Abstract
Objective:
The expression of B-cell lymphoma 2 (Bcl-2) seems to be influenced by the endocrine environment. Numerous reports demonstrate the diverse expression of Bcl-2 family members under sex steroid regulation. With the exception of estrogen-related tumors, androgen-related tumors have shown their characteristics in Bcl-2 expression. In this study, the status of Bcl-2 expression in male hepatocellular carcinoma (HCC) patients was examined to verify the high incidence of HCC in males.
Methods:
Tumor tissue microarray was used to examine Bcl-2 expression levels in 374 HCC cases including 306 males and 68 females. Kaplan-Meier method, log-rank test, and Cox proportional hazards model were applied to investigate the predictive value of Bcl-2 in HCC patients.
Results:
Immunohistochemistry analysis showed that male patients with higher Bcl-2 levels had significantly longer median survival time and recurrence time than those with lower levels. However, no significant differences in outcomes were found between different Bcl-2 levels in female patients. When the male patients were stratified into several age points, the level of Bcl-2 expression showed poorer predictive efficiency in the 45–49 and 55–60 age groups in andropause-age patients compared with other age groups. Bcl-2 was an independent prognostic factor for both overall survival (P < 0.0001) and recurrence time (P = 0.0001) in male patients. After excluding male patients in the 45–60 age group, the predictive efficiency was enhanced (n = 147, OS, P = 0.0002, TTR, P < 0.0001).
Conclusions:
Bcl-2 expression is an independent predictor of survival and recurrence in male HCC. Bcl-2 levels may also be regulated by androgens or androgen receptors in male HCC patients. Bcl-2 levels change and exhibit poor predictive efficiency when androgen levels vary dramatically (andropause age).
Keywords: Hepatocellular carcinoma, andropause, Bcl-2, prognosis, androgen
Introduction
Hepatocellular carcinoma (HCC) is known as a major cancer killer in China. Liver cancer is the most commonly diagnosed cancer and the leading cause of cancer death in men before the age of 601. Surgical resection, liver transplantation, and ablation by radiofrequency or ethanol injection are the conventional therapies at the early stages of this disease. These options offer a 50%–70% chance of survival for five years. The outcomes of resection and local ablation are hampered by high incidences of recurrence, which cannot be prevented by adjuvant therapies2. Therefore, the predictive factor for the prognosis of HCC patients can provide valuable guidance to post-surgery therapy or be a new target for HCC treatment. B-cell lymphoma 2 (Bcl-2) is one of the antiapoptotic proteins. Bcl-2 family proteins regulate apoptosis along the intrinsic mitochondrial apoptosis pathway. Although tumor cells can avoid apoptosis, the major role of Bcl-2 in tumor genesis and progression is more complicated. The expression of Bcl-2 in some tumor cell types inhibits cell adhesion, spreading, and motility by enhancing actin polymerization3. Our team has reported the expression profile of osteopontin and Bcl-2 in HCC4. This prior research also found that high Bcl-2 expression is an effective predictor of HCC prognosis.
In both men and women, gonadal function declines with age. However, in middle-aged women, a relatively abrupt and universal loss of ovarian function, whereas in men, gonadal function appears to develop a more gradual, incomplete, and age-associated decline, which shows a high degree of interindividual variability. This age of men is called andropause. “andras” in Greek means human male, and “pause” in Greek means a cessation5. The liver is a sexually dimorphic organ, with gender differences in gene expression, mitochondrial function, microsomal enzyme activity, membrane lipid composition, and immune responses6. Yang et al.7 found that the pattern of liver Apo A-I isoforms is altered in male HBV-Tg mice but not in female HBV-Tg mice. Their results further indicated that the basic Apo A-I isoform increases in male chronic hepatitis B patients but not in female ones. Another report revealed that hepatocytes expressing the longer androgen receptor (AR) allele in female hepatocarcinogenesis seem to be selected favorably for autonomous growth and transformation, especially in synergy with hepatitis B virus (HBV) infection8. Naugler et al.9 proposed that the estrogen-mediated inhibition of IL-6 production by Kupffer cells reduces liver cancer risk in females, and that these findings may be used to prevent HCC in males. In all the examples provided above, the expression and function of Bcl-2 in HCC may be different from those of other types of tumors, especially in male patients.
Huang et al.10 demonstrated that the suppression of Bcl-2 expression by androgens in prostate cancer cells is mediated by the androgen-induced activation of the CDKI-RB axis. Such suppression decreases the binding of the E2F1 protein to the E2F site in the Bcl-2 promoter. The level of Bcl-2 expression in the tumor tissues of HCC patients was tested. These studies suggest that some factors have disparity in distribution because of the different backgrounds of the sex hormone. Additionally, androgens can inhibit Bcl-2 expression regardless of whether they are normal or malignant. However, the mechanisms underlying Bcl-2 regulation by age and androgens in HCC patients remain unclear. The current study aims to dissect the characteristics of Bcl-2 distribution in terms of age and background of the sex hormone. Bcl-2 can be a more powerful prognostic indicator when stratified by age and gender. The therapy may also be combined with the simultaneous targeting of Bcl-2 and hormone or be fit for a specific group of patients.
Materials and methods
Patients and specimens
A total of 374 patients undergoing curative resection for HCC between 2004 and 2006 at the authors’ institutions were enrolled in this study. For each patient, the diagnosis of HCC was confirmed by pathologic examination, and complete follow-up data were available. The clinicopathologic characteristics of patients TMA are summarized inTable 1. The follow-up was completed in March 2012. Median survival was 55.8±1.7 months. The follow-up procedures and treatment modalities after relapse were described in our previous studies. The diagnosis of recurrence was based on typical imaging appearance in computed tomography (CT) and/or magnetic resonance imaging (MRI) scan and an elevated alpha fetoprotein (AFP) level. Overall survival (OS) was defined as the interval between the dates of surgery and death. Time to recurrence (TTR) was defined as the time between the start of surgery and the first report of recurrence11.
1.
Correlations of Bcl-2 immunohistochemical density of tissue microarrays and age specific groups with clinicopathological features of HCC patients (n = 374)
| Variable | Bcl-2 density | P | Age specific groups | P | |||
| Low (n = 153) | High (n = 153) | 22-44 y (n = 84) | 45-60 y (n = 159) | 61-76 y (n = 63) | |||
| ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer staging system; HBsAg, hepatitis B surface antigen; χ2-tests were conducted for all analyses. | |||||||
| Age, years | |||||||
| ≤44 | 43 | 41 | 0.888 | ||||
| 45-60 | 81 | 78 | |||||
| ≥61 | 29 | 32 | |||||
| Preoperative AFP (ng/mL) | 0.150 | ||||||
| ≤20 | 64 | 58 | 0.484 | 28 | 63 | 31 | |
| >20 | 89 | 95 | 56 | 96 | 32 | ||
| HBsAg | <0.0001 | ||||||
| Negative | 9 | 13 | 0.784 | 2 | 6 | 14 | |
| Positive | 144 | 140 | 82 | 153 | 49 | ||
| Liver cirrhosis | 0.012 | ||||||
| No | 14 | 21 | 0.209 | 15 | 10 | 10 | |
| Yes | 139 | 132 | 69 | 149 | 53 | ||
| ALT (U/L) | 0.965 | ||||||
| ≤75 | 130 | 138 | 0.166 | 73 | 140 | 55 | |
| >75 | 23 | 15 | 11 | 19 | 8 | ||
| Tumor size (cm) | 0.723 | ||||||
| ≤5 | 116 | 119 | 0.685 | 65 | 124 | 46 | |
| >5 | 37 | 34 | 19 | 35 | 17 | ||
| Tumor encapsulation | 0.200 | ||||||
| None | 80 | 77 | 0.732 | 37 | 83 | 37 | |
| Complete | 73 | 76 | 47 | 76 | 26 | ||
| Vascular invasion | 0.824 | ||||||
| No | 99 | 106 | 0.395 | 54 | 108 | 43 | |
| Yes | 54 | 47 | 30 | 51 | 20 | ||
| BCLC stage | 0.995 | ||||||
| 0/A | 83 | 86 | 0.730 | 46 | 88 | 35 | |
| B/C | 70 | 67 | 38 | 71 | 28 | ||
| Tumor differentiation | 0.378 | ||||||
| I-II | 123 | 106 | 0.025 | 53 | 126 | 50 | |
| III-IV | 30 | 47 | 31 | 33 | 13 | ||
This study was approved by the institutional ethics review committee of Huashan Hospital, Fudan University (Shanghai, China). Informed consent was obtained from each patient. Fresh tissues for Western blot were collected immediately after resection, frozen in liquid nitrogen, and then stored at – 80°C.
Tissue microarray immunohistochemistry analysis for Bcl-2
Tissue microarrays were constructed as previously described12. All HCC cases were histologically reviewed by H&E staining, and representative areas were premarked in the paraffin blocks, away from necrotic and hemorrhagic materials. Duplicate cylinders of 1 mm in diameter were taken from two areas of the donor blocks and transferred to the recipient paraffin block at defined array positions (Shanghai Biochip Co., Ltd.). Thus, four tissue microarray blocks were constructed, two with 188 cores, and the other two with 186 cores. Consecutive 4-μm-thick sections were placed on 3-aminopropyltriethoxysilane-coated slides.
Rabbit anti-human Bcl-2 antibody (1:100; Abcam) was purchased. Immunohistochemistry of serial tissue microarrays was carried out as described previously12. Briefly, the sections were dewaxed, hydrated, and washed. After neutralization of endogenous peroxidase and microwave antigen retrieval, slides were preincubated with blocking serum and then incubated with primary antibodies for 12 hours in a moist chamber at 4°C. Subsequently, the sections were serially rinsed, incubated with second antibodies, and treated with horseradish peroxidase-conjugated streptavidin. The components of the Envision Plus detection system were applied (EnVision+/HRP/Mo; Dako, USA). Reaction products were visualized by incubation with 3, 3-diaminobenzidine solution. Negative controls were treated identically but with the primary antibodies omitted.
The photographs of four representative fields were captured by the Leica QWin Plus version 3 software, and the identical settings were used for each photograph under high-power magnification (×100). The density of positive staining was evaluated using a Leica CCD camera DFC420 connected to a Leica DM IRE2 microscope (Leica Microsystems Imaging Solutions, Cambridge, UK) and a computer. The Bcl-2 densities were determined by Image-Pro Plus software (version 5.0, Media Cybernetics, USA) as previously described. The integrated optical density (IOD) of all the positive staining in each photograph was measured, and the density was calculated as the product of IOD/total area13.
Statistical analysis
Statistical analysis was performed using SPSS 15.0 for Windows (SPSS, Chicago, USA). Values were expressed as the mean ± standard deviation. Kaplan-Meier method was used to calculate the survival and recurrence curves, as well as to estimate OS and TTR. Log-rank test was used to compare the TTR and OS between patients in different groups. Spearman rank and Fisher’s exact tests were applied to demonstrate clinicopathological correlations. Univariate and multivariate analyses were performed with Cox proportional hazards model. Student’s t-test was used to compare the data between groups; if variances within groups were not homogeneous, the nonparametric Mann-Whitney U test and Kruskal-Wallis H test were used. P < 0.05 was considered statistically significant. For Bcl-2 density, the cut-off for the definition of subgroups was the median value.
Results
Clinicopathological features of patients
The characteristics of the studied cases are shown inTable 1. Tumor differentiation had a lower grade in the low Bcl-2 group than in the high ones. The younger group had more patients with positive HBsAg than the older groups (97.6%, 96.2%, and 77.8%, respectively; P < 0.0001). Moreover, the oldest group had fewer patients with liver cirrhosis compared with the younger ones (93.2%, 93.7%, and 84.1%, respectively; P = 0.012).
Bcl-2 expression levels in liver tumor tissue detected by immunohistochemistry in TMA
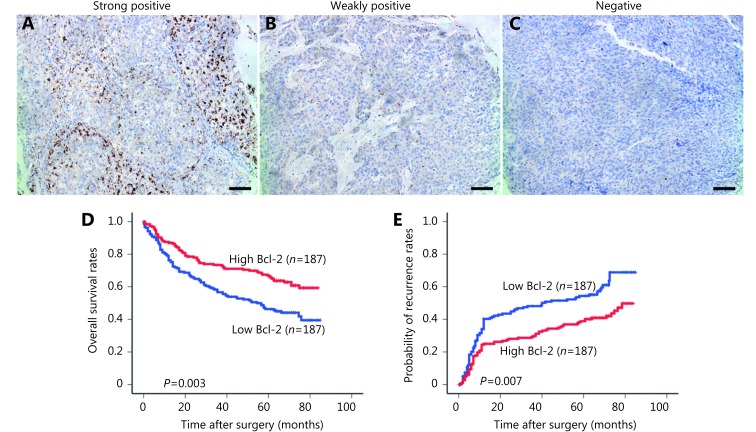
Immunoreactivity for Bcl-2 was observed in both the cytoplasm and the nucleus of tumor cells (Figure 1). Most of the stroma cells presented negative staining, although sporadic positive staining on these cells was also observed (Figure 1A-C).
1.
(A) Different expression status of Bcl-2 in tumor tissues detected by immunohistochemical staining and its correlation with the prognosis of HCC patients. (B) The graph was from a strong positive case in Bcl-2 expression. (C) The photograph shows a weekly positive expression level. (D) The graph was a totally negative one that showed little staining of Bcl-2. Positive cells were stained brown (100×); Bar = 100 μm. (E) High Bcl-2 density in tumor was associated with prolonged OS and TTR. (E) Different expression status of Bcl-2 in tumor tissues detected by immunohistochemical staining and its correlation with the prognosis of HCC patients.
Association between the Bcl-2 level and the prognosis of all HCC patients
The 1, 3, 5 and 7 year OS rates of high Bcl-2 patients (86.9%, 73.4%, 66.8%, and 60.8%, respectively) were significantly higher than those of low Bcl-2 patients (76.2%, 56.6%, 46.3%, and 39.5%, respectively; P = 0.0003) (Figure 1D). The 1, 3, 5 and 7 year tumor cumulative recurrence rates of high Bcl-2 patients (25.0%, 29.9%, 38.9%, and 43.7%, respectively) were significantly lower than those of low Bcl-2 patients (40.3%, 48.1%, 54.4%, and 68.9%, respectively;P = 0.0007) (Figure 1E).
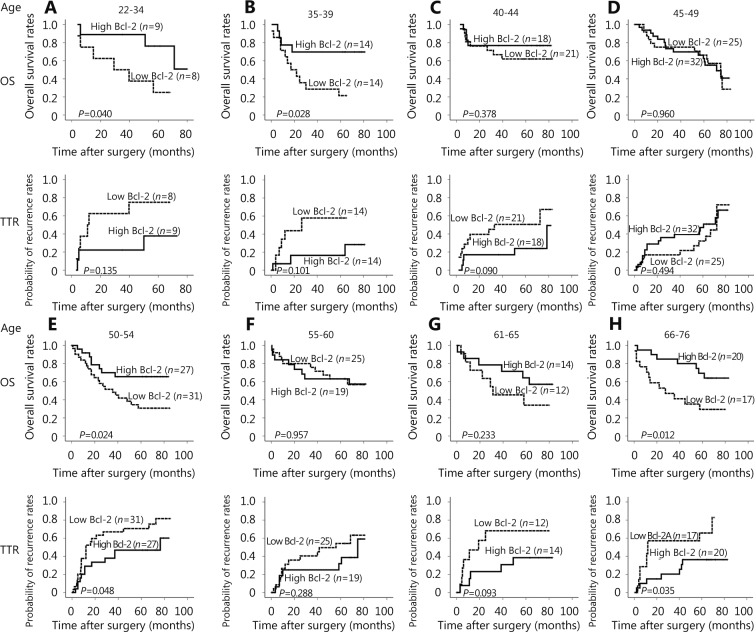
Kaplan-Meier curves of survival differences among HCC male patients in different age groups
For male patients in the 22–34, 35–39, 50–54 and 66–76 age groups, the P values of their OS by Log-rank analysis for Bcl-2 expression were less than 0.05. For male patients in the 50–54 and 66–76 age groups, the log-rank P values of TTR were less than 0.05. Remarkably, for the 45–49 and 55–60 age groups, the P values of both OS and TTR were higher than 0.1. The values are different from other age groups (Figure 2). The results revealed that the expression characteristics of Bcl-2 of male patients in the 45–49 and 55–60 age groups were different from those of other age groups. The age of andropause was approximately 45–60 years. This finding suggested that Bcl-2 expression level may be intervened in the age of andropause.
2.
Kaplan-Meier curves of survival differences among HCC patients in different age groups. (A) OS and TTR for Bcl-2 expression of male patients in the 22–34 age group. (B) OS and TTR for Bcl-2 expression in 35–39-year-old male patients. (C) OS and TTR for Bcl-2 expression in 40–44-year-old male patients. (D) OS and TTR for the Bcl-2 expression in 45–49-year-old male patients. (E) OS and TTR for Bcl-2 expression in 50–54-year-old male patients. (F) OS and TTR for Bcl-2 expression in 55–60-year-old male patients. (G) OS and TTR for Bcl-2 expression in 61–65-year-old male patients. (H) OS and TTR for the Bcl-2 expression in 66–76-year-old male patients.
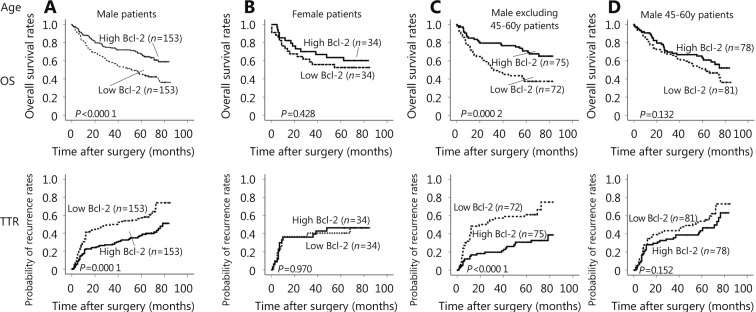
Kaplan-Meier curves were different among HCC patients in different age groups. The OS and TTR for Bcl-2 expression in male patients showed the OS and recurrence time of high Bcl-2 level patients were significantly longer than those of the low Bcl-2 level patients (OS, P < 0.0001; TTR, P = 0.0001) (Figure 3A). Contrarily, the OS and TTR in female patients did not show any difference between the two groups (OS, P = 0.428; TTR, P = 0.970) (Figure 3B). The difference of Bcl-2 expression between the male and female patients implies that the expression of Bcl-2 may be correlated with the sex hormone. The OS and TTR for Bcl-2 expression in male patients, excluding the 45–60 age group, showed the overall survival and recurrence time of high Bcl-2 level patients were significantly longer than those of the low Bcl-2 level patients (OS, P = 0.0002; TTR, P < 0.0001) (Figure 3C). Inversely, the OS and TTR of male patients in the 45–60 age group did not show any difference between the two groups (OS, P = 0.132; TTR, P = 0.152) (Figure 3D). This result further demon-strates that androgen may play an important role in the Bcl-2 expression of HCC patients, especially in male patients of andropause age.
3.
Kaplan-Meier curves of survival differences among HCC patients. (A) OS and TTR for Bcl-2 expression in male patients. (B) OS and TTR for Bcl-2 expression in female patients. (C) OS and TTR for Bcl-2 expression in male patients, excluding those in the 45–60-year-age group. (D) OS and TTR for Bcl-2 expression in 45–60-year-old male patients.
Differences between patients in andropause and other age groups by several indexes
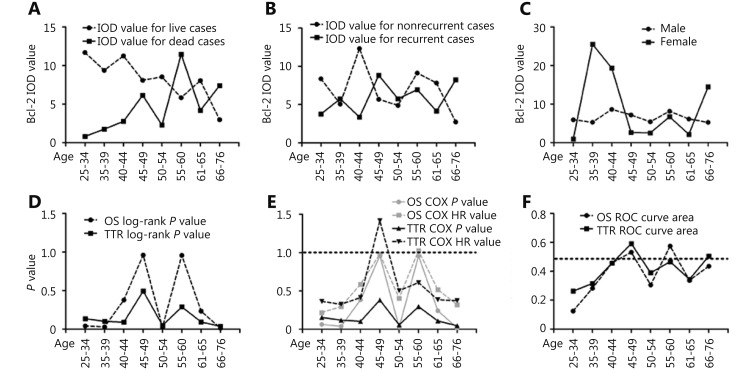
The Bcl-2 IOD value of live and dead cases in different age groups showed that the 45–49 and 55–60 age groups of dead cases had irregularly higher IOD values than the other groups (Figure 4A). The Bcl-2 IOD value of the non-recurrent cases and the recurrent ones in different age groups showed that the 45–54 age groups of recurrent cases had irregularly higher IOD values than the other groups (Figure 4B). The Bcl-2 IOD value of the male and female cases in different age groups showed that male patients had a smoother curve than the female patients (Figure 4C). Unlike other age groups, the P values of OS and TTR in the 45–49 and 55–60 age groups were also much higher than 0.1 (Figure 4D). Meanwhile, hazard ratios analyzed by Cox proportional hazards regression model with survival and recurrence in different age groups showed that the 45–49 and 55–60 age groups had peculiarly higher hazard ratios than the other groups (Figure 4E). The areas under the curve for death and recurrence in different age groups showed that the 45–49 and 55–60 age groups were closer to 0.5 than the other age groups (Figure 4F).
4.
Differences between patients in andropause and other age groups by several indexes. (A) Difference of Bcl-2 IOD value between the live and dead cases in different age groups. (B) Difference of Bcl-2 IOD value between the non-recurrent and recurrent cases in different age groups. (C) Difference of Bcl-2 IOD value between the male and female cases in different age groups. (D) P values of OS and TTR in different age groups. (E) P values and hazard ratios analyzed by Cox proportional hazards regression model with survival and recurrence in different age groups. (F) Area under the curve for death and recurrence in different age groups.
Univariate and multivariate analyses of the prognostic values of Bcl-2 in HCC patients
To further evaluate the prognostic value of Bcl-2 for HCC patients, univariate and multivariate analyses were performed with the clinicopathological characteristics and the Bcl-2 levels (Tables 2 and3). In the univariate analysis, serum AFP and ALT level, tumor size, tumor number, vascular invasion, and Barcelona Clinic Liver Cancer (BCLC) stage were revealed to associate with the OS of HCC patients, tumor size, and vascular invasion with TTR. The Bcl-2 expression level in HCC tissues was also significantly associated with both OS and TTR.
2.
Univariate analyses of factors associated with OS and TTR
| Variable | OS | TTR | |||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| 95% CI, 95% confidence interval; AFP, alpha-fetoprotein; NS, not significant (Cox proportional hazards regression model); BCLC, Barcelona Clinic Liver Cancer Staging System. | |||||
| Age, years (> 52 vs. ≤ 52) | 0.531 (0.723-1.875) | 0.531 | 1.122 (0.683-1.842) | 0.650 | |
| HBsAg (positive vs. negative) | 2.554 (0.929-7.021) | 0.069 | 3.187 (0.997-10.182) | 0.051 | |
| AFP (ng/mL; > 20 vs. ≤ 20) | 1.717 (1.033-2.856) | 0.037 | 1.539 (0.916-2.585) | 0.104 | |
| Liver cirrhosis (yes vs. no) | 1.211 (0.619-2.371) | 0.576 | 1.693 (0.770-3.722) | 0.190 | |
| ALT (U/L; > 75 vs. ≤ 75) | 1.964 (1.072-3.597) | 0.029 | 1.668 (0.848-3.282) | 0.138 | |
| Tumor size (cm; > 5 vs. ≤ 5) | 2.198 (1.328-3.639) | 0.002 | 1.927 ( 1.120-3.318) | 0.018 | |
| Tumor number (multiple vs. single) | 2.915 (1.057-8.042) | 0.039 | 1.273 (0.310-5.221) | 0.738 | |
| Vascular invasion (yes vs. no) | 2.435 (1.509-3.931) | <0.001 | 1.915 (1.153-3.182) | 0.012 | |
| BCLC stage (B/C vs. A) | 2.046 (1.096-3.821) | 0.025 | 1.767 (0.958-3.261) | 0.068 | |
| Tumor differentiation (III-IV vs. I-II) | 1.440 (0.871-2.381) | 0.156 | 1.533 (0.912-2.577) | 0.107 | |
| Tumor encapsulation (none vs. complete) | 1.419 (0.880-2.287) | 0.151 | 1.278 (0.779-2.096) | 0.331 | |
| Bcl-2 (high vs. low) | 0.403 (0.245-0.665) | <0.001 | 0.368 (0.219-0.618) | <0.001 | |
3.
Multivariate analyses of factors associated with OS and TTR
| Variable | OS | TTR | |||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| 95% CI, 95% confidence interval; AFP, alpha-fetoprotein; NS, not significant (Cox proportional hazards regression model). | |||||
| AFP (ng/mL; > 20 vs. ≤ 20) | 1.333 (0.785-2.265) | 0.035 | |||
| ALT (U/L; > 75 vs. ≤ 75) | 1.960 (1.059-3.629) | 0.287 | |||
| Tumor size (cm; > 5 vs. ≤ 5) | 1.687 (1.007-2.825) | 0.047 | 1.667 (0.959-2.897) | 0.070 | |
| Tumor number (multiple vs. single) | 1.585 (0.557-4.506) | 0.388 | |||
| Vascular invasion (yes vs. no) | 2.033 (1.230-3.359) | 0.006 | 1.664 (0.991-2.792) | 0.054 | |
| Bcl-2 (high vs. low) | 0.458 (0.275-0.761) | 0.003 | 0.391 (0.232-0.659) | <0.001 | |
Individual features that showed significance by univariate analysis were adopted as covariates in a multivariate Cox proportional hazards model, and then combined variables were further analyzed. Bcl-2 was demonstrated to be an independent prognostic indicator for OS (P = 0.003) and TTR (P < 0.001).
Discussion
Bcl-2 is an oncogenic protein that acts by inhibiting programmed cell death14. Bcl-2 expression in endothelial cells was reported to enhance tumor metastasis15. However, increasing evidence showed that Bcl-2 expression may, in some cases, be associated with the improved prognosis of patients diagnosed with non-small cell lung cancer16, renal cell carcinoma17, colorectal cancer18, melanoma19, and breast cancer20. The physiological role of Bcl-2 expression in cell motility has not been well characterized3.
The current work analyzed Bcl-2 expression in predominantly HBV-related HCC in men and women, as well as the association of Bcl-2 expression levels with survival and recurrence. The expression of Bcl-2 in tumoral tissues was found to be associated with the prognosis of male HCC patients but not female ones. Male patients whose tumors had low Bcl-2 expression had shorter survival and recurrence time compared with patients whose tumors had high Bcl-2 expression. The disparity of Bcl-2 expression between male and female suggest that the phenomenon may have some relationship with the sex hormone or their receptors in HCC patients. Androgens have been suggested to induce and promote HCC21, and altered androgen metabolism has been reported to be associated with HCC22. In a recent report, androgens enhanced DNA damage and oxidative stress during hepatocarcinogenesis23. A report several years ago demonstrated that AR mRNA levels are related to histological tumoral differentiation and are lower in highly dedifferen-tiated HCCs compared to well-differentiated ones24. One study indicated that androgen/AR facilitates TPA-induced apoptosis by interrupting the NF-κB signaling pathway, leading to the activation of JNK in LNCaP cells25. Elevated AR levels in transformed cells could have a tumor-promoting effect by stimulating cell growth26. Another study suggests that FoxA factors serve as a scaffold for steroid hormone receptors to regulate gene transcription in the liver on a genome-wide scale; such scaffold extends to at least three nuclear hormone receptors as well. The study identified a set of regulatory units in which the juxtaposition of FoxA binding sites next to both EREs and AREs allows for the mediation of the gender-specific effects of sex hormones in the liver27. A report demonstrated that AR promotes HBV induced hepatocarcinogenesis through modulation of the HBV RNA transcription; targeting the AR, rather than the androgen, could likewise be developed as a new therapy to battle HBV-induced HCC28. Thus, AR is a new potential therapeutic target for the treatment of HCC29.
Our results suggest that Bcl-2 expression level had poorer predictive efficiency in male patients in the 45–49 and 55–60 age groups. Bcl-2 expression in the 45–49 and 55–60 age groups was not related to the prognosis of the male HCC patients, who are thought to be in andropause age. In a study of 434 men aged 50–86 years, the prevalence of symptoms increases with decreasing testosterone concentrations30. Some findings suggested that a significant proportion of men over 60 years have circulating testosterone concentrations in the range conventionally considered hypogonadal31.
The Bcl-2 levels in tumor tissues are possibly influenced by some factors that vary in the andropause of male HCC patients. These hypotheses are supported by the following findings. Bcl-2 expression was associated with the prognosis only in male HCC patients but not in female patients. Meanwhile, Bcl-2 expression in the 45–49 and 55–60 age groups was not related to the prognosis of male HCC patients.
The mechanisms behind the Bcl-2 disability of predictive power to HCC prognosis are unclear. Nevertheless, Ohigashi's data indicated that herbimycin A-sensitive tyrosine kinase(s) can regulate apoptosis and inhibit Bcl-2 expression in SC2G mouse androgen-dependent cells32. Fuzio et al.33 analyzed Bcl-2 expression in vivo in prostate cancer tissues obtained from patients who underwent radical prostatectomy after neoadjuvant androgen deprivation therapy (ADT). They found that short-term administration of ADT interferes with Bcl-2 expression, thereby suggesting that androgen-mediated mechanisms may act through Bcl-2-mediated apoptotic pathways. Another study used antisense oligonucleotide treatment. The treatment directed against Bcl-2 can be evaded through compensatory changes in AR expression and some coactivators, promoting tumor growth, and promoting transformation of the tumor to a more aggressive phenotype34. Consistently, another group suggested that the down-regulation of Bcl-2 by antisense oligodeoxynucleotides chemosensitizes androgen-independent Shionogi tumors to taxanes, over and above the effects of taxane-induced phosphorylation of Bcl-235. These hypotheses require evaluation.
In conclusion, Bcl-2 expression was identified as an independent predictor of recurrence and survival in male HCC. And Bcl-2 levels may be regulated by androgens or AR in male HCC patients. When androgen or AR levels varied dramatically (andropause age), the Bcl-2 level changed and had poorer predictive efficiency.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:15–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614–6. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 3.Ke H, Parron VI, Reece J, Zhang JY, Akiyama SK, French JE. BCL2 inhibits cell adhesion, spreading, and motility by enhancing actin polymerization. Cell Res. 2010;20:458–69. doi: 10.1038/cr.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng B, Zhang XF, Zhu XC, Huang H, Jia HL, Ye QH, et al. Correlation and prognostic value of osteopontin and Bcl-2 in hepatocellular carcinoma patients after curative resection. Oncol Rep. 2013;30:2795–803. doi: 10.3892/or.2013.2737. [DOI] [PubMed] [Google Scholar]
- 5.Singh P. Andropause: current concepts. Indian J Endocrinol Metab. 2013;17:S621–9. doi: 10.4103/2230-8210.123552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers AB, Theve EJ, Feng Y, Fry RC, Taghizadeh K, Clapp KM, et al. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 2007;67:11536–46. doi: 10.1158/0008-5472.CAN-07-1479. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Yin YX, Wang F, Zhang L, Wang YQ, Sun SH. An altered pattern of liver apolipoprotein A-I isoforms is implicated in male chronic hepatitis B progression. J Proteome Res. 2010;9:134–43. doi: 10.1021/pr900593r. [DOI] [PubMed] [Google Scholar]
- 8.Yeh SH, Chang CF, Shau WY, Chen YW, Hsu HC, Lee PH, et al. Dominance of functional androgen receptor allele with longer CAG repeat in hepatitis B virus-related female hepatocarcinogenesis. Cancer Res. 2002;62:4346–51. [PubMed] [Google Scholar]
- 9.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 10.Huang HJ, Zegarra-Moro OL, Benson D, Tindall DJ. Androgens repress Bcl-2 expression via activation of the retinoblastoma (RB) protein in prostate cancer cells. Oncogene. 2004;23:2161–76. doi: 10.1038/sj.onc.1207326. [DOI] [PubMed] [Google Scholar]
- 11.Zhou HJ, Huang H, Shi J, Zhao Y, Dong QZ, Jia HL, et al. Prognostic value of interleukin 2 and interleukin 15 in peritumoral hepatic tissues for patients with hepatitis B-related hepatocellular carcinoma after curative resection. Gut. 2010;59:1699–708. doi: 10.1136/gut.2010.218404. [DOI] [PubMed] [Google Scholar]
- 12.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–16. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 14.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–6. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 15.Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest. 2008;88:740–9. doi: 10.1038/labinvest.2008.46. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz A, Savaş I, Dizbay Sak S, Güngör A, Kaya A, Serinsöz E, et al. Distribution of Bcl-2 gene expression and its prognostic value in non-small cell lung cancer. Tuberk Toraks. 2005;53:323–9. [PubMed] [Google Scholar]
- 17.Itoi T, Yamana K, Bilim V, Takahashi K, Tomita F. Impact of frequent Bcl-2 expression on better prognosis in renal cell carcinoma patients. Br J Cancer. 2004;90:200–5. doi: 10.1038/sj.bjc.6601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leahy DT, Mulcahy HE, O'Donoghue DP, Parfrey NA. bcl-2 protein expression is associated with better prognosis in colorectal cancer. Histopathology. 1999;35:360–7. doi: 10.1046/j.1365-2559.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 19.Divito KA, Berger AJ, Camp RL, Dolled-Filhart M, Rimm DL, Kluger HM. Automated quantitative analysis of tissue microarrays reveals an association between high Bcl-2 expression and improved outcome in melanoma. Cancer Res. 2004;64:8773–7. doi: 10.1158/0008-5472.CAN-04-1387. [DOI] [PubMed] [Google Scholar]
- 20.Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010;103:668–75. doi: 10.1038/sj.bjc.6605736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol. 2002;193:59–63. doi: 10.1016/s0303-7207(02)00096-5. [DOI] [PubMed] [Google Scholar]
- 22.Granata OM, Carruba G, Montalto G, Miele M, Bellavia V, Modica G, et al. Altered androgen metabolism eventually leads hepatocellular carcinoma to an impaired hormone responsiveness. Mol Cell Endocrinol. 2002;193:51–8. doi: 10.1016/s0303-7207(02)00095-3. [DOI] [PubMed] [Google Scholar]
- 23.Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947–55. e5. doi: 10.1053/j.gastro.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavian D, De Petro G, Pitozzi A, Portolani N, Giulini SM, Barlati S. Androgen receptor mRNA under-expression in poorly differentiated human hepatocellular carcinoma. Histol Histopathol. 2002;17:1113–9. doi: 10.14670/HH-17.1113. [DOI] [PubMed] [Google Scholar]
- 25.Altuwaijri S, Lin HK, Chuang KH, Lin WJ, Yeh S, Hanchett LA, et al. Interruption of nuclear factor kappaB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res. 2003;63:7106–12. [PubMed] [Google Scholar]
- 26.Barone M, Margiotta M, Scavo MP, Gentile A, Francioso D, Papagni S, et al. Possible involvement of androgen receptor alterations in hepatocarcinogenesis. Dig Liver Dis. 2009;41:665–70. doi: 10.1016/j.dld.2008.12.099. [DOI] [PubMed] [Google Scholar]
- 27.Li ZY, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu MH, Ma WL, Hsu CL, Chen YL, Ou JH, Ryan CK, et al. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2:32ra35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi L, Lin H, Li G, Jin RA, Xu J, Sun Y, et al. Targeting Androgen Receptor (AR)→IL12A Signal Enhances Efficacy of Sorafenib plus NK Cells Immunotherapy to Better Suppress HCC Progression. Mol Cancer Ther. 2016;15:731–42. doi: 10.1158/1535-7163.MCT-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–43. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 31.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 32.Ohigashi T, Ueno M, Nonaka S, Nakanoma T, Furukawa Y, Deguchi N, et al. Tyrosine kinase inhibitors reduce bcl-2 expression and induce apoptosis in androgen-dependent cells. Am J Physiol Cell Physiol. 2000;278:C66–72. doi: 10.1152/ajpcell.2000.278.1.C66. [DOI] [PubMed] [Google Scholar]
- 33.Fuzio P, Ditonno P, Lucarelli G, Battaglia M, Bettocchi C, Senia T, et al. Androgen deprivation therapy affects BCL-2 expression in human prostate cancer. Int J Oncol. 2011;39:1233–42. doi: 10.3892/ijo.2011.1140. [DOI] [PubMed] [Google Scholar]
- 34.Rubenstein M, Hollowell CMP, Guinan P. Increased expression of the androgen receptor with p300 and interleukin-6 coactivators compensate for oligonucleotide suppression of bcl-2: no increased CREB binding protein or interleukin-4 expression. Ther Adv Urol. 2013;5:85–93. doi: 10.1177/1756287212466281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleave ME, Miayake H, Goldie J, Nelson C, Tolcher A. Targeting bcl-2 gene to delay androgen-independent progression and enhance chemosensitivity in prostate cancer using antisense bcl-2 oligodeoxynucleotides. Urology. 1999;54:36–46. doi: 10.1016/s0090-4295(99)00453-7. [DOI] [PubMed] [Google Scholar]