Abstract
Human endogenous retroviruses (HERVs) are retroviruses that infected human genome millions of years ago and have persisted throughout human evolution. About 8% of our genome is composed of HERVs, most of which are nonfunctional because of epigenetic control or deactivating mutations. However, a correlation between HERVs and human cancer has been described and many tumors, such as melanoma, breast cancer, germ cell tumors, renal cancer or ovarian cancer, express HERV proteins, mainly HERV-K (HML6) and HERV-K (HML2). Although the causative role of HERVs in cancer is controversial, data from animal models demonstrated that endogenous retroviruses are potentially oncogenic. HERV protein expression in human cells generates an immune response by activating innate and adaptive immunities. Some HERV-derived peptides have antigenic properties. For example, HERV-K (HML-6) encodes the HER-K MEL peptide recognized by CD8+ lymphocytes. In addition, HERVs are two-edged immunomodulators. HERVs show immunosuppressive activity. The presence of genomic retroviral elements in host-cell cytosol may activate an interferon type I response. Therefore, targeting HERVs through cellular vaccines or immunomodulatory drugs combined with checkpoint inhibitors is attracting interest because they could be active in human tumors.
Keywords: HERVs, cancer, interferon, immunotherapy
Introduction
Retroviruses are a subgroup of viruses characterized by their ability to integrate their genome into host-cell DNA. Retroviruses are double-stranded positive-sense RNA viruses that use reverse transcriptase enzyme to transcribe their RNAs to DNAs. The resultant DNA is later integrated into host DNA through viral integrase enzyme. Viral-integrated DNA (proviral DNA) is translated and transcribed to proteins as part of host genome using the genetic machinery of infected cell1-3.
The genomic structure of retroviruses is composed of gene gag, pol/pro, and env flanked by two long terminal repeats (LTRs). These open reading frames (ORFs) encode structural and functional viral proteins, and LTRs encode promoter and polyadenylation signals4.
Most retroviruses, such as HIV-1, cause infectious diseases in humans. Some of them can also cause cancer in animals and humans. For instance, Rous sarcoma virus incorporates proto-oncogene c-Src into the viral genome, and v-Src triggers tumor formation in hosts5.
Endogenous retroviruses (ERVs) were described in the late 1960s as retroviral sequences integrated in genome of some animals millions of years ago, following the integration of viral genome into germinal cells6,7. ERVs are commonly inactive and unable to replicate; they play an important role in species evolution. Among the most relevant example is the discovery of the major role of env gene of HERV-W syncytin in the development of syncytiotrophoblast of placenta8.
Human ERVs (HERVs) account for 8% of the human genome; they are classified as class I or II depending on whether their sequences are homologous to mammalian type C retroviruses or mammalian type B and D retroviruses, respectively7. These HERV genomes are not replication-competent because of the acquisition of mutations or loss of relevant genes during host evolution9. Furthermore, HERV transcription is controlled by epigenetic mechanisms10. Therefore, uncontrolled HERV activation may induce relevant physiological consequences11. HERV MER41.AIM2 regulates the transcription of gene absent in melanoma 2 (AIM2) that encodes a sensor of free cytosolic DNA for immune response to viral infections12.
Potentially, HERVs could cause diseases, such as autoimmune disease or cancer, through several mechanisms. HERV-related cancer could be induced by the activation of HERV sequences through hypomethylation; expression of oncogenes, such as HERV-encoded Rec and NP9; inactivation of tumor suppressor genes via mutational insertion; homologous recombination; transcription of close oncogenes or growth factors via LTRs; and induction of syncytial formation by Env protein that could aid the dissemination and progression of cancer cells. However, such induction has not been clearly demonstrated. Evidence supports a possible role of HERVs in human cancer because many proteins from HERVs, such as the proteins from HERV-K MHL1 and MLH2 in melanoma and other tumors, are found in certain tumors. The causative role is uncertain, but its important role in the development of a cross-effective immune response against cancer is recently demonstrated. Consequently, the relationship between virus and cancer attracts interest. In this paper, we summarize the current knowledge on this topic.
HERVs: structure and function
Similar to any integrated retrovirus, a complete HERV sequence is flanked by two LTRs that are genetic regulatory sequences. The ORF gag, pro/pol, and env are present between the LTRs and codified structural and functional viral proteins. LTRs can regulate the transcription of HERV and host genes because LTRs contain a transcriptional promoter and enhancer core13.
Integrated HERV sequences accumulate mutations or recombination events that eliminate the infectious capacity of retrovirus. One LTR is commonly lost by recombination of two LTRs (“single LTR”), thereby losing its capacity for replication14. HERVs also exhibit important alterations in their ORFs that cause their replication defective, thereby losing their capacity to move into the genome15.
HERVs are classified into 22 families according to their sequence identity; their nomenclature refers to the first-letter amino-acid core of the tRNA of the primary binding site used by HERV to start reverse transcription16. HERV-K is the most recent HERV family obtained from humans at around three million years ago. HERV-Ks are formed by 11 subgroups (HML-1-HML-11); the most-studied cancer is HERV-K (HML-2), which is the only one with intact full-length ORFs17.
HERV and cancer
Expression of HERVs in cancer cells
Many studies have identified the expression of HERV protein in cancer tissues, but its causative role in cancer development remains controversial. Several factors, such as UV radiation, estrogen hormone, and smoking, have been proposed as a cause of HERV protein expression in cancer tissues. Furthermore, intrinsic activation of the MAPK and p16INK4A-CDK4 pathways lead to HERV protein expression in melanoma18.
HERV-K Env protein has been identified in melanoma by immunohistochemistry using HERV-K Env-specific monoclonal antibody 6H519. The antigen HERV-K-MEL is expressed in 85% of malignant melanocytes from normal nevi and dysplastic lesions to metastatic melanoma. The expression of HERV-K Env in melanomas is higher than that in benign lesions, especially in metastatic tumors19. HERV-K Env is also found in other tumors, such as breast cancer, ovarian cancer, teratocarcinoma, sarcoma, and bladder cancer20-24. Transcripts from HERV-E (CT-RCC) are expressed in von Hippel-Lindau (VHL)-deficient renal carcinomas25. Regulation of HERV activation in renal cell carcinoma by VHL gene is of particular interest. When VHL transgenes are introduced into VHL-deficient carcinoma cell lines, HERV-E expression is suppressed. This result demonstrates that VHL controls provirus activity25.
High plasma levels of the mRNA of various HERV env genes (HERV-K, HERV-R, and HERV-H) have been found in primary breast cancer patients comparing with normal controls26-28. The levels of these genes decrease during treatment with adjuvant chemotherapy, thereby suggesting a close relation with clinical cancer evolution and a possible role for identification of the persistence of microscopic disease in primary breast cancer patients29. Anti-HERV-E, anti-HERV-K (HML-2), and anti-ERV3 antibodies are also detected in more than 30% of ovarian cancer patients30. The levels of these antibodies are higher in patients with lymph-node-positive breast tumors28. In addition, the presence of serum antibodies against HERV-K proteins in stages I-III melanoma patients has also been described and is a prognostic factor for poor survival31.
Possible mechanisms of oncogenesis
The association between cancer and ERV was first observed in a high incidence of thymic lymphomas in mice, which specifically involves AKR, HRS, and C58 strains. Over the years, subsequent studies demonstrated a viral etiology, which probably results from enhanced-mediated insertional activation of a proto-oncogene. Young et al.32 described the potential of Rag1-mouse-associated retroviruses (RARVs) to replicate in T and B lymphocyte-deficient Rag1 mice compared with purified macrophages from B6 wild type. They observed that almost 67% of the mice show signs of morbidity, and all mice present large tumors and anemia. Tumor analysis revealed high expression of eMLV Env and pol DNA copy numbers; therefore, RARVs had infected cells that cause lymphomas.
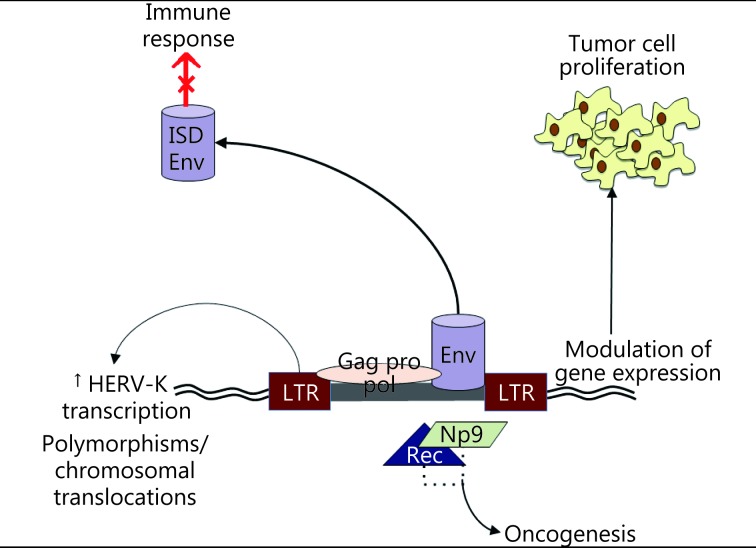
HERVs can transform benign cells through different mechanisms (Figure 1). First, HERVs possess two LTRs that recruit transcription factors from the infected cell for retroviral gene transcription. These LTRs can also enhance transcription of host cell genes, which leads to uncontrolled cell proliferation. A high transcription of the colony stimulation factor receptor 1 in Hodgkin’s lymphoma is caused by reduced expression of transcriptional co-repressor CBFA2T3 (also called MTG16 or ETO2) that inhibits the activity of LTR THE1B in normal cells. Reduced CBFA2T3 expression is caused by the CpG methylation of this gene’s locus33.
1.
Mechanisms of oncogenesis. LTRs recruit transcription factors from the infected cell for retroviral gene transcription. These LTRs can also enhance transcription of the host cell genes, leading to uncontrolled tumor cell proliferation. Some HERVs encode potentially oncogenic proteins like Np9 and Rec that interact with transcription factors and activate immunosuppressor pathways, promoting oncogenesis. HERVs can also induce chromosomal translocations in somatic cells that could lead to tumor proliferation. HERVs also can promote an immunosuppressive response that may lead to cancer formation and spreading, because the Env protein has an immunosuppressive domain (ISD).
As a second oncogenic mechanism, some HERVs, such as HERV-K types I and II, encode potentially oncogenic proteins, such as Np9 and Rec, which interact with transcription factors, including promyelocytic leukemia zinc finger, and activate immunosupressor pathways, such as the beta-catenin pathway34. Conversely, the transcription factor MITF, which is commonly over-activated in melanoma cells, is necessary for the transcriptional activity of the LTR of HERV-K, as demonstrated when forced MITF expression in non-melanoma HEK293 cells activates the HERV-K35.
HERV-induced chromosomal translocation in somatic cells is a third oncogenic mechanism. In prostate cancer, HERV-K and oncogene E26 transformation-specific displays fusion(36), and insertion polymorphisms of HERV-K are correlated with the risk of developing lung adenocarcinoma in non-smokers37.
Finally, HERVs can also promote an immunosuppressive response that may lead to cancer formation and spreading. Env protein contains an immunosuppressive domain, which is confirmed in animal models as a cause of tumor growth for tumor cells harboring the insertion of Moloney MLV (which is not recognized by the animal’s immune system) and in env knockdown in B16 melanoma cells and Neuro-2a neuroblastoma cell lines38.
In conclusion, several potential mechanisms could be used by HERVs for cancer development in animals. Patients with altered immune vigilance system against viruses show increased cancer risk. When there are alterations in key elements of the innate immune system, such as pattern recognition receptors (PRRs), RIG-1, Toll-like receptors (TLRs), or AIM2, increased cancer risk is probably caused by uncontrolled HERV expression. TLR9 or TLR3 deficiency leads to the development of acute lymphoblastic leukemia39. Furthermore, a correlation exists between mutations in retroviral restriction factor SAMHD1 and human cancer40,41.
HERVs as targets for cancer treatment
The frequent overexpression of HERV proteins in cancer cells has been proposed as a target for immunotherapy. Several studies and case reports have described responses to Bacillus Calmette-Guerin (BCG), yellow fever vaccine, or following a febrile process in melanoma patients42,43. Although the mechanism of action is unclear, homology between BCG sequence and yellow fever virus vaccine and the sequence of HERV-K-MEL protein was described44. A case-control study demonstrated that people who received BCG vaccine during childhood or suffered from acute infection present a lower risk of melanoma than those in other members of the population45. Immunoreactivity against melanoma is observed in vitro using sera from Rhesus macaques that received yellow fever vaccine. Furthermore, yellow fever vaccine has been proposed as a profilactic vaccine against melanoma (European Patent EP1586330A1).
Proteins codified by the env gene of HERVs, such as HERV-K and HERV-H, are immunogenic, and humoral and cellular responses are detectable against HERVs. Antibodies against HERV-K inhibit cancer cell growth in vitro and in animal models46. Tumors expressing antigens from HERV env genes are recognized by CD8+ lymphocytes25.
In ovarian22 and breast cancer patients47, the activity of a dendritic vaccine combined with HERV-K Env antigens has been demonstrated in vitro. Other cellular vaccines are prepared using HERV-K Env-specific chimeric antigen receptor (CAR) T-cell vaccines using the Sleeping Beauty system for introducing HERV-K Env-specific CAR derived from mouse monoclonal antibody into T cells48. Recombinant cellular vaccines using the modified vaccine virus Ankara expressing HERV-K Env glycoprotein (MVA-HERV-K Env)49 were also developed, and activity is demonstrated in vitro and in animal models. However, possible secondary effects in humans are concerned. In particular, vaccinating against HERVs antigens could be unsafe because these HERV proteins could play a role in the physiological functions of host.
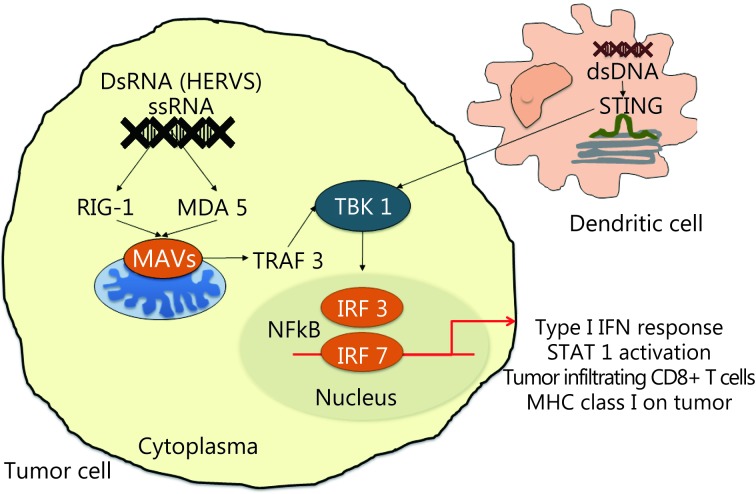
Recently, a new treatment strategy has been proposed using the combination of histone deacetylase inhibitor (HDACi) and checkpoint inhibitors, such as anti-CTLA-4 antibody ipilimumab50. This method is based on the possible reactivation of HERV gene transcription using HDACi or DNA methyltransferase inhibitors that eliminate the epigenetic repression of HERV transcription. HERV expression activates the innate sensor response (PRRs) of single RNA strand (RIG1 and MDA5) and double RNA strand (TLR3) in cytosol that activates the interferon (IFN) type I response by secondary STAT1 activation51. PRR binding to their ligands activates the signaling pathways dependent on adaptor protein mitochondrial antiviral signaling protein (otherwise known as IPS-1). Consequently, this occurrence leads to the activation of the TRAF family member-associated NF-κB activator (TANK)-binding kinase 1 (TBK1) that induces IFN-regulatory factor-3 and 7 (IRF-3 and IRF-7), NF-KB-dependent gene expression, and subsequent production of IFN-beta. IFN-beta, when linked to its membrane receptor (IFNAR1/2), activates IRF9 and STATs, thereby the transcriptional activation of IFN-stimulated genes with cytokine production and increased expression of major histocompatibility complex type I on cancer cells, which potentially increase cancer cell recognition by CD8 T cells50,52,53(Figure 2). When a checkpoint inhibitor is used in combination, these drugs activate CD8 T cells and increase the IFN-γ gamma production by lymphocytes that increase the transcription of IFN-stimulated genes in tumor cells50.
2.
Retranscription of HERVs would activate the innate response of sensors (pattern-recognition receptors or PRRs) of single RNA strand (RIG1 and MDA5) in cytosol of the cancer cells. This activates the signaling pathways leading to activation of TRAF family member-associated NF-κB activator (TANK)-binding kinase 1 (TBK1) that causes induction of the IFN-regulatory factor-3 and 7 (IRF-3 and IRF-7), NF-KB-dependent gene expression and subsequent production of IFN beta. This results in transcriptional activation of interferon stimulated genes with the production of cytokines, and increased expression of MHC type I on cancer cells.
Synergy between epigenetic drugs and immunotherapy has also been proposed54. In HDACi-treated animal models, this phenomenon promotes the production of CD8 effector cells and increases antitumor activity55. Combining hypomethylating agents with anti-CTLA-4 antibodies also increases antitumor activity56.
Conclusions
The discovery of HERV expression in several tumors results in novel cancer treatment strategies based mainly on manipulating immune response against these proteins that are selectively expressed in tumor cells and not transcribed in normal cells.
Immunotherapy for cancer treatment has recently achieved significant results. Several antibodies blocking checkpoint inhibitors, such as anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab and pembrolizumab) drugs, have been approved for treating advanced tumors, including melanoma and non-small cell lung cancer. Nevertheless, the efficacy of this strategy could be increased when combined with other drugs or radiotherapy. Combining drugs that block checkpoint inhibitors with epigenetic drugs is a promising approach. These drug combinations are based on preclinical model results on antitumoral immune responses targeting proteins derived from HERV genes in cancer cells.
References
- 1.Yu P. The potential role of retroviruses in autoimmunity. Immunol Rev. 2016;269:85–99. doi: 10.1111/imr.12371. [DOI] [PubMed] [Google Scholar]
- 2.Stoye JP. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat Rev Microbiol. 2012;10:395–406. doi: 10.1038/nrmicro2783. [DOI] [PubMed] [Google Scholar]
- 3.Weiss RA. The discovery of endogenous retroviruses. Retrovirology. 2006;3:67. doi: 10.1186/1742-4690-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffin JM, Hughes SH, Varmus HE. The Interactions of Retroviruses and their Hosts. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997.
- 5.Martin GS. The road to Src. Oncogene. 2004;23:7910–7. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R. Spontaneous virus production from "non-virus producing" Rous sarcoma cells. Virology. 1967;32:719–23. doi: 10.1016/0042-6822(67)90049-9. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2:REVIEWS1017. doi: 10.1186/gb-2001-2-6-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denner J. Expression and function of endogenous retroviruses in the placenta. APMIS. 2016;124:31–43. doi: 10.1111/apm.12474. [DOI] [PubMed] [Google Scholar]
- 9.Vargiu L, Rodriguez-Tomé P, Sperber GO, Cadeddu M, Grandi N, Blikstad V, et al. Classification and characterization of human endogenous retroviruses; mosaic forms are common. Retrovirology. 2016;13:7. doi: 10.1186/s12977-015-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkman HE, Stetson DB. The enemy within: endogenous retroelements and autoimmune disease. Nat Immunol. 2014;15:415–22. doi: 10.1038/ni.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flockerzi A, Ruggieri A, Frank O, Sauter M, Maldener E, Kopper B, et al. Expression patterns of transcribed human endogenous retrovirus HERV-K(HML-2) loci in human tissues and the need for a HERV Transcriptome Project. BMC Genomics. 2008;9:354. doi: 10.1186/1471-2164-9-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuong EB, Elde NC, Feschotte C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science. 2016;351:1083–7. doi: 10.1126/science.aad5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jern P, Sperber GO, Ahlsén G, Blomberg J. Sequence variability, gene structure, and expression of full-length human endogenous retrovirus H. J Virol. 2005;79:6325–37. doi: 10.1128/JVI.79.10.6325-6337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian RP, Wildschutte JH, Russo C, Coffin JM. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology. 2011;8:90. doi: 10.1186/1742-4690-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villesen P, Aagaard L, Wiuf C, Pedersen FS. Identification of endogenous retroviral reading frames in the human genome. Retrovirology. 2004;1:32. doi: 10.1186/1742-4690-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tristem M. Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J Virol. 2000;74:3715–30. doi: 10.1128/jvi.74.8.3715-3730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bannert N, Kurth R. The evolutionary dynamics of human endogenous retroviral families. Annu Rev Genomics Hum Genet. 2006;7:149–73. doi: 10.1146/annurev.genom.7.080505.115700. [DOI] [PubMed] [Google Scholar]
- 18.Li ZW, Sheng T, Wan XH, Liu TS, Wu H, Dong JL. Expression of HERV-K correlates with status of MEK-ERK and p16INK4A-CDK4 pathways in melanoma cells. Cancer Invest. 2010;28:1031–7. doi: 10.3109/07357907.2010.512604. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy J, Rabinovich BA, Mi TJ, Switzer KC, Olivares S, Maiti SN, et al. Genetic Engineering of T Cells to Target HERV-K, an Ancient Retrovirus on Melanoma. Clin Cancer Res. 2015;21:3241–51. doi: 10.1158/1078-0432.CCR-14-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiavetti F, Thonnard J, Colau D, Boon T, Coulie PG. A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer Res. 2002;62:5510–6. [PubMed] [Google Scholar]
- 21.Zhao J, Rycaj K, Geng SS, Li M, Plummer JB, Yin BN, et al. Expression of human endogenous retrovirus type K envelope protein is a novel candidate prognostic marker for human breast cancer. Genes Cancer. 2011;2:914–22. doi: 10.1177/1947601911431841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rycaj K, Plummer JB, Yin BN, Li M, Garza J, Radvanyi L, et al. Cytotoxicity of human endogenous retrovirus K-specific T cells toward autologous ovarian cancer cells. Clin Cancer Res. 2015;21:471–83. doi: 10.1158/1078-0432.CCR-14-0388. [DOI] [PubMed] [Google Scholar]
- 23.Boller K, König H, Sauter M, Mueller-Lantzsch N, Löwer R, Löwer J, et al. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology. 1993;196:349–53. doi: 10.1006/viro.1993.1487. [DOI] [PubMed] [Google Scholar]
- 24.Cegolon L, Salata C, Weiderpass E, Vineis P, Palù G, Mastrangelo G. Human endogenous retroviruses and cancer prevention: evidence and prospects. BMC Cancer. 2013;13:4. doi: 10.1186/1471-2407-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherkasova E, Scrivani C, Doh S, Weisman Q, Takahashi Y, Harashima N, et al. Detection of an Immunogenic HERV-E Envelope with Selective Expression in Clear Cell Kidney Cancer. Cancer Res. 2016;76:2177–85. doi: 10.1158/0008-5472.CAN-15-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contreras-Galindo R, Kaplan MH, Leissner P, Verjat T, Ferlenghi I, Bagnoli F, et al. Human endogenous retrovirus K (HML-2) elements in the plasma of people with lymphoma and breast cancer. J Virol. 2008;82:9329–36. doi: 10.1128/JVI.00646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang-Johanning F, Li M, Esteva FJ, Hess KR, Yin BN, Rycaj K, et al. Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. Int J Cancer. 2014;134:587–95. doi: 10.1002/ijc.28389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang-Johanning F, Rycaj K, Plummer JB, Li M, Yin BN, Frerich K, et al. Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. J Natl Cancer Inst. 2012;104:189–210. doi: 10.1093/jnci/djr540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhyu DW, Kang YJ, Ock MS, Eo JW, Choi YH, Kim WJ, et al. Expression of human endogenous retrovirus env genes in the blood of breast cancer patients. Int J Mol Sci. 2014;15:9173–83. doi: 10.3390/ijms15069173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang-Johanning F, Liu JS, Rycaj K, Huang M, Tsai K, Rosen DG, et al. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int J Cancer. 2007;120:81–90. doi: 10.1002/ijc.22256. [DOI] [PubMed] [Google Scholar]
- 31.Hahn S, Ugurel S, Hanschmann KM, Strobel H, Tondera C, Schadendorf D, et al. Serological response to human endogenous retrovirus K in melanoma patients correlates with survival probability. AIDS Res Hum Retroviruses. 2008;24:717–23. doi: 10.1089/aid.2007.0286. [DOI] [PubMed] [Google Scholar]
- 32.Young GR, Eksmond U, Salcedo R, Alexopoulou L, Stoye JP, Kassiotis G. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature. 2012;491:774–8. doi: 10.1038/nature11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warming S, Liu PT, Suzuki T, Akagi K, Lindtner S, Pavlakis GN, et al. Evi3, a common retroviral integration site in murine B-cell lymphoma, encodes an EBFAZ-related Kruppel-like zinc finger protein. Blood. 2003;101:1934–40. doi: 10.1182/blood-2002-08-2652. [DOI] [PubMed] [Google Scholar]
- 34.Lin DY, Huang CC, Hsieh YT, Lin HC, Pao PC, Tsou JH, et al. Analysis of the interaction between Zinc finger protein 179 (Znf179) and promyelocytic leukemia zinc finger (Plzf) J Biomed Sci. 2013;20:98. doi: 10.1186/1423-0127-20-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katoh I, Mírová A, Kurata S, Murakami Y, Horikawa K, Nakakuki N, et al. Activation of the long terminal repeat of human endogenous retrovirus K by melanoma-specific transcription factor MITF-M. Neoplasia. 2011;13:1081–92. doi: 10.1593/neo.11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao XH, Morris DS, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–9. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 37.Kahyo T, Tao H, Shinmura K, Yamada H, Mori H, Funai K, et al. Identification and association study with lung cancer for novel insertion polymorphisms of human endogenous retrovirus. Carcinogenesis. 2013;34:2531–8. doi: 10.1093/carcin/bgt253. [DOI] [PubMed] [Google Scholar]
- 38.Mangeney M, Heidmann T. Tumor cells expressing a retroviral envelope escape immune rejection in vivo . Proc Natl Acad Sci U S A. 1998;95:14920–5. doi: 10.1073/pnas.95.25.14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu HR, Huang HC, Kuo HC, Sheen JM, Ou CY, Hsu TY, et al. IFN-α production by human mononuclear cells infected with varicella-zoster virus through TLR9-dependent and -independent pathways. Cell Mol Immunol. 2011;8:181–8. doi: 10.1038/cmi.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–26. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen WX, Soo JSS, Kwan PY, Hong E, Khang TF, Mariapun S, et al. Germline APOBEC3B deletion is associated with breast cancer risk in an Asian multi-ethnic cohort and with immune cell presentation . Breast Cancer Res. 2016;18:56. doi: 10.1186/s13058-016-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran T, Burt D, Eapen L, Keller OR. Spontaneous regression of metastatic melanoma after inoculation with tetanus-diphtheria-pertussis vaccine. Curr Oncol. 2013;20:e270–3. doi: 10.3747/co.20.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maurer H, McIntyre OR, Rueckert F. Spontaneous regression of malignant melanoma. Pathologic and immunologic study in a ten year survivor. Am J Surg. 1974;127:397–403. doi: 10.1016/0002-9610(74)90286-4. [DOI] [PubMed] [Google Scholar]
- 44.Mastrangelo G, Krone B, Fadda E, Buja A, Grange JM, Rausa G, et al. Does yellow fever 17D vaccine protect against melanoma? Vaccine. 2009;27:588–91. doi: 10.1016/j.vaccine.2008.10.076. [DOI] [PubMed] [Google Scholar]
- 45.Krone B, Kölmel KF, Henz BM, Grange JM. Protection against melanoma by vaccination with Bacille Calmette-Guérin (BCG) and/or vaccinia: an epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur J Cancer. 2005;41:104–17. doi: 10.1016/j.ejca.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Kraus B, Fischer K, Büchner SM, Wels WS, Löwer R, Sliva K, et al. Vaccination directed against the human endogenous retrovirus-K envelope protein inhibits tumor growth in a murine model system. PLoS One. 2013;8:e72756. doi: 10.1371/journal.pone.0072756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang-Johanning F, Radvanyi L, Rycaj K, Plummer JB, Yan PS, Sastry KJ, et al. Human endogenous retrovirus K triggers an antigen-specific immune response in breast cancer patients. Cancer Res. 2008;68:5869–77. doi: 10.1158/0008-5472.CAN-07-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou FL, Krishnamurthy J, Wei YC, Li M, Hunt K, Johanning GL, et al. Chimeric antigen receptor T cells targeting HERV-K inhibit breast cancer and its metastasis through downregulation of Ras. Oncoimmunology. 2015;4:e1047582. doi: 10.1080/2162402X.2015.1047582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraus B, Fischer K, Sliva K, Schnierle BS. Vaccination directed against the human endogenous retrovirus-K (HERV-K) gag protein slows HERV-K gag expressing cell growth in a murine model system. Virol J. 2014;11:58. doi: 10.1186/1743-422X-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiappinelli KB, Strissel PL, Desrichard A, Li HL, Henke C, Akman B, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162:974–86. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karpf AR, Peterson PW, Rawlins JT, Dalley BK, Yang Q, Albertsen H, et al. Inhibition of DNA methyltransferase stimulates the expression of signal transducer and activator of transcription 1, 2, and 3 genes in colon tumor cells. Proc Natl Acad Sci U S A. 1999;96:14007–12. doi: 10.1073/pnas.96.24.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123–32. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Feng H, Liu H, Kong RQ, Wang L, Wang YP, Hu W, et al. Expression profiles of carp IRF-3/-7 correlate with the up-regulation of RIG-I/MAVS/TRAF3/TBK1, four pivotal molecules in RIG-I signaling pathway. Fish Shellfish Immunol. 2011;30:1159–69. doi: 10.1016/j.fsi.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Weintraub K. Take two: Combining immunotherapy with epigenetic drugs to tackle cancer. Nat Med. 2016;22:8–10. doi: 10.1038/nm0116-8. [DOI] [PubMed] [Google Scholar]
- 55.Gameiro SR, Malamas AS, Tsang KY, Ferrone S, Hodge JW. Inhibitors of histone deacetylase 1 reverse the immune evasion phenotype to enhance T-cell mediated lysis of prostate and breast carcinoma cells. Oncotarget. 2016;7:7390–402. doi: 10.18632/oncotarget.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Covre A, Coral S, Nicolay H, Parisi G, Fazio C, Colizzi F, et al. Antitumor activity of epigenetic immunomodulation combined with CTLA-4 blockade in syngeneic mouse models. Oncoimmunology. 2015;4:e1019978. doi: 10.1080/2162402X.2015.1019978. [DOI] [PMC free article] [PubMed] [Google Scholar]