Abstract
Steroid-induced IOP elevation affects a significant number of patients. It results from a decrease in outflow facility of the aqueous humor. To understand the pathophysiology of this condition a number of model systems have been created. These include ex-vivo cell and organ cultures as well as in-vivo animal models in organisms ranging from rodents to primates. These model systems can be used to investigate specific aspects of steroid-induced IOP elevation. This brief review summarizes the strengths and limitations of the various model systems and provides examples of where these systems have been successfully used to advance our understanding of steroid-induced IOP elevation.
Keywords: steroids, intraocular pressure, glaucoma, model
Introduction
Steroid-induced ocular hypertension is a frequent complication of chronic treatment with corticosteroids (Jones and Rhee, 2006). Although usually reversible with steroid discontinuation it has been increasingly prevalent because of the use of potent corticosteroids in or around the eye as treatment for various eye diseases (Kiddee et al., 2013). Steroid-induced IOP elevation occurs within weeks in susceptible individuals and is dependent on steroid potency, pharmacokinetics, duration of treatment and route of administration (Becker and Mills, 1963). Approximately one in three individuals on chronic topical steroid therapy will experience IOP elevation and in ~20% of those, IOP elevation will be significant (Becker and Mills, 1963). Intraocular steroids increase the risk for IOP elevation dramatically. In trials of sustained release fluocinolone acetonide implant, over 75% of patients receiving the steroid intravitreally required IOP lowering therapy and 40% required surgical intervention for IOP control (Goldstein et al., 2007). The prevalence of steroid–induced IOP elevation is even higher among patients with glaucoma. Approximately 90% of these patients and 30% of glaucoma suspects will develop moderate IOP elevation after 4 weeks of treatment with steroid drops (Armaly, 1963b).
If unrecognized, prolonged steroid-induced IOP elevation can lead to (or exacerbate) glaucomatous optic neuropathy (Goldmann, 1966) and result in loss of vision. Although it is well established that steroid-induced IOP elevation is the result of increased aqueous humor outflow resistance (Armaly, 1963a; Bernstein and Schwartz, 1962) the exact pathogenetic mechanism remains unknown. However, the similarities in the pathology and the increased prevalence with primary OAG (POAG) suggest that common mechanisms are operational in both conditions.
The rise in IOP caused by steroids is often attributed to alterations in cell cytoskeletal dynamics, and a dysregulation in extracellular matrix (ECM) deposition and remodeling (Clark et al., 1994; Jones and Rhee, 2006; Raghunathan et al., 2015) which result in morphological changes (Tektas and Lutjen-Drecoll, 2009) and reduction in facility of outflow. To try to understand changes at the cellular and molecular level that lead to steroid induced ocular hypertension, a number of ex-vivo and in vivo models for the condition have been created. These model systems have to date provided relevant information but many details of the pathophysiology of this condition still remain unclear. This review summarizes some of the work performed in the various steroid-induced IOP elevation models, highlights some of the strengths and limitations of each one and provides some examples of successful application of model systems in addressing relevant questions.
Ex-vivo model systems
1. Tissue culture models
Some of the earliest attempts to understand steroid-induced IOP elevation at the cellular and molecular level led to the isolation of primary trabecular meshwork (TM) cells (Polansky et al., 1979). These cells can be easily isolated from both human post-mortem eyes as well as animal eyes (Crean et al., 1986; Grierson et al., 1985; Yue et al., 1988) and can be used to study the physiology (Clark and Wordinger, 2009; Gasiorowski and Russell, 2009) and gene expression (Paylakhi et al., 2012; Rozsa et al., 2006) of the cells that in large part control ECM deposition and either directly or indirectly ultimately affect outflow facility. The use of TM cells has resulted in some spectacular success stories in the quest to identify genes related to glaucoma. Most notably work on HTM cultures identified the protein encoded by the first glaucoma gene: TIGR/myocilin (Polansky et al., 1997; Stone et al., 1997). Other relevant important findings that come from work on TM cultures treated with steroids include the increased stiffness in trabecular meshwork (McKee et al., 2011), the activation and proliferation of the endoplasmic reticulum and Golgi apparatus and increased extracellular matrix (ECM) deposition (Wilson et al., 1993), the increase in fusion vesicles on TM cell membranes (McCartney et al., 2006), the increase in HTM cell size (Tripathi et al., 1989), the changes in TM cell cytoskeleton (Wilson et al., 1993) and the decreases in phagocytotic activity (Yang and Li, 1996; Zhang et al., 2007), as well as cell migration and proliferation (Clark et al., 1994).
Although transformed lines of TM cells were generated and used in the 90s (Liu et al., 2002; Pang et al., 1994; Tamm et al., 1999) they have fallen out of favor as they seem to deviate significantly in their physiology and gene expression from primary cells (Liu et al., 2002). In fact even primary cells appear to senesce and change as they progress through different passages (Schachtschabel and Binninger, 1990). The number of viable usable passages depends on donor age with cells from older individuals having decreased replicative capacity. Some TM cultures stop dividing after only a few passages. Typically primary TM cells grown in culture, reflect the physiology of TM cells in vivo only up to passage five. Use of cells beyond this passage may potentially provide unreliable results and should be avoided. Because isolation of TM cells involves dissection of tissue and can potentially result in contamination with other cells from surrounding tissues and because of gene expression changes with passaging, it is advisable to verify the identity of TM cells in primary culture. Most investigators rely for such verification on confirmation of expression of a panel of genes and/or their respective proteins that are typically expressed in TM cells. Prominent among them are myocilin (MYOC), matrix protein Gla (MGP), caveolin 1 (Cav1), collagen 4 alpha 5 (Col4A5) and tissue inhibitor of metalloproteinase 3 (TIMP3) (Du et al., 2012; Hernandez et al., 1987; Kuehn et al., 2011; Mao et al., 2012; Ueda et al., 2000; Xue et al., 2006). Other cell markers include αβ-Crystallin (Welge-Lussen et al., 1999), tissue plasminogen activator (Seftor et al., 1994) and smooth muscle actin (Pang et al., 1994). Significant upregulation of MYOC by steroid treatment is considered also to be strongly indicative of the TM nature of human cells in culture (Polansky et al., 2000) although MYOC mRNA transcription upregulation occurs 12 to 24 hours after exposure to steroids (Joe et al., 2011). An important caveat is that myocilin upregulation (which is universal in humans) does not occur in a number of animal species (e.g. (Sawaguchi et al., 2005)). Since TM cells have avid phagocytic activity (Rohen and van der Zypen, 1968), a phagocytosis assay can also be used as further confirmatory proof that cells in culture are TM cells. In addition contractility assays have been recently developed to characterize and positively identify HTM cells (Dismuke et al., 2014).
Isolation and nutritional requirements of TM cells have been well described (Polansky et al., 1979; Stamer et al., 1998) and involve relatively straightforward manipulations and methods. A number of investigators use rings of tissue left over after keratoplasty to isolate these cells from human post-mortem eyes. If using such tissue source it is important to thoroughly rinse tissue with culture medium prior to putting tissue fragments into culture.
The recent development of differentiated HTM cells from stem cells (Abu-Hassan et al., 2015; Ding et al., 2014; Du et al., 2012) opens up the possibility of generating large amounts of TM cells in culture that will be identical and can be used for experimentation (provided they express all relevant markers and behave similarly to primary HTM cells). This development would make generation of cells that can be readily shared between labs a reality. In addition it holds the promise of allowing generation of disease specific cells.
The other important cellular component of the outflow pathways in the area of the juxtacanalicular tissue (JCT) are Schlemm’s canal (SC) cells. Contrary to TM cells that are fairly easy to culture, SC cells are notoriously hard to obtain in culture (Dautriche et al., 2014). The method most often used for generating such primary cultures involves the placement of a suture in the SC and lengthy incubations (Stamer et al., 1998). Verification of the nature of the cells involves screening for a panel of markers that are normally expressed on these cells including fibulin-2, VE-Cadherin, integrin-α6 (Perkumas and Stamer, 2012) and PECAM-1 (Dautriche et al., 2015) and the absence of LYVE-1 expression (van der Merwe and Kidson, 2014). SC cells in culture also over time change their gene and protein expression (Lei et al., 2014a, b). Furthermore, Human Schlemm’s canal (HSC) cells in traditional culture lose spatial, mechanical, and biochemical cues resulting in altered gene expression and cell signaling compared to in-vivo (Dautriche et al., 2015). This in combination with the difficulty in isolating them creates a bottleneck in terms of experimentation with these cells. To date, generation of SC cells from stem cells has not been reported.
Culturing TM and SC cells allows for the observation and study of structural characteristics, biological properties, as well as the growth patterns of the cells (Dautriche et al., 2014). Gene and protein expression after exposure to steroids have been reported (for examples see (Bollinger et al., 2011, 2012; Zhao et al., 2004)) and contribute to our current understanding of some of the processes that lead to steroid-induced IOP elevation. In addition TM and SC cells in cultures have been used to study the biomechanical properties of these cells (Raghunathan et al., 2015). However, use of TM and SC cells in traditional culture to study steroid induced IOP elevation is limited by the lack of a physiologic parameter that one can monitor to indicate functional effects on outflow facility. Thus, even though traditionally cultured TM and SC cells can be used to dissect molecular pathways in these cells, relationship of these pathways to IOP control has to be established in other models.
Notwithstanding the above caveats, traditional cultures of TM cells and SC cells have been used as systems to study steroid-induced IOP elevation. Cells have been treated with a number of steroids including dexamethasone, triamcinolone, prednisolone, cortisol, progesterone, cortexolone, methyltestosterone, fluorometholone, and rimexolone (for examples see (Clark et al., 1994; Raghunathan et al., 2015; Sharma et al., 2014; Sohn et al., 2010)).
It appears that drug partitioning in the TM increases as lipophilicity increases. The rank order of lipophilicity and subsequently observed steroid partitioning was triamcinolone < prednisolone < dexamethasone < triamcinolone acetonide < fluocinolone acetonide < budesonide (Thakur et al., 2011). Although the effects on gene and protein expression are generally similar, the various steroids should not necessarily be considered interchangeable in their effects on outflow cells in culture.
Culturing TM and SC cells on membranes rather than at the bottom of culture dishes has been a significant improvement as it has enabled the use of these cells in flow studies. Initial attempts to utilize commercially available mixed cellulose ester (HATF) filters for growing the TM cells were only met with limited success (Perkins et al., 1988) although HTM cells grown on such filters responded to steroid treatment by changing transendothelial flow (Underwood et al., 1999). Similarly polyester trans-well inserts do not provide optimal support (Torrejon et al., 2015) as cells tend to orient on these membranes randomly and membranes provide significant resistance to flow because of low porosity. More recently, trans-well inserts have also been used to culture HSC cells for physiological studies (Pedrigi et al., 2011). In addition photolithographically patterned SU-8 biocompatible (Kotzar et al., 2002) epoxy membranes have been used to culture both HTM and HSC cells (Torrejon et al., 2013). HTM cells grown on such well-defined membranes highly resemble cells in vivo in their morphology, assume specific orientation in relationship to the pores and exhibit the characteristic actin stress fibers. Because of the high porosity of these membranes resistance to flow is minimal. The material is also transparent, making observations of cell behavior and growth simpler. Growth of HSC cells on SU-8 scaffolds appears to restore biochemical and spatial cues ordinarily lost in traditional HSC cultures. Thus HSC cells grown on Extracel coated SU-8 have been shown to express VE-Cadherin and CD31, which are often lost in traditional cultures. Furthermore, similar to HTM cells, HSC cells grown on SU-8 display improved alignment (Dautriche et al., 2015).
Confluent HTM and HSC cultures on SU-8 scaffolds have been used in flow through studies and have allowed the calculation of simulated outflow facility of these cells. Although this simulated outflow facility appears to be higher than the outflow facility in vivo, it responds to a variety of pharmacologic agents that are known to modulate outflow facility in vivo. More importantly cells grown on SU-8 respond to steroid (prednisolone acetate) in a physiologic manner by decreasing simulated outflow facility and decreasing their phagocytic activity. These changes were also associated with an increase in production of various ECM components (Torrejon et al., 2015).
Importantly, culturing of HTM cells and HSC cells on SU-8 membranes has allowed the development of basal to basal co-cultures of the two cell types, thus creating a 3D biomimetic culture of the JCT area (Torrejon, 2015b). These cultures are responsive to steroid treatment and thus represent an ideal in-vitro system for studies in the pathogenesis of steroid-induced IOP elevation as they allow for modeling of the complex interactions between cells in the outflow pathways.
2. Organ culture models
Perfused Organ Culture Anterior Segments (OCAS) from humans post-mortem or from a number of different animal species have been a standard model of examining the aqueous outflow pathway for nearly 30 years (Erickson-Lamy et al., 1990; Gottanka et al., 2004; Johnson et al., 1990). This ex vivo model preserves the architecture of the aqueous outflow pathway and enables studies to be performed in a near physiologic state. In this model, eyes are bisected at the equator, and the iris, lens, choroid, ciliary body and vitreous are removed. The anterior segment is clamped into a culture dish carefully machined out of Plexiglas that creates a closed environment between the culture dish and the anterior segment. Media containing antibiotics and antifungal agents is perfused at the normal aqueous flow rate of 2.5 μl/min into the anterior segment through a cannula. Pressures are continuously monitored with a pressure transducer connected to a second access cannula built into the dish, and recorded with an automated computerized system. Typically OCAS are perfused in pairs from the same individual with one eye serving as control, while the other as experimental. This reduces somewhat the variability in the outflow facility measurements.
Human OCAS pairs are limited in number. Although valuable because they represent human material their use in the study of steroid induced IOP elevation has been limited. In perfused human OCAS IOP elevation due to exposure to steroids occurs approximately 5-6 days later in some human OCAS (Clark et al., 1995). The responder rate of these eyes, roughly 30%, coincides with response rates observed clinically. However, other studies have failed to always detect such a high rate of IOP elevation despite evidence of accumulation of glycosaminoglycans resistant to degradation (Johnson et al., 1990).
In contrast bovine OCAS are plentiful and have been proposed as an excellent model of steroid induced IOP elevation (Mao et al., 2011). In unselected cow eyes from the Holstein and Angus strains a significant steroid response in ~40% of the eyes was reported. However, it is interesting to note that all eyes tested exhibited some increase in IOP over contralateral eyes when treated with dexamethasone. Vehicle treated eyes from “steroid respondent animals” also exhibited some (lesser) IOP elevation. It is also intriguing that IOP elevation after exposure to steroids occurred within 24-48 hours. Early elevations are typically not present in Human OCAS and have not been reported to occur in vivo in either humans (except very rarely) or other animal species (including bovine) (Gerometta et al., 2004). It is thus unclear whether this early onset IOP elevation in bovine OCAS is truly reflective of the physiologic changes leading to steroid induced IOP elevation.
Porcine (pig) eyes have also been used for OCAS as they are readily available. Although not as popular as human, bovine and monkey OCAS porcine OCAS also respond to steroids by a decrease in outflow facility (Fujimoto et al., 2012). As with the bovine TM, porcine TM is reticular and both species have an angular aqueous plexus (Mao et al., 2011). Finally it should be noted that although monkey OCAS have been used to study the effect of various medications and genes on outflow facility there are no published reports on the effects of steroids in that system.
For all perfused OCAS studies (irrespective of the species used) it is important to perform histological examination of the outflow tissues after perfusion to ensure no gross disruption of the outflow pathways which can dramatically affect the results (Johnson et al., 1990; Mao et al., 2011). Histological examination should also be used to verify the TM cellularity is within acceptable bounds as loss of TM cells can lead to preparations that are not responsive to pharmacologic agents (Johnson and Tschumper, 1989). It is also important to point out that perfused OCAS from many animal species (including those from primates) exhibit the “washout effect”. This is an increase in outflow facility over time during the initial period of perfusion which occurs in perfusion models and has been attributed to the removal of extracellular cells or ECM components in outflow pathway tissues (Li et al., 2015). Whether washout is time or volume dependent is still not clear (Mao et al., 2011). Although different animal species show varying amounts of this effect (Li et al., 2015; Overby et al., 2002; Scott et al., 2007), the washout effect is absent in human (Erickson-Lamy et al., 1990) and probably mouse eyes (Lei et al., 2011).
In vivo-models
1. Mouse and rat models
Rodents are the phylogenetically lowest organisms that have been used as in-vivo models for the study of steroid-induced IOP elevation. Mice offer the advantages of experimentation in vivo in an organism that can be genetically manipulated, while rats allow for easier manipulation of the eye itself (which is larger in these animals). Both provide models in animals that are readily available in most laboratories and can be easily and inexpensively implemented. However, because mice and rats have small size and are sensitive to the systemic effects of steroids, these models have only been developed within the last decade.
Three different mouse models of SIOHT have been reported:
a. Topical steroid treatment model. In this model dexamethasone phosphate drop (0.1%) are applied to the eyes three times daily for 6 weeks (Zode et al., 2014). Mice are reported to develop IOP elevation starting at 2 weeks after initiation of treatment. Although the amount of IOP elevation seems to be variable between individual eyes it appears to be substantial (6-7mmHg). This is the only mouse model of steroid-induced IOP elevation to be reported to develop glaucomatous optic nerve changes (Zode et al., 2014).
b. Systemic steroid treatment model. In this model animals are exposed to systemic dexamethasone delivered via osmotic minipumps for periods of up to 4 weeks at doses of 0.09 mg DEX/day (Overby et al., 2014b; Whitlock et al., 2010). IOP elevations at 4 weeks are small (2-4mmHg). Some animals do not survive because of systemic effects from steroid treatment.
c. Periocular steroid treatment model. In this model animals are exposed to periocular triamcinolone acetonide (40mg/ml – 20ul per eye) (Kumar et al., 2013). Although changes in IOP are not detectable for the first two weeks, changes in outflow facility of enucleated mouse eyes are large and readily detectable.
Differences between the three models likely reflect differences in the potency of steroids used, difference in the route of administration and bioavailability and differences in the effects of the experimental intervention on parameters that affect measurements (for example instillation of multiple eye drops may affect the corneal epithelium and thus IOP measurements). In general however, it is clear that IOP may not be the best parameter to follow when one studies steroid induced glaucoma in mice. Classical outflow facility on the other hand is clearly affected by steroids in mice.
Despite that, mice are very valuable in the study of steroid-induced effect on outflow physiology. Aqueous physiology is similar to that in humans (Aihara et al., 2003) and the mouse TM shares many similarities with the human TM (Overby et al., 2014a). A recent study in mice suggests that they develop the characteristic plaque like material that have previously been reported for human steroid-induced IOP elevation (Overby et al., 2014b). The two things that remain unsettled are whether mouse upregulate MYOC in response to steroid treatment (Kumar et al., 2013; Zode et al., 2014) and what percentage of total outflow is uveoscleral vs classical (Aihara et al., 2003; Boussommier-Calleja et al., 2012; Boussommier-Calleja et al., 2015; Crowston et al., 2004; Lee et al., 2011; Lei et al., 2011; Millar et al., 2011; Millar et al., 2015; Overby et al., 2014b; Sherwood et al., 2016; Stamer et al., 2011; Zhang et al., 2009). The power of genetic manipulation in these animals however is expected to provide answers to many important questions in this field in the near future.
Similar to the mouse, rats also have been reported to develop steroid-induced IOP elevation after topical treatment with dexamethasone four times daily for periods of 4 weeks (Sawaguchi et al., 2005). Although the rat eye is larger and thus can allow for easier dissection and intraocular interventions, rats at this stage provide limited ability to manipulate genes and thus do not offer significant advantages over mice in the study of this condition. They have however been used in a few studies (Miyara et al., 2008; Razali et al., 2015; Shinzato et al., 2007). Myocilin does not appear to be upregulated by steroids in rats (Sawaguchi et al., 2005).
2. Rabbit models
Rabbits have for historical reasons been used and continue to be used in eye research. Traditionally, for IOP lowering therapies, rabbits have been used as one of the two species for toxicological testing. Corticosteroids will cause ocular hypertension (OHT) in rabbits, but results are variable and the doses required to achieve IOP elevation in rabbits are close to the LD50 dose (Gelatt et al., 1998b). In addition because aqueous humor physiology in these animals is quite different from that in humans the mechanism of IOP elevation may be different (Gelatt et al., 1998a). Notably such IOP elevation cannot often be sustained for long periods of time. In addition IOP elevation was shown to occur only when treatment began during the developmental period (8-10 weeks of age), while even relatively young, mature rabbits (3 years of age) were shown to be refractory to this effect even when dexamethasone was applied every 6 hours for 4 weeks (Knepper et al., 1978; Qin et al., 2012). Thus despite the fact that glucocorticoid receptor concentration is high in ocular tissues of rabbits and intravenously administered steroid has been found to bind specifically to the nuclei of cells in the outflow channels (Knepper et al., 1985), rabbits have fallen out of favor as a model for steroid-induced IOP elevation.
3. Cat models
Moderate elevations of IOP may be produced in cats by topical administration of corticosteroids (either dexamethasone or prednisolone)(Zhan et al., 1992). Furthermore, elevated IOP was maintained as long as corticosteroid treatment was continued. Some cats, like in humans, appeared to develop ocular hypertension more rapidly than others. Cataract development in feline eyes during long-term corticosteroid treatment also suggests that corticosteroids have a similar effect on feline and human eyes.
However, cats have a rate of aqueous humor flow that are several times greater than those of human eyes. In this study it was assumed, therefore, that more dexamethasone would have to be applied to the corneal surface to achieve a concentration in the aqueous humor of cat eyes comparable to that achieved clinically in humans (Zhan et al., 1992). On the other hand, since cats have a body size about one-fortieth that of patients, the risk of systemic effects are obviously greater. Cats are readily available and easier to handle than non-human primates. Ease of handling is an especially important consideration in such studies when treatment has to be administered daily for many weeks or months.
A similar study by another group in normal cats similarly detected a high rate of steroid-induced IOP elevation among these animals (Bhattacherjee et al., 1999). However, more recent work indicated that the responsiveness of normal cats may be lower than initially reported (Gosling et al., 2016). Thus, the feline steroid induced IOP elevation continues to be a potentially useful, albeit somewhat underutilized animal model.
4. Ruminant models (Cows and sheep)
The motivation behind development of the bovine steroid induce model of OHT was the similarity of bovine aqueous physiology to that of humans (Gerometta et al., 2004). In addition a rich literature from experimentation on bovine TM cells suggested that steroid treatment induced changes similar to those seen in human TM cells (exposure of bovine TM to dexamethasone produces alterations in the ECM proteins and cell contacts (Zhou et al., 1998)). Treatment of cows of the Bradford strain with prednisolone acetate drops three times daily has been reported to lead to IOP elevation in 100% of the animals tested within 4 weeks. IOP remains elevated for the duration of steroid treatment and slowly declines over a period of 2-4 weeks after discontinuation of steroids (Gerometta et al., 2004). Morphologic alterations of the outflow tissues resemble those seen in humans (Tektas et al., 2010) despite some differences in the anatomy between the human and bovine TM. The model has been used to study gene expression changes induced by steroid treatment in the TM (Danias et al., 2011) and has led to the identification of a number of relevant genes in this process. Despite the significant advantage of the availability of large amounts of ocular tissues that can be used for further analysis, this model is limited in the types of therapeutic or experimental interventions that can be performed. Although the uniformity in the amount of steroid response among animals of this strain is not necessarily reflective of the variability of human responses, it offers an advantage by reducing biological variability.
Sheep also exhibit 100% incidence of elevated IOP in response to topical prednisolone administration (either twice or three times a day) and are thus potentially suitable for characterizations of the biochemical and molecular factors involved in the increase in aqueous outflow resistance (Gerometta et al., 2009). However IOP elevation is sheep occurs within 7-10 days from initiation of treatment. As sheep are docile animals that can be housed relatively inexpensively they are particularly well suited for in vivo experiments. This advantage outweighs the current lack of extensive genetic information on these animals (although the sheep genome project has been completed).
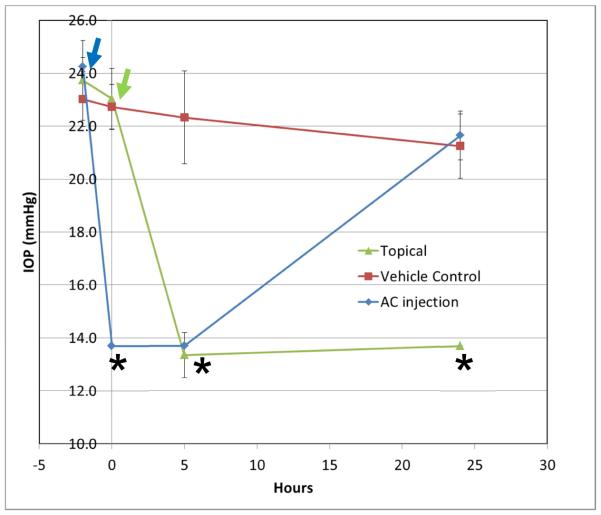
Given these advantages it is no surprise that sheep have been the first large animal model of application of gene therapeutic intervention for the treatment of steroid-induced IOP elevation. Adenovirally (AdV) mediated MMP1 overexpression lead to a reversal of steroid-induced IOP elevation (Gerometta et al., 2010). Similarly, treatment with anterior juxtascleral administration of anecortave acetate has been shown to prevent IOP elevation in sheep (Candia et al., 2010). The sheep model has also been used to determine that tPA can prevent as well as reverse steroid-induced IOP elevation (Candia et al., 2014; Gerometta et al., 2013). More recently we have utilized tPA topically (in the form of eye-drops) to achieve robust IOP lowering in the ovine steroid-induced IOP elevation model (see Figure 1).
Figure 1.
Topical and intracameral tPA reduces IOP in steroid-induced ocular hypertensive sheep. 4 mg of lyophilized tPA (Actilyse®, Boehringer Ingelheim SA BuenosAires, Argentina) were dissolved in 0.4 mL artificial tears (Refresh Plus). Because tPA is formulated with arginine (43.3mg per mg of tPA) an equivalent amount of arginine was dissolved in artificial tears and was used as control. Eyes of 6 sheep received topical application of tPA (N=6 eyes) or vehicle control (N=4 eyes)) at time 0h. Some eyes (AC injection − N=2) received 10ug of tPA intracamerally in 50ul of balanced salt solution 2-3h prior to time 0h. All eyes had been treated with steroids topically (two drops of 1.0% prednisolone acetate (Alcon, Fort Worth, Texas) 3-times daily (7AM, 2PM, and 7PM) for 8 days) to induce IOP elevation (baseline IOP prior to steroid induced IOP elevation was 11.3 ± 0.2 mmHg). Treatment with steroids continued for the duration of the experiment. Error bars indicate standard deviation of measurements. Statistically significant differences (p<0.05, ANOVA) are indicated by an asterisk (*). Arrow indicate the points of administration of tPA topically (green arrow) or intracamerally (blue arrow).
Despite the multiple advantages of these large animal models it should be noted that the anatomy of the sheep and cow angle although similar, is not identical to that of humans. For example bovine and ovine TMs are reticular in appearance and instead of a well formed Schlemm’s canal ruminants have an angular aqueous plexus (Mao et al., 2011). Furthermore contrary to the human steroid-induced IOP elevation myocilin is not upregulated in vivo by steroids in cows (Danias et al., 2011). Finally contrary to humans, younger sheep are less susceptible to steroid induced IOP elevation.
5. Primate model
The high homology between the human and monkey eye makes primates potentially valuable in developing models to elucidate the pathogenesis of steroid induced glaucoma in humans. In a study of pigtail macaque monkeys (Clark et al., 2001) that had received orally administered cortisol acetate for 12 months showed ultrastructural changes in the TM (including beam and basement membrane thickening, reduction of the inter-trabecular space, upregulation of myocilin and ECM deposition) similar to those observed in patients with steroid-induced IOP elevation (Bernstein and Schwartz, 1962). Unfortunately no IOP measurements were reported in that study.
The issue of whether monkeys actually develop IOP elevation remains somewhat controversial. Although an earlier study (Armaly, 1964) failed to induce OHT in three species of monkeys (pigtail, rhesus and cynomolgus) administered steroids either topically or subconjunctivally for periods of 3 months, another study (Fingert et al., 2001) reported that 45% of the animals tested developed a significant IOP elevation. No other reports have confirmed or refuted these findings. The high cost of primates and the difficulty and risks involved in handling monkeys makes such experiments difficult despite the obvious appeal of working with animals that share so many characteristics with humans.
Conclusions
A number of ex-vivo and in vivo models for the study of steroid-induced IOP elevation have been developed. Although ex-vivo systems fail to fully simulate the in-vivo complexity, they are useful for dissecting molecular pathways that are involved in this condition as well as for screening purposes in the effort to develop medications that are tailored to the treatment of steroid-induced IOP elevation. Animal models have specific strengths and weaknesses. Understanding the limitations of each model organism is critically important in selecting the optimal system to address questions that will move our understanding of the pathophysiology of steroid-induced IOP elevation forward.
Acknowledgments
Grant support: R01 EY025543, RPB challenge grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Hassan DW, Li X, Ryan EI, Acott TS, Kelley MJ. Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma. Stem cells. 2015;33:751–761. doi: 10.1002/stem.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara M, Lindsey JD, Weinreb RN. Aqueous humor dynamics in mice. Investigative ophthalmology & visual science. 2003;44:5168–5173. doi: 10.1167/iovs.03-0504. [DOI] [PubMed] [Google Scholar]
- Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. I. The Effect of Dexamethasone in the Normal Eye. Archives of ophthalmology. 1963a;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. Ii. The Effect of Dexamethasone in the Glaucomatous Eye. Archives of ophthalmology. 1963b;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- Armaly MF. Aqueous Outflow Facility in Monkeys and the Effect of Topical Corticoids. Invest Ophthalmol. 1964;3:534–538. [PubMed] [Google Scholar]
- Becker B, Mills DW. Corticosteroids and Intraocular Pressure. Archives of ophthalmology. 1963;70:500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- Bernstein HN, Schwartz B. Effects of long-term systemic steroids on ocular pressure and tonographic values. Archives of ophthalmology. 1962;68:742–753. doi: 10.1001/archopht.1962.00960030746009. [DOI] [PubMed] [Google Scholar]
- Bhattacherjee P, Paterson CA, Spellman JM, Graff G, Yanni JM. Pharmacological validation of a feline model of steroid-induced ocular hypertension. Archives of ophthalmology. 1999;117:361–364. doi: 10.1001/archopht.117.3.361. [DOI] [PubMed] [Google Scholar]
- Bollinger KE, Crabb JS, Yuan X, Putliwala T, Clark AF, Crabb JW. Quantitative proteomics: TGFbeta(2) signaling in trabecular meshwork cells. Investigative ophthalmology & visual science. 2011;52:8287–8294. doi: 10.1167/iovs.11-8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger KE, Crabb JS, Yuan X, Putliwala T, Clark AF, Crabb JW. Proteomic similarities in steroid responsiveness in normal and glaucomatous trabecular meshwork cells. Molecular vision. 2012;18:2001–2011. [PMC free article] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Bertrand J, Woodward DF, Ethier CR, Stamer WD, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Investigative ophthalmology & visual science. 2012;53:5838–5845. doi: 10.1167/iovs.12-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Li G, Wilson A, Ziskind T, Scinteie OE, Ashpole NE, Sherwood JM, Farsiu S, Challa P, Gonzalez P, Downs JC, Ethier CR, Stamer WD, Overby DR. Physical Factors Affecting Outflow Facility Measurements in Mice. Investigative ophthalmology & visual science. 2015;56:8331–8339. doi: 10.1167/iovs.15-17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia OA, Gerometta R, Millar JC, Podos SM. Suppression of corticosteroid-induced ocular hypertension in sheep by anecortave. Archives of ophthalmology. 2010;128:338–343. doi: 10.1001/archophthalmol.2009.387. [DOI] [PubMed] [Google Scholar]
- Candia OA, Gerometta RM, Danias J. Tissue plasminogen activator reduces the elevated intraocular pressure induced by prednisolone in sheep. Exp Eye Res. 2014;128:114–116. doi: 10.1016/j.exer.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Steely HT, Dickerson JE, Jr., English-Wright S, Stropki K, McCartney MD, Jacobson N, Shepard AR, Clark JI, Matsushima H, Peskind ER, Leverenz JB, Wilkinson CW, Swiderski RE, Fingert JH, Sheffield VC, Stone EM. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Investigative ophthalmology & visual science. 2001;42:1769–1780. [PubMed] [Google Scholar]
- Clark AF, Wilson K, de Kater AW, Allingham RR, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Investigative ophthalmology & visual science. 1995;36:478–489. [PubMed] [Google Scholar]
- Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Investigative ophthalmology & visual science. 1994;35:281–294. [PubMed] [Google Scholar]
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Crean EV, Sherwood ME, Casey R, Miller MW, Richardson TM. Establishment of calf trabecular meshwork cell cultures. Exp Eye Res. 1986;43:503–517. doi: 10.1016/s0014-4835(86)80019-7. [DOI] [PubMed] [Google Scholar]
- Crowston JG, Aihara M, Lindsey JD, Weinreb RN. Effect of latanoprost on outflow facility in the mouse. Investigative ophthalmology & visual science. 2004;45:2240–2245. doi: 10.1167/iovs.03-0990. [DOI] [PubMed] [Google Scholar]
- Danias J, Gerometta R, Ge Y, Ren L, Panagis L, Mittag TW, Candia OA, Podos SM. Gene expression changes in steroid-induced IOP elevation in bovine trabecular meshwork. Investigative ophthalmology & visual science. 2011;52:8636–8645. doi: 10.1167/iovs.11-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautriche CN, Szymanski D, Kerr M, Torrejon KY, Bergkvist M, Xie Y, Danias J, Stamer WD, Sharfstein ST. A biomimetic Schlemm's canal inner wall: A model to study outflow physiology, glaucoma pathology and high-throughput drug screening. Biomaterials. 2015;65:86–92. doi: 10.1016/j.biomaterials.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautriche CN, Xie Y, Sharfstein ST. Walking through trabecular meshwork biology: Toward engineering design of outflow physiology. Biotechnol Adv. 2014;32:971–983. doi: 10.1016/j.biotechadv.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Ding QJ, Zhu W, Cook AC, Anfinson KR, Tucker BA, Kuehn MH. Induction of trabecular meshwork cells from induced pluripotent stem cells. Investigative ophthalmology & visual science. 2014;55:7065–7072. doi: 10.1167/iovs.14-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismuke WM, Liang J, Overby DR, Stamer WD. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp Eye Res. 2014;120:28–35. doi: 10.1016/j.exer.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Roh DS, Mann MM, Funderburgh ML, Funderburgh JL, Schuman JS. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Investigative ophthalmology & visual science. 2012;53:1566–1575. doi: 10.1167/iovs.11-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase ("washout") in the perfused enucleated human eye. Investigative ophthalmology & visual science. 1990;31:2384–2388. [PubMed] [Google Scholar]
- Fingert JH, Clark AF, Craig JE, Alward WL, Snibson GR, McLaughlin M, Tuttle L, Mackey DA, Sheffield VC, Stone EM. Evaluation of the myocilin (MYOC) glaucoma gene in monkey and human steroid-induced ocular hypertension. Investigative ophthalmology & visual science. 2001;42:145–152. [PubMed] [Google Scholar]
- Fujimoto T, Inoue T, Kameda T, Kasaoka N, Inoue-Mochita M, Tsuboi N, Tanihara H. Involvement of RhoA/Rho-associated kinase signal transduction pathway in dexamethasone-induced alterations in aqueous outflow. Investigative ophthalmology & visual science. 2012;53:7097–7108. doi: 10.1167/iovs.12-9989. [DOI] [PubMed] [Google Scholar]
- Gasiorowski JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009;88:671–675. doi: 10.1016/j.exer.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelatt KN, Brooks DE, Samuelson DA. Comparative glaucomatology. I: The spontaneous glaucomas. J Glaucoma. 1998a;7:187–201. [PubMed] [Google Scholar]
- Gelatt KN, Brooks DE, Samuelson DA. Comparative glaucomatology. II: The experimental glaucomas. J Glaucoma. 1998b;7:282–294. [PubMed] [Google Scholar]
- Gerometta R, Kumar S, Shah S, Alvarez LJ, Candia OA, Danias J. Reduction of steroid-induced intraocular pressure elevation in sheep by tissue plasminogen activator. Investigative ophthalmology & visual science. 2013 doi: 10.1167/iovs.13-12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerometta R, Podos SM, Candia OA, Wu B, Malgor LA, Mittag T, Danias J. Steroid-induced ocular hypertension in normal cattle. Archives of ophthalmology. 2004;122:1492–1497. doi: 10.1001/archopht.122.10.1492. [DOI] [PubMed] [Google Scholar]
- Gerometta R, Podos SM, Danias J, Candia OA. Steroid-induced ocular hypertension in normal sheep. Investigative ophthalmology & visual science. 2009;50:669–673. doi: 10.1167/iovs.08-2410. [DOI] [PubMed] [Google Scholar]
- Gerometta R, Spiga MG, Borras T, Candia OA. Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Investigative ophthalmology & visual science. 2010;51:3042–3048. doi: 10.1167/iovs.09-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann H. Cortisone glaucoma. International ophthalmology clinics. 1966;6:991–1003. doi: 10.1097/00004397-196606040-00011. [DOI] [PubMed] [Google Scholar]
- Goldstein DA, Godfrey DG, Hall A, Callanan DG, Jaffe GJ, Pearson PA, Usner DW, Comstock TL. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Archives of ophthalmology. 2007;125:1478–1485. doi: 10.1001/archopht.125.11.ecs70063. [DOI] [PubMed] [Google Scholar]
- Gosling AA, Kiland JA, Rutkowski LE, Hoefs A, Ellinwood NM, McLellan GJ. Effects of topical corticosteroid administration on intraocular pressure in normal and glaucomatous cats. Vet Ophthalmol. 2016 doi: 10.1111/vop.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Investigative ophthalmology & visual science. 2004;45:153–158. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- Grierson I, Robins E, Unger W, Millar L, Ahmed A. The cells of the bovine outflow system in tissue culture. Exp Eye Res. 1985;40:35–46. doi: 10.1016/0014-4835(85)90106-x. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Weinstein BI, Schwartz J, Ritch R, Gordon GG, Southren AL. Human trabecular meshwork cells in culture: morphology and extracellular matrix components. Investigative ophthalmology & visual science. 1987;28:1655–1660. [PubMed] [Google Scholar]
- Joe MK, Sohn S, Kim TE, Im JE, Choi YR, Kee C. Analysis of glucocorticoid-induced MYOC expression in human trabecular meshwork cells. Vision Res. 2011;51:1033–1038. doi: 10.1016/j.visres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Bradley JM, Acott TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Investigative ophthalmology & visual science. 1990;31:2568–2571. [PubMed] [Google Scholar]
- Johnson DH, Tschumper RC. The effect of organ culture on human trabecular meshwork. Exp Eye Res. 1989;49:113–127. doi: 10.1016/0014-4835(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Current opinion in ophthalmology. 2006;17:163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, Baath J, Buys YM. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Survey of ophthalmology. 2013;58:291–310. doi: 10.1016/j.survophthal.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Knepper PA, Breen M, Weinstein HG, Blacik JL. Intraocular pressure and glycosaminoglycan distribution in the rabbit eye: effect of age and dexamethasone. Exp Eye Res. 1978;27:567–575. doi: 10.1016/0014-4835(78)90141-0. [DOI] [PubMed] [Google Scholar]
- Knepper PA, Collins JA, Frederick R. Effects of dexamethasone, progesterone, and testosterone on IOP and GAGs in the rabbit eye. Investigative ophthalmology & visual science. 1985;26:1093–1100. [PubMed] [Google Scholar]
- Kotzar G, Freas M, Abel P, Fleischman A, Roy S, Zorman C, Moran JM, Melzak J. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials. 2002;23:2737–2750. doi: 10.1016/s0142-9612(02)00007-8. [DOI] [PubMed] [Google Scholar]
- Kuehn MH, Wang K, Roos B, Stone EM, Kwon YH, Alward WL, Mullins RF, Fingert JH. Chromosome 7q31 POAG locus: ocular expression of caveolins and lack of association with POAG in a US cohort. Molecular vision. 2011;17:430–435. [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Shah S, Deutsch ER, Tang HM, Danias J. Triamcinolone acetonide decreases outflow facility in C57BL/6 mouse eyes. Investigative ophthalmology & visual science. 2013;54:1280–1287. doi: 10.1167/iovs.12-11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Tresguerres M, Hess K, Marmorstein LY, Levin LR, Buck J, Marmorstein AD. Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. The Journal of biological chemistry. 2011;286:41353–41358. doi: 10.1074/jbc.M111.284679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Overby DR, Boussommier-Calleja A, Stamer WD, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Investigative ophthalmology & visual science. 2011;52:1865–1871. doi: 10.1167/iovs.10-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Stamer WD, Wu J, Sun X. Cell senescence reduced the mechanotransduction sensitivity of porcine angular aqueous plexus cells to elevation of pressure. Investigative ophthalmology & visual science. 2014a;55:2324–2328. doi: 10.1167/iovs.13-13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Stamer WD, Wu J, Sun X. Endothelial nitric oxide synthase-related mechanotransduction changes in aged porcine angular aqueous plexus cells. Investigative ophthalmology & visual science. 2014b;55:8402–8408. doi: 10.1167/iovs.14-14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Shi HM, Cong L, Lu ZZ, Ye W, Zhang YY. Outflow facility efficacy of five drugs in enucleated porcine eyes by a method of constant-pressure perfusion. Int J Clin Exp Med. 2015;8:7184–7191. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang CY, Cai S, Polansky JR, Kaufman PL, Brandt CR. Transformation of human trabecular meshwork cells with SV40 TAg alters promoter utilization. Current eye research. 2002;25:347–353. doi: 10.1076/ceyr.25.6.347.14226. [DOI] [PubMed] [Google Scholar]
- Mao W, Liu Y, Mody A, Montecchi-Palmer M, Wordinger RJ, Clark AF. Characterization of a spontaneously immortalized bovine trabecular meshwork cell line. Exp Eye Res. 2012;105:53–59. doi: 10.1016/j.exer.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Mao W, Tovar-Vidales T, Yorio T, Wordinger RJ, Clark AF. Perfusion-cultured bovine anterior segments as an ex vivo model for studying glucocorticoid-induced ocular hypertension and glaucoma. Investigative ophthalmology & visual science. 2011;52:8068–8075. doi: 10.1167/iovs.11-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney MD, Cantu-Crouch D, Clark AF. Freeze-fracture examination of cultured human trabecular meshwork cells: effect of dexamethasone. Exp Eye Res. 2006;82:994–1001. doi: 10.1016/j.exer.2005.10.010. [DOI] [PubMed] [Google Scholar]
- McKee CT, Wood JA, Shah NM, Fischer ME, Reilly CM, Murphy CJ, Russell P. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011;32:2417–2423. doi: 10.1016/j.biomaterials.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JC, Clark AF, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Investigative ophthalmology & visual science. 2011;52:685–694. doi: 10.1167/iovs.10-6069. [DOI] [PubMed] [Google Scholar]
- Millar JC, Phan TN, Pang IH, Clark AF. Strain and Age Effects on Aqueous Humor Dynamics in the Mouse. Investigative ophthalmology & visual science. 2015;56:5764–5776. doi: 10.1167/iovs.15-16720. [DOI] [PubMed] [Google Scholar]
- Miyara N, Shinzato M, Yamashiro Y, Iwamatsu A, Kariya K, Sawaguchi S. Proteomic analysis of rat retina in a steroid-induced ocular hypertension model: potential vulnerability to oxidative stress. Japanese journal of ophthalmology. 2008;52:84–90. doi: 10.1007/s10384-007-0507-5. [DOI] [PubMed] [Google Scholar]
- Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Investigative ophthalmology & visual science. 2002;43:3455–3464. [PubMed] [Google Scholar]
- Overby DR, Bertrand J, Schicht M, Paulsen F, Stamer WD, Lutjen-Drecoll E. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Investigative ophthalmology & visual science. 2014a;55:3727–3736. doi: 10.1167/iovs.13-13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby DR, Bertrand J, Tektas OY, Boussommier-Calleja A, Schicht M, Ethier CR, Woodward DF, Stamer WD, Lutjen-Drecoll E. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Investigative ophthalmology & visual science. 2014b;55:4922–4933. doi: 10.1167/iovs.14-14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Current eye research. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- Paylakhi SH, Yazdani S, April C, Fan JB, Moazzeni H, Ronaghi M, Elahi E. Non-housekeeping genes expressed in human trabecular meshwork cell cultures. Molecular vision. 2012;18:241–254. [PMC free article] [PubMed] [Google Scholar]
- Pedrigi RM, Simon D, Reed A, Stamer WD, Overby DR. A model of giant vacuole dynamics in human Schlemm's canal endothelial cells. Exp Eye Res. 2011;92:57–66. doi: 10.1016/j.exer.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins TW, Alvarado JA, Polansky JR, Stilwell L, Maglio M, Juster R. Trabecular meshwork cells grown on filters. Conductivity and cytochalasin effects. Investigative ophthalmology & visual science. 1988;29:1836–1846. [PubMed] [Google Scholar]
- Perkumas KM, Stamer WD. Protein markers and differentiation in culture for Schlemm's canal endothelial cells. Exp Eye Res. 2012;96:82–87. doi: 10.1016/j.exer.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Zimmerman CC. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye. 2000;14(3B):503–514. doi: 10.1038/eye.2000.137. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Investigative ophthalmology & visual science. 1979;18:1043–1049. [PubMed] [Google Scholar]
- Qin Y, Lam S, Yam GH, Choy KW, Liu DT, Chiu TY, Li WY, Lam DS, Pang CP, Fan DS. A rabbit model of age-dependant ocular hypertensive response to topical corticosteroids. Acta Ophthalmol. 2012;90:559–563. doi: 10.1111/j.1755-3768.2010.02016.x. [DOI] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investigative ophthalmology & visual science. 2015;56:4447–4459. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razali N, Agarwal R, Agarwal P, Kapitonova MY, Kannan Kutty M, Smirnov A, Salmah Bakar N, Ismail NM. Anterior and posterior segment changes in rat eyes with chronic steroid administration and their responsiveness to antiglaucoma drugs. European journal of pharmacology. 2015;749:73–80. doi: 10.1016/j.ejphar.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Rohen JW, van der Zypen E. The phagocytic activity of the trabecularmeshwork endothelium. An electron-microscopic study of the vervet (Cercopithecus aethiops) Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1968;175:143–160. doi: 10.1007/BF02385060. [DOI] [PubMed] [Google Scholar]
- Rozsa FW, Reed DM, Scott KM, Pawar H, Moroi SE, Kijek TG, Krafchak CM, Othman MI, Vollrath D, Elner VM, Richards JE. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Molecular vision. 2006;12:125–141. [PubMed] [Google Scholar]
- Sawaguchi K, Nakamura Y, Nakamura Y, Sakai H, Sawaguchi S. Myocilin gene expression in the trabecular meshwork of rats in a steroid-induced ocular hypertension model. Ophthalmic Res. 2005;37:235–242. doi: 10.1159/000086946. [DOI] [PubMed] [Google Scholar]
- Schachtschabel DO, Binninger E. Aging of trabecular meshwork cells of the human eye in vitro. Zeitschrift fur Gerontologie. 1990;23:133–135. [PubMed] [Google Scholar]
- Scott PA, Overby DR, Freddo TF, Gong H. Comparative studies between species that do and do not exhibit the washout effect. Exp Eye Res. 2007;84:435–443. doi: 10.1016/j.exer.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor RE, Stamer WD, Seftor EA, Snyder RW. Dexamethasone decreases tissue plasminogen activator activity in trabecular meshwork organ and cell cultures. J Glaucoma. 1994;3:323–328. [PubMed] [Google Scholar]
- Sharma A, Patil AJ, Gupta N, Estrago-Franco MF, Mansoor S, Raymond V, Kenney MC, Kuppermann BD. Effects of triamcinolone acetonide on human trabecular meshwork cells in vitro. Indian J Ophthalmol. 2014;62:429–436. doi: 10.4103/0301-4738.121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR. Measurement of Outflow Facility Using iPerfusion. PloS one. 2016;11:e0150694. doi: 10.1371/journal.pone.0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato M, Yamashiro Y, Miyara N, Iwamatsu A, Takeuchi K, Umikawa M, Bayarjargal M, Kariya K, Sawaguchi S. Proteomic analysis of the trabecular meshwork of rats in a steroid-induced ocular hypertension model: downregulation of type I collagen C-propeptides. Ophthalmic Res. 2007;39:330–337. doi: 10.1159/000109989. [DOI] [PubMed] [Google Scholar]
- Sohn S, Hur W, Choi YR, Chung YS, Ki CS, Kee C. Little evidence for association of the glaucoma gene MYOC with open-angle glaucoma. Br J Ophthalmol. 2010;94:639–642. doi: 10.1136/bjo.2009.158261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Investigative ophthalmology & visual science. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm's canal. Investigative ophthalmology & visual science. 1998;39:1804–1812. [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Piatigorsky J. Development of characterization of a immortal and differentiated murine trabecular meshwork cell line. Investigative ophthalmology & visual science. 1999;40:1392–1403. [PubMed] [Google Scholar]
- Tektas OY, Hammer CM, Danias J, Candia O, Gerometta R, Podos SM, Lutjen-Drecoll E. Morphologic changes in the outflow pathways of bovine eyes treated with corticosteroids. Investigative ophthalmology & visual science. 2010;51:4060–4066. doi: 10.1167/iovs.09-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Thakur A, Kadam R, Kompella UB. Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Archives of ophthalmology. 2011;129:914–920. doi: 10.1001/archophthalmol.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrejon KY. Bioengineering In Vitro Human Trabecular Meshwork Models for Glaucoma Therapeutic Screening, Colleges of Nanoscale Science and Engineering. UMI; University at Albany: 2015b. [Google Scholar]
- Torrejon KY, Papke EL, Halman JR, Stolwijk J, Dautriche CN, Bergkvist M, Danias J, Sharfstein ST, Xie Y. Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnology and bioengineering. 2015 doi: 10.1002/bit.25899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrejon KY, Pu D, Bergkvist M, Danias J, Sharfstein ST, Xie Y. Recreating a human trabecular meshwork outflow system on microfabricated porous structures. Biotechnology and bioengineering. 2013;110:3205–3218. doi: 10.1002/bit.24977. [DOI] [PubMed] [Google Scholar]
- Tripathi BJ, Tripathi RC, Swift HH. Hydrocortisone-induced DNA endoreplication in human trabecular cells in vitro. Exp Eye Res. 1989;49:259–270. doi: 10.1016/0014-4835(89)90095-x. [DOI] [PubMed] [Google Scholar]
- Ueda J, Wentz-Hunter KK, Cheng EL, Fukuchi T, Abe H, Yue BY. Ultrastructural localization of myocilin in human trabecular meshwork cells and tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2000;48:1321–1330. doi: 10.1177/002215540004801003. [DOI] [PubMed] [Google Scholar]
- Underwood JL, Murphy CG, Chen J, Franse-Carman L, Wood I, Epstein DL, Alvarado JA. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. The American journal of physiology. 1999;277:C330–342. doi: 10.1152/ajpcell.1999.277.2.C330. [DOI] [PubMed] [Google Scholar]
- van der Merwe EL, Kidson SH. The three-dimensional organisation of the post-trabecular aqueous outflow pathway and limbal vasculature in the mouse. Exp Eye Res. 2014;125:226–235. doi: 10.1016/j.exer.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Welge-Lussen U, May CA, Eichhorn M, Bloemendal H, Lutjen-Drecoll E. AlphaB-crystallin in the trabecular meshwork is inducible by transforming growth factor-beta. Investigative ophthalmology & visual science. 1999;40:2235–2241. [PubMed] [Google Scholar]
- Whitlock NA, McKnight B, Corcoran KN, Rodriguez LA, Rice DS. Increased intraocular pressure in mice treated with dexamethasone. Investigative ophthalmology & visual science. 2010;51:6496–6503. doi: 10.1167/iovs.10-5430. [DOI] [PubMed] [Google Scholar]
- Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Current eye research. 1993;12:783–793. doi: 10.3109/02713689309020383. [DOI] [PubMed] [Google Scholar]
- Xue W, Wallin R, Olmsted-Davis EA, Borras T. Matrix GLA protein function in human trabecular meshwork cells: inhibition of BMP2-induced calcification process. Investigative ophthalmology & visual science. 2006;47:997–1007. doi: 10.1167/iovs.05-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li M. [Establishment of in vitro culture of bovine trabecular meshwork cells and their phagocytosis] [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 1996;32:136–139. [PubMed] [Google Scholar]
- Yue BY, Kurosawa A, Elvart JL, Elner VM, Tso MO. Monkey trabecular meshwork cells in culture: growth, morphologic, and biochemical characteristics. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1988;226:262–268. doi: 10.1007/BF02181193. [DOI] [PubMed] [Google Scholar]
- Zhan GL, Miranda OC, Bito LZ. Steroid glaucoma: corticosteroid-induced ocular hypertension in cats. Exp Eye Res. 1992;54:211–218. doi: 10.1016/s0014-4835(05)80210-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res. 2007;84:275–284. doi: 10.1016/j.exer.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Davidson BR, Stamer WD, Barton JK, Marmorstein LY, Marmorstein AD. Enhanced inflow and outflow rates despite lower IOP in bestrophin-2-deficient mice. Investigative ophthalmology & visual science. 2009;50:765–770. doi: 10.1167/iovs.08-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ramsey KE, Stephan DA, Russell P. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Investigative ophthalmology & visual science. 2004;45:4023–4034. doi: 10.1167/iovs.04-0535. [DOI] [PubMed] [Google Scholar]
- Zhou L, Li Y, Yue BY. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med. 1998;1:339–346. [PubMed] [Google Scholar]
- Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]