Abstract
Fibrocytes are circulating mesenchymal precursors (CD45+, col 1+) recruited to fibrotic areas. Fibrocytes secrete profibrotic mediators including periostin; a matricellular protein that regulates cellular interactions with extracellular matrix (ECM) components. In bleomycin-induced fibrosis, periostin deficiency in structural or hematopoietic cells limits development of pulmonary fibrosis. To determine if hematopoietic-derived fibrocytes might secrete soluble factors to activate structural myofibroblast differentiation, wild-type (WT) fibroblasts were treated with conditioned medium from fibrocytes isolated from bleomycin-treated WT or periostin−/− mice. After 24 hours we saw less α-smooth muscle actin expression in cells treated with conditioned medium from periostin−/− fibrocytes. Adoptive transfer of WT fibrocytes augmented lung fibrosis to a greater extent than transfer of fibrocytes from periostin−/− mice. In vitro analysis of fibrocytes and fibroblasts isolated from WT and periostin −/− mice treated with TGFβ1 or periostin demonstrated co-regulation of mesenchymal activation and beta 1 integrin as a potential receptor for periostin on fibrocytes. Additionally, connective tissue growth factor (CTGF) mRNA expression was increased in fibrocytes treated with periostin whereas CTGF and lysl oxidase (LOX) mRNA expression was low in bleomycin-treated periostin−/− fibrocytes. These data suggest fibrocytes may augment bleomycin-induced fibrosis via secretion of periostin and other soluble factors that promote myofibroblast differentiation.
Keywords: fibroblast, fibrocyte, fibrosis, periostin, mesenchymal, lung
Introduction
Fibrosis can be triggered by various known insults (e.g., infection, allergens, toxins, or radiation), or can occur for unknown reasons as in the case of idiopathic pulmonary fibrosis (IPF) 1. IPF is a chronic progressive parenchymal lung disease of unknown origin, with mean survival rate of 3-5 years after diagnosis. Prevalence and mortality rates are increasing globally 2-4. The cellular mechanisms leading to IPF remain unclear but a commonly held paradigm describes continuous cycles of injury to the alveolar epithelium leading to dysregulated repair. Prominent features of this dysregulated repair include fibroblast differentiation, myofibroblast accumulation and excessive collagen deposition in the alveolar space 5-7. Mediators such as growth factors and cytokines from different cell types contribute to the persistence of activated fibroblasts and myofibroblasts8. Based on our current knowledge, bone marrow-derived collagen producing cells (e.g. fibrocytes and other collagen producing cells) play a significant role in the development of lung fibrosis 9-17. Fibrocytes respond to a number of cytokines and chemokines that are typically associated with migration and activation of inflammatory cells18. Fibrocytes are hematopoietic bone marrow-derived cells that express both mesenchymal and leukocyte markers. They circulate in the peripheral blood and can be isolated from tissues. Cultured fibrocytes have been shown to express a number of ECM proteins including collagen 1, collagen 3 and fibronectin 18-22. In addition, fibrocytes also express a number of chemokine receptors, including CXCR4, CCR7 and CCR2, which may contribute to the recruitment and activation of fibrocytes resulting in migration to damaged tissues 23-25. Although several reports indicate that fibrocytes express type I collagen, others have suggested that uptake of secreted type I collagen by hematopoietic cells characterizes the fibrocyte population 26, 27.
A more recent study demonstrated that cells of hematopoietic origin produce type I collagen but are not a necessary source of type I collagen during experimental lung fibrosis 28. Kleaveland et al. illustrated that fibrocytes can efficiently uptake type I collagen even though the underlying mechanism is not clearly understood 28. Adoptive transfer of fibrocytes leads to augmented fibrosis in a FITC-induced mouse model and several studies have found a correlation between increased numbers of fibrocytes and worse disease progression22, 24, 29-31. Recently, fibrocytes were shown to contribute to the progression of pulmonary fibrosis without differentiating to myofibroblasts themselves using a transforming growth factor alpha model of fibrosis 32. Additionally, Garcia de alba et al. also showed that fibrocytes contributed to increased mRNA levels of ECM proteins in both fibrosis and chronic pneumonitis 31. Work from our laboratory demonstrated that fibrocytes in circulation of IPF patients are major producers of the matricellular protein, periostin, and that periostin accumulates in the lung tissue of IPF patients 33.
Periostin, also known as osteoblast-specific factor 2, is a recently characterized matricellular protein that binds to components of the ECM including type I collagen and fibronectin and has been shown to be involved in collagen fibrillogenesis34. Periostin protein transmits signals from the ECM to the cell by binding to cellular receptors such as integrins that affect cell adhesion, proliferation, migration and tissue angiogenesis 35. It has been reported that periostin promotes cancer cell invasion and metastasis through the integrin/phosphatidylinositol 3-kinase/AKT pathway, leading to the development of various tumors 36. Work from our laboratory and others have shown that there are increased circulating periostin levels in IPF patients compared to controls 33, 37. Our work further showed that the increased periostin expression in IPF tissues was localized to active areas of fibrosis and that IPF patients also showed increased percentages of periostin-expressing fibrocytes and monocytes in the blood 33. Chimeric mouse studies showed that both structural and hematopoietic sources of periostin were important in protecting periostin-deficient mice from bleomycin-induced fibrosis 33. Although periostin has diverse functions, it is becoming increasingly clear that periostin is upregulated in lung tissue in the context of several respiratory diseases 38. At present, the precise function of periostin in the lung has not been fully elucidated. In this study we used bleomycin to establish fibrosis in mice, and then analyzed how hematopoietic-derived fibrocytes might signal structural myofibroblast differentiation through their ability to secrete periostin or other periostin-regulated soluble factors. We explored the use of periostin-deficient fibrocytes in adoptive transfer experiments as well as the use of integrin blocking antibodies to demonstrate that periostin interacts with different receptors on fibrocytes and fibroblasts and that periostin secretion by fibrocytes is important for the exacerbation of bleomycin-induced lung fibrosis.
Materials and Methods
Animals
Wild-type C57BL/6 (B6) age and sex-matched mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Periostin−/− mice were bred at the University of Michigan. In previous experiments we verified that C57Bl/6 mice purchased from Jackson Laboratory behave similarly with respect to the periostin−/− mice as do control mice generated by heterozygous breeding in house. Mice were housed under pathogen-free conditions and provided food and water ad libitum. All animal experiments complied with university and federal guidelines for humane use and care. The University of Michigan Institutional Animal Care and Use Committee approved these experiments.
Bleomycin Model of Pulmonary Fibrosis
Mice were given bleomycin (0.025 U (Sigma) dissolved in sterile saline in a 50-μl volume) intratracheally as described previously 39 or via oropharyngeal aspiration.
Lung Collagen Measurements
Collagen deposition was measured using a hydroxyproline assay on day 21 post-bleomycin challenge as described previously 39.
Semi quantitative real-time RT-PCR
RNA was extracted from lung mesenchymal cells using TRIzol reagent (Invitrogen, Carlsbad, CA) then analyzed by real-time RT-PCR on an Applied Biosystems StepOne Plus thermocycler (Applied Biosystems, Foster City, CA). Gene specific primers and probes were purchased from Sigma-Aldrich (St Louis, MO). Relative expression was calculated using the comparative CT method as previously described 40. Additionally, RNA isolated from bleomycin-treated WT and periostin −/− fibrocytes was analyzed using the Mouse Fibrosis RT2 Profiler ™ PCR Array and then confirmed using gene specific primers and probes (see on line supplement Table 1) as previously described. We have determined that β-actin expression did not change significantly in response to TGFβ stimulation (data not shown) making this an appropriate normalization for our gene expression data.
Enzyme-linked Immunoassay/ELISA
Whole lung homogenates were prepared for analysis of cytokines as described previously 39. Each ELISA assay was done using a Duoset ELISA kit (R&D systems, Minneapolis, MN) according to manufacturer’s instructions.
Mesenchymal Cell Isolation
Lung mesenchymal cells were grown for two weeks from lung minces. At this time, all cells expressed collagen I and were designated lung mesenchymal cells. In some experiments, mesenchymal cells were magnetically sorted for expression of CD45 to obtain CD45+ collagen I+ fibrocytes or CD45− collagen I+ fibroblasts as previously described 24. Briefly, labeled cells were sorted using LS-positive selection columns using a SuperMacs apparatus (Miltenyi Biotech) according to manufacturer's instructions. For extra purity, CD45+ cells were sometimes reapplied to a second LS-positive selection column. For experiments where fibrocyte supernatants were collected, purified fibrocytes were cultured overnight in serum-free media (SFM) with or without anti-periostin Ab (AF-2955, R&D systems). These cell-free supernatants were then added to purified fibroblasts.
Adoptive Transfer
Fibrocytes were purified by magnetic separation from lung mince cultures from untreated wild-type or periostin−/− mice as described above. 5 × 105 fibrocytes were injected via tail vein in a 200-μl volume into mice that had received bleomycin inoculation intratracheally 4 d previously. Mice were harvested and lungs collected on day 21 after bleomycin, and lung collagen content was determined by hydroxyproline assay.
Flow Cytometry
Lung mesenchymal cells were grown for two weeks from lung minces from saline and bleomycin-treated wild-type or periostin−/− mice as previously described. On day 14 cells were collected and incubated with Fc block (1:100) clone 24G2 (BD Pharmingen, San Diego, CA) for 15mins, then stained with combinations of anti-mouse CD45 (BD Pharmingen), anti-mouse CD29 (Beta 1), anti-mouse CD49a (Alpha 1) and anti-mouse CD51 directly-conjugated antibodies available from Biolegend. Cells were run on a FACS Canto (BD Biosciences, Mountain View, CA) and further analyzed using the Flowjo single cell analysis software.
Western Blot Analysis
Mesenchymal cells were lysed in radioimmunoprecipitation assay buffer with protease inhibitor cocktail [Sigma]) for 15 min at 4°C and centrifuged at 10,000g for 10 mins. Total protein concentrations were determined by the Bicinchoninic acid assay (Pierce). Equal amounts of protein from each sample were separated on a 4-20% gradient SDS-polyacrylamide gel and transferred to a PVDF membrane (Amersham/GE Healthcare, Pittsburgh, PA). PVDF membrane was probed for alpha smooth muscle actin (α-SMA) (Sigma), beta1 integrin (ab52971 Abcam) and rabbit polyclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell signaling, Beverly, MA) as a loading control. Densitometry analyses of Western blot pixel densities were performed using the NIH Image J program.
Statistical Analysis
Statistical significance was assessed by analysis of variance (3 or more comparisons) with a Bonferroni post-hoc test or Student t test (2 comparisons) using GraphPad Prism 6 software (San Diego, CA). Data shown represents mean ± SEM, p< 0.05 was considered significant.
Results
Mesenchymal cells increase periostin mRNA expression after bleomycin treatment
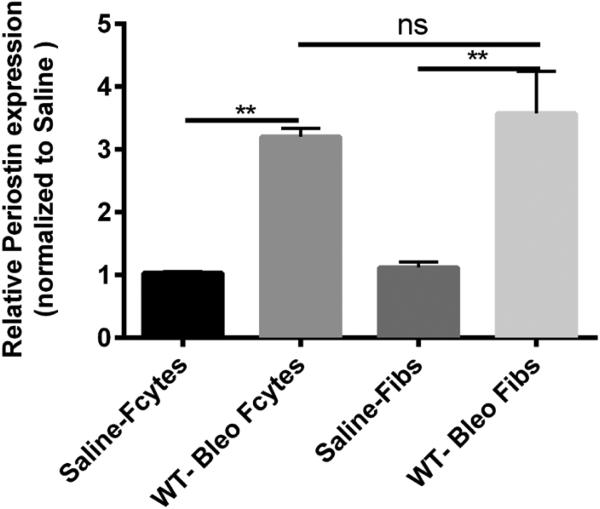
To specifically assess the upregulation of periostin expression in lung mesenchymal cells after bleomycin treatment, WT mice were given intratracheal saline or bleomycin on day 0. All lungs were harvested on day 14 and mesenchymal cells were cultured for 14 days then sorted into fibrocytes (CD45 positive) and fibroblasts (CD45 negative). RNA was isolated for real-time RT-PCR analysis. We observed that both fibroblasts and fibrocytes had significant increases in periostin mRNA expression post bleomycin treatment (Figure 1).
Figure 1. Increased mRNA expression of periostin in lung mesenchymal cells post-bleomycin treatment.
Wild type mice were given 0.025U of bleomycin or saline intratracheally on day 0. On day 14, lung mesenchymal cells were cultured and sorted by Magnetic bead separation for fibroblasts and fibrocytes. Total RNA was isolated and by real time RT-PCR we measured the expression of periostin and β-actin in fibrocytes and fibroblasts . Data represent n=3 per group pooled from multiple mice. **p < 0.01, *p< 0.05, ns=not significant.
TGFβ1 and periostin co-regulate each other in lung mesenchymal cells
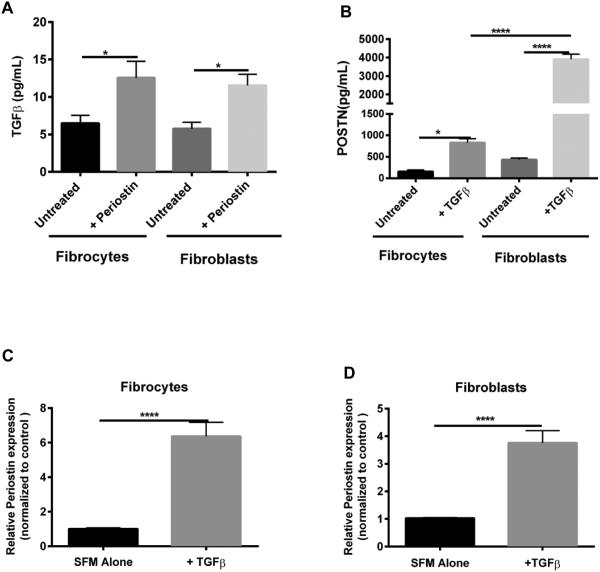
We previously showed that treatment of WT mesenchymal cells with TGFβ showed a significant increase in periostin production 33. To assess whether periostin also up-regulates TGFβ1 production in purified fibroblasts and fibrocytes, we isolated these cell types from lung mince cultures from naïve mouse lungs and treated with recombinant periostin (500 ng/mL) in SFM. Treatment of WT fibrocytes and fibroblasts with periostin lead to increased TGFβ1 production as measured by ELISA (Figure 2A). In addition, treatment of fibroblasts and fibrocytes with recombinant TGF-β (2ng/mL) increased protein production of periostin as measured by ELISA (Figure 2B), as well as increased mRNA expression of periostin (Figure 2C-D).
Figure 2. Periostin and TGFβ regulate each other in fibroblasts and fibrocytes.
Fibroblasts and fibrocytes from wild-type mice were treated with periostin (500ng/mL) or TGFβ (2ng/mL) for 48 hours. Cell-free supernatants were collected and analyzed by ELISA for TGFβ (A) and periostin (B). Total RNA was isolated and by real time RT-PCR we measured the expression of periostin and β-actin in fibrocytes (C) and fibroblasts (D). Data are representative of mean ±SEM, n=3 wells/treatment per group. *p< 0.05, **p < 0.01 ,****p<0.0001, ns=not significant
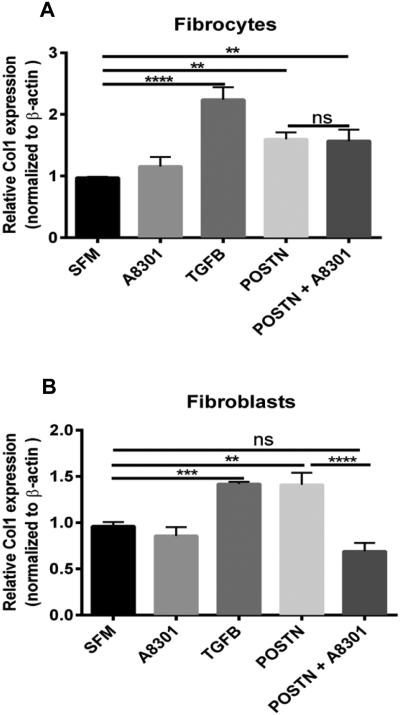
Since, periostin and TGFβ1 seem to be co-regulating each other, we wanted to assess whether the production of collagen I from fibroblasts and fibrocytes was different in the presence of periostin and if this effect was dependent on TGF-β1 signaling. Figure 3 illustrates that treatment of murine lung mesenchymal cells with periostin for 48hrs led to a significant increase in mRNA expression for collagen I. Because collagen I mRNA is extremely stable41, small increases in transcription can lead to significant enhancement of translated protein. However, the increase in collagen I expression was independent of TGFβ1 signaling in the fibrocytes compared to the fibroblasts as indicated by treatment with the A8301 TGF-β1 receptor. Additionally, supplemental Figure 1 demonstrates that periostin can influence other extracellular matrix components as well including collagen 3 and fibronectin. Overall these data suggest that TGFβ1 and periostin are co-regulating each other but periostin can signal to fibrocytes independently of TGFβ1 and that the receptor needed for periostin signaling may be different on fibrocytes compared to fibroblasts.
Figure 3. Periostin induces collagen 1 expression in fibrocytes independently of TGFβ signaling.
Mesenchymal cells were cultured in complete media, sorted into fibrocytes and fibroblasts, then switched to SFM where they were treated with TGFB (2ng/mL), A8301 (ALK5 inhibitor) or periostin (500ng/mL) for 48hours. Total RNA was isolated and by real time RT-PCR we measured the expression of collagen I and β-actin in fibrocytes (A) and fibroblasts (B). Data are representative of mean ±SEM, n=3 wells/treatment per group from 2 independent experiments ****p<0.0001, ***p<0.001,**p<0.01, *p<0.05 and ns + not significant.
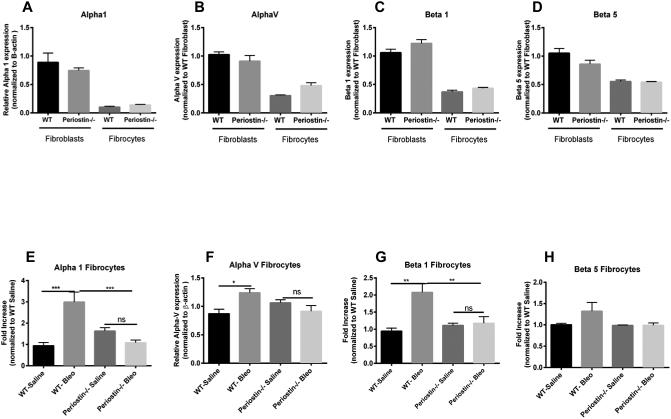
Decreased integrin expression in fibrocytes from periostin−/− mice post-bleomycin treatment
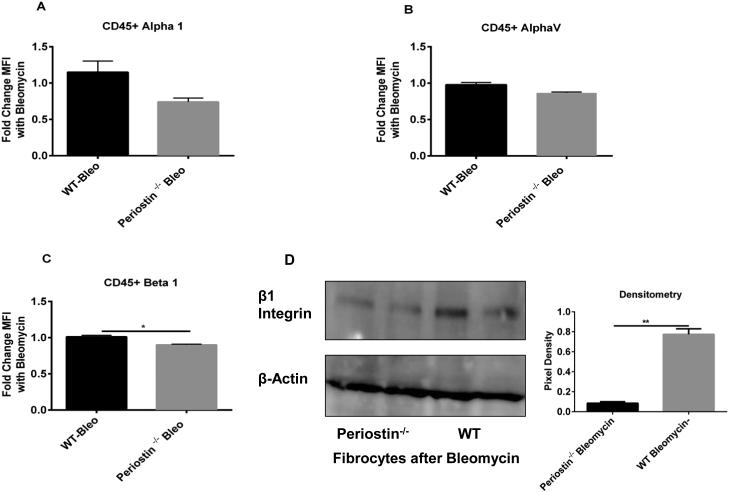
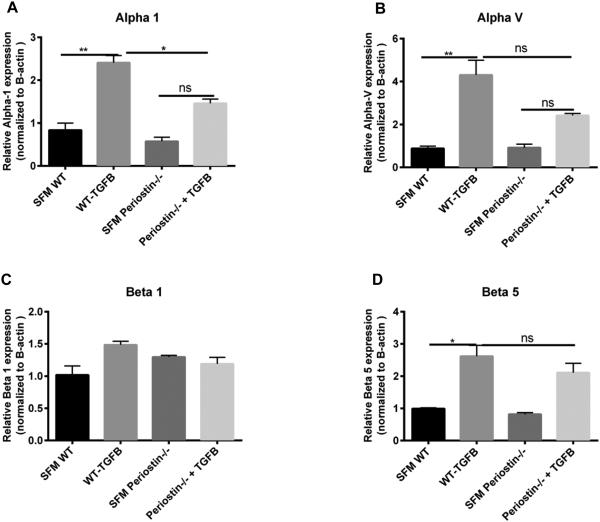
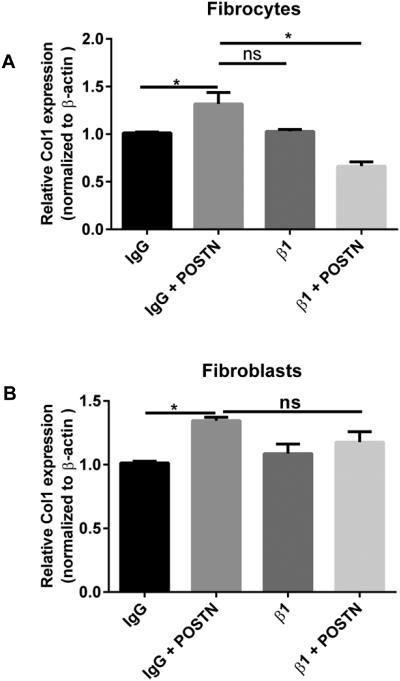
In many cancers, periostin binds to integrins activating the AKT/PKB and FAK-mediated signaling pathways. This leads to increased cell survival, invasion, angiogenesis, metastasis and epithelial-mesenchymal transition 42. Periostin also functions as a ligand for integrin alphaVbeta3 and alphaVbeta 5 to support adhesion and migration of epithelial cells 43. Because our data suggest that periostin might be signaling differently in fibrocytes vs fibroblasts, we wanted to examine the expression levels of integrins in WT and periostin−/− naïve lung mesenchymal cells. As illustrated in figure 4A-D, there is no significant difference in the baseline mRNA expression levels of alpha 1, alpha V, beta 1 and beta 5 integrin levels on fibrocytes and fibroblasts from WT or periostin−/− mice, although in most cases, the expression levels of integrins were lower in fibrocytes than fibroblasts. We next treated WT and periostin−/− mice intratracheally with saline or bleomycin for 14 days then cultured lung mesenchymal cells, sorted for fibrocytes and assessed mRNA expression for different integrins post-bleomycin treatment. Alpha 1, alpha V and beta 1 were found to be significantly upregulated in WT fibrocytes post bleomycin treatment but beta 5 expression was not altered. None of the integrins were upregulated post-bleomycin in the periostin−/− cells (Figure 4E-H). To compare the differences in integrin expression on fibrocytes at the protein level, fibrocytes were isolated from bleomycin-treated WT or periostin−/− mice and integrin profiles were analyzed by flow cytometry (Figure 5 A-C). Levels of alpha 1 were lower in periostin−/− fibrocytes but did not reach significance in this experiment. In contrast, the decrease in β1 integrin noted in periostin−/− mice did show a significant reduction (Figure 5C). We also confirmed lower levels of β1 integrin in periostin−/− fibrocytes by Western blot (Figure. 5D). There were no statistically significant differences in integrin expression in fibroblasts from WT or periostin−/− mice at the mRNA or protein levels (Supplemental Figure 2). To determine how integrin expression profiles correlated with TGFβ1 production, we treated fibrocytes with exogenous TGFβ1 and found increased mRNA expression for alpha V, alpha 1 and beta 5 in wild-type cells (Figure 6). Expression of these integrins was similarly increased in the periostin−/− cells treated with TGFβ1. In contrast, TGFβ1 treatment caused a modest, but not significant increase in beta 1 expression in WT cells, but did not in periostin−/− cells providing further support that beta 1 integrin expression may be necessary for periostin signaling to fibrocytes in murine lungs (Figure 6). Next, we treated WT fibrocytes and fibroblasts with periostin in the presence of a beta 1 integrin blocking antibody (102209-Biolegend) and after 48h assessed collagen1 mRNA expression as an indication of mesenchymal cell activation (Figure 7). Fibrocytes treated with periostin in the presence of beta 1 blocking antibody had markedly less collagen I mRNA expression compared to samples treated with periostin and isotype control (Figure 7A). In contrast, addition of beta 1 integrin blocking antibody to fibroblasts showed no change in collagen I expression (Figure 7B). Taken together our data suggest periostin signals collagen expression through beta 1 integrin to activate fibrocytes and this is independent of TGFβ signaling.
Figure 4. Loss of periostin decreases the expression of integrins post-bleomycin treatment A-D).
Wild type or periostin−/− mice were given bleomycin or saline intratracheally on day 0. On day 14 lungs were harvested and mesenchymal cells were cultured and sorted as previously described. Total RNA was isolated and by real time RT-PCR we measured the expression of alpha1, alpha V, beta 1 and beta 5 expression in saline treated fibrocytes and fibroblasts (A-D) and bleomycin treated (E-H) fibrocytes. Values are expressed as mean ± SEM, and represent n=3 animals/group from two independent experiments ns=not significant, ***p<0.001,**p<0.01 and *p<0.05.
Figure 5. Periostin−/− fibrocytes express significantly less Beta 1 integrin post-bleomycin treatment.
(A-C) Wild type or periostin−/− mice were given bleomycin or saline intratracheally on day 0. On day 14 lungs were harvested and mesenchymal cells were incubated with a pan CD45 antibody, alpha 1, alpha V and beta 1-integrin antibodies. Cells were analyzed by flow cytometry to assess surface expression of integrins on CD45+ and CD45− cells. (D) Fibrocytes from bleomycin-treated WT and periostin−/− mice were lysed in RIPA buffer with protease inhibitor and we assessed the expression of Beta 1 and β-actin by Western blot The intensity of the bands were also quantitated using the NIH Image J software from duplicate cultures, **p<0.01 and *p<0.05.
Figure 6. TGFβ treatment did not induce of β1 integrin mRNA expression in fibrocytes in the absence of periostin.
Fibrocytes from wild-type and periostin−/− mice were treated with recombinant TGFβ (2ng/mL) for 48 hours. Total RNA was isolated and by real time RT-PCR we measured the expression of different integrins and β-actin in fibrocytes (A) Alpha 1, (B) Alpha V, (C) Beta 1 and (D) Beta 5. Data are representative of mean ±SEM, n=3 wells/treatment per group ,**p<0.01, *p<0.05 and ns=not significant.
Figure 7. Beta 1 integrin blockade in WT fibrocytes caused decreased Collagen 1 expression with periostin treatment but showed no effect in fibroblasts.
Mesenchymal cells were cultured in complete media, sorted into fibrocytes and fibroblasts, then switched to SFM where they were treated with purified rat anti-mouse beta 1 (β1)(HMβ1-1, rat CD29) blocking antibody or purified armenian Hamster IgG (400916) isotype control for 30mins, then incubated with or without periostin (500ng/mL) for 48hours. Total RNA was isolated and by real time RT-PCR we measured the expression of collagen 1 and β-actin in fibrocytes (A) and fibroblasts (B). Data are representative of mean ±SEM, n=3 wells/treatment per group *p<0.05. and ns=not significant.
Fibrocytes exacerbate bleomycin-induced fibrosis through paracrine effects and in a periostin-dependent manner
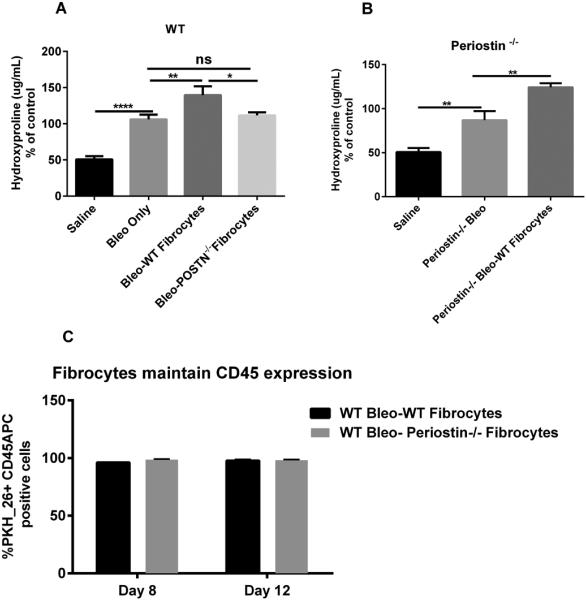
We previously showed that fibrocytes augment FITC-induced lung fibrosis 24. In addition, fibrocytes and monocytes were the major producers of periostin in circulation and a hematopoietic source of periostin was important for fibrogenesis in the bleomycin model of lung fibrosis 33. To specifically assess the role of periostin produced by fibrocytes in lung fibrogenesis, WT or periostin−/− mice were given intratracheal saline or bleomycin on day 0. On day 4 post-bleomycin, mice were give 5×105 additional cultured WT or periostin−/− lung fibrocytes. Lungs from all mice were harvested on day 21 post-bleomycin and lung collagen content was measured by hydroxyproline assay. As expected, bleomycin-treated WT mice showed a significant increase in collagen content when compared to saline controls but WT mice that received the additional WT fibrocytes had significantly higher amounts of collagen in the lungs compared to bleomycin alone (Figure 8A). Interestingly, bleomycin-treated WT mice given the periostin−/− fibrocytes showed similar levels of lung collagen as the bleomycin-treated wild-type mice with no added cells (Figure 8A). Figure 8B demonstrates that periostin−/− mice treated with bleomycin then given additional WT lung fibrocytes have significantly more collagen content in the lungs compared to periostin−/− mice treated with saline or bleomycin alone. Additionally, we analyzed the ability of fibrocytes to maintain their CD45 expression post-transfer over time. Using flow cytometry analysis we found that all the labeled PKH26 positive fibrocytes were detected as CD45 positive cells 4-8 days after transfer (Figure 8C). These data demonstrate that fibrocytes were not differentiating into CD45-negative fibroblasts after transfer.
Figure 8. Adoptive transfer of WT but not periostin−/− fibrocytes augments bleomycin-induced fibrosis but both maintained CD45 expression in vivo.
(A) Wild type mice were given 0.025U of bleomycin or PBS intratracheally on day 0. On day 5, post-bleomycin treatment half of each group received 5×105 WT or periostin−/− fibrocytes by intravenous tail vein injection. Lungs were harvested on day 21 post-bleomycin for hydroxyproline quantification. Data shown are pooled from two independent experiments, with n = 4-6 mice per group in each experiment. **p<0.01, *p<0.05 and ns=not significant (B) Periostin−/− mice were given 0.025U of bleomycin intratracheally on day 0. On day 5 post-bleomycin treatment, half of each group received 5×105 WT fibrocytes, lungs were harvested and assessed collagen content on day 21 by hydroxyproline assay *p<0.05. (C) Wild type mice were given 0.025U of bleomycin or PBS intratracheally on day 0. On day 5, post-bleomycin treatment half of each group received 5×105 PKH-26 labeled WT or periostin−/− fibrocytes by intravenous tail vein injection. On day 3 and 7 post-transfer, lungs were then harvested and total lung leukocytes were enumerated and then labeled with CD45 APC to determine the percentages of PKH-26+CD45+APC fibrocytes. Data shown represents mean ± SEM, n=3 animals/group, ns, not significant.
Periostin production by fibrocytes promotes their profibrotic effects on myofibroblasts via other mediators
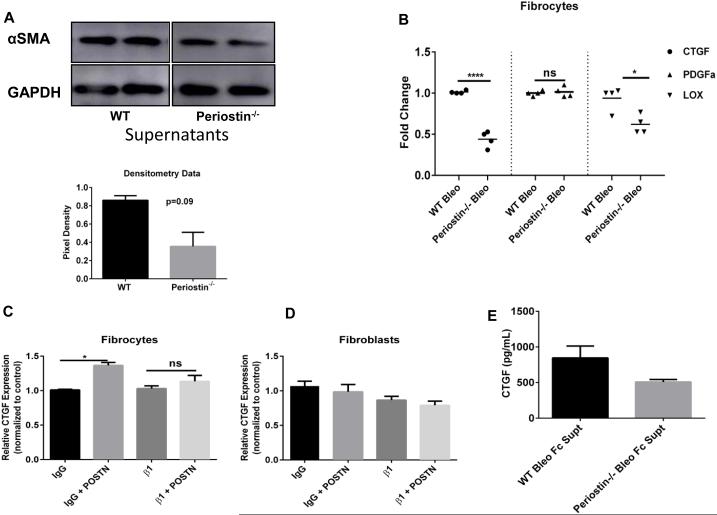
Myofibroblast differentiation is characterized, in part, by induction of αSMA gene expression as well as by increased production of ECM components and fibrogenic cytokines such as TGFβ1 42-44. To assess how fibrocyte-derived periostin affects myofibroblast differentiation, we analyzed protein expression of αSMA in WT untreated fibroblasts that were incubated with cell-free supernatants from CD45 positive fibrocytes from bleomycin-treated WT and periostin−/− mice. Cells were incubated in this media for 24h. After 24h we analyzed expression of αSMA by Western blot and saw less αSMA expression in WT fibroblasts that were incubated with bleomycin-treated supernatants from periostin−/− fibrocytes (Figure 9A). To address whether this effect was solely due to periostin or possibly involved other downstream signaling proteins we performed a microarray using a targeted mouse fibrosis array with the goal of identifying differences between the WT and periostin−/− fibrocytes. We saw significant decreases in connective tissue growth factor (CTGF) and Lysyl oxidase (LOX) but not platelet-derived growth factor (PDGF)α in periostin−/− fibrocytes. Findings were confirmed by RT-qPCR (Figure 9B). In addition, there was a significant increase in CTGF mRNA expression in fibrocytes treated with exogenous periostin; however, in the presence of a beta 1 blocking antibody, periostin could no longer induce a significant increase in CTGF (Figure 9C). There was no change in CTGF mRNA expression in fibroblasts (Figure 9D). Given the importance of CTGF in fibrogenesis, we measured the amount of CTGF protein in the supernatants used in the experiments described in Figure 9A by ELISA and saw less CTGF in the periostin−/− fibrocytes supernatants (Figure 9E). Taken together these data suggest that fibrocytes in the presence of periostin secrete additional pro-fibrotic mediators such as CTGF that contribute to myofibroblast differentiation.
Figure 9. WT fibrocytes secrete CTGF in the presence of periostin and increase αSMA protein expression in fibroblasts in the presence of periostin.
(A) Wild type mice were given 0.025U of bleomycin or PBS intratracheally on day 0. On day 14, post-bleomycin treatment lung mesenchymal cells were cultured for 14 days. After 14 days in culture cells were sorted and CD45 positive fibrocytes were incubated in SFM overnight. Cell-free supernatants were collected from both bleomycin-WT and periostin−/− cells and added 1:1 with SFM onto WT untreated fibroblasts (CD45 negative) cells for 24h. Cells were lysed in RIPA buffer with protease inhibitor and we assessed the expression of αSMA and GAPDH by western blot. Densitometry data is also shown. (B) Total RNA was isolated from the WT and periostin−/− fibrocytes after overnight incubation in serum-free media and by real time RT-PCR we measured the mRNA expression of CTGF, PDGFα and LOX. Data shown represents mean ± SEM, n=3 wells/group, ns=not significant, ****p<0.0001, **p<0.01 and *p<0.05. (C-D) Total RNA was isolated from WT mesenchymal cells treated with periostin (500ng/mL) and by real time RT-PCR we measured the mRNA expression of CTGF. Data shown represents mean ± SEM, n=3 wells/group, ns=not significant and *p<0.05. (E)) Cell free supernatants were analyzed by ELISA for CTGF.
Periostin signaling is critical for fibrocyte amplification of a profibrotic response
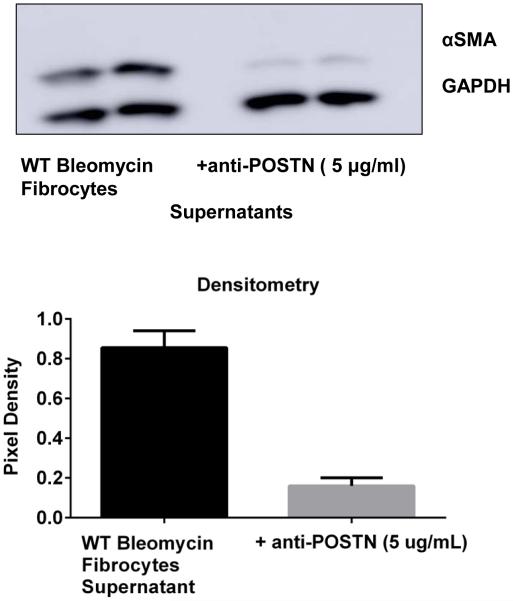
We next wanted to determine the impact of periostin signaling to the fibrocytes to induce their profibrotic paracrine mediators. We isolated fibrocytes from bleomycin-treated wild-type mice and cultured them in SFM overnight in the presence or absence of an anti-periostin Ab to neutralize the periostin that is secreted into the supernatant by fibrocytes. When these supernatants were collected and incubated with fibroblasts, the supernatant collected from fibrocytes treated with anti-periostin antibodies could not induce expression of α-SMA to the same extent in the fibroblasts as supernatant from untreated fibrocytes (Figure 10). These results suggest that autocrine periostin signaling to the fibrocytes themselves is a critical upstream mediator of the release of fibrocyte pro-fibrotic paracrine mediators.
Figure 10. Neutralization of periostin secretion by fibrocytes post-bleomycin treatment limited their ability to promote myofibrolast differentiation.
WT mice were given 0.025U of bleomycin or PBS intratracheally on day 0. On day 14, post-bleomycin treatment lung mesenchymal cells were cultured for 14 days. After 14 days in culture cells were sorted and CD45 positive fibrocytes were incubated in SFM overnight in presence or absence of a mouse periostin neutralizing antibody (AF-2955, R&D systems). Cell-free supernatants were collected from both and added 1:1 with SFM onto WT untreated fibroblasts (CD45 negative) for 24h. Cells were lysed in RIPA buffer with protease inhibitor and we assessed the expression of αSMA and GAPDH by western blot. Data was quantitated using Image J software and pixel density is represented on bar graph.
Discussion
Previous studies have shown that periostin −/− mice treated with bleomycin intratracheally had decreased collagen content compared with WT littermates 33, 45. Both monocytes and fibrocytes likely contribute to the pool of circulating periostin in IPF patients and chimeric mouse studies demonstrated that both the hematopoietic-derived and structural sources of periostin contributed similarly to fibrogenesis 33. These studies suggested that a circulating source of periostin could be playing a significant role in the exacerbation of lung fibrosis, but it was unclear how the lower levels of periostin produced by circulating fibrocytes vs. structural cells (e.g. fibroblasts, see Figure 2B) were influencing disease. Our current findings in vivo, and in vitro, suggest that fibrocyte profibrotic function is likely via multiple paracrine mechanisms.
We examined the effects of fibrocytes in the exacerbation of bleomycin-induced fibrosis in WT and periostin−/− mice. Adoptive transfer of WT fibrocytes during established lung fibrosis augmented bleomycin-induced fibrosis in recipient mice, corroborating the earlier studies in a fluorescein isothiocyanate (FITC)-induced model of pulmonary fibrosis 24. Furthermore, in periostin−/− mice receiving WT fibrocytes there was a significant increase in lung hydroxyproline, which is evidence of enhanced lung fibrosis relative to the periostin−/− mice that did not receive additional fibrocytes (Figure 8). We first wondered whether there was a defect in migration of periostin−/− fibrocytes to the injured lung. However, Figure 8C shows that periostin−/− fibrocytes could be identified in the lungs as easily as WT fibrocytes. Furthermore, we found no defect in the expression of chemokine receptors on the WT vs. the periostin−/− fibrocytes (data not shown). Therefore, the decrease in fibrosis in mice treated with the periostin−/− fibrocytes could not be attributed to a migration defect. Furthermore, we considered the possibility that WT fibrocytes might better be able to differentiate into fibroblasts or myofibroblasts once arriving in the injured lung. While in vitro work has clearly shown that fibrocytes can differentiate into myofibroblasts 25, 46, 47, our in vivo work corroborates recent findings in another model system to suggest fibrocytes are not differentiating into myofibroblasts during fibrosis (Figure 8C) 32. We also previously showed that fibrocytes migrate to areas in the lungs adjacent to fibroblasts 23. Given that collagen production by circulating cells is also dispensable for development of lung fibrosis 28, these data, when taken together, suggest that fibrocytes work mostly by paracrine mechanisms to induce fibrogenesis. We believe that their ability to migrate to the site of injury by virtue of their chemokine receptors makes them uniquely poised to deliver their pro-fibrotic paracrine factors where they are most effective at stimulating wound repair; yet in the setting of chronic lung disease, these fibrocytes may promote exaggerated wound repair and fibrosis.
It is likely that some of the paracrine effects of periostin-producing fibrocytes are due to periostin itself. We have previously shown that periostin can directly influence ECM deposition and migration of lung fibroblasts 33 (and Supplemental Figure 1). Another feature of myofibroblasts is their ability to resist apoptosis, likely due to upregulation of anti-apoptotic proteins such as X-linked inhibitor of apoptosis (XIAP) expression 48. Periostin may directly contribute to myofibroblast resistance to apoptosis via stimulating XIAP protein expression (Supplemental Figure 3). We recently demonstrated that blocking inhibitor of apoptosis family proteins using pharmacologic interventions attenuated bleomycin-induced fibrosis 48, 49. Thus, periostin likely contributes to the exacerbation of bleomycin-induced fibrosis at least in part via direct effects on fibroblasts.
Another possibility is that periostin signals back to the fibrocytes themselves to influence production of other profibrotic mediators. Figure 10 demonstrates that secretion of pro-fibrotic mediators by fibrocytes is blunted in the presence of a neutralizing antibody against periostin. This suggests that periostin plays an important autocrine signaling role to fibrocytes to promote pro-fibrotic mediator release. Additionally, we demonstrate that periostin production by fibrocytes promotes expression of CTGF and LOX post bleomycin-treatment (Figure 9B) and fibrocytes secrete less CTGF in the absence of periostin post-bleomycin treatment (Figure 9E). CTGF has previously been described as an activator of mesenchymal cells 50-52, while LOX is known to promote collagen crosslinking, likely leading to stiffer ECM and more robust matrix-dependent fibroblast activation 34, 53. CTGF can induce a variety of cytokines such as TGFβ and vascular endothelial growth factor (reviewed in 50), which in turn induce more expression of CTGF, indicating a positive feedback loop involving CTGF expression that can contribute to the progression of fibrosis. It is also likely that a similar feedback loop is occurring with periostin. Inhibiting this positive feedback loop would be ideal for understanding the mechanism by which CTGF from fibrocytes enables persistent lung fibrosis and promotes myofibroblast differentiation in the presence of periostin. However, there is not a specific receptor antagonist for CTGF due to the complex mechanism of CTGF signaling. Treatment of duplicate cultures of WT untreated fibroblasts with 10μM of an MRTF inhibitor (CCG 203971, shown to inhibit CTGF expression), in the presence of WT bleomycin fibrocyte supernatant resulted in a decrease in αSMA protein expression similar to what was seen with the periostin−/− supernatants demonstrating the importance of autocrine CTGF expression in fibroblasts (Supplemental Figure 4).
To further examine the effects of periostin on fibrocytes we designed experiments using integrin blocking antibodies. Our results suggest that periostin signals uniquely to fibrocytes via beta 1 integrin. A recent study by Reed and colleagues 54 suggested that inhibition of the alphaVbeta1 integrin using a small molecule inhibitor significantly attenuated bleomycin-induced pulmonary fibrosis. In that study the authors only looked at lung mesenchymal cells, which are a combination of fibroblasts and fibrocytes. Given our findings that beta1 integrins are critical for periostin signaling to fibrocytes, it will be important to determine if the alphav-beta1 heterodimers are responsible for periostin signaling to fibrocytes.
One very interesting experiment would have been to deplete fibrocytes during fibrosis; however this strategy proved technically unsuccessful. We generated a collagenIα2-Cre-DTR transgenic mouse and used these animals to generate bone-marrow chimeras in a CD45.2 recipient mouse so that the bone-marrow derived cells (but not lung resident cells) would be expressing CD45.1 and the collagenIα2-Cre-DTR transgene. These mice were then given bleomycin and treated with diphtheria toxin on days 11 and 18 to deplete the recruited collagen Iα2-Cre-DTR expressing cells. Despite excellent chimerism in the lung on day 21, surprisingly, there was a high percentage of recipient fibrocytes present post-bleomycin treatment that could not be eliminated by diphtheria toxin treatment (Supplemental Figure 5). Additionally, the low level of DTR expression driven by the collagen promoter in fibrocytes likely impaired the ability to deplete these cells with diphtheria toxin. We feel it is important for the lung community to know that this strategy to create a fibrocyte-knock-out mouse was not effective. Definitive proof of the role of fibrocytes in lung fibrosis awaits the characterization of a fibrocyte-specific promoter that can be used in future cellular depletion studies.
In conclusion, the current study showed that once recruited to the lungs, fibrocytes did not differentiate into fibroblasts directly. Rather, fibrocyte-derived periostin signals via beta 1 integrin to cause release of profibrotic mediators such as CTGF from the fibrocytes themselves to promote myofibroblast differentiation.
Supplementary Material
Acknowledgements
We thank Amanda Huber for technical assistance and comments on the manuscript.
Funding sources; NIH/NHLBI HL115618 (B.B.M), UNCF/MERCK Dissertation Fellowship (SLA), T32 Training grant AI007413 (SLA), NIH/NHLBI HL108904 (K.K.K)
References
- 1.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2(2):103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King TE, Jr., Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc. 2014;11(8):1176–1185. doi: 10.1513/AnnalsATS.201404-145OC. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr., et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12):963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. Pt 1. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou KM, Pataka A, Bouros D, Siafakas NM. Pathogenetic pathways and novel pharmacotherapeutic targets in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2007;20(5):453–461. doi: 10.1016/j.pupt.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380(9842):680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 7.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, et al. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. American journal of respiratory and critical care medicine. 2014;189(2):214–222. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 12.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3(4):364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43(2):161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188(7):820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19(8):1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, et al. Implicating exudate macrophages and Ly-6C(high) monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190(7):3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleaveland KR, Moore BB, Kim KK. Paracrine functions of fibrocytes to promote lung fibrosis. Expert Rev Respir Med. 2014;8(2):163–172. doi: 10.1586/17476348.2014.862154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 20.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94(12):6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 22.Moore BB, Kolb M. Fibrocytes and progression of fibrotic lung disease. Ready for showtime? Am J Respir Crit Care Med. 2014;190(12):1338–1339. doi: 10.1164/rccm.201411-2013ED. [DOI] [PubMed] [Google Scholar]
- 23.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madsen DH, Ingvarsen S, Jurgensen HJ, Melander MC, Kjoller L, Moyer A, et al. The non-phagocytic route of collagen uptake: a distinct degradation pathway. J Biol Chem. 2011;286(30):26996–27010. doi: 10.1074/jbc.M110.208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianchetti L, Barczyk M, Cardoso J, Schmidt M, Bellini A, Mattoli S. Extracellular matrix remodelling properties of human fibrocytes. J Cell Mol Med. 2012;16(3):483–495. doi: 10.1111/j.1582-4934.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleaveland KR, Velikoff M, Yang J, Agarwal M, Rippe RA, Moore BB, et al. Fibrocytes are not an essential source of type I collagen during lung fibrosis. J Immunol. 2014;193(10):5229–5239. doi: 10.4049/jimmunol.1400753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Kisseleva T. Bone marrow-derived fibrocytes contribute to liver fibrosis. Exp Biol Med (Maywood) 2015;240(6):691–700. doi: 10.1177/1535370215584933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Cong M, Park TJ, Scholten D, Brenner DA, Kisseleva T. Contribution of bone marrow-derived fibrocytes to liver fibrosis. Hepatobiliary Surg Nutr. 2015;4(1):34–47. doi: 10.3978/j.issn.2304-3881.2015.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia de Alba C, Buendia-Roldan I, Salgado A, Becerril C, Ramirez R, Gonzalez Y, et al. Fibrocytes contribute to inflammation and fibrosis in chronic hypersensitivity pneumonitis through paracrine effects. Am J Respir Crit Care Med. 2015;191(4):427–436. doi: 10.1164/rccm.201407-1334OC. [DOI] [PubMed] [Google Scholar]
- 32.Madala SK, Edukulla R, Schmidt S, Davidson C, Ikegami M, Hardie WD. Bone marrow-derived stromal cells are invasive and hyperproliferative and alter transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50(4):777–786. doi: 10.1165/rcmb.2013-0042OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2012;303(12):L1046–1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68(19):3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 36.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66(14):2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37(5):1119–1127. doi: 10.1183/09031936.00059810. [DOI] [PubMed] [Google Scholar]
- 38.Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, et al. Roles of Periostin in Respiratory Disorders. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201510-2032PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitterding S, Ngan R, et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167(8):4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 40.Ashley SL, Jegal Y, Moore TA, van Dyk LF, Laouar Y, Moore BB. gamma-Herpes virus-68, but not Pseudomonas aeruginosa or influenza A (H1N1), exacerbates established murine lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2014;307(3):L219–230. doi: 10.1152/ajplung.00300.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maatta A, Ekholm E, Penttinen RP. Effect of the 3'-untranslated region on the expression levels and mRNA stability of alpha 1(I) collagen gene. Biochimica et biophysica acta. 1995;1260(3):294–300. doi: 10.1016/0167-4781(94)00207-j. [DOI] [PubMed] [Google Scholar]
- 42.Bonner JC. Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis Tissue Repair. 2010;3:15. doi: 10.1186/1755-1536-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(6 Suppl):286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 45.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46(5):677–686. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171(1):380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 48.Ajayi IO, Sisson TH, Higgins PD, Booth AJ, Sagana RL, Huang SK, et al. X-linked inhibitor of apoptosis regulates lung fibroblast resistance to Fas-mediated apoptosis. Am J Respir Cell Mol Biol. 2013;49(1):86–95. doi: 10.1165/rcmb.2012-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashley SL, Sisson TH, Wheaton AK, Kim KK, Wilke CA, Ajayi IO, et al. Targeting Inhibitor of Apoptosis Proteins Protects from Bleomycin-induced Lung Fibrosis. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2015-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circulation research. 2010;106(11):1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 51.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Velikoff M, Canalis E, Horowitz JC, Kim KK. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. Am J Physiol Lung Cell Mol Physiol. 2014;306(8):L786–796. doi: 10.1152/ajplung.00243.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285(17):13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, et al. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7(288):288ra279. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.