Abstract
Objective:
Ki-67 plays an important function in cell division, but its exact role is still unknown. Moreover, few works regarding its overall function were published. The present study evaluated the clinical significance of Ki-67 index as a prognostic marker and predictor of recurrence in different molecular subtypes of breast cancer. The relationship of Ki-67 index with different clinicopathological factors was also analyzed.
Methods:
Ki-67 index was measured in 107 cases of primary breast cancer from 2010-2012. These patients were evaluated for estrogen receptor, progesterone receptor, and HER2. Ki-67 was divided according to percentage levels: < 15% and > 15%. Follow-up ranged from 32 months up to 6 years.
Results:
Approximately 44, 23, 15, and 25 cases were grouped as luminal A, luminal B, HER2 subtype, and triple-negative (TN), respectively. No luminal A patients showed Ki-67 level higher than 15%, and their recurrence was 20%. In luminal B group, Ki-67 level higher than 15% was observed in 69% of patients, and recurrence was 39%. In HER2 subtype, Ki-67 was higher than 15% in 34% of cases, and recurrence was 40%. In triple-negative cases, Ki-67 was higher than 15% in 60% of cases, and recurrence was detected in 32% of patients. Patients with Ki-67 less than 15% displayed better overall survival than those with Ki-67 higher than 15% (P = 0.01). Patients with Ki-67 higher than 15% exhibited higher incidence of metastasis and recurrence than those with Ki-67 less than 15% (P = 0.000).
Conclusions:
Ki-67 may be considered as a valuable biomarker in breast cancer patients.
Keywords: Ki-67, prognostic, molecular subtypes, breast cancer
Introduction
Breast cancer is a heterogeneous disease with several biological subtypes1. Conventional clinical factors, such as tumor grade, size, lymph node involvement, and surgical margin, are not sufficient as the only prognostic factors; therefore, breast cancer subtype should be considered in making treatment decisions2.
Four main breast cancer subtypes have been identified according to estrogen receptor (ER), progesterone receptor (PR), and HER2. These subtypes include luminal types A and B, basal-like, and HER2-enriched subtype3. Luminal A is the most common breast cancer subtype and characterized by ER+ and/or PR+/HER2− status, low-grade tumor, and good prognosis4-6. Luminal B subtype accounts for approximately 10% of all breast cancers and is distinguished by ER+ and/or PR/HER2− status7. Luminal B-like (HER2 positive) is characterized by ER+, HER2 overexpression or amplification, and any Ki-67 or PR8.
Differentiation of luminal A from luminal B/HER2– breast cancers results in important therapeutic implications. Hence, the Saint Gallen Guidelines recommended the assessment of the Ki-67 proliferation index9. Luminal B breast cancer should show a higher proliferation index than Luminal A; however, the Ki-67 cut-off point for differentiating these two categories has changed over time7. Breast cancer subtypes with negative ER, PR, and HER2 status are typically called “triple-negative” breast cancers and approximate the basal-like category. The basal-like subtype is common in premenopausal, young, and overweight patients. This subtype is also associated with high-grade tumors4,6,10. HER2-enriched subtype (HER2+/ER−/PR−) is less common but is similarly characterized by high-grade tumors and poor outcomes4.
Uncontrolled proliferation is a distinct characteristic of malignancy and may be assessed through various methods, including counting mitotic figures in stained tissue sections, incorporation of labeled nucleotides into DNA, and flow cytometric evaluation of cell fraction in S phase11. Dowsett et al.12 reviewed that the most common measurement involves immunohistochemical assessment of Ki-67 antigen.
Ki-67 is present in all proliferating cells, and its role as a proliferation marker attracts considerable interest. Ki-67 is a nuclear nonhistone protein present in all active phases of cell cycle, except the G0 phase13. Moreover, Ki-67 is among the 21 prospectively selected genes included in the Oncotype DXTM assay used to predict the risk of recurrence and extent of chemotherapy benefits in women with node-negative, ER+ breast cancers14,15. The proliferation biomarker Ki-67 is also considered a prognostic factor for breast cancer and has been investigated in several studies16,17.
In spite of consistent data on Ki-67 as a prognostic marker in early breast cancer, its role in breast cancer management remains uncertain. Potential uses of Ki-67 include prognosis of relative responsiveness, resistance to chemotherapy or endocrine therapy, estimation of residual risk in patients on standard therapy, and as a dynamic biomarker of treatment efficacy in samples obtained before, during, and after neoadjuvant therapy, particularly neoadjuvant endocrine therapy12.
In the present study, we analyzed the relationship of Ki-67 index with clinicopathological factors in 107 cases of breast cancer, as well as with prognosis [disease-free survival (DFS) and overall survival (OS)], according to breast cancer subtypes, namely, luminal, HER2, and triple-negative.
Materials and methods
A total of 107 selected cases of invasive breast carcinoma were collected retrospectively from Mansoura University, Faculty of Medicine, Oncology Center, Egypt between January 2010 and December 2012. All cases underwent modified radical mastectomy operations and received postoperative hormonal, chemotherapy, or radiotherapy. Postoperative follow-up was performed periodically, and data were collected until August 2015. Follow-up period ranged within 32–68 months, with a median follow-up of 37 ± 20.51 months. This study was approved by the ethics committee of Mansoura University.
Hematoxylin and eosin-stained slides (cut from formalin-fixed, paraffin wax-embedded specimens) were retrieved from the archive of the oncology center and reviewed. Tumors were diagnosed according to the WHO classification 201218. A total of 101 (94.4%) cases were diagnosed as invasive ductal carcinoma (IDC), not otherwise specified (NOS). Five (4.7%) cases were diagnosed as invasive lobular carcinoma. One case was diagnosed as mucinous carcinoma (0.9%). Tumors were graded according to Nottingham Grading System19.
Tissue microarray construction
Manual tissue microarray (TMA) was assembled using a mechanical pencil tip20,21. Cores from the surrounding normal breast tissue were also taken as an internal control.
Immunohistochemistry (IHC)
The constructed TMA blocks were recut at a thickness of 3–4 µm on coated slides, deparaffinized, and rehydrated in descending grades of alcohol into water. Antigen retrieval was conducted using citrate buffer at pH according to the type of primary antibody and via microwave heating for 10 min. Subsequently, the sections were incubated in 3% H2O2 blocking medium for 5 min, washed with distilled water, and incubated for 60 min at room temperature with mouse monoclonal primary antibodies against the following antigens: ER (1D5, 1:50; pH, 7.3; Dako, San Jose, USA), PR (PR 636, 1:50; pH, 7.3; Dako, San Jose, USA), HER2/neu (CB11, 1:50; pH, 7.3; Novocastra, Newcastle, U.K), and cell marque Ki-67 (sp6) rabbit monoclonal antibody (REF275R-18). Immunodetection was performed using Dako RealTM EnVision TM system, peroxidase/DAB+, Rabbit/Mouse (Code: K5007, Dako, Glostrup, Denmark) with Dako automated immunostaining instruments. Staining was performed according to the manufacturer’s instructions. Immunoreaction was visualized through adding DAB (Code: K5007) for 3 min. The slides were counterstained with Dako REAL hematoxylin (Code: S2020) for 1 min and cover slipped with mounting media. Internal positive controls were normal breast duct epithelia for ER and PR. Positive external controls were ER, PR, and HER2/neu-positive breast carcinomas for ER, PR, and HER2/neu, respectively. Negative controls were assessed via replacing primary antibody with PBS.
IHC evaluation
Tumors were considered positive for ER and PR when at least 1% of the tumor cells showed unequivocal nuclear staining according to ASCO/CAP guidelines22. HER2/neu was scored according to the pattern of membranous staining and percentage of stained malignant cells as follows: 0, no staining or faint incomplete staining in < 10% of cells; 1, faint incomplete staining in > 10% of cells; 2, weak to moderate complete staining in > 10% of cells; and 3, strong complete staining in > 10% of cells. Only score 3 was considered positive23. Different molecular subtypes were assessed after evaluating ER, PR, and HER2/neu based on IHC results.
Interpretation of Ki-67 staining and scoring
Ki-67 is a nuclear protein. Cytoplasmic staining and occasional membrane staining of Ki-67 can occur with MIB1 antibody and should be ignored when scoring Ki-67. Only nuclear staining (plus mitotic figures stained with Ki-67) should be incorporated into the Ki-67 score that is defined as the percentage of positively stained cells among the total number of malignant cells scored. Similar to other IHC stains, internal positive controls, such as mitotic figures, normal ducts, and lymphocytes, and endothelial and stromal cells (lesser extent), are helpful11,12.
When staining is homogenous, at least three randomly selected, high-power (×40 objective) fields should be counted. However, biological heterogeneity of Ki-67 staining can occur across specimens; in this case, scoring should be from the tumor edge or hot spots. For the former, three fields should be scored at tumor periphery because the invasive edge is widely considered the most biologically active part and most probable to drive the disease outcome. Hot spots are areas where Ki-67 staining is particularly prevalent11,12.
The fraction of proliferating cells was based on a count of at least 500 tumor cells. The Ki-67 values were expressed as the percentage of positive cells in each case. Cases with > 15% positive nuclei were classified as high Ki-67 expression, and those with < 15% were classified as low Ki-67 expression24,25.
Statistical analysis
Data were tabulated, coded, and analyzed using SPSS version 17.0. Descriptive statistics was presented as mean±standard deviation and frequency (number-percent). Chi square test (χ2-value) was used for intergroup comparison of categorical data. Kaplan-Meier test was used to test the equality of survival distribution among Ki-67 categories. In addition, prognostic significance of the Ki-67 index in each molecular subtype was investigated.
The IHC expression of Ki-67 was correlated with clinical and histopathological features of breast carcinoma, including the patient’s age, tumor size, histological type, tumor grade, nodal status, and patient outcome.
Results
This study was carried out retrospectively on 107 patients with invasive breast carcinomas. The mean age of the patients was 54.6±12 years, with an age range of 31–88 years. The different clinicopathological features of cases are shown inTable 1.
1.
The clinicopathological features of the studied cases
| Characteristics | n | % |
| Tumor grade | ||
| G1 | 38 | 35.5 |
| G2 | 45 | 42.1 |
| G3 | 24 | 22.4 |
| Mitotic count | ||
| M1 | 48 | 44.9 |
| M2 | 54 | 50.5 |
| M3 | 5 | 4.7 |
| Tumor size, cm | ||
| <2 | 5 | 4.7 |
| >2 | 102 | 95.3 |
| Lymph node | ||
| Negative | 26 | 24.3 |
| Positive | 81 | 75.7 |
| Tumor stage | ||
| Stage I | 2 | 1.9 |
| Stage II | 48 | 44.9 |
| Stage III | 57 | 53.3 |
| Alive or dead | ||
| Alive | 84 | 79.0 |
| Dead | 23 | 21.0 |
| Metastasis or recurrence | ||
| Negative | 75 | 70.0 |
| Positive | 32 | 30.0 |
| Histological type | ||
| IDC | 101 | 94.4 |
| ILC | 5 | 4.7 |
| Mucinous | 1 | 0.9 |
| ER | ||
| Negative | 50 | 46.7 |
| Positive | 57 | 53.3 |
| PR | ||
| Negative | 48 | 44.9 |
| Positive | 59 | 55.1 |
| HER2 | ||
| Negative | 86 | 80.4 |
| Positive | 21 | 19.6 |
| Ki-67 | ||
| <15 | 71 | 66.2 |
| >15 | 36 | 33.8 |
| Molecular type | ||
| HER2 | 15 | 14.0 |
| Lumial A | 44 | 41.1 |
| Lumial B | 23 | 21.5 |
| Triple -negative | 25 | 23.4 |
Table 1 shows that among the 107 cases, 101 cases were IDC NOS (94.4%), 5 cases were invasive lobular carcinoma (ILC) (4.9%) cases, and only 1 case was mucinous carcinoma (0.9%). Approximately 42% of the cases were grade 2, and 95% of the cases displayed tumor size of more than 2 cm. About 50% of the cases showed a mitotic count of 11-22/10 HPF. Approximately 75% of the patients exhibited pathologically positive lymph nodes, and 53% of the cases were in stage III. Additionally, 30% of the cases developed distant metastasis and recurrence, and 20% of the cases were dead. In terms of biological markers, the ER+ and PR+ rates were 57% and 59%, respectively. Approximately 19% of cases were HER2+ (score, 3+). Ki-67 nuclear positivity of more than 15% was detected in 34% of the cases (Figure 1). According to this immunophenotyping, the cases used in this study were classified as luminal A, luminal B, HER2, and triple-negative in 41%, 21%, 14%, and 23% of the cases, respectively.
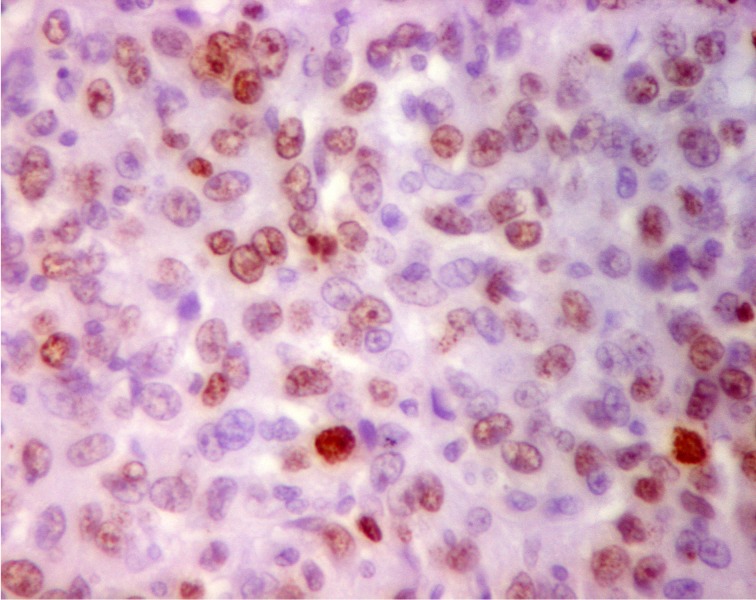
1.
Nuclear positivity for Ki-67 in more than 15% of tumor cells with variable staining intensity (IHC, 400×).
Patient and tumor characteristics in relation to different molecular subtypes
Table 2 reveals statistically significant association among molecular subtypes of cases with both tumor grade and Ki-67 positivity (P values of 0.005 and 0.00, respectively). Luminal A cases showed the highest proportion of grades 1 and 2 cases (45% and 50%, respectively). HER2 and triple-negative subtypes comprised a high proportion of grades 3 (53% and 32%, respectively) and 2 cases (20% and 44%, respectively). Ki-67 < 15% was present in 100% of luminal A, 31% of luminal B, 66% of HER2, and 40% of triple-negative cases. Ki-67 > 15% was present in 0% of luminal A, 69% of luminal B, 34% of HER2, and 60% of triple-negative cases. No statistically significant association was observed among molecular subtypes of the studied cases and age, mitotic count, tumor size, nodal status, stage, histological subtype, or patient outcome (death, recurrence, or metastasis).
2.
Patient and tumor characteristics in relation to different molecular subtypes, n (%).
| Characteristics | Luminal A | Luminal B | HER2 | Triple-negative | P |
| Age, years | 0.9 | ||||
| <55 | 20 (46) | 12 (52) | 8 (53) | 13 (52) | |
| >55 | 24 (54) | 11 (48) | 7 (47) | 12 (48) | |
| Mitotic count | 0.3 | ||||
| <11 | 24 (55) | 12 (52) | 4 (26) | 8 (32) | |
| 11-22 | 18 (41) | 11 (48) | 10 (67) | 15 (60) | |
| > 22 | 2 (4) | 0 (0) | 1 (7) | 2 (8) | |
| Tumor grade | 0.005 * | ||||
| G1 | 20 (45) | 8 (35) | 4 (27) | 6 (24) | |
| G2 | 22 (50) | 9 (39) | 3 (20) | 11 (44) | |
| G3 | 2 (5) | 6 (26) | 8 (53) | 8 (32) | |
| Tumor size, cm | 0.7 | ||||
| <2 | 2 (5) | 1 (4) | 0 (0) | 2 (8) | |
| >2 | 42 (95) | 22 (96) | 15 (100) | 23 (92) | |
| Lymph node | 0.8 | ||||
| Negative | 12 | 6 (26) | 3 (20) | 5 (20) | |
| Positive | 32 (73) | 17 (74) | 12 (80) | 20 (80) | |
| Stage | 0.6 | ||||
| I | 0 (0) | 1 (5) | 0 (0) | 1 (4) | |
| II | 23 (52) | 9 (39) | 5 (33) | 11 (44) | |
| III | 21 (46) | 13 (56) | 10 (67) | 13 (52) | |
| Histological type | 0.8 | ||||
| IDC | 40 (91) | 22 (96) | 15 (100) | 24 (96) | |
| ILC | 3 (7) | 1 (4) | 0 (0) | 1 (4) | |
| Mucinous | 1 (2) | 0 (0) | 0 (0) | 0 (0) | |
| Ki-67 | 0.00 * | ||||
| <15% | 44 (100) | 7 (31) | 10 (66) | 10 (40) | |
| >15% | 0 (0) | 16 (69) | 5 (34) | 15 (60) | |
| Alive or dead | 0.6 | ||||
| Alive | 37 (84) | 17 (74) | 11 (73) | 19 (76) | |
| Dead | 7 (16) | 6 (26) | 4 (17) | 6 (24) | |
| Recurrence & metastasis | 0.3 | ||||
| Negative | 35 (80) | 14 (61) | 10 (60) | 17 (68) | |
| Positive | 9 (20) | 9 (39) | 5 (40) | 8 (32) |
Relationship of Ki-67 expression with clinicopathological characteristics of breast carcinoma
Table 3 shows that high Ki-67 expression (> 15%) was present in 36 cases (33%). Furthermore, 39% and 44% of Ki-67 > 15% positive cases were grades II and III, respectively, with statistically significant associations between Ki-67 > 15% expression and tumor grade (P = 0.00). A statistically significant association was also observed between mitotic count (M) and Ki-67 positivity, where 25%, 67%, and 8% of Ki-67 cases with > 15% positivity were M1, M2, and M3, respectively (P = 0.01). No statistically significant association was observed among age, tumor size, lymph node status, tumor stage, histological type, and Ki-67 positivity.
3.
The relationship of Ki-67 expression with the clinicopathological characteristics of breast cancer,n (%).
| Characteristics | Ki-67 | P | |
| <15% | >15% | ||
| Age, years | 0.6 | ||
| <55 | 34 (64) | 14 (36) | |
| >55 | 37 (69) | 17 (31) | |
| Tumor grade | 0.00 * | ||
| G1 | 32 (45) | 6 (17) | |
| G2 | 31 (44) | 14 (39) | |
| G3 | 8 (11) | 16 (44) | |
| Mitotic count | 0.01 * | ||
| <11 | 39 (55) | 9 (25) | |
| 11-22 | 30 (42) | 24 (67) | |
| >22 | 2 (3) | 3 (8) | |
| Tumor size, cm | 0.5 | ||
| <2 | 3 (4) | 2 (6) | |
| >2 | 68 (96) | 34 (94) | |
| Lymph node | |||
| Negative | 20 (28) | 6 (17) | 0.1 |
| Positive | 51 (72) | 30 (83) | |
| Stage | 0.2 | ||
| I | 1 (1) | 1 (3) | |
| II | 36 (51) | 12 (33) | |
| III | 34 (48) | 23 (64) | |
| Histological type | 0.6 | ||
| IDC | 66 (93) | 35 (97) | |
| ILC | 4 (6) | 1 (4) | |
| Mucinous | 1 (1) | 0 (0) | |
Relationship of Ki-67 expression with molecular subtypes, IHC characteristics of breast carcinoma, and patient outcomes
Table 4 shows that high Ki-67 expression (> 15%) was negatively associated with ER and PR, with P values of < 0.02 and 0.01, respectively. A statistically significant association was also found among Ki-67 expression and molecular subtypes, incidence of recurrence and metastasis (P = 0.000), and OS (P = 0.01).
4.
The relationship of Ki-67 expression with the molecular subtypes, immunohistochemical characteristics of breast cancer and patient outcome, n (%).
| Ki-67 | P | ||
| <15% | >15% | ||
| ER | 0.027 * | ||
| Negative | 28 (39) | 22 (61) | |
| Positive | 43 (61) | 14 (39) | |
| PR | 0.014 * | ||
| Negative | 26 (37) | 22 (61) | |
| Positive | 45 (63) | 14 (39) | |
| HER2 | 0.4 | ||
| Negative | 58 (82) | 28 (78) | |
| Positive | 13 (18) | 8 (22) | |
| Molecular subtypes | 0.00 * | ||
| Luminal A | 44 (100) | 0 (0) | |
| Luminal B | 7 (30) | 16 (70) | |
| HER2 | 10 (67) | 5 (33) | |
| Triple-negative | 10 (40) | 15 (60) | |
| Alive or dead | 0.01 * | ||
| Alive | 61 (86) | 23 (64) | |
| Dead | 10 (14) | 13 (36) | |
| Recurrence & metastasis | 0.00 * | ||
| Negative | 59 (83) | 16 (44) | |
| Positive | 12 (17) | 20 (56) | |
Test of equality of survival distributions for different levels of Ki-67 in the studied cases
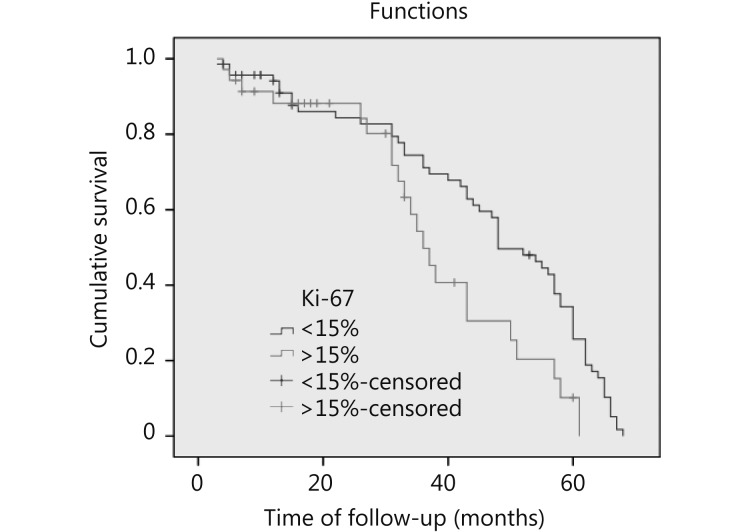
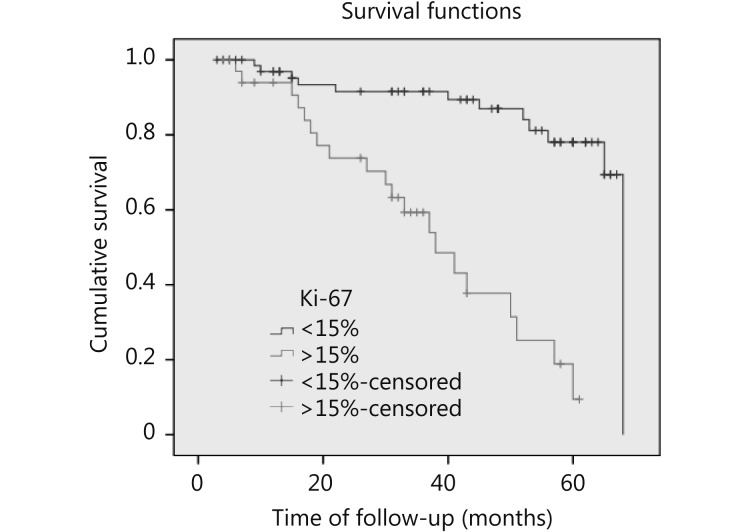
Figure 2 shows that patients with Ki-67 < 15% exhibited better OS than those with Ki-67 > 15% (P = 0.012).Figure 3 illustrates that patients with Ki-67 > 15% were more likely to develop recurrence and distant metastasis than those with Ki-67 < 15% (P = 0.000).
2.
Survival curves of breast cancer patients. Patients with Ki-67 < 15% have better OS than those with Ki-67 > 15% (P = 0.012, HR 6.3)
3.
Disease free survival of breast cancer patients. Patients with Ki-67 > 15% are more likely to develop recurrence and distant metastasis than those with Ki-67 < 15% (P = 0.000, HR 30.47).
Test of equality of survival distributions for different levels of Ki-67 in each molecular subtype
Table 5 reveals that Ki-67 index is not significantly correlated with DFS in any molecular subtype. Additionally, in luminal A, estimation was limited to the longest survival time.
5.
Test of equality of survival distributions (DFS) for the different levels of Ki-67 in each molecular subtype
| Molecular subtype | Chi-square | df | Sig. |
| Luminal A | Estimation is limited | ||
| Luminal B | 1.114 | 1 | 0.299 |
| HER2 neu | 0.021 | 1 | 0.885 |
| Triple-negative | 1.942 | 1 | 0.163 |
Discussion
This study evaluated the clinical significance of Ki-67 index as a prognostic marker in relation to breast cancer molecular subtypes of 107 breast cancer cases. Moreover, the relationships between the Ki-67 index and clinicopa-thological factors reflecting prognosis were investigated.
The appropriate cut-off point is still a matter of debate among oncologists. Hence, the most suitable cut-off point for Ki-67 in clinical practice is widely investigated26.
In our study, the cut-off point for Ki-67 status was more than 14% of positively stained cells, which approximated that of Fasching et al.27 and was in accordance with the biological analysis presented by Cheang et al.28. This cut-off point was used because it was in the range reported by Yerushalmi et al.16. Additionally, this cut-off point was correlated with the molecular subtypes of breast cancer, as reported by Cheang et al.28. Furthermore, in our study, no variability was observed in the Ki-67 index. In addition, no significant prognostic difference existed between patients with Ki-67 < 14% and those with Ki-67 14%-20% because no results were obtained in the latter category. These results agreed with those concluded by Bustreo et al.7.
A high Ki-67 index (≥ 15%) was significantly correlated with adverse prognostic factors. High Ki-67 index (≥ 15%) was significantly correlated with ER−/PR−. These results were also in accordance with those of Inwald et al.29. High Ki-67 index (≥ 15%) is significantly correlated with high tumor grade29,30. These results were in accordance with our results. In the present study, high Ki-67 index (≥ 15%) was significantly correlated with high mitotic count, which was in agreement with the results of Nishimura et al.13 and Yerushalmi et al.16. Therefore, no significant association existed between high Ki-67 positivity and positive HER2/neu. This result may be explained by the considerably small number of HER2+ positive cases (only 21), in which eight cases showed Ki-67 ≥ 15%. Another possible explanation for this difference is attributed to the methods of interpretation. We used manual interpretation of Ki-67, whereas most studies utilized image analysis, which is more accurate11. Furthermore, the heterogeneity of Ki-67 tumor expression agreed with tissue microarray result and may explain our results regarding HER2/neu. This result was also reported by Yang et al.31. On the basis of the above results, we conclude that assessing Ki-67 on whole tumor is better than that of microarray. In triple-negative cases (25 cases), 15 were characterized by > 15% Ki-67 positivity (60%). Ricciardi et al.32 showed that 37.7% of triple-negative cases (45 case) are characterized by > 20% Ki-67 positivity.
The present analysis confirmed that Ki-67 expression is a predictive factor for DFS and OS, which was also proven by Albarracin and Dhamne33 and Inwald et al.29. Despite numerous investigations on the possible use of Ki-67 as a prognostic marker for breast cancer, the optimal cut-off point and scoring protocol have not yet been standardized. The present data included 107 tumors, but the Ki-67 index of luminal A type tumors was low at < 15% in 100% of the cases. This result was in agreement with that of Nishimura et al.13 and Yerushalmi et al.16. However, results regarding the other molecular types were different because of the small number of cases and different cut-off values for Ki-67 index used in each study.
A prognostic significance of the Ki-67 index in each molecular subtype was investigated. The Ki-67 index was not significantly correlated with DFS in any subtype. This result was in contrast to that reported by Nishimura et al.13, which confirmed the significant correlation of the Ki-67 index with DFS only in luminal A type tumors13. The difference can be explained by the limited number of luminal A cases (n = 44) in our study compared with that of Nishimura et al.13 (n = 625). All of our cases showed low Ki-67 expression. Thus, we cannot test the equality of survival distribution of different Ki-67 levels despite the data revealing that 37 patients, out of 44 luminal A patients, were still alive.
Conclusions
The present results indicated that Ki-67 level may be considered a valuable biomarker in breast cancer patients and be used in treatment and follow-up. Future work should focus on standardization of Ki-67 assessment and specifi-cation of its role in making treatment decisions.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 2.Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21:1848–55. doi: 10.1158/1055-9965.EPI-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Børresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol. 2011;3:pii: a003293. doi: 10.1101/cshperspect.a003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 7.Bustreo S, Osella-Abate S, Cassoni P, Donadio M, Airoldi M, Pedani F, et al. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157:363–71. doi: 10.1007/s10549-016-3817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inic Z, Zegarac M, Inic M, Markovic I, Kozomara Z, Djurisic I, et al. Difference between luminal A and luminal B subtypes according to Ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clin Med Insights Oncol. 2014;8:107–11. doi: 10.4137/CMO.S18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies--improving the management of early breast cancer: St gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–46. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suciu C, Muresan A, Cornea R, Suciu O, Dema A, Raica M. Semi-automated evaluation of Ki-67 index in invasive ductal carcinoma of the breast. Oncol Lett. 2014;7:107–114. doi: 10.3892/ol.2013.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med. 2010;1:747–54. doi: 10.3892/etm.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 16.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–83. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 17.de Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. Br J Cancer. 2007;96:1504–13. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. Lyon: IARC Press; 2012. [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Shebl AM, Zalata KR, Amin MM, El-Hawary AK. An inexpensive method of small paraffin tissue microarrays using mechanical pencil tips. Diagn Pathol. 2011;6:117. doi: 10.1186/1746-1596-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foda AA. No-cost manual method for preparation of tissue microarrays having high quality comparable to semiautomated methods. Appl Immunohistochem Mol Morphol. 2013;21:271–4. doi: 10.1097/PAI.0b013e318268a93f. [DOI] [PubMed] [Google Scholar]
- 22.Deyarmin B, Kane JL, Valente AL, van Laar R, Gallagher C, Shriver CD, et al. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;20:87–93. doi: 10.1245/s10434-012-2588-8. [DOI] [PubMed] [Google Scholar]
- 23.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 24.Engstrom MJ, Opdahl S, Hagen AI, Romundstad PR, Akslen LA, Haugen OA, et al. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat. 2013;140:463–73. doi: 10.1007/s10549-013-2647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–29. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153:477–91. doi: 10.1007/s10549-015-3559-0. [DOI] [PubMed] [Google Scholar]
- 27.Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal b breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–52. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors--a surrogate marker? Cancer. 2003;97:1321–31. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZH, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–60. doi: 10.1097/PAS.0b013e31821a0696. [DOI] [PubMed] [Google Scholar]
- 32.Ricciardi GR, Adamo B, Ieni A, Licata L, Cardia R, Ferraro G, et al. Androgen receptor (AR), E-cadherin, and Ki-67 as emerging targets and novel prognostic markers in triple-negative breast cancer (TNBC) patients. PLoS One. 2015;10:e0128368. doi: 10.1371/journal.pone.0128368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albarracin C, Dhamne S. Evolving role of Ki67 as a predictive and prognostic marker in breast cancer. J Clin Exp Pathol. 2014;4:e117. [Google Scholar]