible for two stage hepatectomy with assistance of the ALPPS procedure. Proponents of ALPPS suggest that greater, more rapid liver hypertrophy occurs with the technique as compared to PVE6,32. However, recent studies have raised concerns over increased morbidity and mortality associated with the procedure as compared to PVE; histologically the regenerative hepatocytes observed in ALPPS are immature as compared to those observed with PVE33. These findings suggest that though ALPPS is associated with greater absolute size of FLR, the size does not correlate with greater functional increase as compared to PVE. A meta-analysis comparing ALPPS vs. portal vein occlusion was recently performed by Eshmuminov et al.34. Data from 4, 352 patients pooled from 90 studies demonstrated that though ALPPS was associated with greater hypertrophy of the future liver remnant (76% vs. 37%; P < 0.001) and completion of second stage hepatectomy (100%vs. 77%; P < 0.001); ALPPS demonstrated trends towards higher morbidity (73% vs. 59%; P = 0.16) and mortality (14% vs. 7%; P = 0.19) as compared to PVE34.
Transhepatic LVD is in its infancy, and only limited reports are available to support its clinical adoption. Hwang et al.35,36 first reported on a series of patients who initially underwent PVE with inadequate hypertrophy of the FLR, and then underwent subsequent hepatic vein embolization with improved hypertrophy that resulted in surgical candidacy. From their work, the concept of total venous deprivation was developed. In LVD, both portal and hepatic vein embolization is carried out during the same procedure. The potential benefit of LVD as compared to PVE is more massive and rapid hypertrophy, similar to ALPPS. Guiu et al.5 reported on a series of 7 patients who underwent LVD, with technical success achieved in all patients. FLR was reported to increase from 28.2% (22.4%–33.3%) to 40.9% (33.6%–59.3%%) over a 23-day period. Histologic evaluation of the resected liver after LVD demonstrated features similar to those expected for PVE, with the authors concluding that important atrophy was achieved. Greater clinical experience needs to be cumulated to define the role of LVD as compared to PVE to hypertrophy the FLR.
PVE technique
FLR volumetry and predicting liver function after PVE
PVE is only indicated when the anticipated FLR is insufficient to support hepatic function after liver resection. The presence or absence of underlying liver disease has a major impact on the volume of liver remnant needed for adequate function, hence baseline Child Pugh status is critical in predicting liver function (discussed in detail in the outcomes section). Accurate calculation of the FLR is essential for triaging appropriate hepactectomy candidates for which PVE is indicated. Liver volume is directly correlated with a patient’s size; hence, normalizing the anticipated liver volume to a patient’s size results in a more accurate assessment of the FLR10,37. This principle led to the proposal and clinical validation of a standardized FLR (sFLR) by Vauthey et al.10 expressed as a ratio of the FLR over the total estimated functioning liver volume (TELV): sFLR=FLR/TELV.
Several methods have been used to measure TELV including those based upon body surface area (BSA), computed tomography (CT) volumetry, or body weight. Through the analysis of a Western population with normal livers, Vauthey et al.38,39 derived the following formula for estimating TELV based upon BSA: TELV=-794.41 + 1, 267.28 × (BSA). This formula is commonly used in practice due to its relative accuracy and ease of obtaining measurements as compared to other techniques (Figure 2). Alternative methods that have been used to measure TLV include CT volumetry and body weight. CT volumetry is accurate within ± 5% of estimating normal liver parenchymal volumes10,40. However, determining TLV from CT volumetry can be tedious since measurements of the tumor volume must be performed and excluded from the overall liver volume. Ribero et al.41 identified a subset of patients for whom CT volumetry underestimated the risk of hepatic insufficiency, suggesting BSA as the more accurate method. However, a more recent study by Leung et al.42 demonstrated that measured volumetrics using CT volumetry with subtraction methods correlated with outcomes better than the estimated volumetrics using the BSA method; however their experience was reported from a single tertiary center.
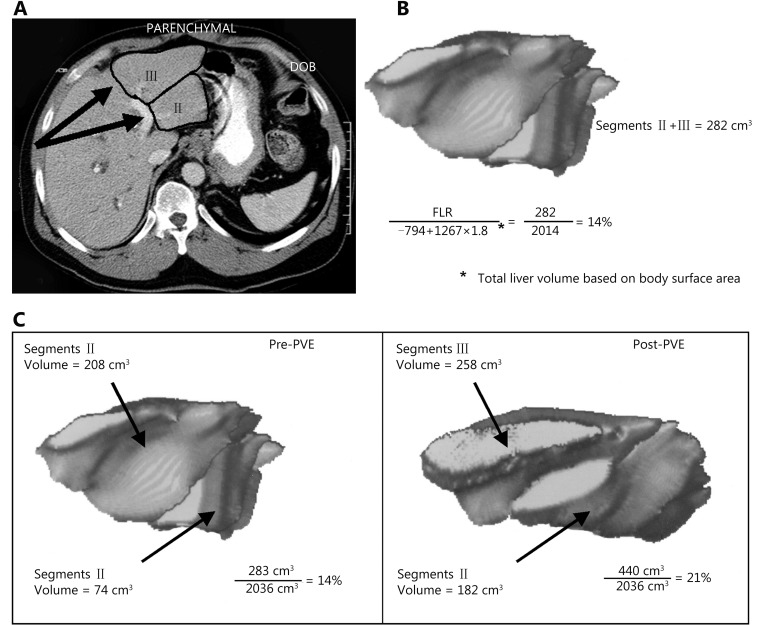
2.
Hypertrophy of the FLR after PVE as determined by three-dimensional reconstruction of CT images. (A) Three-dimensional volumetric measurements are determined by outlining the hepatic segmental contours and then calculating the volumes from the surface measurements of each slice. (B) The formula for calculating total liver volume is based on the patient’s body surface area. (C) Before embolization, the volume of segments 2 and 3 was 283 cm3, or 14% of the total liver volume (2, 036 cm3). After embolization, the volume of segments 2 and 4 was 440 cm3, or 21% of the total liver volume (a degree of hypertrophy of 7%). B was modified from Vauthey et al.39 and C was modified from Vauthey et al.10 with permission.
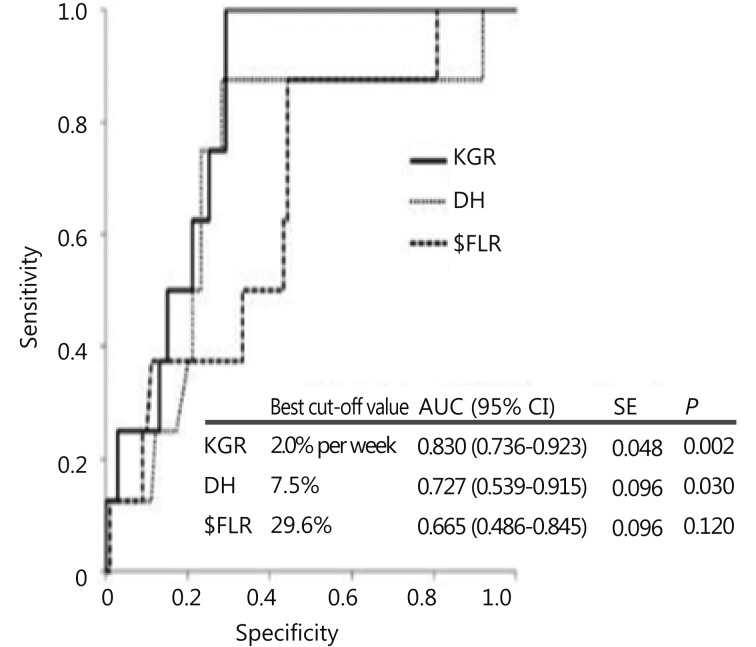
Recent studies have expanded on alternative predictors of postoperative liver function in addition to FLR. Through the analysis of a series of 107 patients who underwent right PVE and subsequent resection, Shindoh et al.43 proposed the kinetic growth rate (defined as degree of hypertrophy at initial volume assessment divided by number of weeks elapsed after PVE) as a predictor of postoperative complications after hepatectomy as compared to the sFLR. The kinetic growth rate was found to be the most accurate predictor of postoperative hepatic insufficiency and mortality when compared to sFLR or degree of hypertrophy measurements using receiver operating characteristic analysis. Of the three measures, a kinetic growth rate cutoff value of < 2.0%/week demonstrated the highest accuracy (81%) with sensitivity of 100% and specificity of 71% in predicting postoperative hepatic insufficiency (Figure 3).
3.
Receiver operating characteristic curves for measured volume parameters in the prediction of postoperative hepatic insufficiency. Area under the curve (AUC) calculated for kinetic growth rate (KGR), degree of hypertrophy (DH), and sFLR. P values represent asymptotic significance (null hypothesis, AUC = 0.500). Modified with permission from Ref.43
Several groups have begun investigating the effect of PVE on parameters of liver function in addition to liver volumetrics. Indocyanine green is a dye that binds to plasma proteins that is almost exclusively removed from the body by the liver via a carrier mediated mechanism44. As such, indocyanine green retention at fifteen minutes (ICGR15) serves as a surrogate quantitative measure of liver function and has been validated in clinical series to be helpful in prediction of post-surgical outcomes. ICGR15 is incorporated as a pivotal parameter in the seminal criteria for safe liver resection proposed by Makuuchi et al.45 in 1993. In a retrospective analysis by Mihara et al.46, indocyanine green plasma clearance rate (KICG) was incorporated with anticipated future liver remnant (FRLV): (KICG × FRLV)/total liver volume to create a new predictor of anticipated liver function (Krem) that correlated well with expected postoperative liver insufficiency in a series of 172 patients. Interestingly, a study by Meier et al.47 demonstrated that preoperative PVE positively influenced postoperative liver function independently from changes expected from increase in liver volume alone, suggesting that PVE not only increases the postoperative volume but also the functional capacity of the FLR. This result is congruent with the improved postoperative hepatic function outcomes associated with PVE as compared to ALPPS despite the smaller relative hypertrophy noted in the PVE as compared to ALPPS cohorts.
Approaches
PVE is performed to redirect portal blood flow toward the liver that remains after surgery (i.e. the FLR) to promote its hypertrophy prior to resection of the tumor bearing liver. To ensure adequate hypertrophy, embolization of portal branches must be as complete as possible so that recanalization of the occluded portal system is minimized. The entire portal system to be resected must be occluded to avoid the development of intrahepatic portoportal collaterals that may limit regeneration.
PVE is typically performed percutaneously and access gained to the portal venous system through several approaches. The most commonly performed technique is transhepatic portal access; either via the FLR (contralateral) or via the liver to be resected (ipsilateral) approaches. These approaches are chosen based on operator preference, type of hepatic resection planned, extent of embolization [e.g., right PVE without or with extension to segment 4 (RPVE or RPVE + 4)] and type of embolic agent used.
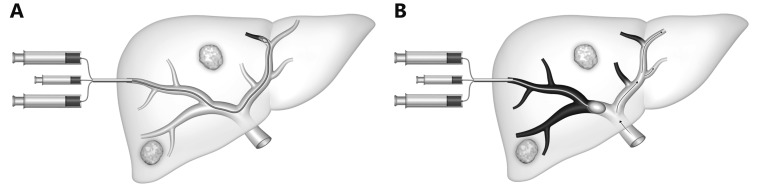
In the transhepatic contralateral approach, developed by Kinoshita et al.48, a branch of the left portal system (usually segment 3) is accessed, and the catheter is advanced into the right portal venous system for embolization (Figure 4). The major advantage of this approach is that catheterization of the desired right portal vein branches is more direct via the left system than via the right, making the procedure technically easier. However, the disadvantage of this technique is the risk of injury to the FLR parenchyma and the left portal vein.
4.
Schematic representation of the contralateral approach. An occlusion balloon catheter is placed from the left lobe into right portal branch, with delivery of the embolic agent in the anterograde direction.
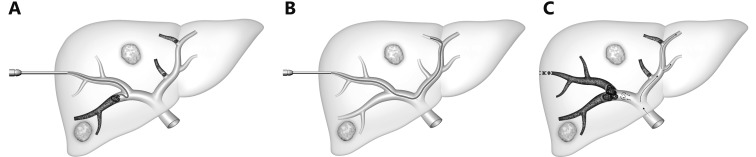
In the transhepatic ipsilateral approach, first described by Nagino et al.49, a peripheral portal vein branch in the liver to be resected is accessed, through which the embolic material is administered (Figure 5). Because Nagino’s ipsilateral approach required the use of specialized catheters, modifications of the ipsilateral technique have been developed with standard angiographic catheters used for combined particulate and coil embolization (Figure 6)50-52. When right hepatectomy is planned, RPVE is performed (Figure 7). One advantage of the ipsilateral approach is that the anticipated liver remnant is not instrumented. However, catheterization of the right portal vein branches may be more difficult because of severe angulations between right portal branches, necessitating the use of reverse-curved catheters. Another potential disadvantage of this approach is that some embolic material could be displaced upon catheter removal.
5.
Schematic representation of the ipsilateral approach for RPVE and segment 4 as described by Nagino et al.17. Different portions of the balloon catheter are used for antegrade embolization of segment 4 veins (A) and for retrograde delivery of the embolic agent into the right portal system (B).
6.
Schematic representation shows modification of the ipsilateral technique for RPVE extended to segment 4. (A) Placement of a 6F vascular sheath into the right portal branch. An angled 5F catheter is placed into the left portal system with coaxial placement of a microcatheter into a segment 4 branch. Particulate embolization is performed, followed by placement of coils, until all the branches are occluded. (B) After segment 4 embolization is completely occluded, a 5F reverse-curve catheter is used for RPVE. (C) After PVE is complete, the access tract is embolized with coils and/or gelfoam to prevent subcapsular hemorrhage.
7.
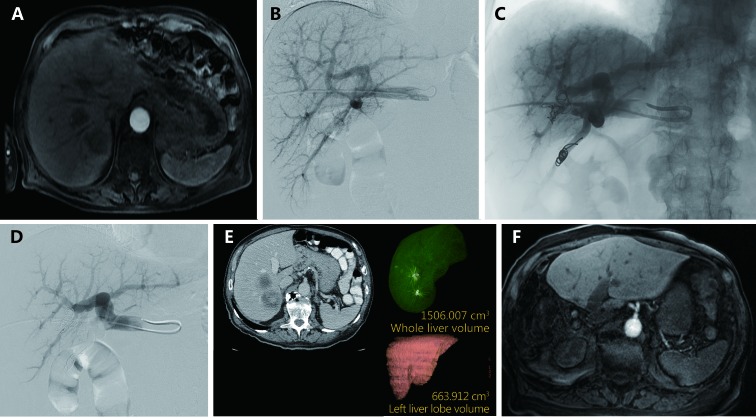
Transhepatic ipsilateral RPVE in an 87 year old male with two segment 7 colorectal metastases prior to right hepactectomy. (A) Axial contrast enhanced MRI demonstrating two colorectal metastases in segment 7 prior to RPVE. (B) Pre-embolization portogram demonstrates patent conventional portal anatomy. (C) Intra-procedural fluoroscopic image demonstrating reduction of segment 7 flow after embolization with tris-acryl microspheres and coils. (D) Post-embolization portogram shows complete occlusion of branches of the right portal vein and patency of the left portal vein. (E) A single image from post-PVE contrast-enhanced CT scan shows hypertrophy of the left liver. The FLR/TELV increased to 43%. (F) Axial contrast enhanced MRI status after right hepatectomy demonstrates hypertrophied left liver with no evidence of disease.
The ipsilateral approach also allows operators to more readily perform segment 4 embolization without the sharp angulations encountered when trying to cannulate segment 4 from a contralateral approach. When two stage or extended right hepatectomy is planned, RPVE is extended to segment 4 (RPVE + 4) (Figure 8). Ipsilateral RPVE ± 4 is performed after a 5- or 6F sheath is placed into a distal right portal vein branch. When RPVE + 4 is needed, segment 4 embolization is performed first so as to not manipulate catheters through previously embolized segments. A microcatheter is advanced coaxially through an angled catheter into the portal vein branches in segment 4 so that particulate embolics followed by coils can be delivered. Once segment 4 embolization is completed, a reverse-curve catheter is often needed for RPVE. After complete occlusion of the right portal vein, embolization of the access tract is performed with coils and/or gelfoam to reduce the risk of perihepatic hemorrhage at the puncture site.
8.
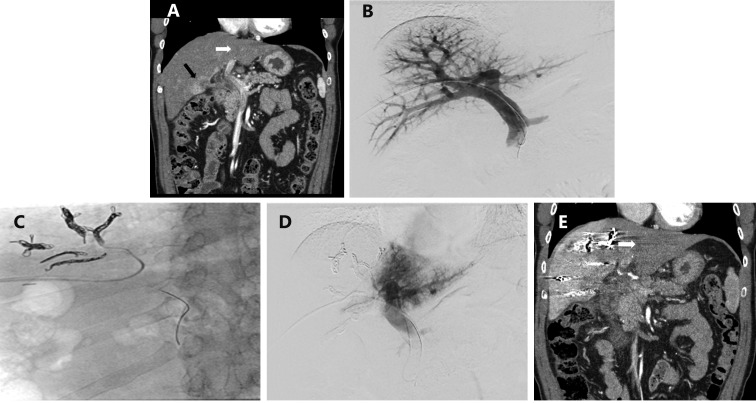
Transhepatic ipsilateral right PVE extended to segment 4 using tris-acryl particles and coils performed in a 48 year old male with cholangiocarcinoma involving segments 4 and 5. (A) Contrast-enhanced coronal CT image of the liver demonstrated an enhancing mass centered in segment 5 (black arrow) and the normal appearing left lateral liver (white arrow) prior to PVE. (B) Anteroposterior flush portogram obtained through a 5-F flush catheter within the main portal vein via ipsilateral approach demonstrates patent conventional portal anatomy. (C) Intraprocedural fluoroscopic image from PVE depicts coil placement into segment 4 branches via a microcatheter. (D) Final portogram shows occlusion of the portal vein branches to segments 4-8 with continued patency of the veins supplying the left lateral liver. (E) CT obtained after PVE demonstrates massive hypertrophy of the FLR (White arrow; Left lateral liver volume increased from 157 to 457 mL).
Published complication rates between the contralateral vs. ipsilateral approaches are relatively similar. Di Stefano et al.53 reported on 188 patients who underwent contralateral approach PVE and found a 12.8% adverse event rate and only one major complication (complete portal vein thrombosis) directly related to the contralateral approach that precluded surgery. Ribero et al.21 reported on 112 patients who underwent ipsilateral approach PVE and found an 8.9% adverse event rate21. Accounting for the fact that Di Stefano et al.53 included clinically occult CT findings in their complications, the rates are comparable between the two studies. Kodama et al.54 compared complication rates between contralateral (n = 11) and ipsilateral approaches (n = 36) in a series of 47 patients who underwent PVE. Contralateral approach PVE was associated with an 18.1% complication rate as compared to 13.9% for ipsilateral PVE. Though the difference did not reach statistical significance, the authors recommended ipsilateral approach due to the potential for injury to the FLR during contralateral approach.
Additional approaches
A recent report by Sarwar et al.55 highlighted the use of trans-splenic approach access to the portal venous system in two patients whose tumor burden prohibited ipsilateral transhepatic access. Both patients underwent successful right portal vein embolization with a combination of microspheres and coils and subsequent extended right hepatectomy. No adverse bleeding events were noted. This approach is attractive as there is no potential for damage to the FLR from direct transhepatic access; however larger series are needed to ensure lack of bleeding complications from the splenic access.
PVE using a transjugular approach has been reported in a series of 15 patients56. In this approach, a right or left portal branch was punctured from a right, middle, or left hepatic vein and a catheter placed near the portal bifurcation through which embolization was performed using a mixture of n-butyl-2-cyanoacrylate (NBCA) and iodized oil. FLR hypertrophy was adequate, and right hepatectomy was performed in 12/15 patients with no PVE related complications.
PVE can also be performed via a transileocolic venous approach during laparotomy by direct cannulation of the ileocolic vein and advancement of a balloon catheter into the portal vein for embolization15. This approach is not commonly used in current practice as improvements in experience, imaging equipment, catheter systems, and embolic agents have led to greater use of minimally invasive approaches. The transileocolic venous approach necessitates general anesthesia and laparotomy, and is typically only performed when a percutaneous approach is not considered feasible or additional treatment is needed during the same surgical exploration57,58.
Embolic agents
A variety of materials and devices exist for embolization and some of these have been adapted for the portal system. Commonly reported agents include polyvinyl alcohol (PVA), gelfoam, fibrin glue, NBCA, polidocanol foam, microspheres, lipiodol, coils and Amplatzer plugs50,59-62. An ideal material will provide permanent portal venous embolization that is safe and well tolerated by the patient8. The two agents most commonly discussed currently are NBCA and microspheres in combination with coils.
NBCA induces an inflammatory reaction resulting in peribilliary fibrosis and rates of liver regeneration are believed to be as good as or better than other embolic agents. In early studies, NBCA was shown to induce a larger FLR when compared coils and gelatin sponge and produce durable occlusion of greater than four weeks63,64. Recently, Jaberi et al.65 also demonstrate increased hypertrophy with NBCA and Amplatzer Plug as compared to PVA and coils in their single institution retrospective series. However, plugs and coils were used earlier in the institution’s experience, and overall surgical outcomes and complications were no different in the two groups.
Jaberi et al.65 also demonstrated decreased contrast utilization and fluoroscopy time in the NBCA and Amplatzer plug group as compared to PVA and coils. However, preparation and administration of NBCA requires expertise.63 Great care must be taken to prevent embolization of NBCA to non-target areas. As such, NBCA is routinely administered through a contralateral approach, and embolization is not routinely extended to segment 4 when using this agent. NBCA is delivered through an end hole angiographic catheter from second or third order portal branches to prevent non-target embolization. In addition, many groups advocate for the deployment of an Amplatzer plug to prevent backflow of glue61,65. Straight catheters are preferred by some operators to prevent gluing of catheters into the liver.
Multiple studies have demonstrated the safety and effectiveness of small particle embolization of the liver with both PVA particles and microspheres50,66. After catheterization of the portal system, embolization of distal small veins is performed with 100–300 micron particles. More proximal veins are embolized with larger particles with a goal of near stasis of flow or stasis. Coils are placed behind particles to prevent later particle dislodgement and recanalization, improving hypertrophy of the FLR. A study by Geisel et al.67 demonstrated superior FLR hypertrophy with reported percentage volume gain of (53.3 ± 34.5)% with the use of coils and plugs in combination with particles vs. (30.9 +/- 28.8)% with the use of particles alone (P = 0.002).
Extent of embolization
Extending right PVE to include segment 4 (RPVE + 4) prior to extended right hepatectomy has been supported by several studies68-71. Institutions with the capability to perform RPVE + 4 had statistically significant higher ratings for both likelihood of technical success and likelihood of subsequent hypertrophy as compared to those without in a multicenter survey of surgical preferences72. Possible benefits include improved hypertrophy of segments 2 + 3, embolization of the entire tumor bearing liver, and the reduction of potentially challenging surgical resections in the setting segment 4 hypertrophy69,70. Kishi et al.70 compared patients who underwent RPVE (n = 15) vs. those that underwent RPVE + 4 (n = 58) and demonstrated statistically significant increases in both absolute volume and hypertrophy rate of segment 2 + 3 in the RPVE + 4 group. Mise et al.69 reported on the clinical utility of RPVE + 4 performed during two stage hepatectomy; they found that the dynamics of liver regeneration of segments 2 + 3 was impaired after RPVE alone but not RPVE + 4 after the first stage resection. The drawback of extension of embolization to segment 4 is the inadvertent reflux of embolic material to the FLR73,74. Manipulation of the catheter to segment 4 to prevent inadvertent non-target embolization can be technically challenging.
Complications
Complications of PVE are similar to other image guided transhepatic procedures and include subscapular hematoma, hemoperitoneum, hemobilia, abscess formation, cholangitis and sepsis, arterioportal shunts, arterioportal fistula, and pneumothorax. In addition, PVE-specific complications include non-target embolization, recanalization of embolized segments, and extension of portal vein thrombosis to involve the left or main branches75. The Society of Interventional Radiology established quality improvement guidelines, including a suggested threshold for PVE related major complications of 6% and morbidity of 11%76. Most published complication rates fall well below this range77. A meta-analysis by Abulkhir et al.7 pooling 1,088 total subjects who underwent PVE found procedure-related morbidity and mortality to be 2.2% and 0%, respectively. In their analysis, percutaneous PVE was performed in the majority of cases (72%); the remainder performed via the transilecolic technique. There are similarly low complication rates for both RPVE and RPVE + 4.
PVE clinical considerations
Indications and contraindications
PVE allows for safe, potentially curative hepatectomy in patients with anticipated small remnant livers previously considered ineligible for resection9,11-15,18,39. Candidates for PVE include hepatic resection candidates with primary or metastatic liver disease, who have anticipated FLR that are too small for adequate function perioperatively. If too little liver remains after resection, immediate post-resection hepatic failure leads to multisystem organ failure and death. If a marginal volume of liver remains, cirrhotic or not, the lack of reserve often leads to a cascade of complications, prolonged hospital and intensive-care unit stays, and slow recovery or slowly progressive liver failure over weeks to months, with eventual death2,3,10.
Several factors are considered to determine clinical candidacy for PVE8. First, the presence or absence of underlying liver disease will have a major impact on the volume of liver remnant needed for adequate function. Normal liver has a greater regenerative capacity than a cirrhotic liver, functions more efficiently and tolerates injury better. Patients can survive resection of up to 90% of the liver in the absence of underlying liver disease, but survival after resection beyond 60% of the functional parenchyma in patients with cirrhosis is unlikely11. Second, patient size must be considered; larger patients require larger liver remnants than do smaller patients; hence normalization of the FLR to the sFLR in practice is needed10,39. Third, the extent and complexity of the planned resection and the possibility that associated non-hepatic surgery will be performed at the time of liver resection (e.g., hepatectomy plus pancreatico-duodenectomy) must be considered. These three factors are considered in the setting of the patient’s age and comorbidities (e.g., diabetes) that may affect hypertrophy. Thus, once the procedure type and extent of resection necessary to treat the patient have been determined, appropriate liver volumetry is performed so that the sFLR volume expressed as a percentage of the estimated TLV can be used to determine the need for PVE. Candidates for PVE include patients with primary or metastatic liver disease, who are otherwise hepatic resection candidates, except for the following: (a) normal underlying liver and a standardized FLR (sFLR) less than 20%11,21,78; (b) cirrhosis and/or advanced fibrosis and a sFLR less than 40%3,79; (c) extensive chemotherapy and a sFLR less than 30%80.
PVE is an adjunctive procedure to major hepatectomy. Hence, contraindications to PVE mirror those of hepatectomy. Severe portal hypertension precluding surgery is the only absolute contraindication to PVE. Objective measures of severe portal hypertension include elevated hepatic venous pressure gradients >12 mmHg, refractory ascites, and variceal bleeding. Also, in cases where tumor obstructs the portal system in the liver to be resected, PVE is not necessary as portal flow is already redirected to the FLR11,52,81. Two stage hepatectomy has expanded the patients with bilobar hepatic disease burden eligible for PVE and potential curative resection; however diffuse hepatic disease burden remains a contraindication to PVE. Relative contraindications include uncorrectable coagulopathy, renal failure and extrahepatic metastasis.
Outcomes
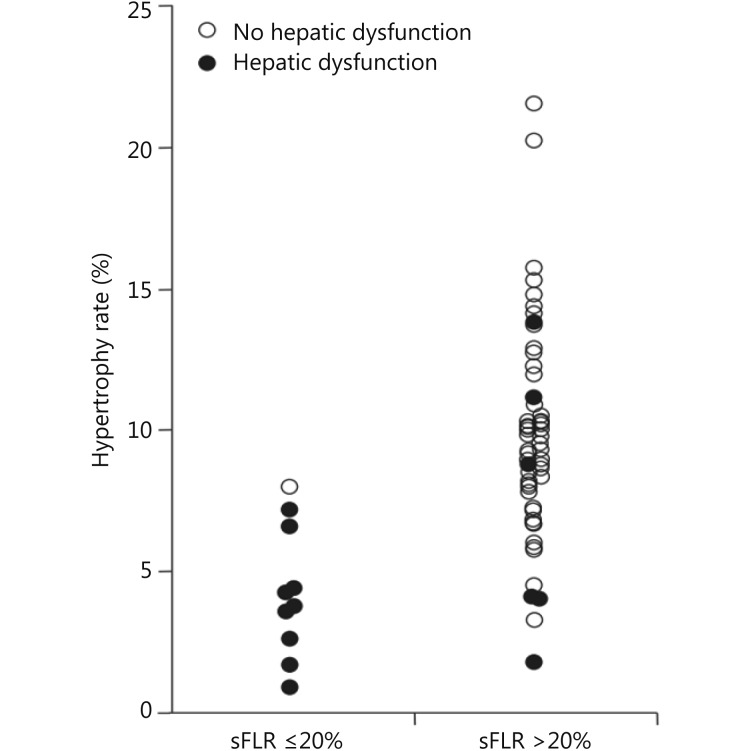
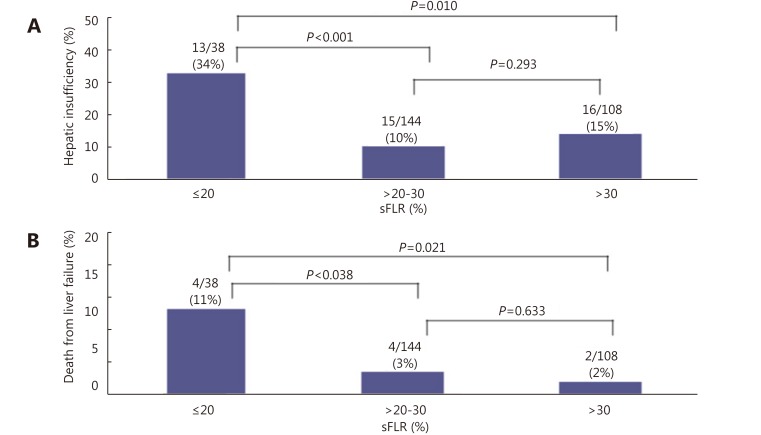
PVE is indicated with normal underlying liver and sFLR less than 20%. Multiple studies have demonstrated that hepatectomy in a setting of sFLR < 20% is associated with increased postoperative complications11,21,78. Ribero et al.21 found that both sFLR less than 20% and degree of sFLR hypertrophy after PVE less than 5% predicted outcome after resection in a series of 112 patients (Figure 9) . Kishi et al.78 found that patients with a preoperative sFLR < 20% had significantly higher rates of postoperative liver insufficiency and death from liver failure compared with patients with sFLR > 20% in a series of 301 patients who underwent hepatic resection (P < 0.05). In addition, patients who underwent PVE before surgery to increase their sFLR from < 20% to > 20% had statistically equivalent rates of liver insufficiency as patients with sFLR > 20% at baseline (Figure 10). This study confirmed both the sFLR threshold of < 20% being associated with increased perioperative complications and the beneficial role of PVE in reducing perioperative complication rates in those patients who hypertrophy their liver to a sFLR > 20%.
9.
Presence of hepatic dysfunction by sFLR volume and degree of hypertrophy. Used with permission from Ref.21.
10.
Rates of hepatic insufficiency (A) and death (B) by preoperative sFLR volume. Modified with permission from Ref.78.
Liver regeneration occurs at a reduced rate and capacity in diseased livers, an observation directly correlated to clinical outcomes. Patients with cirrhosis with marginal liver remnants are at high risk for complications and mortality from liver failure3. As such, the recommended sFLR cutoff in the setting of cirrhosis is 40%; higher than the 20% sFLR cutoff recommended in the setting of normal underlying liver parenchyma9,82,83. In patients with chronic liver disease, hepatectomy outcomes, including the number and severity of complications and the incidence of postoperative liver failure and death, are better with PVE than without79,84,85.
Azoulay et al.84 reported on a series of cirrhotic patients who underwent PVE for sFLR < 40% prior to extended hepactectomy. There was significant increase in the FLR volumes in all patients who underwent PVE that correlated with reduced incidence of liver failure and death without differences in disease-free survival rates. Tanaka et al.85 reported several benefits of PVE in a larger study of patients with HCC and cirrhosis. Disease-free survival rates were similar, but cumulative survival rates were significantly higher in the PVE group than in the non-PVE group. In addition, patients with recurrence following PVE plus resection were more often candidates for further treatments such as TAE. However, the complications of PVE are higher in patients with chronic liver disease than in those with an otherwise normal liver because of an increased risk of secondary portal vein thrombosis, presumably from slow flow in the portal vein trunk after PVE53,86.
In patients with chronic liver disease such as chronic hepatitis, fibrosis or cirrhosis, the increase in nonembolized liver volumes after PVE varies (range, 28%–46%), and hypertrophy after PVE may take more than 4 weeks because of slower regeneration rates22,87. The degree of parenchymal fibrosis is thought to limit regeneration, possibly as a result of reduced portal blood flow86. Hence, treatment strategies combining transarterial therapy with PVE are particularly useful in cirrhotic populations to maximize potential liver regeneration and prevent disease progression; as discussed in more detail below.
In conjunction with chemotherapy
PVE has been reported to accelerate tumor growth for both primary and metastatic liver tumors88-91. Progression of disease after PVE may preclude curative intent surgery; in two stage hepactectomy series 20% drop out rates due to progression of disease have been reported after first stage resection92,93. Administration of neoadjuvant chemotherapy is helpful in lowering the rate of disease progression and does not interfere with liver regeneration; however concerns have been raised regarding its potential deleterious effects on liver function. Two separate series, one by Pawlik et al.94 and another by Vauthey et al.95 have demonstrated an association of oxaliplatin with sinusoidal dilation and irinotecan with steatohepatitis. In the series by Vauthey et al.95, the presence of steatohepatitis in patients who had undergone resection was correlated to increased 90-day mortality (14.7% vs. 1.6%; P = 0.001; OR = 10.5; 95% CI, 2.0 to 36.4).
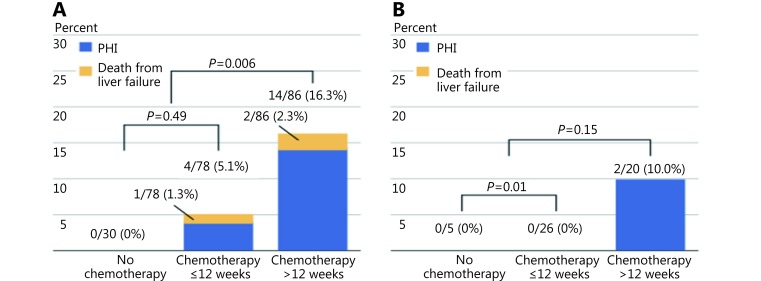
When long term chemotherapy is administered (> 12 weeks), a sFLR cutoff of > 30% should be considered given the potential deleterious effects of chemotherapy on native liver function. Shindoh et al.80 performed a retrospective analysis of 194 patients with colorectal liver metastasis to determine the optimal FLR in patients treated with neoadjuvant chemotherapy. The authors found that both long duration of chemotherapy (defined as > 12 weeks) and sFLR ≤ 30% were predictors of hepatic insufficiency (OR = 5.4, P = 0.004; OR = 6.3, P = 0.019, respectively) (Figure 11). No cases of postoperative mortality and only two cases of postoperative hepatic insufficiency were reported if the sFLR > 30%.
11.
Postoperative liver insufficiency and mortality from liver failure in patients who underwent extended right hepatectomy in the setting of colorectal metastases, stratified to those undergoing no chemotherapy, chemotherapy, or long duration (> 12weeks) chemotherapy. sFLR > 20% cut-off (A) and sFLR > 30% cut-off (B) for resection eligibility. Used with permission from Ref.80.
The effect of systemic neoadjuvant chemotherapy on liver hypertrophy after PVE has been addressed by several studies, with varying results96-99. Zorzi et al.98 reviewed FLR hypertrophy after PVE in patients with colorectal liver metastases who underwent PVE either with (n = 43) or without neoadjuvant chemotherapy (n = 22) prior to resection. The chemotherapy group, demonstrated similar rates of hypertrophy when compared to the no chemotherapy group at 4 weeks after PVE. Similarly, Covey et al.96 also reported on patients with colorectal liver metastases who underwent PVE either with (n = 47) or without (n = 53) neoadjuvant chemotherapy with no significant difference in median contralateral liver growth after PVE. However, Aussilhou et al.99 demonstrated decreased liver hypertrophy rates after portal vein occlusion in patients who received both bevacizumab in addition to FOLFOX or FOLFIRI as compared to FOLFOX/FOLFIRI alone.
Several studies have examined the effect of chemotherapy on disease progression after PVE prior to hepatectomy100-103. Spelt et al.103 found that the rate of tumor progression in patients who underwent PVE was low when concomitant chemotherapy was administered, and was associated with keeping the interval between completion of chemotherapy and PVE short. Fischer et al.100 reported on a series of 64 consecutive patients who underwent PVE stratified into two groups: those that received chemotherapy (n = 25) and those that did not (n = 39), in anticipation of extended right hepatic resection. Though there was no statistical difference between the proportion of patients who ultimately underwent hepatic resection between the two groups, the chemotherapy group had statistically lower rate of progression by RECIST criteria (18.9% vs. 34.2%; P = 0.03). Of greater importance, the chemotherapy group demonstrated a clear survival benefit as compared to the no chemotherapy group (49% vs. 24% of 5-year survival; P = 0.006) in both the surgical resection and non-surgical cohorts.
In combination with TAE
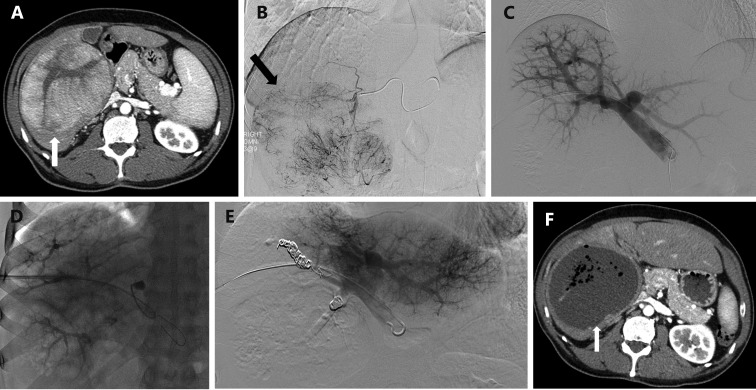
PVE can be combined with other interventional radiology techniques such as TAE (Figure 12) in patients who are not anticipated to have sufficient hypertrophy after PVE alone68,104. The mechanism of TAE is complementary as a component of inflammation and necrosis is added to the apoptosis mediated cell death induced by PVE to stimulate liver hypertrophy. Nagino et al.68 first described the use of TAE to improve FLR volume in two patients with cholangiocarcinoma who demonstrated inadequate hypertrophy following PVE104. In both patients, PVE in the setting of underlying liver disease led to negligible hypertrophy of the FLR. After interval TAE, the FLR volume demonstrated adequate increase, and both patients under-went successful curative resection. A second report by Gruttadauria et al.104 demonstrated similar results: two patients with inadequate hypertrophy after PVE demon-strated improved hypertrophy after subsequent TAE allowing for subsequent successful hepatectomy.
12.
A 55-year-old man with hepatitis C cirrhosis complicated by a 12 cm hepatocellular carcinoma replacing the entire right liver who underwent sequential TAE followed 1 month later by RPVE prior to a right hepatectomy. (A) Contrast enhanced axial image of the liver demonstrated a 12 cm enhancing mass replacing the right liver and normal appearing of left lateral liver prior to embolization and PVE. (B) Intraprocedural digital subtraction selective angiography demonstrates hypervascular tumor with successful particle embolization. (C) Anteroposterior flush portogram obtained through a 5-F flush catheter within the main portal vein via ipsilateral approach demonstrates patent conventional portal anatomy. (D) Intraprocedural fluoroscopic image from PVE depicts complete occlusion of all branches to right portal vein. (E) Final portogram shows occlusion of the portal vein branches to segments 4–8 with continued patency of the veins supplying the left lateral liver. (F) A single image from post-PVE contrast-enhanced CT scan demonstrates profound necrosis of the tumor (white arrow) and massive hypertrophy of the left lateral liver. The patient underwent uncomplicated right hepatectomy.
Since the original reports, TAE is more commonly performed as a staged procedure prior to PVE. Care must be taken to reduce the risk of hepatic infarction including staging the procedures at least 2 to 3 weeks apart. Embolization should not be carried out to complete stasis and use of non-particulate embolic agents such as chemoembolization should be favoured over particulate agents. Following embolization, the interventional radiologist should confirm patency of the hepatic artery supplying the targeted liver segment, in order to avoid occlusion of arterial and portal hepatopetal flow and the potential of parenchymal necrosis.
TAE followed by PVE has been advocated for in the setting of cirrhosis complicated by HCC. In this patient population, the rationale for performing TAE prior to PVE includes prevention of tumor progression after PVE, reduction of arterioportal shunts that may limit the effectiveness of the subsequent PVE, and boosting the regenerative stimulus in chronically diseased livers105,106. Using this regimen, Aoki et al.105 demonstrated increased profound tumor necrosis without substantial injury to the noncancerous liver in patients with large HCC and chronically injured livers. Similarly, Ogata et al.106 found increased incidence of complete tumor necrosis (83% vs. 6%; P < 0.001) and increased 5-year disease-free survival rate (37% vs. 19%; P = 0.041) in patients who underwent TACE and PVE as compared to PVE alone.
Areas of future study
Augmenting PVE with additional agents can potentially further promote hypertrophy and postoperative liver function. am Esch et al.107 investigated the treatment of patients with both PVE and bone marrow-derived stem cells (PVE + SC; n = 11) as compared to patients undergoing PVE alone (PVE; n = 11) in a series of extended right hepatectomy patients. They demonstrated mean hepatic growth of segments 2 and 3 to be significantly higher in the PVE + SC as compared to the PVE groups (138.7 mL ± 66.3 mL vs. 63.0 mL ± 40.0 mL; P = 0.004) after a waiting period of only 14 days. In their investigation, PVE was performed using a surgical transileocolic approach; a subsequent animal (porcine model) study by Avritscher et al.108 demonstrated the ability to administer mesenchymal stem cells using percutaneous endovascular approaches. Using an alternative method of PVE augmentation, Beppu et al.109 prospectively evaluated the effect of adding branched amino acids to patients’ diets on functional liver regeneration following PVE. Branched chain amino acid diet supplementation was concluded to improve functional liver regeneration in patients undergoing PVE followed by major resection, as evidenced by ultimately increased liver serum albumin scintigraphy values 6 months after resection (266.7% vs. 77.6%; P = 0.04). Overall, augmentation of PVE using various physiologic aspects of liver regeneration is a promising area of future research, and may improve upon the already well established efficacy of PVE.
Conclusions
PVE is well established as an invaluable adjunctive procedure to increase candidacy for and the safety of major hepatic resections. Numerous studies have validated PVE’s clinical utility in surgical hepatic resection candidates with limited anticipated FLRs. PVE is incorporated into standard of care paradigms due to its low associated complication rates in combination with its efficacy in promoting hypertrophy of the FLR that is directly correlated with improved surgical outcomes. Increased data regarding appropriate usage of PVE in complex multidisciplinary treatment plans exists. Information improving upon correlates of functional assessment of the FLR as compared to volumetry alone, appropriate FLR cutoffs in diseased livers, the combination of TAE with PVE, and how to best incorporate chemotherapy with PVE have all been reported. Exciting areas of future research include augmenting the regenerative potential of PVE with stem cells or medications. PVE remains an important aspect of oncologic care; in large part due to the substantial foundation of information available demonstrating its clear clinical benefit for hepatic resection candidates with small anticipated FLRs.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Tjandra JJ, Fan ST, Wong J. Peri-operative mortality in hepatic resection. Aust N Z J Surg. 1991;61:201–6. doi: 10.1111/j.1445-2197.1991.tb07592.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsao JI, Loftus JP, Nagorney DM, Adson MA, Ilstrup DM. Trends in morbidity and mortality of hepatic resection for malignancy. A matched comparative analysis. Ann Surg. 1994;220:199–205. doi: 10.1097/00000658-199408000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304–9. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 4.Aloia TA. Associating liver partition and portal vein ligation for staged hepatectomy: portal vein embolization should remain the gold standard. JAMA Surg. 2015;150:927–8. doi: 10.1001/jamasurg.2015.1646. [DOI] [PubMed] [Google Scholar]
- 5.Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26:4259–67. doi: 10.1007/s00330-016-4291-9. [DOI] [PubMed] [Google Scholar]
- 6.Pandanaboyana S, Bell R, Hidalgo E, Toogood G, Prasad KR, Bartlett A, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015;157:690–8. doi: 10.1016/j.surg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 8.Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–90. doi: 10.1097/01.RVI.0000159543.28222.73. [DOI] [PubMed] [Google Scholar]
- 9.May BJ, Talenfeld AD, Madoff DC. Update on portal vein embolization: evidence-based outcomes, controversies, and novel strategies. J Vasc Interv Radiol. 2013;24:241–54. doi: 10.1016/j.jvir.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–9. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 11.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–80; discussion 680–1. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–6. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Baere T, Roche A, Vavasseur D, Therasse E, Indushekar S, Elias D, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993;188:73–7. doi: 10.1148/radiology.188.1.8511321. [DOI] [PubMed] [Google Scholar]
- 14.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–81. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 15.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–7. [PubMed] [Google Scholar]
- 16.Nagino M, Kamiya J, Kanai M, Uesaka K, Sano T, Yamamoto H, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155–60. doi: 10.1067/msy.2000.101273. [DOI] [PubMed] [Google Scholar]
- 17.Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, et al. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677–81. doi: 10.1016/s0039-6060(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 18.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–30; discussion 730-2. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koniaris LG, McKillop IH, Schwartz SI, Zimmers TA. Liver regeneration. J Am Coll Surg. 2003;197:634–59. doi: 10.1016/S1072-7515(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Behrns KE. A scientist revisits the atrophy-hypertrophy complex: hepatic apoptosis and regeneration. Surg Oncol Clin N Am. 2002;11:849–64. doi: 10.1016/s1055-3207(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 21.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–94. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 22.Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21:434–9. [PubMed] [Google Scholar]
- 23.Starzl TE, Francavilla A, Porter KA, Benichou J, Jones AF. The effect of splanchnic viscera removal upon canine liver regeneration. Surg Gynecol Obstet. 1978;147:193–207. [PMC free article] [PubMed] [Google Scholar]
- 24.Kock NG, Hahnloser P, Roding B, Schenk WG Jr. Interaction between portal venous and hepatic arterial blood flow: an experimental study in the dog. Surgery. 1972;72:414–9. [PubMed] [Google Scholar]
- 25.Michalopoulos GK, De Frances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 26.Michalopoulos GK, Zarnegav R. Hepatocyte growth factor. Hepatology. 1992;15:149–55. doi: 10.1002/hep.1840150125. [DOI] [PubMed] [Google Scholar]
- 27.Ponfick VA. Ueber leberresection und leberreaction. Verhandl Deutsch Gesellsch Chir. 1980;19:28. [Google Scholar]
- 28.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–75. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 29.Goto Y, Nagino M, Nimura Y. Doppler estimation of portal blood flow after percutaneous transhepatic portal vein embolization. Ann Surg. 1998;228:209–13. doi: 10.1097/00000658-199808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan JR, Hicks ME, Cai SR, Brunt EM, Ponder KP. Embolization of portal vein branches induces hepatocyte replication in swine: a potential step in hepatic gene therapy. Radiology. 1999;210:467–77. doi: 10.1148/radiology.210.2.r99fe10467. [DOI] [PubMed] [Google Scholar]
- 31.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–14. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 32.Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection . Br J Surg. 2013;100:388–94. doi: 10.1002/bjs.8955. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Yamazaki K, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery. 2016;159:1289–98. doi: 10.1016/j.surg.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Eshmuminov D, Raptis DA, Linecker M, Wirsching A, Lesurtel M, Clavien PA. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg. 2016 doi: 10.1002/bjs.10290. [DOI] [PubMed] [Google Scholar]
- 35.Hwang S, Ha TY, Ko GY, Kwon DI, Song GW, Jung DH, et al. Preoperative sequential portal and hepatic vein embolization in patients with hepatobiliary malignancy. World J Surg. 2015;39:2990–8. doi: 10.1007/s00268-015-3194-2. [DOI] [PubMed] [Google Scholar]
- 36.Hwang S, Lee SG, Ko GY, Kim BS, Sung KB, Kim MH, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249:608–16. doi: 10.1097/SLA.0b013e31819ecc5c. [DOI] [PubMed] [Google Scholar]
- 37.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11:1481–93. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
- 38.Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol. 2008;25:104–9. doi: 10.1055/s-2008-1076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–40. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 40.Soyer P, Roche A, Elias D, Levesque M. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology. 1992;184:695–7. doi: 10.1148/radiology.184.3.1509051. [DOI] [PubMed] [Google Scholar]
- 41.Ribero D, Amisano M, Bertuzzo F, Langella S, Lo Tesoriere R, Ferrero A, et al. Measured versus estimated total liver volume to preoperatively assess the adequacy of the future liver remnant: which method should we use? Ann Surg. 2013;258:801–6; discussion 806-7. doi: 10.1097/SLA.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 42.Leung U, Simpson AL, Araujo RLC, Gönen M, McAuliffe C, Miga MI, et al. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014;219:620–30. doi: 10.1016/j.jamcollsurg.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201–9. doi: 10.1016/j.jamcollsurg.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107–16. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 45.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 46.Mihara K, Sugiura T, Okamura Y, Kanemoto H, Mizuno T, Moriguchi M, et al. A predictive factor of insufficient liver regeneration after preoperative portal vein embolization. Eur Surg Res. 2013;51:118–28. doi: 10.1159/000356368. [DOI] [PubMed] [Google Scholar]
- 47.Meier RPH, Toso C, Terraz S, Breguet R, Berney T, Andres A, et al. Improved liver function after portal vein embolization and an elective right hepatectomy. HPB (Oxford) 2015;17:1009–18. doi: 10.1111/hpb.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–8. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 49.Nagino M, Nimura Y, Kamiya J, Kondo S, Kanai M. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology. 1996;200:559–63. doi: 10.1148/radiology.200.2.8685357. [DOI] [PubMed] [Google Scholar]
- 50.Madoff DC, Abdalla EK, Gupta S, Wu TT, Morris JS, Denys A, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–25. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 51.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness--study in 26 patients. Radiology. 2003;227:251–60. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 52.Madoff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA, Ahrar K, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22:1063–76. doi: 10.1148/radiographics.22.5.g02se161063. [DOI] [PubMed] [Google Scholar]
- 53.Di Stefano DR, de Baere T, Denys A, Hakime A, Gorin G, Gillet M, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625–30. doi: 10.1148/radiol.2342031996. [DOI] [PubMed] [Google Scholar]
- 54.Kodama Y, Shimizu T, Endo H, Miyamoto N, Miyasaka K. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13:1233–7. doi: 10.1016/s1051-0443(07)61970-8. [DOI] [PubMed] [Google Scholar]
- 55.Sarwar A, Brook OR, Weinstein JL, Khwaja K, Ahmed M. Trans-splenic portal vein embolization: a technique to avoid damage to the future liver remnant. Cardiovasc Intervent Radiol. 2016;39:1514–8. doi: 10.1007/s00270-016-1359-5. [DOI] [PubMed] [Google Scholar]
- 56.Perarnau JM, Daradkeh S, Johann M, Deneuville M, Weinling P, Coniel C. Transjugular preoperative portal embolization (TJPE) a pilot study. Hepatogastroenterology. 2003;50:610–3. [PubMed] [Google Scholar]
- 57.Azoulay D, Raccuia JS, Castaing D, Bismuth H. Right portal vein embolization in preparation for major hepatic resection. J Am Coll Surg. 1995;181:266–9. [PubMed] [Google Scholar]
- 58.Denys A, Madoff DC, Doenz F, Schneider F, Gillet M, Vauthey JN, et al. Indications for and limitations of portal vein embolization before major hepatic resection for hepatobiliary malignancy. Surg Oncol Clin N Am. 2002;11:955–68. doi: 10.1016/s1055-3207(02)00039-x. [DOI] [PubMed] [Google Scholar]
- 59.Madoff DC. Portal vein embolization: the continued search for the ideal embolic agent. J Vasc Interv Radiol. 2014;25:1053–5. doi: 10.1016/j.jvir.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Guiu B, Bize P, Gunthern D, Demartines N, Halkic N, Denys A. Portal vein embolization before right hepatectomy: improved results using n-butyl-cyanoacrylate compared to microparticles plus coils. Cardiovasc Intervent Radiol. 2013;36:1306–12. doi: 10.1007/s00270-013-0565-7. [DOI] [PubMed] [Google Scholar]
- 61.Bent CL, Low D, Matson MB, Renfrew I, Fotheringham T. Portal vein embolization using a nitinol plug (Amplatzer vascular plug) in combination with histoacryl glue and iodinized oil: adequate hypertrophy with a reduced risk of nontarget embolization. Cardiovasc Intervent Radiol. 2009;32:471–7. doi: 10.1007/s00270-009-9515-9. [DOI] [PubMed] [Google Scholar]
- 62.Fischman AM, Ward TJ, Horn JC, Kim E, Patel RS, Nowakowski FS, et al. Portal vein embolization before right hepatectomy or extended right hepatectomy using sodium tetradecyl sulfate foam: technique and initial results. J Vasc Interv Radiol. 2014;25:1045–53. doi: 10.1016/j.jvir.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 63.de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–91. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- 64.Matsuoka T, Nakatsuka H, Nakamura K, Kaminou T, Manabe T, Yamada T, et al. Long-term embolization of the portal vein with isobutyl-2-cyanoacrylate in hepatoma. Nihon Igaku Hoshasen Gakkai Zasshi. 1986;46:72–4. [PubMed] [Google Scholar]
- 65.Jaberi A, Toor SS, Rajan DK, Mironov O, Kachura JR, Cleary SP, et al. Comparison of clinical outcomes following glue versus polyvinyl alcohol portal vein embolization for hypertrophy of the future liver remnant prior to right hepatectomy. J Vasc Interv Radiol. 2016 doi: 10.1016/j.jvir.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 66.Cazejust J, Bessoud B, Le Bail M, Menu Y. Preoperative portal vein embolization with a combination of trisacryl microspheres, gelfoam and coils. Diagn Interv Imaging. 2015;96:57–64. doi: 10.1016/j.diii.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Geisel D, Malinowski M, Powerski MJ, Wüstefeld J, Heller V, Denecke T, et al. Improved hypertrophy of future remnant liver after portal vein embolization with plugs, coils and particles. Cardiovasc Intervent Radiol. 2014;37:1251–8. doi: 10.1007/s00270-013-0810-0. [DOI] [PubMed] [Google Scholar]
- 68.Nagino M, Kanai M, Morioka A, Yamamoto H, Kawabata Y, Hayakawa N, et al. Portal and arterial embolization before extensive liver resection in patients with markedly poor functional reserve. J Vasc Interv Radiol. 2000;11:1063–8. doi: 10.1016/s1051-0443(07)61340-2. [DOI] [PubMed] [Google Scholar]
- 69.Mise Y, Aloia TA, Conrad C, Huang SY, Wallace MJ, Vauthey JN. Volume regeneration of segments 2 and 3 after right portal vein embolization in patients undergoing two-stage hepatectomy. J Gastrointest Surg. 2015;19:133–41; discussion 141. doi: 10.1007/s11605-014-2617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kishi Y, Madoff DC, Abdalla EK, Palavecino M, Ribero D, Chun YS, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744–51. doi: 10.1016/j.surg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller L, Hillert C, Möller L, Krupski-Berdien G, Rogiers X, Broering DC. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol. 2008;15:1908–17. doi: 10.1245/s10434-008-9925-y. [DOI] [PubMed] [Google Scholar]
- 72.Day RW, Conrad C, Vauthey JN, Aloia TA. Evaluating surgeon attitudes towards the safety and efficacy of portal vein occlusion and associating liver partition and portal vein ligation: a report of the MALINSA survey. HPB (Oxford) 2015;17:936–41. doi: 10.1111/hpb.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capussotti L, Muratore A, Ferrero A, Anselmetti GC, Corgnier A, Regge D. Extension of right portal vein embolization to segment IV portal branches. Arch Surg. 2005;140:1100–3. doi: 10.1001/archsurg.140.11.1100. [DOI] [PubMed] [Google Scholar]
- 74.van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch ORC, Heger M, et al. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436–44. doi: 10.1159/000184735. [DOI] [PubMed] [Google Scholar]
- 75.Yeom YK, Shin JH. Complications of portal vein embolization: evaluation on cross-sectional imaging. Korean J Radiol. 2015;16:1079–85. doi: 10.3348/kjr.2015.16.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angle JF, Siddiqi NH, Wallace MJ, Kundu S, Stokes L, Wojak JC, et al. Quality improvement guidelines for percutaneous transcatheter embolization: society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2010;21:1479–86. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Denys A, Bize P, Demartines N, Deschamps F, De Baere T. Quality improvement for portal vein embolization. Cardiovasc Intervent Radiol. 2010;33:452–6. doi: 10.1007/s00270-009-9737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–8. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 79.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–17. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shindoh J, Tzeng CWD, Aloia TA, Curley SA, Zimmitti G, Wei SH, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol. 2013;20:2493–500. doi: 10.1245/s10434-012-2864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thakrar PD, Madoff DC. Preoperative portal vein embolization: an approach to improve the safety of major hepatic resection. Semin Roentgenol. 2011;46:142–53. doi: 10.1053/j.ro.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 82.Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–91. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palavecino M, Chun YS, Madoff DC, Zorzi D, Kishi Y, Kaseb AO, et al. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: perioperative outcome and survival. Surgery. 2009;145:399–405. doi: 10.1016/j.surg.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 84.Azoulay D, Castaing D, Krissat J, Smail A, Marin HG, Lemoine A, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–72. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka H, Hirohashi K, Kubo S, Shuto T, Higaki I, Kinoshita H. Preoperative portal vein embolization improves prognosis after right hepatectomy for hepatocellular carcinoma in patients with impaired hepatic function. Br J Surg. 2000;87:879–82. doi: 10.1046/j.1365-2168.2000.01438.x. [DOI] [PubMed] [Google Scholar]
- 86.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–44. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 87.Shimamura T, Nakajima Y, Une Y, Namieno T, Ogasawara K, Yamashita K, et al. Efficacy and safety of preoperative percutaneous transhepatic portal embolization with absolute ethanol: a clinical study. Surgery. 1997;121:135–41. doi: 10.1016/s0039-6060(97)90282-8. [DOI] [PubMed] [Google Scholar]
- 88.Al-Sharif E, Simoneau E, Hassanain M. Portal vein embolization effect on colorectal cancer liver metastasis progression: lessons learned. World J Clin Oncol. 2015;6:142–6. doi: 10.5306/wjco.v6.i5.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elias D, De Baere T, Roche A, Ducreux M, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–8. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 90.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–72. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 91.Simoneau E, Aljiffry M, Salman A, Abualhassan N, Cabrera T, Valenti D, et al. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastases. HPB (Oxford) 2012;14:461–8. doi: 10.1111/j.1477-2574.2012.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–90. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Narita M, Oussoultzoglou E, Jaeck D, Fuchschuber P, Rosso E, Pessaux P, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg. 2011;98:1463–75. doi: 10.1002/bjs.7580. [DOI] [PubMed] [Google Scholar]
- 94.Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860–8. doi: 10.1007/s11605-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 95.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–72. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 96.Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451–5. doi: 10.1097/SLA.0b013e31815ed693. [DOI] [PubMed] [Google Scholar]
- 97.Simoneau E, Alanazi R, Alshenaifi J, Molla N, Aljiffry M, Medkhali A, et al. Neoadjuvant chemotherapy does not impair liver regeneration following hepatectomy or portal vein embolization for colorectal cancer liver metastases. J Surg Oncol. 2016;113:449–55. doi: 10.1002/jso.24139. [DOI] [PubMed] [Google Scholar]
- 98.Zorzi D, Chun YS, Madoff DC, Abdalla EK, Vauthey JN. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann Surg Oncol. 2008;15:2765–72. doi: 10.1245/s10434-008-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aussilhou B, Dokmak S, Faivre S, Paradis V, Vilgrain V, Belghiti J. Preoperative liver hypertrophy induced by portal flow occlusion before major hepatic resection for colorectal metastases can be impaired by bevacizumab. Ann Surg Oncol. 2009;16:1553–9. doi: 10.1245/s10434-009-0447-z. [DOI] [PubMed] [Google Scholar]
- 100.Fischer C, Melstrom LG, Arnaoutakis D, Jarnagin W, Brown K, D'Angelica M, et al. Chemotherapy after portal vein embolization to protect against tumor growth during liver hypertrophy before hepatectomy. JAMA Surg. 2013;148:1103–8. doi: 10.1001/jamasurg.2013.2126. [DOI] [PubMed] [Google Scholar]
- 101.Muratore A, Zimmitti G, Ribero D, Mellano A, Viganò L, Capussotti L. Chemotherapy between the first and second stages of a two-stage hepatectomy for colorectal liver metastases: should we routinely recommend it? Ann Surg Oncol. 2012;19:1310–5. doi: 10.1245/s10434-011-2069-5. [DOI] [PubMed] [Google Scholar]
- 102.Simoneau E, Hassanain M, Shaheen M, Aljiffry M, Molla N, Chaudhury P, et al. Portal vein embolization and its effect on tumour progression for colorectal cancer liver metastases. Br J Surg. 2015;102:1240–9. doi: 10.1002/bjs.9872. [DOI] [PubMed] [Google Scholar]
- 103.Spelt L, Sparrelid E, Isaksson B, Andersson RG, Sturesson C. Tumour growth after portal vein embolization with pre-procedural chemotherapy for colorectal liver metastases. HPB (Oxford) 2015;17:529–35. doi: 10.1111/hpb.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gruttadauria S, Luca A, Mandala L, Miraglia R, Gridelli B. Sequential preoperative ipsilateral portal and arterial embolization in patients with colorectal liver metastases. World J Surg. 2006;30:576–8. doi: 10.1007/s00268-005-0423-0. [DOI] [PubMed] [Google Scholar]
- 105.Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–74. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]
- 106.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–8. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 107.am Esch JS, Schmelzle M, Fürst G, Robson SC, Krieg A, Duhme C, et al. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances functional hepatic reserves after extended right hepatectomy: a retrospective single-center study. Ann Surg. 2012;255:79–85. doi: 10.1097/SLA.0b013e31823d7d08. [DOI] [PubMed] [Google Scholar]
- 108.Avritscher R, Abdelsalam ME, Javadi S, Ensor J, Wallace MJ, Alt E, et al. Percutaneous intraportal application of adipose tissue-derived mesenchymal stem cells using a balloon occlusion catheter in a porcine model of liver fibrosis. J Vasc Interv Radiol. 2013;24:1871–8. doi: 10.1016/j.jvir.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 109.Beppu T, Nitta H, Hayashi H, Imai K, Okabe H, Nakagawa S, et al. Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol. 2015;50:1197–205. doi: 10.1007/s00535-015-1067-y. [DOI] [PubMed] [Google Scholar]