Abstract
Background
This study explored the safety of using real-time sensor glucose (SG) data for treatment decisions in adolescents with poorly-controlled type 1 diabetes.
Methods
10 adolescents with type 1 diabetes, HbA1c ≥9% on insulin pumps were admitted to the clinical research center and a continuous glucose sensor was inserted. Plasma glucose was measured at least hourly using Yellow Springs Instrument’s (YSI) glucose analyzer. Starting at dinner, SG rather than YSI was used for treatment decisions unless YSI was <70 mg/dL (<3.9 mmol/L) or specific criteria indicating SG and YSI were very discordant were met. Participants were discharged after lunch the next day.
Results
10 pts (7 males; 15.2–17.8 years old) completed the study. The range of differences between high glucose correction doses using SG vs. YSI for calculations was −2 (SG<YSI dose) to +1 (SG>YSI dose). In only two of 23 correction doses was the difference two units (all SG<YSI dose). There were five episodes of mild hypoglycemia in two patients, two of which occurred after using SG for dose calculations. There was no severe hypoglycemia and no YSI glucose >350 mg/dL (19.4 mmol/L). Mean (±SE) pre- and post-meal YSI glucose were 163±11 and 183±12 mg/dL (9.1±0.6 and 10.2±0.7 mmol/L), respectively.
Conclusion
Use of real-time continuous glucose monitoring for treatment decisions was safe and did not result in significant over- or under-treatment. Use of SG for treatment decisions under supervised inpatient conditions is a suitable alternative to repeated fingerstick glucose monitoring. Outpatient studies using SG in real-time are needed.
Keywords: type 1 diabetes, pediatrics, continuous glucose monitoring
Many children and adolescents that have poorly controlled type 1 diabetes are noncompliant with home blood glucose monitoring. The lack of glucose data prevents proper insulin dosing. Continuous glucose monitors (CGM) have been used extensively but to date are limited in improving glycemic control in children and adolescents, in part due to lack of consistent wear (1, 2). CGM provide interstitial glucose data but are only U.S. Food and Drug Administration (FDA)-approved to be used for evaluating glucose trends – therapeutic decisions are supposed to be based on fingerstick self blood glucose monitoring (SBGM). This dual burden lessens the potential use of CGM in those patients not willing to perform SBGM regularly. Whether sensor glucose (SG) data generated via CGM and used in real-time (i.e., without confirmatory fingerstick glucose) would be safe remains unknown. We therefore performed a pilot study to investigate whether using real-time SG data for treatment decisions would be safe in children and adolescents with poorly-controlled type 1 diabetes in a controlled inpatient environment.
I. Research Design and Methods
Prior to enrollment, an Investigational Device Exemption (IDE) was obtained from the FDA because the CGM device use (i.e., using SG data in real time) was off label. Institutional Review Board approval was obtained at Wolfson Children’s Hospital, Jacksonville, FL. Participants 15 to <18 years old with type 1 diabetes for ≥1 year, on insulin pump therapy but under poor control (A1c ≥9%) and with poor compliance with SBGM (averaging <4 times per day) were recruited. Participants had been fully educated regarding basal-bolus therapy including necessary SBGM. Pump and meter downloads at enrollment indicated infrequent SBGM (range 0–3 times per day) and infrequent meal and/or correction boluses. Furthermore, inadequate carbohydrate counting also likely contributed to the poor control.
Participants were admitted to the clinical research center (CRC) after lunch for a 26-hour stay. The Medtronic MiniMed Paradigm® REAL-Time CGM System (Medtronic, Inc.; Northridge, CA, USA) replaced their current pump upon admission. Calibrations were done as per manufacturer recommendations, and SG data were available by their evening meal. Fingerstick blood sugars were checked for calibrations using a Bayer Contour Next meter and whenever patient and family desired. At the CRC we structured the study so that participants and/or family members were responsible for all treatment decisions (i.e., meal and correction dose calculations and decisions regarding treatment of hypoglycemia) as if they were in their own home environment. Beginning with dinner, SG was used for all treatment decisions, with five exceptions, requested by FDA, listed in Table 1. Patients were encouraged to be active during the CRC stay but they were not required to exercise.
Table 1.
Using YSI glucose result for treatment decisions. YSI glucose, not sensor glucose, was used for treatment decisions if any of the following criteria were met.
| • Absolute difference between YSI and SG was ≥100 mg/dL |
| • The absolute difference between YSI and SG was more than the participant’s correction factor (ISF) used for high glucose correction dose |
| • YSI and sensor trends were in opposite directions (i.e., one indicated rising whereas the other indicated declining glucose levels) |
| • Rate of change for SG was low (<60 mg/dL/hr), but YSI rate of change was high (≥60 mg/dL/hr), or vice versa |
Plasma glucose measurements using the Yellow Springs Instrument glucose analyzer (YSI 2300 STAT Plus™ Biochemistry Analyzer, Yellow Springs Instruments, Inc., Yellow Springs, OH) were done hourly using venous blood, on the hour, starting at 2 PM, and within 15 min of insulin dosing. Participants and family were blinded to YSI results unless YSI had to be used for treatment decisions. Urine ketones were checked every six hours, once randomly during the CRC stay as determined by CRC staff, plus at any time YSI was ≥240 mg/dL (13.3 mmol/L).
For safety purposes, an algorithm was used to determine when CRC staff would intervene for hypoglycemia (Figure 1) or hyperglycemia (YSI glucose >300 mg/dL [16.7 mmol/L], urine ketones were small or higher).
Figure 1.
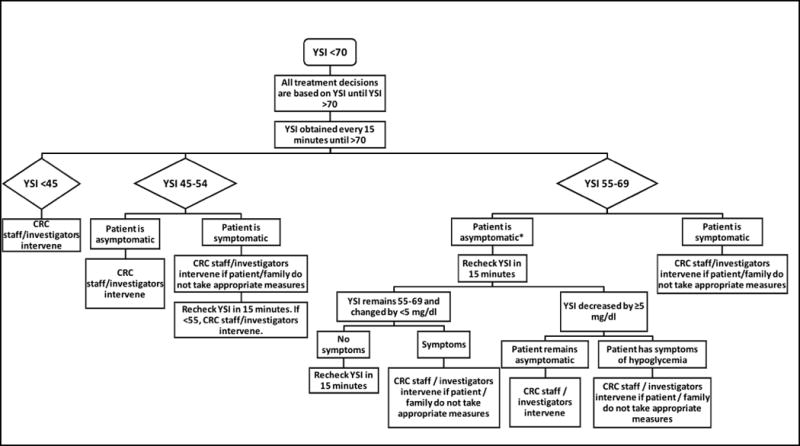
Algorithm for treatment of hypoglycemia in Clinical Research Center. YSI refers to glucose using YSI 2300 STAT Plus™ Biochemistry Analyzer (Yellow Springs Instruments, Inc., Yellow Springs, OH). *For asymptomatic patients with YSI glucose <70 mg/dl, YSI glucose was obtained every 30 minutes for the remainder of the study once YSI is >70 mg/dl. All glucose results in mg/dl; divide mg/dl by 18 for mol/L.
Principal study outcome was the effect of using SG for insulin doses by comparing pre- and post-meal (between 2–3 hours) YSI glucoses. Post-meal blood sugars that overlapped with the use of a second meal bolus were excluded from post-meal analysis. The frequency that YSI glucose readings were required for treatment decisions was also recorded. We also compared doses used for high blood sugar corrections when calculated using the SG to doses if the YSI glucose was used for calculation. In addition, the frequency of hyperglycemia (>180 mg/dL; 10 mmol/L) was assessed, as was hypoglycemia (YSI or SG <70 mg/dL [3.9 mmol/L]). Separate episodes of hypoglycemia were considered if the glucose increased after treatment and decreased again to <70 mg/dL (3.9 mmol/L). Area under the curve for glucose concentrations between 70 and 180 mg/dL (3.9 and 10.0 mmol/L; AUC70–180) was calculated for YSI and SG. Lastly, mean absolute difference (MAD) and mean absolute relative difference (MARD) of sensor/YSI glucose concentration pairs were calculated.
Statistical analysis
Demographic, anthropometric and paired YSI and SG values were summarized. Quantitative variables were reported using mean and standard error of mean (SE); categorical variables were reported using number and percentages. For each patient, AUC70–180 of YSI and SG values were computed for all static time points for which paired values of YSI and SG were collected. A paired t-test was used to compare mean YSI and SG. Pearson as well as intra-class correlation coefficients (ICC) of AUC70–180 of YSI and SG were used to examine the agreement of YSI and SG. A mixed effects model was used to estimate the ICC and compare the mean AUC70–180 of YSI and SG.
We calculated the absolute difference and percent difference of sensor vs. YSI glucose levels at each time point and estimated MAD and MARD of sensor glucose reading from the YSI glucose values. A linear mixed effects model was used to compare the mean differences between YSI and SG values. Subject ID was used as the random variable. Pre- or post- meal, glucose type (YSI or SG), meal (dinner, snack, and breakfast), and two-way and three-way interactions of these three variables were used as fixed factors. In addition, the model was adjusted for sex and BMI z-score. Furthermore, AUC70–180 of YSI and SG were ordered and categorized at median to form ordinal groups. Kappa test was used to measure the agreement between two categorical variables. A sensitivity and specificity analysis was also performed of these two variables to test the consistency in the detection above or below median of these two variables. All tests were two tailed at the level of significance of 0.05. Statistical software SAS, version 9.3, and SPSS, version 22, were used for data analysis.
II. Results
The clinical characteristics of the 10 participants (all Caucasian) that completed the study are shown in Table 2. The MAD and MARD (±SE) for sensor/YSI glucose pairs were 30.4±4.1 mg/dL (range 14.0–53.3) (1.7±0.2 mmol/L; 0.8–3.0), and 17.1±2.0% (9.4–27.4), respectively.
Table 2.
Clinical characteristics of study subjects. Values are mean ± SE (range). All participants were Caucasian.
| N (males) | 10 (7) |
| Age at enrollment | 16.3 ± 0.3 years (15.2–17.8) |
| HbA1c | 9.9 ± 0.3% (9.1–11.7) (85 ± 3.3 mmol/mol [76–104]) |
| Duration of diabetes | 10.1 ± 1.1 years (5.4–14.4) |
| Total daily dose | 0.9 ± 0.04 u/kg/d (0.7–1.2) |
| Body mass index (BMI) | 21.4 ± 1.1 kg/m2 (15.8–26.4) |
| BMI z-score | −0.03 ± 0.39 (−2.21–1.38) |
A total of 30 meal or snack insulin doses (carbohydrate dose with or without correction dose) were administered to the 10 subjects. The use of YSI vs. SG for dose calculations is described in Table 3. Eight of 23 high glucose corrections required YSI for dosing: six of these because the absolute difference between SG & YSI was >ISF, and two were because the rate of change of YSI was low (<60 mg/dl/hr [3.3 mmol/L/hr]) but the rate of change of SG was high (≥60 mg/dl/hr [3.3 mmol/L/hr]).
Table 3.
Number of meal and correction doses given during the study, and whether YSI or SG were used for dose calculations. See text for description of the 8 mealtime doses with correction requiring YSI for calculations.
| Total | Used SG for Dose Calculation | Required YSI for Dose Calculation | |
|---|---|---|---|
| Mealtime doses | 30 | 26 | 4 |
| Without correction dose | 14 | 14 | 0 |
| With correction dose | 16 | 12 | 4 |
| Correction dose alone (not given with meal or snack) | 7 | 3 | 4 |
Table 4 shows the differences between high glucose correction doses when calculated using SG vs. YSI. Calculation differences are provided for when patients actually used SG for calculations (n=15), when the correction dose calculation required YSI (n=8), and all correction doses combined. The range of differences between the two calculations (SG dose minus YSI dose) was from −2 units (SG < YSI dose) to +1 unit (SG > YSI dose). There were only two of 23 correction doses where the difference was two units (all SG < YSI). In 91% of dose calculation comparisons, the difference between correction doses calculated using SG vs. YSI was within one unit (Table 4).
Table 4.
Difference between high glucose correction doses when using SG vs. YSI for calculations. Calculation differences are provided for when patients actually used SG for calculations, when the correction dose required YSI, and all correction doses combined. Difference is calculated by subtracting SG dose minus YSI dose. Negative differences (<0) indicate SG dose is lower dose than YSI; differences >0 indicate SG dose is higher than YSI.
| Correction Doses When Using SG | Correction Doses Requiring YSI | All Correction Doses Combined | ||||
|---|---|---|---|---|---|---|
| Difference between SG dose and YSI dose | n | Percent | n | Percent | n | Percent |
| −2 units | 0 | 0% | 2 | 25% | 2 | 8.7% |
| −1 units | 5 | 33.3% | 5 | 62.5% | 10 | 43.5% |
| 0 units | 7 | 46.7% | 1 | 12.5% | 8 | 34.8% |
| +1 units | 3 | 20% | 0 | 0 | 3 | 13% |
| +2 units | 0 | 0% | 0 | 0 | 0 | 0% |
| TOTAL | 15 | 100% | 8 | 100% | 23 | 100% |
Twenty-six post-meal dose assessments of plasma (YSI) glucose changes were able to be made, after excluding four because less than two hours had passed before another meal bolus was given by the family. Mean (±SE) pre- and post-meal YSI glucose were 163±18 mg/dL (9.1±1.0 mmol/L) and 183±19 mg/dL (10.2±1.1 mmol/L), respectively, with a change ranging from −117 to +122 mg/dL (−6.5 to +6.8 mmol/L). Four of the 26 mealtime doses (one in each of four patients) resulted in an increase of YSI by ≥100 mg/dL (range 102–122 mg/dL) (≥5.6 mmol/L; 5.7–6.8) within three hours, but none resulted in a glucose ≥350 mg/dL (19.4 mmol/L). Only two insulin doses using SG for calculations resulted in a decrease in YSI by ≥100 mg/dL (5.6 mmol/L) (YSI decreased by 117 mg/dL [6.5 mmol/L] for one subject at breakfast and by 111 mg/dL [6.2 mmol/L] at dinner for another participant.
Pre- and post meal YSI and SG values are described in Figure 2. Mean (±SE) pre- and post-meal YSI glucose were 163±11 and 183±12 mg/dL (9.1±0.5 and 10.2±0.7 mmol/L), respectively; pre- and post-meal SG were 157±9 and 174±10 mg/dL (8.7±0.5 mmol/L and 9.7±0.6 mmol/L), respectively. The number of postprandial YSI glucose levels >180 after dinner, snack and breakfast were three of 8, five of 8 and five of 10, respectively. There were no significant differences in overall mean glucose between pre- and post-meal (p=0.10), YSI vs. SG (p=0.21) and meal (dinner, breakfast or snack), after accounting for subject level heterogeneity (data not shown). There was some variability in the difference between mean pre- and post-meal over the meals (p=0.06). Overall mean glucose (YSI and SG) was higher in females than males (202±19 vs. 155±13 mg/dL [11.2±1.1 vs. 8.6±0.7 mmol/L]; p=0.04).
Figure 2.
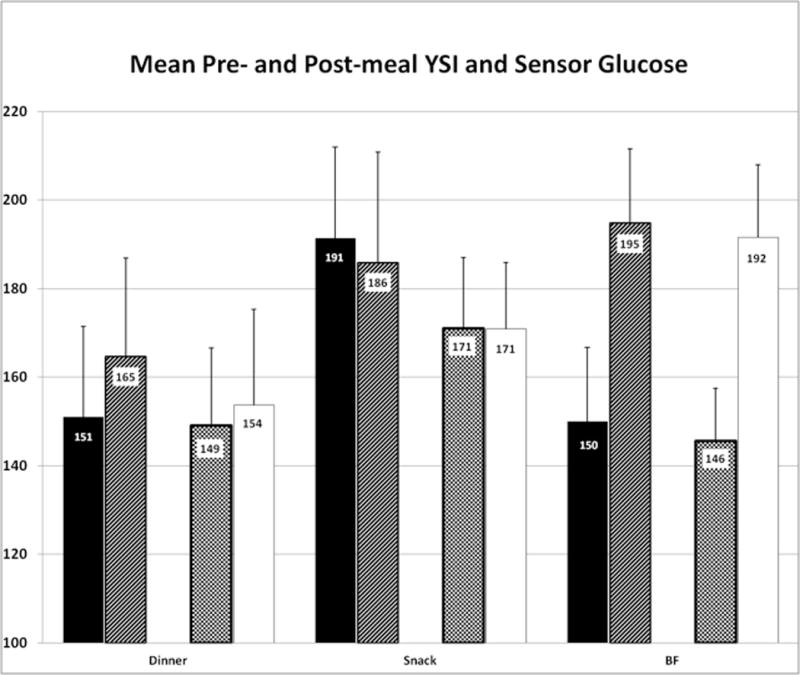
Mean (+SE) YSI and sensor pre- and post-meal glucose concentrations for all dinner (n=8), bedtime snack (n=8), and breakfast (BF) (n=10) during the study period. Study procedures ended immediately after lunch, and thus no lunch postprandial sugars were obtained. Solid black = YSI premeal; upward hash = YSI post meal; checker board = SG premeal; solid white = SG post meal.
Five episodes of hypoglycemia occurred in three patients (two with YSI <70 mg/dL [3.9 mmol/L], one with SG <70 mg/dL) (Table 5). Two episodes in one patient were symptomatic (YSI was 61–68 mg/dL [3.4–3.8 mmol/L]) and one patient with SG of 47 mg/dL (2.6 mmol/L) had symptoms but concurrent YSI was 148 mg/dL (8.2 mmol/L). The remaining two low blood sugars were asymptomatic. Two of the five low glucose levels occurred after using SG for insulin dose calculations. A comparison of the YSI and SG for the five episodes of hypoglycemia is provided in Table 5. There was no severe hypoglycemia, with no YSI glucose under 60 mg/dl for any of the subjects at any time during the study. There were no episodes of diabetic ketoacidosis.
Table 5.
Comparison of sensor vs. YSI glucose in three participants that experienced hypoglycemia during the study. All values are in mg/dL (divide by 18 to convert to mmol/L).
| Time | Sensor | YSI | |
|---|---|---|---|
| Patient #1 | 00:59 | 73 | 57 |
| 06:00 | 79 | 68 | |
| Patient #2 | 07:00 | 47 | 148 |
| Patient #3 | 21:30 | 79 | 61 |
| 01:44 | 79 | 68 |
Table 6 (online supplement) compares sensor vs. YSI glucose in subjects experiencing hyperglycemia (SG or YSI >240 mg/dL [13.3 mmol/L]) at anytime throughout the duration of the study.
The mean AUC70–180 was slightly but not significantly lower in SG vs. YSI (mean±SE 139.1±7.6 vs. 148.7±10.2 mg/dL [7.7±0.4 vs. 8.3±0.6 mmol/L], respectively; paired t-test p=0.13). There was a strong linear relationship between AUC70–180 of YSI and SG (Pearson r=0.68, p=0.03) and there was a strong agreement between AUC70–180 of YSI and SG (ICC=0.75, p=0.03). There was a moderate to strong agreement between two ordinal variables of median-split of AUC70–180 (kappa=0.6, p=0.06). Furthermore, the sensitivity and specificity to detect above or below median of one variable using the corresponding split of the other variable were both at 80%.
III. Conclusions
Our pilot study demonstrated that using sensor data rather than fingerstick or reference glucoses in real time for treatment decisions was fundamentally safe. High glucose correction and mealtime doses using SG data in a controlled hospital environment did not result in significant hyper- or hypoglycemia or dosing errors.
To the best of our knowledge, this is the first study directly assessing the safety of using real-time sensor data for treatment decisions. Outpatient use of real-time CGM has been described, assessing use and effectiveness of an algorithm developed by investigators and coordinators in the Diabetes Research in Children Network (DirecNet), but safety was not formally assessed (3). In that 3-month study, patients and families were taught an algorithm that provided guidelines on making insulin adjustments based on sensor glucose values, taking into account the direction and rate of change of the glucose concentrations. All other published studies of CGM are in accordance with FDA approval of the devices (i.e., treatment decisions are based on fingerstick data, not sensor data).
The current FDA-approved use of CGM devices is for glucose trend pattern assessment and not using SG data for treatment decisions. This is because the accuracy of CGM sensors is less than that of home glucose meters, potentially leading to inaccurate and possibly unsafe dosing. However, published studies indicate that use of SBGM declines with time while on CGM (4, 5), suggesting patients are already using SG for treatment decisions despite the devices not being FDA-approved to do so. Furthermore, in the SWITCH trial (6) the number of fingerstick blood sugars tests performed by study participants decreased while wearing a sensor (4.9 vs 5.5 per day; p <0.001), yet there was improvement in diabetes control. Our pilot study did not suggest that using SG for treatment decisions results in significant treatment errors or dose miscalculations. In fact, the differences between doses using SG vs. YSI for calculating high glucose corrections were minimal, and in all but two of 23 instances the difference was one unit or less, and never more than two units.
Missing or unnecessarily treating low BGs remains a possibility when using SG rather than fingerstick blood sugars, but there was minimal hypoglycemia in our study. However, we had one patient who had a sensor glucose of 47 mg/dL (2.6 mmol/L) but YSI was 148 (8.2 mmol/L). This emphasizes that hypoglycemia based on sensor data is not yet reliable, hence treatment of hypoglycemia needs to be based on confirmatory fingerstick glucoses.
Diabetes control often declines during adolescence (7). Many factors contribute to the poor control in this age group, including noncompliance with many aspects of the diabetes management, such as SBGM. Without SBGM high glucoses cannot be treated appropriately. It is therefore reasonable to postulate that using SG data for treatment decisions, even without the same accuracy as home glucose meters (8, 9), would allow improved control in patients infrequently checking fingerstick BGs (i.e., having SG data, despite the lower accuracy compared to meters, is better than not having any BGs at all). Our data open the possibility that using SG for treatment decisions can potentially improve control in this age group as it would provide data often not available due to lack of compliance with fingerstick BGs, as suggested by prior studies (10, 11). It is important to point out, however, that a minimum of two calibrations per day are necessary, and thus even if SG are used for treatment decisions, patients need to be willing to check fingerstick blood sugars at least twice per day with the current devices available.
CGM has been studied in several settings, including critically ill (12–14), surgical intensive care (15), pregnancy during delivery (16), cardiac surgery (17), and perioperatively (18). Being able to use sensor data in real time in these inpatient settings would help reduce cost (less laboratory or bedside glucose measurements) and allow more timely changes in therapy, but use of real-time CGM for treatment changes in these populations needs further study.
The ultimate goal in diabetes management would be to create an artificial pancreas, and studies with such devices are ongoing (19–23). In fact, several in-home studies of closed loop devices, which inherently use SG for treatment decisions, have demonstrated substantial improvement in many outcomes studied (20–24). When closed loop devices are ultimately approved by the FDA, treatment decisions will inherently be based on sensor data. Until that technology is readily available, however, home blood glucose monitoring remains key to management of type 1 diabetes. Adding CGM to SBGM adds significant patient burden that many adolescents are not willing to have. In adolescents (or any age group) with poorly controlled type 1 diabetes who are not regularly checking fingerstick BGs, using real-time SG to make treatment decisions may safely improve control.
A large number of high BG corrections in our patients required YSI for dose calculations (8 out of 23), and thus it appears that SG cannot frequently be used for treatment decisions. However, the aim of this study was not to assess how frequently SG could be used instead of YSI, but to assess whether problems occurred when using SG for dose calculations. The criteria established for using YSI instead of SG for calculations were somewhat arbitrary, and may not be applicable in real-world settings.
There are potential pitfalls with our study. First, it was short term, lasting <24 hours. Second, although the protocol tried to mimic the home environment with regards to how patients and families would make management decisions, with little intervention by CRC staff for insulin doses and treatment of low BGs, it was in an inpatient controlled setting, and thus results cannot necessarily be generalized for outpatient use. However, the safety of using sensor glucose data for treatment decisions needed to be established, and the hospital, under a controlled environment, is ideally suited for early studies designed to establish such safety. Also, the sample size is small, but appropriate in obtaining preliminary safety data. Data from this pilot study can be used to develop longer and larger inpatient and subsequent outpatient studies.
In summary, using real-time sensor data for treatment decisions in this study was safe, and may provide additional glucose data that can be used for treatment decisions in patients who perform SBGM too infrequently. Additional long term, larger inpatient and outpatient studies are needed to confirm the safety and efficacy of this approach.
Supplementary Material
Acknowledgments
Funded by NIH #RO3 HD067329-01A1 and Nemours Research Programs. CGM devices and supplies were provided by Medtronic, Inc. (Northridge, CA, USA).
LAF has received research support from Medtronic (CGM devices and supplies). NM has received research support in the form of devices and grant support from Medtronic.
Footnotes
This study was registered on ClinicalTrials.gov (#NCT01586065).
Data were presented in part at International Society for Pediatric and Adolescent Diabetes, Toronto, Canada, September, 2014.
We are indebted to the nursing staff of our Clinical Research Center.
Author Disclosure Statement.
EB, KE and JH report no competing financial interests exist.
References
- 1.Mauras N, Fox L, Englert K, Beck RW. Continuous glucose monitoring in type 1 diabetes. Endocrine. 2013;43:41–50. doi: 10.1007/s12020-012-9765-1. [DOI] [PubMed] [Google Scholar]
- 2.Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2012;35:204–10. doi: 10.2337/dc11-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckingham B, Xing D, Weinzimer S, Fiallo-Scharer R, Kollman C, Mauras N, et al. Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator) Pediatr Diabetes. 2008;9:142–7. doi: 10.1111/j.1399-5448.2007.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinzimer S, Xing D, Tansey M, Fiallo-Scharer R, Mauras N, Wysocki T, et al. FreeStyle navigator continuous glucose monitoring system use in children with type 1 diabetes using glargine-based multiple daily dose regimens: results of a pilot trial Diabetes Research in Children Network (DirecNet) Study Group. Diabetes Care. 2008;31:525–7. doi: 10.2337/dc07-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Research in Children Network Study G. Buckingham B, Beck RW, Tamborlane WV, Xing D, Kollman C, et al. Continuous glucose monitoring in children with type 1 diabetes. J Pediatr. 2007;151:388–93. 93 e1–2. doi: 10.1016/j.jpeds.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battelino T, Conget I, Olsen B, Schutz-Fuhrmann I, Hommel E, Hoogma R, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155–62. doi: 10.1007/s00125-012-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971–8. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 8.Weinzimer SA, Beck RW, Chase HP, Fox LA, Buckingham BA, Tamborlane WV, et al. Accuracy of newer-generation home blood glucose meters in a Diabetes Research in Children Network (DirecNet) inpatient exercise study. Diabetes Technol Ther. 2005;7:675–80. doi: 10.1089/dia.2005.7.675. discussion 81–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akintola AA, Noordam R, Jansen SW, de Craen AJ, Ballieux BE, Cobbaert CM, et al. Accuracy of Continuous Glucose Monitoring Measurements in Normo-Glycemic Individuals. PLoS One. 2015;10:e0139973. doi: 10.1371/journal.pone.0139973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 11.Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32:2245–50. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunner R, Adelsmayr G, Herkner H, Madl C, Holzinger U. Glycemic variability and glucose complexity in critically ill patients: a retrospective analysis of continuous glucose monitoring data. Crit Care. 2012;16:R175. doi: 10.1186/cc11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu W, Jiang L, Jiang S, Ma Y, Zhang M. Real-time continuous glucose monitoring versus conventional glucose monitoring in critically ill patients: a systematic review study protocol. BMJ Open. 2015;5:e006579. doi: 10.1136/bmjopen-2014-006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Block CE, Gios J, Verheyen N, Manuel YKB, Rogiers P, Jorens PG, et al. Randomized Evaluation of Glycemic Control in the Medical Intensive Care Unit Using Real-Time Continuous Glucose Monitoring (REGIMEN Trial) Diabetes Technol Ther. 2015 doi: 10.1089/dia.2015.0151. [DOI] [PubMed] [Google Scholar]
- 15.Schuster KM, Barre K, Inzucchi SE, Udelsman R, Davis KA. Continuous glucose monitoring in the surgical intensive care unit: concordance with capillary glucose. J Trauma Acute Care Surg. 2014;76:798–803. doi: 10.1097/TA.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 16.Fresa R, Visalli N, Di Blasi V, Cavallaro V, Ansaldi E, Trifoglio O, et al. Experiences of continuous subcutaneous insulin infusion in pregnant women with type 1 diabetes during delivery from four Italian centers: a retrospective observational study. Diabetes Technol Ther. 2013;15:328–34. doi: 10.1089/dia.2012.0260. [DOI] [PubMed] [Google Scholar]
- 17.Siegelaar SE, Barwari T, Hermanides J, van der Voort PH, Hoekstra JB, DeVries JH. Microcirculation and its relation to continuous subcutaneous glucose sensor accuracy in cardiac surgery patients in the intensive care unit. J Thorac Cardiovasc Surg. 2013;146:1283–9. doi: 10.1016/j.jtcvs.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Poljakova I, Elsikova E, Chlup R, Kalabus S, Hasala P, Zapletalova J. Glucose sensing module - is it time to integrate it into real-time perioperative monitoring? An observational pilot study with subcutaneous sensors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:346–57. doi: 10.5507/bp.2013.049. [DOI] [PubMed] [Google Scholar]
- 19.Del Favero S, Bruttomesso D, Di Palma F, Lanzola G, Visentin R, Filippi A, et al. First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care. 2014;37:1212–5. doi: 10.2337/dc13-1631. [DOI] [PubMed] [Google Scholar]
- 20.Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, El-Khairi R, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204–11. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimri R, Muller I, Atlas E, Miller S, Fogel A, Bratina N, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37:3025–32. doi: 10.2337/dc14-0835. [DOI] [PubMed] [Google Scholar]
- 22.Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313–25. doi: 10.1056/NEJMoa1314474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly TT, Roy A, Grosman B, Shin J, Campbell A, Monirabbasi S, et al. Day and Night Closed-Loop Control Using the Integrated Medtronic Hybrid Closed-Loop System in Type 1 Diabetes at Diabetes Camp. Diabetes Care. 2015;38:1205–11. doi: 10.2337/dc14-3073. [DOI] [PubMed] [Google Scholar]
- 24.Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, et al. Day-and-Night Hybrid Closed-Loop Insulin Delivery in Adolescents With Type 1 Diabetes: A Free-Living, Randomized Clinical Trial. Diabetes Care. 2016 doi: 10.2337/dc15-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


