Abstract
Hydrazide–hydrazone derivatives are present in many bioactive molecules and display a wide variety of biological activities, such as antibacterial, antitubercular, antifungal, anticancer, anti-inflammatory, anticonvulsant, antiviral, and antiprotozoal action. Therefore, many medicinal chemists synthesize various hydrazide–hydrazones and evaluate them for biological activities. Among biological properties of this class of compounds, antimicrobial activity is the most frequently encountered in scientific literature. This paper is focused on the overview of the literature findings of the last six years (2010–2016) covering the research on antimicrobial activity of hydrazide–hydrazone derivatives. This review may also serve as a useful guide for the development of new hydrazide–hydrazones as potential antimicrobial agents.
Keywords: Hydrazide–hydrazone, Antibacterial activity, Antitubercular activity, Antifungal activity, MIC
Introduction
Hydrazide–hydrazones constitute a class of organic compounds, which attracts the attention of medicinal chemists due to the fact that they contain azomethine group (–NH–N=CH–) connected with carbonyl group, which is responsible for their different pharmaceutical applications and makes possible the synthesis of different heterocyclic scaffolds (Rollas and Küçükgüzel 2007), like 1,3,4-oxadiazolines (Doğan et al. 1998), azetidin-2-ones (Kalsi et al. 2006), coumarins (Mohareb et al. 2011), 1,3-thiazolidin-4-ones (Popiołek et al. 2015, 2016a), and 1,3-benzothiazin-4-ones (Popiołek et al. 2016b).
The main route to synthesize hydrazide–hydrazone derivatives is the heating of appropriate hydrazides of carboxylic or heterocarboxylic acids with different aldehydes or ketones in various organic solvents like ethanol, methanol or butanol (Bala et al. 2013; Popiołek et al. 2015, 2016a; Popiołek and Biernasiuk 2016a, b). The molecular stucture of synthesized hydrazide–hydrazone derivatives can be easily confirmed by spectral methods. In the IR spectra, three characteristic bands are observed. The peaks around 1550 cm−1 correspond to the presence of C=N group. Carbonyl group (C=O) gives a characteristic band around 1650 cm−1, whereas the NH group can be found in the area around 3050 cm−1. In the 1H NMR spectra of hydrazide–hydrazones, we can observe a characteristic singlet signal in the range of δ 8–9 ppm, and the second singlet signal around δ 10–13 ppm, which correspond to =CH and NH groups, respectively. In the 13C NMR spectra, the signal for =CH group usually appears around δ 145–160 ppm, whereas in the range of δ 160–170 ppm we can observe the signal for carbonyl group (C=O) (Mohareb et al. 2011, Popiołek and Biernasiuk 2016a, b).
In recent years, a lot of biologically important hydrazide–hydrazone derivatives with a number of functional groups have been synthesized from many different carbonyl compounds. They were found to possess anticancer (Kumar et al. 2012; Yadagiri et al. 2014; Machakanur et al. 2012; Nasr et al. 2014), anti-inflammatory (Kumar et al. 2015), anticonvulsant (Çakır et al. 2001), antiviral (Şenkardes et al. 2016), and antiprotozoal (Siddiqui et al. 2014) activities. Among the biological properties of this class of compounds, the antimicrobial activity is the most frequently encountered one in scientific literature. Additionally, widely used chemotherapeutic agents such as nitrofurazone (McCalla et al. 1970), furazolidone (Chatterjee and Ghosh 1979; Ali 1983), and nitrofurantoin (McOsker and Fitzpatrick 1994; Munoz-Davila 2014) are known to contain typical hydrazide–hydrazone moiety or hydrazide-hydrazone moiety, in which the carbonyl group and nitrogen atom are included in the 1,3-oxazolidine-2-one or imidazolidine-2,4-dione ring (Fig. 1).
Fig. 1.
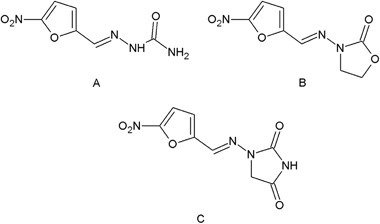
Chemical structures of medicines containing hydrazide–hydrazone moiety: nitrofurazone (a), furazolidone (b) and nitrofurantoin (c)
Encouraged by the above mentioned facts, this study is an attempt to collect the hydrazide–hydrazone derivatives, which can be considered as potential antimicrobial agents, reported in the literature in the years 2010–2016.
Antibacterial activity
Searching for effective and non-toxic chemotherapeutic agents is still a very important issue due to the increase of multi-resistant bacterial strains (Moellering 2011). The treatment of bacterial infections is especially challenging in patients with compromised immune systems or with other associated diseases (Coates et al. 2002). Some of currently used antibacterial agents are known to contain hydrazide–hydrazone moiety (McCalla et al. 1970; Chatterjee and Ghosh 1979; Ali 1983; McOsker and Fitzpatrick 1994; Munoz-Davila 2014) (Fig. 1). Due to this fact, it is reasonable to search for novel antibacterial agents among hydrazide–hydrazone derivatives.
The in vitro screening results of newly synthesized benzimidazole derivatives bearing hydrazone moiety revealed that some of the compounds had significant antimicrobial activity (Özkay et al. 2010). Among synthesized derivatives, compounds 1 and 2 had bactericidal effect on the growth of Salmonella typhimurium, two times better (compound 1: MIC = 6.25 μg/ml) or equal (compound 2: MIC = 12.5 μg/ml) to the activity of chloramphenicol (MIC = 12.5 μg/ml), which was used as positive control (Fig. 2). The activity of these compounds against other Gram-negative bacterial strains, like Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, or Pseudomonas aeruginosa, was good (MIC = 25–100 μg/ml). The activity against Gram-positive bacteria was assessed on four strains: Listeria monocytogenes, Staphylococcus aureus, Enterococcus faecalis, and Bacillus subtilis. The best activity equal to the activity of chloramphenicol was found against E. faecalis (MIC = 12.5 μg/ml). Against other Gram-positive bacterial strains, the activity of compounds 1 and 2 was good to moderate (MIC = 25–200 μg/ml) (Özkay et al. 2010).
Fig. 2.
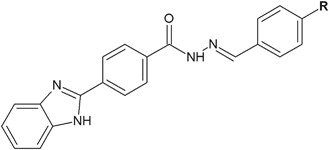
Benzimidazoles showing interesting activity against Salmonella typhimurium. R = Cl (1); Br (2)
In another research, Rasras et al. (2010) synthesized novel hydrazide–hydrazones of cholic acid and tested them for antibacterial activity against three Gram-negative and three Gram-positive bacterial strains (Fig. 3). The activity of five derivatives (3–7) against E. coli was strong (MIC = 3.91–7.81 µg/ml), but weaker than cefixime, which was used as control. Interestingly, none of the tested compounds had any activity against P. aeruginosa and Enterobacter aerogenes. In turn, the activity against Gram-positive bacterium Enterococcus faecalis for the compounds 3 and 6 (MIC = 1.96 µg/ml), 4 and 7 (MIC = 3.91 µg/ml), and 5 (MIC = 7.82 µg/ml) was almost 16, 8, and 4 times higher, respectively, than the activity of cefaclor (MIC = 31.25 µg/ml) and cefixime (MIC = 31.25 µg/ml). The MIC values for tested compounds against two other Gram-positive bacteria Staphyloccocus aureus and Bacillus megaterium were also good (MIC = 7.82–62.5 µg/ml) and comparable to chemotherapeutics used as controls (Table 1) (Rasras et al. 2010).
Fig. 3.
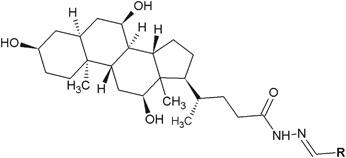
New derivatives of cholic acid with hydrazide–hydrazone moiety. R = 4-ClC6H4 (3); 4-BrC6H4 (4); 4-NO2C6H4 (5); 3-ClC6H4 (6); 4-Cl-3-NO2C6H3 (7)
Table 1.
In vitro antibacterial screening results of novel hydrazide-hydrazones of cholic acid
| No. of compound | R | MIC (μg/ml) | |||
|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | ||||
| E. coli | S. aureus | E. faecalis | B. megaterium | ||
| 3 | 4-ClC6H4 | 3.91 | 62.5 | 1.96 | 31.25 |
| 4 | 4-BrC6H4 | 3.91 | 62.5 | 3.91 | 7.82 |
| 5 | 4-NO2C6H4 | 7.81 | 31.25 | 7.82 | 31.25 |
| 6 | 3-ClC6H4 | 3.91 | 31.25 | 1.96 | 31.25 |
| 7 | 4-Cl-3-NO2C6H3 | 7.81 | 31.25 | 3.91 | 31.25 |
| Cefaclor | – | na | 31.25 | 31.25 | 31.25 |
| Cefixime | – | 1.96 | 31.25 | 31.25 | na |
na not active, – not applicable
Kumar et al. (2011a) evaluated novel hydrazide–hydrazones of 4-chlorophenylsulfonyl acid for in vitro antibacterial activity. The tested compounds (8, 9 and 10) showed moderate to mild antibacterial activity on the basis of the measurement of the zone of the inhibition growth (ZOI = 10–21 mm) against two Gram-positive (Bacillus subtillis and S. aureus) and two Gram-negative (E. coli and Salmonella typhi) bacterial strains, when compared to ampicillin sodium used as a control (ZOI = 20–24 mm) (Fig. 4). The best antibacterial activity was displayed by compound 8 (ZOI = 21 mm) against S. aureus (control ZOI = 22 mm) and compound 10 against E. coli, whose zone of inhibition was 21 mm, whereas for ampicillin it was 20 mm (Table 2.) (Kumar et al. 2011a).
Fig. 4.
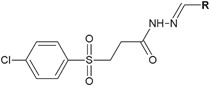
Hydrazide–hydrazones of 4-chlorophenylsulfonyl acid with antibacterial activity. R = 3-OCH3-C6H4 (8); 3-OH-C6H4 (9); 4-OH-3-OCH3-C6H3 (10)
Table 2.
Results of in vitro antibacterial assays of 4-chlorophenylsulfonyl acid derivatives
| No. of compound | R | Zone of inhibition growth (mm) | |||||
|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | Fungi | |||||
| B. subtilis | S. aureus | E. coli | S. typhi | C. albicans | A. niger | ||
| 8 | 3-OCH3-C6H4 | 19 | 21 | 10 | 10 | 31 | 20 |
| 9 | 3-OH-C6H4 | 17 | 20 | 11 | 14 | 26 | 21 |
| 10 | 4-OH-3-OCH3-C6H3 | 19 | 16 | 21 | 19 | 26 | 18 |
| Ampicillin sodium | – | 24 | 22 | 20 | 21 | – | – |
| Clotrimazole | – | – | – | – | – | 30 | 22 |
– not applicable
Among the isonicotinoyl hydrazide analogs synthesized by Moldovan et al. (2011), compound 11 appeared to have the strongest antibacterial activity. This hydrazide–hydrazone derivative possesses similar to ampicillin (ZOI = 16 mm) antibacterial activity (ZOI = 15 mm) against S. aureus (Fig. 5). The activity against other Gram-positive (Bacillus cereus) and Gram-negative bacteria (E. coli, Salmonella thyphimurium, Proteus mirabilis, and Salmonella enterica) was good to moderate (ZOI = 12–17 mm), when compared with chemotherapeutics used as controls: ampicillin, ciprofloxacin, gentamicin, and co-trimoxazole (Moldovan et al. 2011).
Fig. 5.
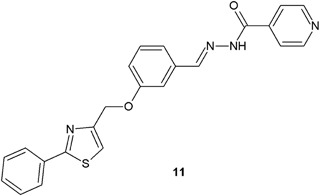
Isonicotinoyl hydrazide analog with significant activity against S. aureus
In another research, indoles containing hydrazide–hydrazone moiety (12, 13) synthesized by Shirinzadeh et al. (2011), showed good to moderate activity (MIC = 50–100 μg/ml) against the tested bacterial strains (Fig. 6).
Fig. 6.
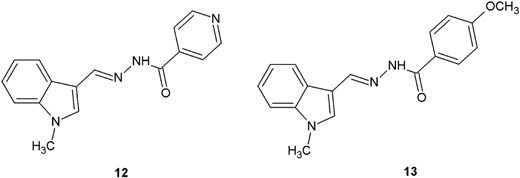
Indoles containing hydrazide–hydrazone moiety
Kumar et al. (2011b) synthesized and evaluated antimicrobial assays, as well as performed QSAR studies of twenty 3-ethoxy-4-hydroxybenzylidene/4-nitrobenzylidene hydrazides. Seven of the new compounds (14–20) showed the antibacterial activity higher than that of ciprofloxacin against S. aureus, B. subtilis, and E. coli (Fig. 7) (Kumar et al. 2011b).
Fig. 7.
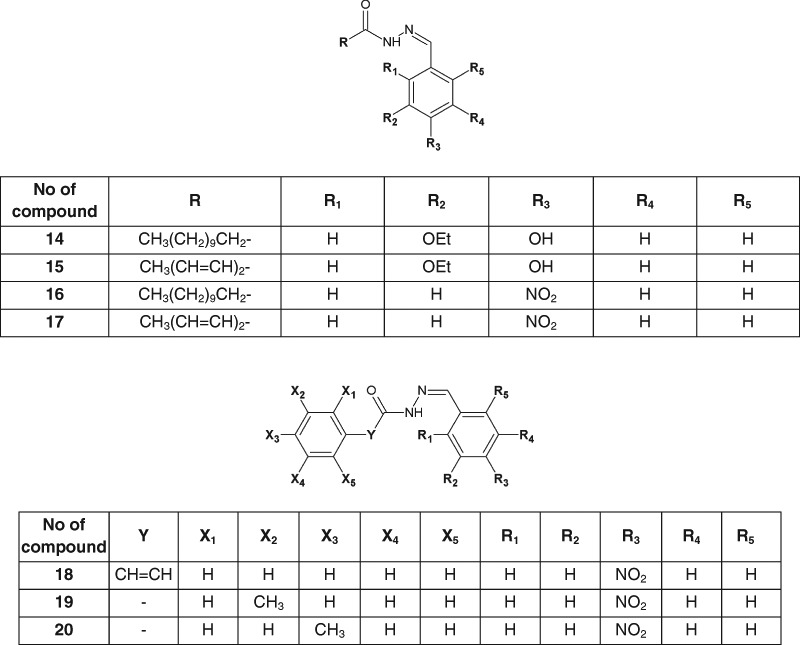
New 3-ethoxy-4-hydroxybenzylidene/4-nitrobenzylidene hydrazide–hydrazones with significant antibacterial activity
Two derivatives of 1,2-dihydropyrimidine (21, 22) synthesized by Al-Sharifi and Patel (2012) showed significant antibacterial activity against a panel of Gram-positive bacteria, including B. subtilis, S. aureus and, Micrococcus luteus, and Gram-negative bacteria, like E. coli and Pseudomonas picketti (Fig. 8) MIC values against these bacterial strains were in the range of 0.08–1 µg/ml, which can be assessed as very strong antibacterial activity. It is worth to underline that the lowest value of MIC was presented by compound 21 against M. luteus (MIC = 0.08 µg/ml) (Al-Sharifi and Patel 2012).
Fig. 8.
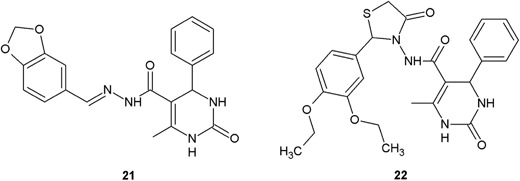
1,2-Dihydropyrimidine derivatives with antibacterial activity
Xaiver et al. (2012) synthesized novel hydrazide–hydrazones as a result of condensation of 2,4-diaryl-3-azabicyclo[3.3.1]nonan-9-ones with 4-aminobenzoic acid hydrazide (Fig. 9). The obtained compounds were tested for in vitro antibacterial activity against eight bacterial strains (Gram negative bacteria: S. thypimurium, E. coli, Vibrio cholerae, S. typhi, P. aeruginosa, and K. pneumonia, and Gram-positive bacteria: B. subtilis and S. aureus). Among the synthesized derivatives, it is worth to mention two compounds 23, 24, which showed good to moderate activity against all bacterial strains (MIC = 50–200 μg/ml) (Table 3) (Xaiver et al. 2012).
Fig. 9.
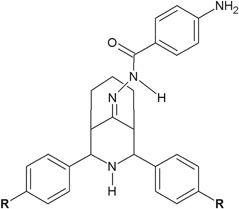
Novel hydrazide–hydrazones obtained from 4-aminobenzoic acid hydrazide. R = Br (23); Cl (24); OCH3 (25)
Table 3.
In vitro antibacterial screening results of 4-aminobenzoic acid hydrazide derivatives
| No. of compound | R | MIC (μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | ||||||||
| S. typhimurium | E. coli | V. cholerae | S. typhi | P. aeruginosa | K. pneumoniae | B. subtilis | S. aureus | ||
| 23 | Br | 200 | 100 | 200 | 50 | 50 | 200 | 200 | 50 |
| 24 | Cl | 200 | 50 | 100 | 200 | 100 | 200 | 50 | 50 |
| Streptomycin | – | 25 | 50 | 50 | 25 | 50 | 20 | 12.5 | 25 |
– not applicable
In another research, Kodisundaram et al. (2013) obtained a series of heterobicyclic methylthiadiazole hydrazones and investigated their activity in vitro against Gram-positive and Gram-negative bacteria. Two of the synthesized compounds, 26 and 27, showed interesting activity, especially against B. subtilis (Fig. 10). Their activity against this bacterium (MIC = 6.25 μg/ml) was two times higher than the activity of streptomycin (MIC = 12.5 μg/ml), which was used as positive control. The activity of these compounds against S. aureus was good (MIC = 25–50 μg/ml). As for the Gram-negative bacteria, the synthesized compounds (26, 27) showed two times better activity (MIC = 6.25 μg/ml) than streptomycin (MIC = 12.5 μg/ml) against K. peneumoniae, whereas against P. aeruginosa, compound 27 showed the activity equal to streptomycin (MIC = 12.5 μg/ml). The activity of the inhibition of the growth of E. coli was also two times higher (MIC = 12.5 μg/ml) in comparison with the control (MIC = 25 μg/ml) (Kodisundaram et al. 2013).
Fig. 10.
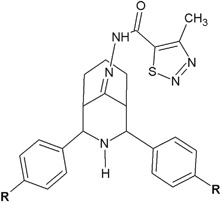
Methylthiadiazoles with significant activity against Bacillus subtilis. R = F (26); Br (27)
Pieczonka et al. (2013) synthesized a series of new imidazole derivatives containing hydrazide–hydrazone moiety and evaluated them for antibacterial activity against a panel of bacterial strains. Two of synthesized compounds (28 and 29) showed the best activity towards Staphylococcus epidermidis ATCC 12228 (MIC = 4 μg/ml) (Fig. 11). The activity of these compounds against this bacterium was two times higher than the activity of nitrofurantoin (MIC = 8 μg/ml). The MICs against the other bacterial strains like, S. aureus ATCC 6538, S. aureus ATCC 29213, and S. aureus ATCC 29213, were also low (MIC = 11–27 μg/ml) (Pieczonka et al. 2013).
Fig. 11.
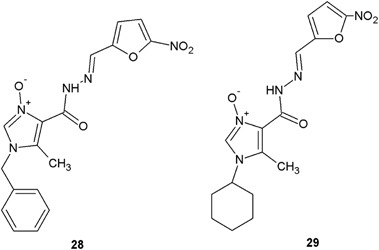
New imidazole derivatives containing hydrazide–hydrazone moiety
The infection caused by P. aeruginosa constitutes a severe problem, especially for the patients with weak immune system. In response to this, hydrazide–hydrazones of 2,5-difluorobenzoic acid were synthesized by Narisetty et al. (2013) (Fig. 12). Among a series of new derivatives, compound 30 showed better activity (ZOI = 21 mm) against P. aeruginosa MTCC 424 than ampicillin (ZOI = 20 mm). Two of other synthesized compounds (31, 32) showed also very good activity (ZOI = 21–24 mm) against E. coli MTCC 443, S. aureus MTCC 96 and Streptococcus pyogenes MTCC 442. The activity of these compounds was higher than or comparable to the activity of ampicillin (ZOI = 19–22 mm) (Table 4) (Narisetty et al. 2013).
Fig. 12.
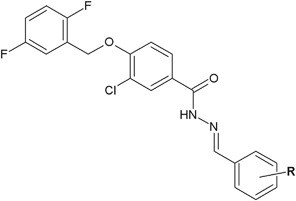
New derivatives of 2,5-difluorobenzoic acid with hydrazide–hydrazone moiety. R = 4-CF3 (30); 2-CF3 (31); 2,4-diF (32)
Table 4.
Measured zones of the inhibition growth of hydrazide-hydrazones of 2,5-difluorobenzoic acid
| No. of compound | R | Zone of inhibition growth (mm) | |||
|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | ||||
| E. coli MTCC 443 | P. aeruginosa MTCC 424 | S. aureus MTCC 96 | S. pyogenes MTCC 442 | ||
| 30 | 4-CF3 | 24 | 21 | 22 | 21 |
| 31 | 2-CF3 | 23 | 22 | 24 | 21 |
| 32 | 2,4-diF | 24 | 21 | 23 | 21 |
| Ampicillin | – | 22 | 20 | 21 | 19 |
Morjan et al. (2014) synthesized novel hydrazide–hydrazones of nicotinic acid by the condensation reaction of nicotinic acid hydrazide with various ketones (Fig. 13). The synthesized derivatives were screened in vitro for antibacterial activity against Gram-positive and Gram-negative bacteria. The results of antimicrobial assay revealed that the synthesized compounds had interesting antibacterial activity against Gram-negative strains. Especially compounds 33 and 34 showed very strong activity against P. aeruginosa (MIC = 0.22 and 0.19 μg/ml, respectively). The activity of these compounds against K. pneumoniae was also high (33: MIC = 3.12 μg/ml, 34: MIC = 14.00 μg/ml). The inhibitory potency against Gram-positive bacteria Streptococcus pneumoniae (33: MIC = 3.12 μg/ml) and against S. aureus (34: MIC = 7.03 μg/ml) was also very significant (Morjan et al. 2014).
Fig. 13.
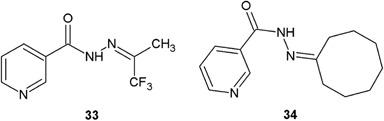
Novel hydrazide–hydrazones of nicotinic acid
Satyanarayana et al. (2014) synthesized novel hydrazide–hydrazone derivatives of 4-(4-chlorophenyl)cyclohexanecarboxylic acid as potential antibacterial agents (Fig. 14). Three of the obtained compounds (35, 36 and 37) showed good antibacterial activity (MIC = 32–64 μg/ml) against a panel of four bacterial strains, including: S. aureus, B. subtilis, E. coli, and P. aeruginosa (Table 5). Unfortunately, the MIC values of the synthesized compounds were higher than the activity of the antibiotic used as control—ciprofloxacin (MIC = 5 μg/ml) (Satyanarayana et al. 2014).
Fig. 14.
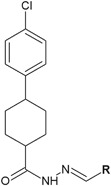
Novel hydrazide–hydrazone derivatives of 4-(4-chlorophenyl)cyclohexanecarboxylic acid as potential antibacterial agents. R = 2,4-diF-C6H3 (35); 2,6-diF-C6H3 (36); 3,4-diF-C6H3 (37)
Table 5.
Results of in vitro screening of novel hydrazide-hydrazone derivatives of 4-(4-chlorophenyl)cyclohexanecarboxylic acid
| No. of compound | R | MIC (μg/ml) | |||
|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | ||||
| S. aureus | B. subtilis | E. coli | P. aeruginosa | ||
| 35 | 2,4-diF-C6H3 | 64 | 64 | 32 | 32 |
| 36 | 2,6-diF-C6H3 | 64 | 64 | 32 | 32 |
| 37 | 3,4-diF-C6H3 | 64 | 64 | 32 | 32 |
| Ciprofloxacin | – | 5 | 5 | 5 | 5 |
– not applicable
2-(2,3-Dihydrobenzofuran-5-yl)acetic acid was used as a starting material for the synthesis of new hydrazide–hydrazone derivatives by Kaki et al. (2014). Among a series of synthesized derivatives, two compounds (38 and 39) possessed interesting antibacterial activity measured by the diameter of the zone of the inhibition growth against two Gram-negative (E. coli and P. aeruginosa) and two Gram-positive (S. aureus and S. pyogenes) bacterial strains (Fig. 15). Compounds (38 and 39) according to the antimicrobial activity assays, had similar values of inhibition zone (ZOI = 19–22 mm) as ampicillin, which was used as positive control (ZOI = 19–22 mm) (Kaki et al. 2014).
Fig. 15.
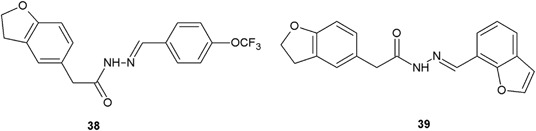
2-(2,3-Dihydrobenzofuran-5-yl)acetic acid derivatives with interesting antibacterial properties
In the research conducted by Rambabu et al. (2015), new anacardic acid hydrazone derivatives were synthesized and subjected to antibacterial screening. The obtained compounds were tested against two Gram-negative (P. aeruginosa and E. coli) and two Gram-positive (S. aureus and S. pyogenes) bacterial strains (Fig. 16). The antibacterial activity was assessed on the basis of the measurement of the zone of inhibition growth. Antimicrobial assays revealed that three of the obtained derivatives (40, 41 and 42) had better antibacterial activity (ZOI = 20–24 mm) than the activity of ampicillin, because their zones of inhibition were larger than the control antibiotic (ZOI = 18–20 mm) (Table 6) (Rambabu et al. 2015).
Fig. 16.
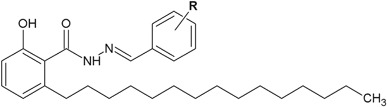
New anacardic acid hydrazide derivatives. R = 3,4-diOCH3 (40); 3,4,5-triOCH3 (41); 4-SO2CH3 (42)
Table 6.
Zone of inhibition growth of tested anacardic acid hydrazide derivatives
| No. of compound | R | Zone of inhibition growth (mm) | |||||
|---|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | Fungi | |||||
| P. aeruginosa | E. coli | S. aureus | S. pyogenes | C. albicans | A. niger | ||
| 40 | 3,4-diOCH3 | 24 | 23 | 21 | 22 | 11 | 12 |
| 41 | 3,4,5-triOCH3 | 22 | 22 | 20 | 21 | 18 | 23 |
| 42 | 4-SO2CH3 | 23 | 22 | 20 | 20 | 18 | 20 |
| Ampicillin | – | 20 | 20 | 18 | 19 | – | – |
| Griseofulvin | – | – | – | – | – | 24 | 28 |
– not applicable
Tejeswara et al. (2016) synthesized a series of novel pefloxacin derivatives and tested them for their in vitro antibacterial activity against four Gram-positive bacterial strains and two Gram-negative bacterial strains by the agar well diffusion method (Fig. 17). On the basis of the measurement of the zone of inhibition growth, antibacterial activity assay revealed that compounds 46 and 47 (ZOI = 24 and 26 mm, respectively) had higher antibacterial activity compared to ciprofloxacin (ZOI = 23 mm) against Bacillus sphaericus. Against other Bacillus strain, B. subtilis compound 47 had a larger zone of inhibition (ZOI = 23 mm) than the reference substance (ZOI = 22 mm). Compound 47 also displayed higher activity against Pseudomonas aeruginosa (ZOI = 25 mm) than ciprofloxacin (ZOI = 21 mm) (Table 7) (Tejeswara et al. 2016).
Fig. 17.
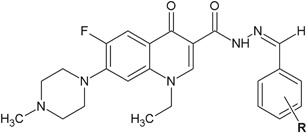
Novel pefloxacin derivatives with interesting antibacterial activity. R = 2,6-diCl (43); 4-NO2 (44); 3,4,5-triOCH3 (45); 5-Br-2-OH (46); 2,5-diOCH3 (47); 3-OH (48)
Table 7.
Measured zones of inhibition growth of pefloxacin derivatives
| Compound | Zone of inhibition growth (mm) | |||||
|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | |||||
| S. aureus | B. sphaericus | B. subtilis | M. luteus | P. aeruginosa | P. vulgaris | |
| 43 | 20 | 15 | 16 | 30 | 15 | 10 |
| 44 | 18 | 16 | 15 | 20 | 14 | 11 |
| 45 | 19 | 22 | 17 | 15 | 21 | 12 |
| 46 | 19 | 24 | 15 | 12 | 14 | 16 |
| 47 | 18 | 26 | 23 | 15 | 25 | 17 |
| 48 | 21 | 21 | 16 | 14 | 14 | 15 |
| Ciprofloxacin | 20 | 23 | 22 | 18 | 21 | 17 |
Novel 1,2,3-triazole carbohydrazide derivatives synthesized by Sreedhar et al. (2016) were tested against four bacterial strains (Fig. 18). The measured zones of growth inhibition for the obtained compounds were similar to those of ciprofoxacin used as control. In the case of compound 50, its zone of inhibition against S. aureus MTCC 96 (ZOI = 23 mm) was larger than for the reference compound (ZOI = 22 mm). A similar situation appeared for compounds 49 and 52, their zones of inhibition (ZOI = 23 mm) were larger than for ciprofloxacin (ZOI = 22 mm) against S. pyogenes MTCC 442 (Table 8) (Sreedhar et al. 2016).
Fig. 18.
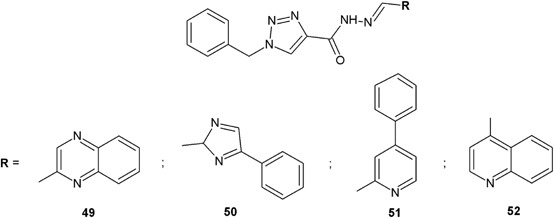
N-substituted-1-benzyl-1H-1,2,3-triazole-carbohydrazide derivatives with potential antibacterial activity
Table 8.
The values of the zone of the inhibition growth of 1,2,3-triazole-carbohydrazide derivatives
| No. of compound | Zone of inhibition growth (mm) | |||
|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | |||
| S. aureus MTCC 96 | S. pyogenes MTCC 442 | E. coli MTCC 443 | P. aeruginosa MTCC 424 | |
| 49 | 21 | 23 | 27 | 26 |
| 50 | 23 | 22 | 26 | 25 |
| 51 | 22 | 21 | 25 | 26 |
| 52 | 22 | 23 | 24 | 23 |
| Ciprofloxacin | 22 | 22 | 28 | 27 |
Dommati et al. (2016) obtained novel benzohydrazide derivatives and evaluated them for in vitro antibacterial activity (Fig. 19). The highest activity, but weaker than control streptomicin, was shown by compounds 53 and 54 against Gram-negative bacteria: E. coli MTCC 2692 and P. aeruginosa MTCC 2453 (ZOI = 5–13 mm), and Gram-positive bacteria: S. aureus MTCC 902 and B. subtilis MTCC 441 (ZOI = 18–21 mm) (Dommati et al. 2016).
Fig. 19.
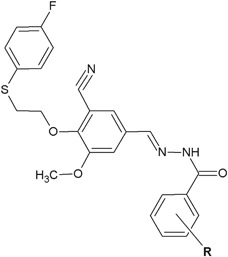
Benzohydrazide derivatives with interesting antibacterial activity. R = OH (53); 3,4,5-triOCH3 (54)
Antitubercular activity
Despite the fact that tuberculosis is a curable and treatable disease, it still remains a major cause of death and illness (WHO 2010), which confirms that seeking and developing new antitubercular agents is needed. A survey of scientific literature reveals that several hydrazide–hydrazones synthesized during last 6 years possess interesting antitubercular activity (Unissa et al. 2016). Additionally, it is worth to mention that according to the most recent article published by John et al. (2016) the 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone may act as a novel inhibitor of methionine aminopeptidases from Mycobacterium tuberculosis.
Among the series of hydrazide–hydrazones synthesized by Pavan et al. (2010), the four compounds (55, 56, 57, 58) showed especially high activity towards M. tuberculosis (MIC = 1.5–12.5 μg/ml) (Fig. 20) (Pavan et al. 2010).
Fig. 20.
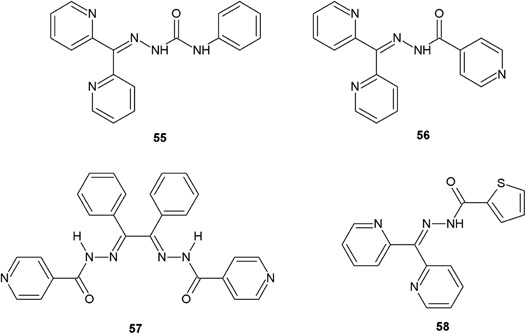
Hydrazide–hydrazones with interesting antitubercular activity
Sriram et al. (2010) tested for antitubercular activity a new series of 5-nitro-2-furoic acid hydrazones. New compounds were obtained by the condensation reaction of 5-nitro-2-furoic hydrazide with appropriate aldehydes and ketones (Fig. 21). In vitro screening of the obtained compounds revealed potent antitubercular activity of synthesized derivatives (59–62). Especially compounds 59 and 60 showed very good activity (MIC = 4.76 and 2.65 µM, respectively) in comparison with isoniazid (MIC = 0.72 µM) and rifampicin (MIC = 0.48 µM) (Sriram et al. 2010).
Fig. 21.
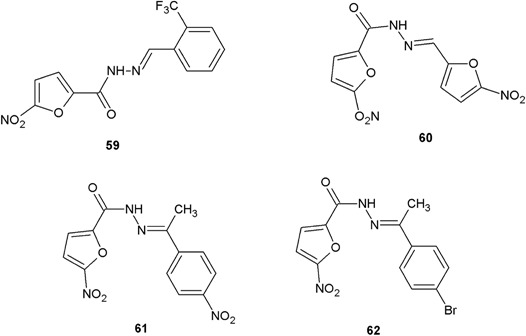
New 5-nitro-2-furoic acid hydrazide derivatives with potent in vitro antitubercular activity
Novel thioureas containing hydrazide–hydrazone moiety were synthesized and tested against M. tuberculosis H37Rv (ATCC 27294) by Çıkla et al. (2010) (Fig. 22). In the antitubercular assays, these compounds showed lower activity (MIC > 6.25 μg/ml) than rifampicin used as a reference substance (Çıkla et al. 2010).
Fig. 22.
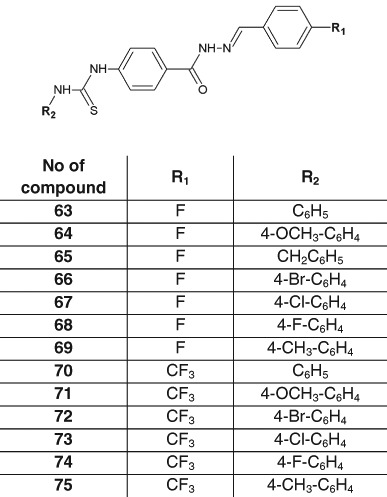
Novel thioureas with activity against Mycobacterium tuberculosis H37Rv
Coelho et al.’s study (2012) evaluated the in vitro antibacterial activity of 23 hydrazide–hydrazones obtained from isonicotinic hydrazide against one M. tuberculosis isoniazid-susceptible strain and three isoniazid-resistant M. tuberculosis clinical isolates (Fig. 23). Interestingly, 13 derivatives showed good activity against isoniazid-resistant strains. The best activity (better than that of isoniazid against isoniazid-susceptible strain) was shown by compound 76 (MIC = 0.98 μg/ml) (Coelho et al. 2012).
Fig. 23.
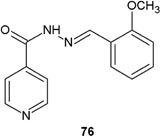
Hydrazide–hydrazone obtained from isonicotinic hydrazide with in vitro activity against M. tuberculosis isoniazid-susceptible strain
Cihan-Üstündağ and Çapan (2012) evaluated a series of indole derivatives containing hydrazide–hydrazone scaffold for in vitro antitubercular activity (Fig. 24). Antimycobacterial activity was tested against M. tuberculosis H37Rv ATCC 27294 with the use of rifampicine as a control. Unfortunately the synthesized compounds showed weaker activity (MIC > 6.25 μg/ml) than the reference substance (MIC = 0.125 μg/ml) (Cihan-Üstündağ and Çapan 2012).
Fig. 24.
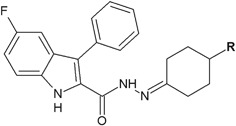
Indole derivatives containing hydrazide–hydrazone scaffold with antitubercular activity. R = H (77); CH3 (78); C2H5 (79); C3H7 (80); C6H5 (81)
Velezheva et al. (2016) designed and synthesized a series indole-pyridine derived hydrazide–hydrazones and evaluated them against two M. tuberculosis strains (H37Rv and CN-40) (Fig. 25). Based on the obtained results, hydrazide–hydrazone derivative 85 appeared to be the most potent among examined compounds (MIC=0.05 µg/ml) against the M. tuberculosis H37Rv strain. Its activity was equal to that of isoniazid used as positive control in this assay. Besides, this compound 85, unlike isoniazid, showed significant activity against isoniazid-resistant M. tuberculosis CN-40 strain. Other synthesized derivatives also displayed high antitubercular activity (Table 9) (Velezheva et al. 2016).
Fig. 25.
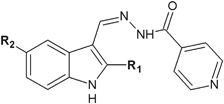
Novel indole derivatives with significant antitubercular activity. 82: R 1 = COOH, R 2 = H; 83: R 1 = COOC2H5, R 2 = H; 84: R 1 = COOC2H5, R 2 = Cl; 85: R 1 = COOC2H5, R 2 = CH3
Table 9.
MIC values of indole derivatives with hydrazide–hydrazone moieties
| No. of compound | MIC (μg/ml) | |
|---|---|---|
| M. tuberculosis H37Rv | M. tuberculosis CN-40 | |
| 82 | 0.1 | 10 |
| 83 | 0.15 | 2–5 |
| 84 | 0.1 | 2–5 |
| 85 | 0.05 | 2–5 |
Antifungal activity
Fungal infections still remain a serious problem, even though there are many available medicines on the market (Lewis 2011). In this section, I will present several examples of hydrazide–hydrazones which possess significant antifungal activity.
Benzimidazole derivatives bearing hydrazone moiety (Özkay et al. 2010) were also screened for antifungal activity against three species of yeasts: Candida albicans, Candida glabrata, and Candida tropicalis. The activity of compounds 1 and 2 against these fungi was good (MIC = 50–100 μg/ml). In the case of C. tropicalis, the MICs of the synthesized compounds (MIC = 50 μg/ml) were equal to the MIC of ketoconazole used as control (MIC = 50 μg/ml) (Özkay et al. 2010).
Similar research was performed by Kumar et al. (2011a). New hydrazide–hydrazones of 4-chlorophenylsulfonyl acid were synthesized and tested for antifungal activity on the basis of the measurement of the zone of inhibition growth against C. albicans and Aspergillus niger. Three of the synthesized compounds (8, 9 and 10) showed promising antifungal activity compared with the clotrimazole, which was used as positive control. In the case of compound 8, the antifungal activity (ZOI = 31 mm) was greater than the activity of clotrimazole (ZOI = 30 mm) against C. albicans (Kumar et al. 2011a).
The compounds synthesized by Shirinzadeh et al. (2011) (12, 13) were subjected to antifungal assays against C. albicans. The revealed antifungal activity was strong for both compounds (12: MIC = 6.25 μg/ml and 13: 12.5 μg/ml, respectively), but weaker than for fluconazole (MIC = 0.78 μg/ml) (Shirinzadeh et al. 2011).
New hydrazide–hydrazone derivatives (23, 24 and 25) (Xaiver et al. 2012) were tested against a panel of five fungi strains including C. albicans, Fusarium oxysporum, Aspergillus flavus, A. niger, and Cryptococcus neoformans. The measured MIC parameters against these fungi (MIC = 50–200 μg/ml) were much higher than the activity of amphotericin B used as control substance (MIC = 25–50 μg/ml), and the activity of these compounds can only be assessed as good against A. niger (MIC = 50–100 μg/ml), and as moderate against other fungi (MIC = 100–200 μg/ml) (Table 10) (Xaiver et al. 2012).
Table 10.
In vitro antifungal data of hydrazide–hydrazone derivatives of 4-aminobenzoic acid hydrazide
| No. of compound | R | MIC (μg/ml) | ||||
|---|---|---|---|---|---|---|
| C. albicans | F. oxysporum | A. flavus | A. niger | C. neoformans | ||
| 23 | Br | 200 | 100 | 100 | 50 | 100 |
| 24 | Cl | 100 | 200 | 100 | 50 | 200 |
| 25 | OCH3 | 200 | 100 | 50 | 100 | 200 |
| Amphotericin B | – | 25 | 25 | 50 | 50 | 25 |
– not applicable
Novel heterobicyclic methylthiadiazole hydrazones synthesized by Kodisundaram et al. (2013) were also tested for antifungal activity against Aspergillus spp. and C. albicans. The activity of compounds 26 and 27 against A. flavus and A. niger was two times higher (MIC = 12.5 μg/ml) than the antifungal activity of fluconazole (MIC = 25 μg/ml). The MICs against C. albicans for synthesized compounds were also two times higher (MIC = 6.25 μg/ml) than the MIC value of fluconazole (12.5 μg/ml) (Kodisundaram et al. 2013).
New anacardic acid hydrazone derivatives were subjected to in vitro antifungal assays against C. albicans and A. niger (Rambabu et al. 2015). Three of the synthesized compounds (40, 41 and 42) showed good to moderate antifungal activity (ZOI = 11–20 mm) based on the measurement of the zone of inhibition growth in comparison with the gryseofulvin used as control (ZOI = 24–28 mm) (Rambabu et al. 2015).
Pyrrolidinones with hydrazone moieties synthesized by Rutkauskas et al. (2013) showed strong (86) or good (87) activity against Candida tenuis (86: MIC = 15.6 µg/ml and 87: 31.2 µg/ml, respectively) (Fig. 26), whereas the activity against A. niger was lower (MIC = 500 µg/ml) (Rutkauskas et al. 2013).
Fig. 26.
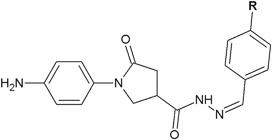
Pyrrolidinones with hydrazone moieties with antifungal activity. R=N(CH3)2 (86); Cl (87)
In the case of compounds 88 and 89 the antifungal activity against C. tenuis and A. niger was much better (Fig. 27). Compounds 88 and 89 against C. tenuis showed the MIC values of 1.9 and 0.9 µg/ml, respectively. Compound 88 displayed strong activity (MIC = 3.9 µg/ml) against A. niger and compound 89 showed moderate activity (MIC = 125 µg/ml) (Rutkauskas et al. 2013).
Fig. 27.
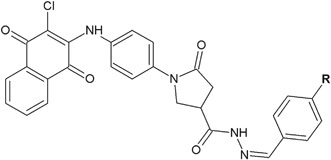
Hydrazide–hydrazones with significant antifungal activity against Candida tenuis and Aspergillus niger. R=N(CH3)2 (88); Cl (89)
Hydrazide–hydrazones of benzoic acid synthesized by Backes et al. (2014) showed interesting antifungal activity against Candida spp. (Fig. 28). The activity of compounds 90–93 was very strong (MIC80 = 0.5–4 µg/ml) against C. albicans. C. glabrata was also very sensitive to the obtained benzoic acid derivatives (MIC80 = 0.5–1.0 µg/ml) (Table 11) (Backes et al. 2014).
Fig. 28.
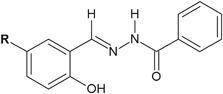
Hydrazide–hydrazones of benzoic acid with antifungal activity. R = H (90); CH3 (91); OCH3 (92); Cl (93)
Table 11.
MIC80 values of hydrazide–hydrazones of benzoic acid
| No. of compound | R | MIC80 (μg/ml) | |
|---|---|---|---|
| C. albicans | C. glabrata | ||
| 90 | H | 2 | 1 |
| 91 | CH3 | 1 | 1 |
| 92 | CH3O | 4 | 0.5 |
| 93 | Cl | 0.5 | 0.5 |
In the case of hydrazide–hydrazones of 4-nitrobenzoic acid, the antifungal activity against the above mentioned fungi strains was even better (Fig. 29). The MIC80 parameters for compounds 94 and 95 were 0.5 µg/ml against C. albicans, whereas for compound 95 it was even 0.125 µg/ml against C. glabrata. The MIC80 value for compound 94 against C. glabrata was also very low (MIC80 = 0.5 µg/ml) (Backes et al. 2014).
Fig. 29.
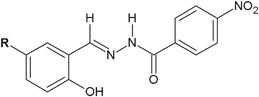
Hydrazide–hydrazones of 4-nitrobenzoic acid with the antifungal activity. R = H (94); CH3 (95)
Additionally, compounds 96 and 97 as hydrazide–hydrazone derivatives of 4-hydroxybenzoic acid displayed activity only against C. glabrata (Fig. 30). The MIC80 values against this fungus were 4 and 1 µg/ml for compounds 96 and 97, respectively (Backes et al. 2014).
Fig. 30.
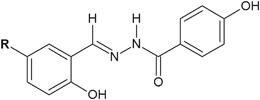
Hydrazide–hydrazone derivatives of 4-hydroxybenzoic acid with activity against Candida glabrata. R = H (96); CH3 (97)
Conclusions
In conclusion, this paper gives an overview of the antibacterial, antitubercular and antifungal properties of hydrazide-hydrazone derivatives. As presented in this study hydrazide–hydrazone moiety may be found and incorporated in various bioactive molecules. Thus this paper appears to be important for further development of hydrazide–hydrazones as potential antimicrobial agents.
Abbreviations
- MIC
Minimal inhibitory concentration
- ZOI
Zone of inhibition
- A. flavus
Aspergillus flavus
- A. niger
Aspergillus niger
- B. megaterium
Bacillus megaterium
- B. sphaericus
Bacillus sphaericus
- B. subtilis
Bacillus subtilis
- C. albicans
Candida albicans
- C. glabrata
Candida glabrata
- C. tropicalis
Candida tropicalis
- C. tenuis
Candida tenuis
- C. neoformans
Cryptococcus neoformans
- E. aerogenes
Enterobacter aerogenes
- E. faecalis
Enterococcus faecalis
- E. coli
Escherichia coli
- F. oxysporum
Fusarium oxysporum
- K. pneumoniae
Klebsiella pneumoniae
- L. monocytogenes
Listeria monocytogenes
- M. luteus
Micrococcus luteus
- M. tuberculosis
Mycobacterium tuberculosis
- P. mirabilis
Proteus mirabilis
- P. vulgaris
Proteus vulgaris
- P. aeruginosa
Pseudomonas aeruginosa
- P. picketti
Pseudomonas picketti
- S. enterica
Salmonella enterica
- S. typhi
Salmonella typhi
- S. typhimurium
Salmonella typhimurium
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- S. pneumoniae
Streptococcus pneumoniae
- V. cholerae
Vibrio cholerae
Compliance with ethical standards
Conflict of interest
The author declares that he has no competing interests.
References
- Ali BH. Some pharmacological and toxicological properties of furazolidone. Vet Res Commun. 1983;6:1–11. doi: 10.1007/BF02214890. [DOI] [PubMed] [Google Scholar]
- Al-Sharifi H, Patel HS. Synthesis, spectral investigation and biological evaluation of novel hydrazones derivative of substituted 1,2-dihydropyrimidine ring. Der Pharm Sin. 2012;3(3):305–311. [Google Scholar]
- Backes GL, Neumann DM, Jursic BS. Synthesis and anifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg Med Chem. 2014;22:4629–4636. doi: 10.1016/j.bmc.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Bala S, Uppal G, Kajal A, Kamboj S, Sharma V. Hydrazones as promising lead with diversity in bioactivity-therapeutic potential in present scenario. Int J Pharm Sci Rev Res. 2013;18(1):65–74. [Google Scholar]
- Chatterjee SN, Ghosh S. Mechanism of action of furazolidone: inter-strand cross-linking in DNA & liquid holding recovery of Vibrio cholerae cells. Indian J Biochem Biophys. 1979;16(3):125–130. [PubMed] [Google Scholar]
- Cihan-Üstündağ G, Çapan G. Synthesis and evaluation of functionalized indoles as antimycobacterial and anticancer agents. Mol Divers. 2012;16:525–539. doi: 10.1007/s11030-012-9385-y. [DOI] [PubMed] [Google Scholar]
- Coates A, Hu Y, Bax R, Page C. The future challenges facing the development of new antimicrobial drugs. Nat Rev Drug Discov. 2002;1:895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- Coelho TS, Cantos JB, Bispo MLF, Gonçalves RSB, Lima CHS, da Silva PEA, Souza MV. In vitro anti-mycobacterial activity of (E)-N′-(monosubstituted-benzylidene) isonicotinohydrazide derivatives against isoniazid-resistant strains. Infect Dis Rep. 2012;4:e13. doi: 10.4081/idr.2012.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakır B, Dağ Ö, Yıldırım E, Erol K, Şahin MF. Synthesis and anticonvulsant activity of some hydrazones of 2-[(3H)-oxobenzoxazolin-3-yl-aceto]hydrazide. J Fac Pharm Gazi. 2001;18:99–106. [Google Scholar]
- Çıkla P, Küçükgüzel SG, Küçükgüzel İ, Rollas S, De Clercq E, Pannecougue Ch, Andrei G, Snoeck R, Şahin F, Bayrak ÖF. Synthesis and evaluation of antiviral, antitubercular and anticancer activities of some novel thioureas derived from 4-aminobenzohydrazide hydrazones. Marmara Pharm J. 2010;14:13–20. [Google Scholar]
- Doğan HN, Duran A, Rollas S, Şener G, Armutak Y, Keyer-Uysal M. Synthesis and structure elucidation of some new hydrazones and oxadiazolines anticonvulsant activities of 2-(3-acetyloxy-2-naphtyl)-4-acetyl-5-substituted-1,3,4-oxadiazolines. Med Sci Res. 1998;26:755–758. [Google Scholar]
- Dommati L, Satyanarayana B, Hela PG, Ram B, Srinivas G. Synthesis and antibacterial activity of (E)-N′-[4-{2-(4-fluorophenylthio)ethoxy}-3-cyano-5-methoxybenzylidene]-substituted benzohydrazide derivatives. Asian J Chem. 2016;28(7):1584–1588. doi: 10.14233/ajchem.2016.19776. [DOI] [Google Scholar]
- John SF, Aniemeke E, Ha NP, Chong CR, Gu P, Zhou J, Zhang Y, Graviss E, Liu JO, Olaleye OA (2016) Characterization of 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazine as a novel inhibitor of methionine aminopeptidases from Mycobacterium tuberculosis. Tuberculosis. doi:10.1016/j.tube.2016.09.025 [DOI] [PubMed]
- Kaki GR, Sreenivasulu B, Islam A, Nageshwar D, Korupolu R, Rao BV. Synthesis, characterization and antimicrobial activity of hydrazone derivatives of 2-(2,3-dihydrobenzofuran-5yl)acetic acid. J Appl Chem. 2014;3(4):1481–1487. [Google Scholar]
- Kalsi R, Shrimali M, Bhalla TN, Barthwal JP. Synthesis and anti-inflammatory activity of indolyl azetidinones. Indian J Pharm Sci. 2006;41:353–359. [Google Scholar]
- Kodisundaram P, Amirthaganesan S, Balasankar T. Antimicrobial evaluation of a set of heterobicyclic methylthiadiazole hydrazones: synthesis, characterization, and SAR studies. J Agric Food Chem. 2013;16:11952–11956. doi: 10.1021/jf404537d. [DOI] [PubMed] [Google Scholar]
- Kumar LV, Naik PJ, Khan PS, Reddy AB, Sekhar TCh, Swamy GN. Synthesis, characterization and biological evaluation of some new hydrazide hydrazones. Der Pharm Chem. 2011;3(3):317–322. [Google Scholar]
- Kumar D, Kapoor A, Thangadurai A, Kumar P, Narasimhan B. Synthesis, antimicrobial evaluation and QSAR studies of 3-ethoxy-4-hydroxybenzylidene/4-nitrobenzylidene hydrazides. Chin Chem Lett. 2011;22:1293–1296. doi: 10.1016/j.cclet.2011.06.014. [DOI] [Google Scholar]
- Kumar D, Kumar NM, Ghosh S, Shah K. Novel bis(indolyl)hydrazide–hydrazones as potent cytotoxic agents. Bioorg Med Chem Lett. 2012;22:212–215. doi: 10.1016/j.bmcl.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Kumar V, Basavarajaswamy G, Rai MV, Poojary B, Pai VR, Shruthi N, Bhat M. Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg Med Chem Lett. 2015;25:1420–1426. doi: 10.1016/j.bmcl.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc. 2011;86(8):805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machakanur SS, Patil BR, Badiger DS, Bakale RP, Gudasi KB, Bligh SWA. Synthesis, characterization and anticancer evaluation of novel tri-arm star shaped 1,3,5-triazine hydrazones. J Mol Struct. 2012;1011:121–127. doi: 10.1016/j.molstruc.2011.12.023. [DOI] [Google Scholar]
- McCalla DR, Reuvers A, Kaiser C. Mode of action of nitrofurazone. J Bacteriol. 1970;104:1126–1134. doi: 10.1128/jb.104.3.1126-1134.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOsker CC, Fitzpatrick PM. Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother. 1994;33:23–30. doi: 10.1093/jac/33.suppl_A.23. [DOI] [PubMed] [Google Scholar]
- Moellering RC., Jr Discovering new antimicrobial agents. Int J Antimicrob Agents. 2011;37:2–9. doi: 10.1016/j.ijantimicag.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Mohareb RM, Fleita DH, Sakka OK. Novel synthesis of hydrazide–hydrazone derivatives and their utilizaiton in the synthesis of coumarin, pyridine, thiazole and thiopehene derivatives with antitumor activity. Molecules. 2011;16:16–27. doi: 10.3390/molecules16010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan C, Oniga O, Meda R, Tiperciuc B, Verite P, Pîrnău A, Crişan O, Bojiṭă M. Synthesis and antimicrobial screening of novel 2,3 or 4-[2-aryl-thiazol-yl-methoxy (oxo-ethoxy)]-benzaldehyde isonicotinoyl hydrazide analogs. Farmacia. 2011;59(5):659–668. [Google Scholar]
- Morjan RY, Mkadmh AM, Beadham J, Elmanama AA, Mattar MR, Raftery J, Pritchard RG, Awadallah A, del M, Gardiner JM. Antibacterial activities of novel nicotinic acid hydrazides and their conversion into N-acetyl-1,3,4-oxadiazoles. Bioorg Med Chem Lett. 2014;24:5796–5800. doi: 10.1016/j.bmcl.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Munoz-Davila MJ. Role of old antibiotics in the Era of antibiotic resistance. Highlighted nitrofurantoin for the treatment of lower urinary tract infections. Antibiotics. 2014;3:39–48. doi: 10.3390/antibiotics3010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisetty R, Chandrasekhar KB, Mohanty S, Rao MR, Balram B. Synthesis and Antimicrobial evaluation of some novel hydrazone derivatives of 2,5-diflurobenzoic acid. J Appl Chem. 2013;2(6):1489–1498. [Google Scholar]
- Nasr T, Bondock S, Youns M. Anticancer activity of new coumarin substituted hydrazide–hydrazone derivatives. Eur J Med Chem. 2014;76:539–548. doi: 10.1016/j.ejmech.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Özkay Y, Tunah Y, Karaca H, Işıkdağ İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur J Med Chem. 2010;45:3293–3298. doi: 10.1016/j.ejmech.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Pavan FR, Maia PI, da S, Leite SRA, Deflon VM, Batista AA, Sato DN, Franzblau SG, Leite CQF. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur J Med Chem. 2010;45:1898–1905. doi: 10.1016/j.ejmech.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Pieczonka AM, Strzelczyk A, Sadowska B, Mlostoń G, Stączek P. Synthesis and evaluation of antimicrobial activity of hydrazones derived from 3-oxido-1H-imidazole-4-carbohydrazides. Eur J Med Chem. 2013;64:389–395. doi: 10.1016/j.ejmech.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Popiołek Ł, Biernasiuk A, Malm A. Synthesis and antimicrobial activity of new 1,3-thiazolidin-4-one derivatives obtained from carboxylic acid hydrazides. Phosphorus Sulfur. 2015;190:251–260. doi: 10.1080/10426507.2014.919293. [DOI] [Google Scholar]
- Popiołek Ł, Stefańska J, Kiełczykowska M, Musik I, Biernasiuk A, Malm A, Wujec M. Synthesis, dissociation constants, and antimicrobial activity of novel 2,3-disubstituted-1,3-Thiazolidin-4-one derivatives. J Heterocycl Chem. 2016;53:393–402. doi: 10.1002/jhet.2418. [DOI] [Google Scholar]
- Popiołek Ł, Biernasiuk A, Malm A. Design, synthesis, and in vitro antimicrobial activity of new Furan/Thiophene-1,3-Benzothiazin-4-one hybrids. J Heterocycl Chem. 2016;53:479–486. doi: 10.1002/jhet.2429. [DOI] [Google Scholar]
- Popiołek Ł, Biernasiuk A (2016a) Hydrazide-hydrazones of 3-methoxybenzoic acid and 4-tert-butylbenzoic acid with promising antibacterial activity against Bacillus spp. J Enzym Inhib Med Chem. doi:10.3109/14756366.2016.1170012 [DOI] [PubMed]
- Popiołek Ł, Biernasiuk A. Design, synthesis, and in vitro antimicrobial activity of hydrazide-hydrazones of 2-substituted acetic acid. Chem Biol Drug Des. 2016;88:873–883. doi: 10.1111/cbdd.12820. [DOI] [PubMed] [Google Scholar]
- Rambabu N, Dubey PK, Ram B, Balram B. Synthesis, characterization and antimicrobial activity of some novel hydrazone derivatives of anacardic acid. Der Pharma Chem. 2015;7(4):90–97. [Google Scholar]
- Rasras AJM, Al-Tel TH, Al-Aboudi AF, Al-Qawasmeh RA. Synthesis and antimicrobial activity of cholic acid hydrazone analogues. Eur J Med Chem. 2010;45:2307–2313. doi: 10.1016/j.ejmech.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Rollas S, Küçükgüzel ŞG. Biological activities of hydrazone derivatives. Molecules. 2007;12:1910–1939. doi: 10.3390/12081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkauskas K, Mickevičius V, Kantminienè K, Stasevych M, Komarovska-Porokhnyavets O, Musyanovych R, Novikov V. Synthesis and antimicrobial activity of 1,3-disubstituted pyrrolidinones with hydrazone and naphthoquinone moieties. Chemija. 2013;24(1):74–80. [Google Scholar]
- Satyanarayana GV, Rao VL, Chary MT, Ram B, Balram B, Chinmaiyee V. Synthesis and antimicrobial activity of novel hydrazone derivatives of 4-(4-chlorophenyl)cyclohexanecarboxylic acid. J Appl Chem. 2014;3(3):1232–1238. [Google Scholar]
- Şenkardes S, Kaushik-Basu N, Durmaz İ, Manvar D, Basu A, Atalay R, Küçükgüzel ŞG. Synthesis of novel diflunisal hydrazide-hydrazones as anti-hepatitis C virus agents and hepatocellular carcinoma inhibitors. Eur J Med Chem. 2016;10:301–308. doi: 10.1016/j.ejmech.2015.10.041. [DOI] [PubMed] [Google Scholar]
- Shirinzadeh H, Altanlar N, Yucel N, Ozden S, Suzen S. Antimicrobial evaluation of indole-containing hydrazone derivatives. Z Naturforsch. 2011;66c:340–344. doi: 10.5560/ZNC.2011.66c0340. [DOI] [PubMed] [Google Scholar]
- Siddiqui AISM, Macedo TS, Moreira DRM, Leite ACL, Soares MBP, Azam A. Design, synthesis and biological evaluation of 3-[4-(7-chloro-quinolin-4-yl)-piperazin-1-yl]-propionic acid hydrazones as antiprotozoal agents. Eur J Med Chem. 2014;75:67–76. doi: 10.1016/j.ejmech.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Sreedhar P, Srinivas G, Raju RM. Synthesis and antibacterial activity of N-substituted-1-benzyl-1H-1,2,3-triazole-carbohydrazide derivatives. Pharm Chem. 2016;8(10):173–178. [Google Scholar]
- Sriram D, Yogeeswari P, Vyas DRK, Senthilkumar P, Srividya PB. 5-Nitro-2-furoic acid hydrazones: design, synthesis and in vitro antimycobacterial evaluation against log and starved phase cultures. Bioorg Med Chem Lett. 2010;20:4313–4316. doi: 10.1016/j.bmcl.2010.06.096. [DOI] [PubMed] [Google Scholar]
- Tejeswara RA, Polkam N, Rayam P, Sridhara J, Garikapati NarahariSastry, Kotapalli SS, Ummanni R, Anireddy JS. Design, synthesis and biological activity evaluation of novel pefloxacin derivatives as potential antibacterial agents. Med Chem Res. 2016;25:977–993. doi: 10.1007/s00044-016-1544-8. [DOI] [Google Scholar]
- Unissa AN, Hanna LE, Swaminatha S. A note on derivatives of isoniazid, Rifampicin, and pyrazinamide showing activity against resistant Mycobacterium tuberculosis. Chem Biol Drug Des. 2016;87:537–550. doi: 10.1111/cbdd.12684. [DOI] [PubMed] [Google Scholar]
- Velezheva V, Brennan P, Ivanov P, Koronienko A, Lyubimov S, Kazarian K, Nikonenko B, Majorov K, Apt A. Synthesis and antituberculosis activity of indole-pyridine derived hydrazides, hydrazide-hydrazones, and thiosemicarbazones. Bioorg Med Chem Lett. 2016;26:978–985. doi: 10.1016/j.bmcl.2015.12.049. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Treatment of tuberculosis guidelines. 4th edn. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- Xaiver JJF, Krishnasamy K, Sankar C. Synthesis and antibacterial, antifungal activities of some 2r,4c-diaryl-3-azabicyclo[3.3.1]nonan-9-one-4-aminobenzoyl hydrazones. Med Chem Res. 2012;21:345–350. doi: 10.1007/s00044-010-9528-6. [DOI] [Google Scholar]
- Yadagiri B, Holagunda UD, Bantu R, Nagarapu L, Guguloth V, Polepally S, Jain N. Rational design, synthesis and anti-proliferative evaluation of novel benzosuberone tethered with hydrazide–hydrazones. Bioorg Med Chem Lett. 2014;24:5041–5044. doi: 10.1016/j.bmcl.2014.09.018. [DOI] [PubMed] [Google Scholar]


