Abstract
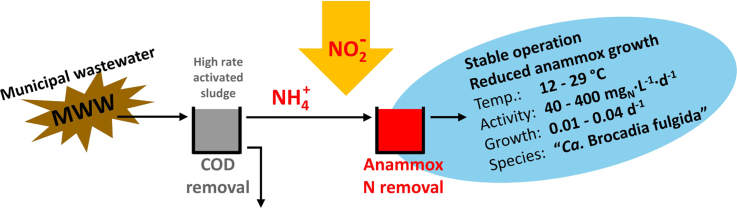
Direct treatment of municipal wastewater (MWW) based on anaerobic ammonium oxidizing (anammox) bacteria holds promise to turn the energy balance of wastewater treatment neutral or even positive. Currently, anammox processes are successfully implemented at full scale for the treatment of high-strength wastewaters, whereas the possibility of their mainstream application still needs to be confirmed. In this study, the growth of anammox organisms on aerobically pre-treated municipal wastewater (MWWpre-treated), amended with nitrite, was proven in three parallel reactors. The reactors were operated at total N concentrations in the range 5–20 mgN∙L−1, as expected for MWW. Anammox activities up to 465 mgN∙L−1∙d−1 were reached at 29 °C, with minimum doubling times of 18 d. Lowering the temperature to 12.5 °C resulted in a marked decrease in activity to 46 mgN∙L−1∙d−1 (79 days doubling time), still in a reasonable range for autotrophic nitrogen removal from MWW. During the experiment, the biomass evolved from a suspended growth inoculum to a hybrid system with suspended flocs and wall-attached biofilm. At the same time, MWWpre-treated had a direct impact on process performance. Changing the influent from synthetic medium to MWWpre-treated resulted in a two-month delay in net anammox growth and a two to three-fold increase in the estimated doubling times of the anammox organisms. Interestingly, anammox remained the primary nitrogen consumption route, and high-throughput 16S rRNA gene-targeted amplicon sequencing analyses revealed that the shift in performance was not associated with a shift in dominant anammox bacteria (“Candidatus Brocadia fulgida”). Furthermore, only limited heterotrophic denitrification was observed in the presence of easily biodegradable organics (acetate, glucose). The observed delays in net anammox growth were thus ascribed to the acclimatization of the initial anammox population or/and the development of a side population beneficial for them. Additionally, by combining microautoradiography and fluorescence in situ hybridization it was confirmed that the anammox organisms involved in the process did not directly incorporate or store the amended acetate and glucose. In conclusion, these investigations strongly support the feasibility of MWW treatment via anammox.
Keywords: Mainstream anammox, Municipal wastewater, “Candidatus Brocadia fulgida”, MAR-FISH, 16S rRNA gene-targeted amplicon sequencing, Denitrification
Graphical abstract
Highlights
-
•
Anammox guild grows on aerobically pre-treated municipal wastewater (MWW) amended with NO2−.
-
•
Anammox activity at 12.5 °C was comparable to conventional N-removal treatments.
-
•
Anammox growth was 2 to 3 times slower on pre-treated compared to synthetic MWW.
-
•
“Ca. Brocadia fulgida” was the dominant anammox strain growing on pre-treated MWW.
-
•
“Ca. Brocadia” affiliates did not directly accumulate organics (acetate, glucose).
1. Introduction
Nitrogen is removed from municipal wastewater (MWW) to reduce its adverse environmental impacts (Galloway et al. 2003). Conventionally, ammonium is first oxidized aerobically to nitrite and nitrate via autotrophic nitrification and subsequently (partly) reduced to di-nitrogen gas through heterotrophic denitrification. This route, while allowing for reliable nitrogen removal, is demanding both in terms of energy (e.g. blowers for aeration, requirement of influent organic load for denitrification and thus reduced biogas production) and costs (e.g. sludge disposal). The anaerobic ammonium-oxidizing (anammox) bacteria, capable of autotrophic ammonium oxidation with nitrite as the terminal electron acceptor, was discovered in the mid-1990s (Mulder et al. 1995). Today, anammox-based processes are considered the most promising alternative for biological nitrogen removal in MWW applications (van Loosdrecht and Brdjanovic, 2014) with the potential, in combination with anaerobic digestion, to turn the energy balance of wastewater treatment neutral or even positive (Siegrist et al. 2008).
In principle, direct MWW treatment based on anammox would allow for the segregation of the removal of organic matter (COD) and nitrogen. COD could indeed be removed in a first step – either via a high-rate activated sludge or a physico-chemical process (Versprille et al. 1985) – and the energetic content of the removed organics could then be valorized in anaerobic digestion. Next, the nitrogen-rich liquid fraction along with the digestion supernatant could be treated autotrophically via anammox after partial nitritation (oxidation of half of the ammonium to nitrite). This would lead to significant reductions in oxygen consumption and to an increase in biogas production (Siegrist et al. 2008). To date, anammox-based processes constitute a robust and reliable treatment for wastewaters with high nitrogen concentrations at mesophilic conditions, and over 100 full-scale plants have been installed worldwide (Lackner et al. 2014). However, the potential for the direct treatment of real MWW with anammox-based processes has still not been fully confirmed despite increasing experimental evidence.
Maintaining nitrogen removal rates above 50 gN∙m−3∙d (e.g. typical values for municipal wastewater treatment) and reliably achieving the required effluent quality at temperatures of 10–25 °C represent two of the current main challenges towards full-scale application (e.g. Gilbert et al. (2014)). A significant impact of temperature decrease on anammox activity has been reported (Isaka et al., 2008, Lotti et al., 2015). Nevertheless, proofs of concept have been obtained with COD-free synthetic wastewater at nitrogen concentrations in the MWW range. Combined partial nitritation/anammox reactors have been stably operated at both 12 °C (Hu et al. 2013) and 10 °C (Gilbert et al. 2014). However, volumetric anammox activities have been reported in a relatively low range (Gilbert et al. 2014).
Moreover, the presence of complex mixtures of organics in real MWW could affect the anammox performance directly (e.g. methanol toxicity (Güven et al. 2005)) or indirectly (e.g. fostering competing microbial species (Lackner et al. 2008)). So far, there is little understanding of the intrinsic effects of MWW composition on process performance, anammox metabolism and the overall structure of the microbial community, as observed for example in the case of source-separated urine treatment (Bürgmann et al. 2011). Only few research efforts have focused on real MWW, namely pre-settled diluted raw MWW (De Clippeleir et al. 2013) or nitrite-amended secondary clarifier effluent (Ma et al. 2013) and aerobically pre-treated MWW (Lotti et al. 2014). All these studies are restricted to granules or biofilms and no information is available on the use of suspended-growth anammox sludges for mainstream applications. Furthermore, the direct effects of MWW are as yet unclear.
Additionally, there is no conclusive evidence as to whether treating real MWW would select for specific anammox species. Recently published results based on fluorescence in situ hybridization (FISH) have highlighted “Candidatus Brocadia fulgida” as the dominant species in MWW (Lotti et al., 2014, Ma et al., 2013). However, to the authors' knowledge, no DNA sequencing data is available for the anammox candidate species in MWW applications.
The goal of the present work was to assess the possibility for the anammox guild to grow on MWW at conditions relevant for mainstream application. Three parallel reactors were inoculated with anammox sludges treating digestor supernatant and initially operated at 29 °C in order to investigate: (i) anammox activity and growth rates on pre-treated MWW and (ii) the potential effects of MWW both on the anammox and the overall population in terms of nitrogen turnover, dominant population dynamics and substrate competition. The effects of a temperature decrease to psychrophilic conditions (12.5 °C) were also assessed. Process performance investigations at reactor level were efficiently complemented by quantitative FISH, microautoradiography combined with FISH and high-throughput 16S rRNA gene-targeted amplicon sequencing to obtain information on microbial composition and ecophysiology.
2. Materials and methods
2.1. Reactor operation
Three anammox-based sequencing batch reactors (SBR; 12 L working volume) were operated. Two of them (R1 and R2) were inoculated with two distinct suspended nitritation/anammox sludges treating digestor supernatant. R1 was seeded with biomass from a full-scale reactor (WWTP Werdhölzli, Zürich, Switzerland). R2 received the biomass from a pilot-scale reactor described elsewhere (Eawag, 400 L; (Joss et al. 2011)). Both reactors were operated at 29 ± 2 °C and total N concentration in the range 5–20 mgN∙L−1, as expected for mainstream applications, with three distinct operational phases:
-
•
Phase I: To first test the possibility for combined nitritation/anammox with suspended sludge, the reactors were run under micro-aerobic conditions (air supply controlled at dissolved O2 concentrations < 0.05 mgO2∙L−1) and fed with pre-treated municipal wastewater (MWWpre-treated, described below). Each SBR cycle consisted of five steps: settling (30 min), feeding (3 L of MWWpre-treated), anoxic mixing (30 min), aeration (variable duration, terminated when NH4+ reached a concentration < 2 mgN∙L−1) and anoxic mixing (30 min). The cycle duration varied between 3 and 10 h, depending on activity.
-
•
Phase II: To specifically test anammox activity at typical MWW conditions, the reactors were operated without O2 supply and fed with synthetic wastewater (MWWsynthetic, described below). Each SBR cycle consisted of three steps: settling (30 min), feeding (1 L of MWWpre-treated), anoxic mixing (duration between 3 and 8 h, depending on activity). NO2− was generally depleted within the first half of the anoxic phase, with the remaining time left for the release of N2 gas bubbles.
-
•
Phase III: To assess the possibility for the anammox guild to grow on MWW, the feeding was changed to MWWpre-treated supplemented with NH4+ and NO2− and the reactors were operated anoxically as in Phase II. According to the set N concentration in MWWpre-treated (see below), feeding volumes were 3 and 1 L in R1 and R2, respectively.
A third reactor (R3) was inoculated with half of the biomass from R2 and was then run at different temperature levels (12.5 ± 1–29 ± 2 °C) on MWWpre-treated supplemented with NH4+ and NO2− as R2 in Phase III. In all reactors, the pH was controlled in the range 7.3 ± 0.2 by the addition of 0.5 mol L−1 HCl.
2.2. Substrates (MWWpre-treated, MWWsynthetic)
Municipal wastewater from the municipality of Dübendorf (Switzerland) was pre-treated after primary sedimentation in a 12 L aerated SBR for COD removal at 1 d sludge retention time (SRT). Removal efficiencies >75% were obtained both for total and soluble COD. The pre-treated municipal wastewater (MWWpre-treated) displayed the following composition: 71 ± 19 mgCODtot∙L−1, 47 ± 13 mgCODsol∙L−1, 19 ± 6 mgNH4-N∙L−1, <0.3 mgN∙L−1 of NO2− and NO3−, EC 1.9–2.4 mS/cm and pH 7.7 ± 0.1. MWWpre-treated was fed directly to R1 and R2 during Phase I. In Phase III, MWWpre-treated was first supplemented with NH4+ and NO2− in an external 50 L bucket in order to reach 20 mgN∙L−1 for each compound in the influent of R1 and 65 mgN∙L−1 in the influents of R2 and R3.
The synthetic municipal wastewater (MWWsynthetic) used in Phase II contained 65 mgN∙L−1 of both NH4+ and NO2− (EC 3.2–3.5 mS/cm; pH 7.5 ± 0.1) and was prepared according to section S1 in Supporting Information. In all phases feeding volumes were adjusted to maintain total N concentrations in the range 5–20 mgN∙L−1 in the reactor.
2.3. Anammox activity
Anammox activity is defined as the nitrogen removal rate (sum of NH4+ and NO2−) in the absence of O2 and non-limiting concentrations of NH4+ and NO2−. In Phase I, the reactor operation was controlled only on the basis of online measurements of the residual NH4+ concentration and anammox activity measured in batch tests twice a week in situ. NH4+ and NO2− were supplied as NH4Cl and NaNO2 (≥15 mgN∙L−1 each) and their consumption rates were calculated by linear regression of off-line measurements of 3–4 bulk liquid-phase grab samples. The time between samples depended on the actual rate, 15–60 min.
During Phases II and III, the anammox activity was calculated on a daily basis directly during operation. Following a normal feeding, two samples were taken during the linear NH4+ depletion phase (sensor signal) and the NH4+ and NO2− consumptions were calculated by linear regression. Anammox activities are reported only as volumetric rates, in mgN∙L−1∙d−1, as irregular biofilm formation on the walls of the reactor (see below) hindered the proper estimation of the total solids content and thus the accurate calculation of biomass specific N removal rates.
2.4. Estimation of anammox growth rate
The anammox growth rate (μmax, d−1) was calculated according to Eq. (1) below:
| (1) |
where, treaction is the reaction time (in the presence of both NH4+ and NO2−), ttotal is the total batch time, and μobs is the average growth rate estimated from the change in anammox activity over time (μobs = ln(r2/r1)/(t2 − t1) in d−1, with ri being the anammox activity in mgN∙L−1∙d−1 at time ti). The uncertainty associated with μmax was estimated by means of Monte Carlo simulations, assuming: ttotal/treaction normally distributed (mean and standard deviation of the values per batch); μobs normally distributed (mean and standard deviation obtained by the maximum-likelihood linear estimation of the log-transformed values).
2.5. Heterotrophic activity
To verify the presence of heterotrophic denitrifying activity, the consumption of NO2− and NO3− was measured in the presence of different easily biodegradable carbon sources after complete NH4+ depletion. This test was performed during Phase III directly in the reactors, at 29 °C, and in the absence of O2. Following a normal feeding step, NH4+ was first consumed via anammox after addition of NO2−. The system was then left with non-limiting concentrations of NO2− and NO3− (4–10 mgN∙L−1), and acetate, glucose, maleate or fresh MWW were added at intervals of 1–2 h in equivalents of 5–15 mgCODsol∙L−1. The substrates were chosen as representatives of easily degradable organics. Finally, the consumption of the remaining NO2− after addition of NH4+ was measured to confirm the occurrence of anammox activity. The experiment was repeated three times in R1 on days 260, 300, 322 and once in R2 on day 300.
2.6. Total solids and biomass sampling
In reactor R1, during the first 145 days, the SRT (>60 d) was controlled by the spontaneous sludge loss via the effluent. From day 145, the biomass found in the collected effluent was regularly returned after decanting to the reactor and thus solids were lost only through sampling. At the same time, biofilm started to form on the walls, detaching irregularly and thus resulting in variations of the measured TSS concentration. After day 145, the total solids content was consequently estimated experimentally only at the end of the experiment. The compositions of the bacterial communities underlying the suspended and wall-attached biomass were assumed to be comparable, as shown by the qFISH and amplicon sequencing analyses (see below). Thus the attached biomass could be estimated by measuring its activity and extrapolating from the activity and amount of the suspended biomass. Similarly, in the case of R2 the SRT was >50 d until day 72. From day 72, the biomass collected in the effluent was regularly reintroduced to the reactor. On day 134, half of the reactor content was removed to inoculate R3 and the effect of biofilm on the walls also began to be significant. As for R1, the total solids content was estimated experimentally only at the end of the experiment.
Suspended biomass samples were taken from R1 and R2 on a weekly basis in triplicates. From day 240, wall-biofilms were also sampled weekly.
In R3, proper quantification of the total solids was allowed because of regular reintroduction of the effluent biomass, a lower sampling frequency, reduced biofilm formation on the walls possibly due to the lower temperatures, and sampling right after re-suspension of wall-biofilms by brushing. No microbiological characterization was performed for R3.
2.7. Analytical methods
NH4+ was analyzed using a flow injection analyzer (Foss FIA star 5000, Rellingen, Germany). NO2− and NO3− were analyzed by ion chromatography (Compact IC 761, Metrohm, Herisau, Switzerland). COD was measured photometrically with test kits (Hach Lange, Düsseldorf, Germany). Prior to analysis, the samples were filtered using 0.45 μm filters. TSS was determined according to standard methods (American Public Health Association, 2005).
2.8. Fluorescence in situ hybridization (FISH)
Fixation and hybridization of biomass samples were conducted as previously described (Nielsen et al. 2009). Prior to hybridization, the samples were homogenized by sonication (20 kHz, amplitude 50%, 18.6 W; Sonoplus HD 3100, Bandelin electronic GmbH, Germany) in order to disrupt large aggregates.
The oligonucleotide probes specific to “Candidatus Brocadia anammoxidans” and “Ca. Kuenenia stuttgartiensis” (Amx820) and “Ca. Brocadia fulgida” (Bfu613) were applied in equimolar mixtures to target the anammox guild. Preliminary screening showed that all other probes specific to different anammox candidate species did not give a significant signal. The probes used to detect the ammonium- (AOB) and nitrite- (NOB) oxidizing and total (EUB) bacteria as well as details of their specificity are shown in Table S1 (Supporting Information). All probes were purchased from Thermo-Fisher Scientific (Ulm, Germany).
2.9. Confocal laser scanning microscopy (CLSM) and quantitative FISH (qFISH)
The hybridized biomass samples were examined with a confocal laser scanning microscope (Leica, SP5, Germany) in order to quantify the relative abundance of microbial guilds in the biomass and to characterize the sludge architectures: 8–15 CLSM imaging z-stacks were acquired per sample with 15–40 images per stack covering a vertical separation of 15–60 μm. The fluorescence signals of the Cy3, Cy5, and FLUOS dyes were detected using 543, 633 and 488 nm laser lines, respectively, with the filter settings recommended by the supplier. The relative abundances of AMX, AOB and NOB were estimated by calculating the ratio of their respective specific bacterial biovolumes to the total bacterial biovolume using the Daime software (Daims et al. 2006). The sensitivity of the quantification method was evaluated by progressive dilution of one sample for each anammox reactor with conventional activated sludge containing negligible amounts of AMX. The observed and expected abundances differed by less than ±10%, with an estimated detection limit of 2% (Figure S1, Supporting Information).
2.10. Microautoradiography and FISH (MAR-FISH)
MAR–FISH was carried out as described by Nielsen and Nielsen (2005). To mimic the conditions of the heterotrophic activity tests, sludge from R1 was pre-incubated overnight at 29 °C to deplete the residual NH4+ in the presence of NO2−. Next, 2 mL aliquots were transferred to 9 mL serum bottles and supplemented with 20 μCi of [3H]acetate or [3H]glucose. The initial concentrations of unlabeled compounds were: 20 mgNO2-N∙L−1, 20 mgNO3-N∙L−1, 20 mgCODsol∙L−1 (as acetate or glucose). Anoxic conditions were reached by alternatively applying flushing/evacuation with oxygen-free N2 (final overpressure of 0.5 bar). Incubations were carried out at 29 °C, on a shaker (150 rpm), and lasted 4.5 h (acetate) or 17.5 h (glucose). The use of 3H, instead of 14C, allowed to specifically focus on the primary uptake (no secondary uptake of CO2 produced by heterotrophs). Biomass samples were then fixed according to the procedure described above, and the same oligonucleotide probes targeting AMX and EUB were used. Prior to hybridization, the biomass samples were mechanically homogenized with a glass tissue grinder for 1 min. Images were acquired and analyzed using a LSM 510 Meta confocal laser scanning microscope (Carl Zeiss, Germany).
2.11. Molecular analysis of bacterial community compositions
The bacterial community compositions were analyzed using the molecular method described in Weissbrodt et al. (2015). Genomic DNA (gDNA) was extracted from biomass samples by bead-beating (four series of 20s each) and purified using the FastDNA SPIN Kit for Soils (MP Biomedicals, USA) according to the manufacturer's instructions. The purified gDNA extracts were diluted to 20 ng μL−1 and sent to Research and Testing Laboratory (Lubbock, TX, USA) for 16S rRNA gene-targeted amplicon sequencing using the MiSeq desktop technology (>10,000 reads per sample, Illumina, USA). The primer pair targeting the v4 region (515F: 5′-GTGCCAGCMGCCGCGGTAA-3′ and 806R: 5′-GGACTACHVGGGTWTCTAAT-3′) was selected for PCR. Comprehensive in silico evaluation and experimental validation of a set of oligonucleotides was conducted in order to cover anammox populations within the overall bacterial community (Weissbrodt et al. 2015). The same primer pair was also used very recently by Gilbert et al. (2014). Greengenes and SILVA were used as databases of reference sequences for identifying the closest bacterial relatives.
3. Results
3.1. Impact of changes in influent composition on anammox performance (reactor R1)
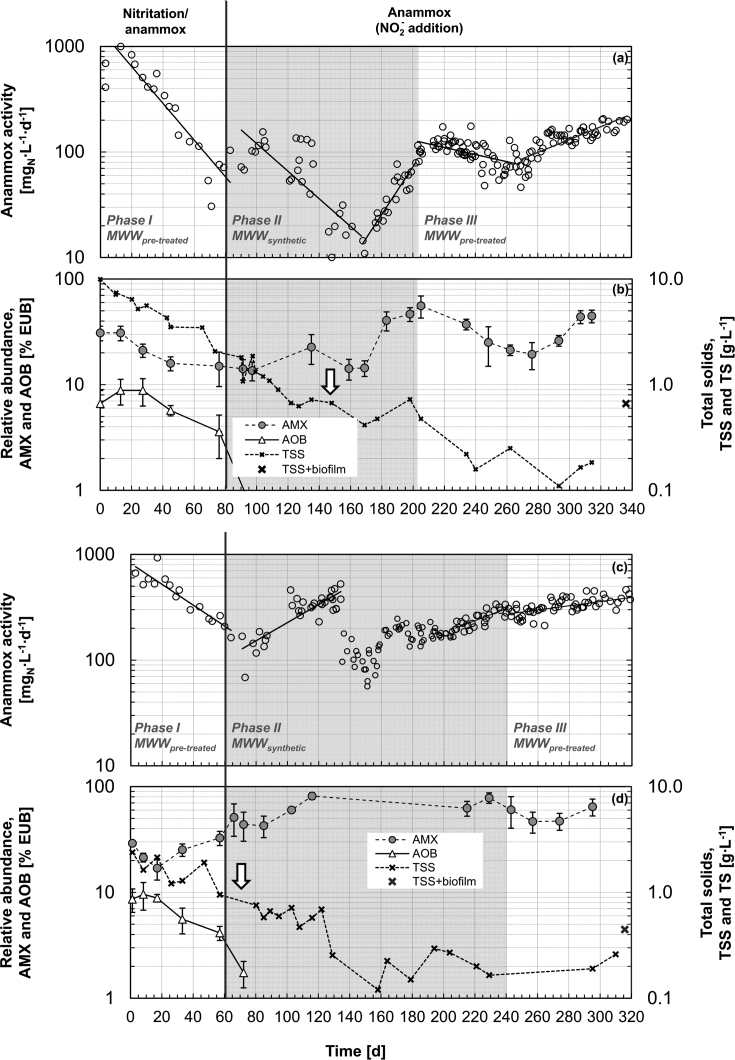
The temporal profile of anammox activity in R1 is presented in Fig. 1(a) on a logarithmic scale. In Phase I, when the reactor was operated as nitritation/anammox with aerobically pre-treated municipal wastewater (MWWpre-treated), the anammox activity decreased exponentially and correlated with the observed loss in total suspended solids (TSS) (Fig. 1(b)). During this phase, nitrite and ammonium were consumed in a ratio of 1.69 ± 0.72 gNO2-N/gNH4-N and the NO3− production was negligible – whereas a ratio of 1.32 gNO2-N/gNH4-N and a production of 0.11 gNO3-N per g(NH4+NO2)-N consumed are expected in the anammox stoichiometry (Strous et al. 1999). This suggests the involvement of residual heterotrophic denitrification.
Fig. 1.
Temporal evolution of the volumetric anammox activity and the relative abundances of anammox populations in the sludge during the three successive experimental phases at 29 °C, for reactors R1 (panel (a), (b)) and R2 (panel (c), (d)). (a, c) Anammox activity expressed as the sum of NH4+ and NO2− consumption. (b, d) Relative abundance of the guilds of known anammox populations (AMX; Cy3-labeled Amx820 and Bfu613 oligonucleotide probes) and aerobic ammonia oxidizing bacteria (AOB; FLUOS-labeled AOB-mix) measured by 16S rRNA-targeted FISH over the total bacterial population (EUB; Cy5-labeled EUB-mix), as well as concentrations of total suspended solids (TSS) and TSS together with wall-biofilms (TSS + biofilm) as estimated at the end of the experiment. The error bars represent the standard deviation of the FISH quantification. In Phase I, the reactor was operated for full nitritation and anammox on municipal wastewater pre-treated for COD removal (MWWpre-treated). In Phases II and III, the reactor was operated for anammox only with synthetic MWW (MWWsynthetic) and MWWpre-treated amended with NH4+ and NO2−, respectively. The vertical arrows indicate the day on which effluent biomass began to be regularly reintroduced to the reactor.
On day 83, to restore anammox activity the aeration was switched off and the feed was changed to synthetic wastewater (MWWsynthetic) (Phase II). This change did not result in an immediate recovery of activity. However, the regular reintroduction of biomass transported with the effluent into the reactor, from day 145, possibly favored the re-establishment of the anammox population and an exponential increase in activity from day 167 onwards. According to Eq. (1), this increase corresponded to an anammox growth rate μmax of 0.095 ± 0.038 d−1, equivalent to a doubling time of 7 days.
When the feeding was reverted back to MWWpre-treated supplemented with NO2− (Phase III), the activity decreased slightly for about two months. From day 268, the activity increased exponentially similarly to Phase II with an estimated growth rate of 0.040 ± 0.013 d−1 (doubling time of 18 days), suggesting an adaptation or a shift in population. In Phase III, anammox activities over 200 mgN∙L−1∙d−1 were reached with a hydraulic retention time (HRT) of 16.5 h. The average ratio of nitrite reduced per ammonium oxidized and the ratio of nitrate produced per ammonium and nitrite consumed were 1.52 ± 0.32 gNO2-N/gNH4-N and 0.13 ± 0.05 gNO3-N/g(NH4+NO2)-N respectively, in good agreement with the values proposed for anammox (Strous et al. 1999), suggesting that only limited heterotrophic denitrification occurred. NO2− was never measured above detection limit (0.1 mgNO2-N∙L−1) in the reactor effluent.
3.2. Impact of changes in influent composition on anammox performance (reactor R2)
In Phase I, R2 was operated as partial nitritation/anammox in the same way as R1. In an analogous way, the anammox activity decreased exponentially during this period (Fig. 1(c)). In this case, the marked activity loss from over 600 to about 100 mgN∙L−1∙d−1 could only partially be explained by the 50% TSS loss (Fig. 1(d)).
Unlike for R1, the anammox activity increased immediately without any delay when aeration stopped and feeding was changed to MWWsynthetic (Phase II), reaching activities higher than 400 mgN∙L−1∙d−1. On day 134, half of the reactor content was removed to inoculate the third reactor (R3). Due to technical problems, the activity remained unstable between 100 and 200 mgN∙L−1∙d−1 until day 207. Once these problems were fixed, the anammox activity increased exponentially, with an estimated growth rate of 0.041 ± 0.009 d−1 (doubling time of 17 days).
When feeding was reverted to MWWpre-treated (Phase III), only a short delay was observed before the activity started to increase, up to values > 400 mgN∙L−1∙d−1. The corresponding estimated growth rate was 0.015 ± 0.004 d−1 (doubling time of 46 days). The average ratios of nitrite reduced per ammonium oxidized and of nitrate produced per ammonium and nitrite consumed were 1.41 ± 0.29 gNO2-N/gNH4-N and 0.13 ± 0.08 gNO3-N/g(NH4+NO2)-N, respectively, suggesting that only limited denitrification occurred. NO2− was never detected in the effluent at any time.
3.3. Impact of step-wise temperature decrease on anammox performance (reactor R3)
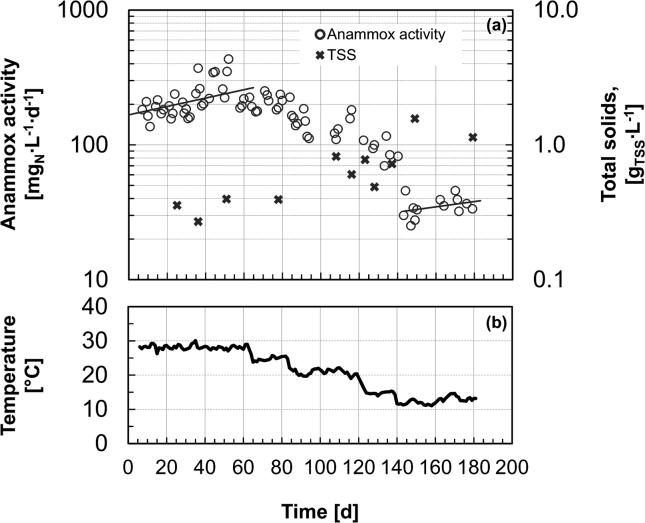
R3 was fed over its entire operational period with MWWpre-treated supplemented with NH4+ and NO2− and the total N concentration in the reactor was always in the range 5–20 mgN∙L−1. The effects of the progressive temperature decrease from 29 °C to 12.5 °C on the anammox activity are presented in Fig. 2. At 29 °C, anammox activity increased from about 200 to 432 mgN∙L−1∙d−1, with an estimated growth rate of 0.029 ± 0.012 d−1 (doubling time of 24 days). Lower anammox activity was observed along with the step-wise decrease in temperatures, notably between 15 and 12.5 °C. During more than one month of operation at 12.5 °C, the anammox activity increased exponentially up to values >40 mgN∙L−1∙d−1 with a corresponding estimated growth rate of 0.009 ± 0.008 d−1 (doubling time of 79 days). In this last phase, the average ratios of nitrite reduced per ammonium oxidized and of nitrate produced per ammonium and nitrite consumed were 1.19 ± 0.28 gNO2-N/gNH4-N and 0.21 ± 0.26 gNO3-N/g(NH4+NO2)-N respectively. In contrast to R1 and R2, the measured TSS concentration increased consistently in R3 throughout the experimental period from about 0.3 to over 1.0 gTSS∙L−1.
Fig. 2.
Temporal evolution of volumetric anammox activity in response to the step-wise decrease in operation temperature from 29 to 12.5 °C. (a) Anammox activity expressed as the sum of NH4+ and NO2− consumption and total suspended solids (TSS) concentration. (b) Profile of the daily average temperature. The reactor was operated on MWWpre-treated amended with NH4+ and NO2−.
3.4. Characterization of microbial compositions and bio-aggregate architectures of the biomasses
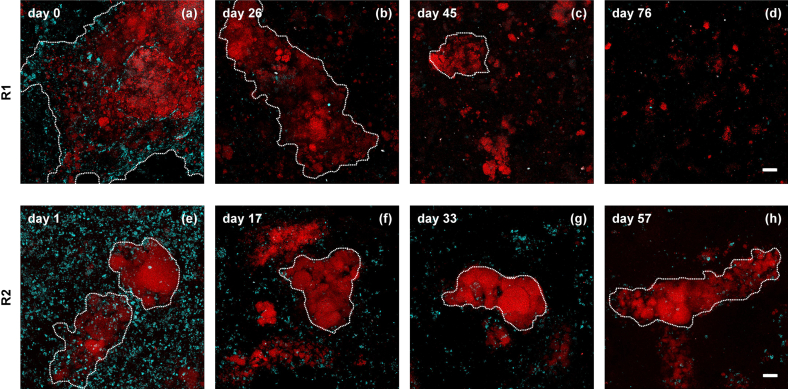
The two inocula displayed comparable relative abundances of AMX (30%) and of AOB (8%) (Fig. 1(b, d)) and the same dominant anammox candidate species (“Ca. Brocadia fulgida”). However, the biomasses featured substantially different floc sizes and compactness (Fig. 3(a, e)).
Fig. 3.
Representative FISH-CLSM digital images illustrating changes in the population composition and architecture of the biomass during the start-up period (Phase I) in R1 (a–d) and R2 (e–h). Anammox populations (AMX; Amx820+Bfu613 oligonucleotides labeled with the fluorescent probe Cy3) are displayed with red color allocation and aerobic ammonium-oxidizing bacteria (AOB; AOB-mix, FLUOS) in cyan. The border of biomass aggregates is highlighted with a dashed white line in order to facilitate the interpretation of the images (scale bars: 20 μm). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
For R1, the dynamics in the relative abundances of AMX and AOB over EUB as measured by qFISH are presented in Fig. 1(b). In Phase I, both populations decreased exponentially, and the decrease was more pronounced for the AOB. At the end of this phase, the AOB were almost completely washed out and subsequently never grew in again during Phases II and III in the absence of O2. The same holds true for the NOB, already present in low relative abundances in the inoculum (<3%; data not shown). According to the FISH analysis, the biomass architecture changed during the same period from big aggregates dominated by AMX to smaller and dispersed flocs (Fig. 3(a–d)). Variations in AMX abundances in Phase II and Phase III correlated well with the measured anammox activities, especially during the exponential growth between days 167–202 and days 269–338 (Fig. 1(b)). According to the 16S rRNA gene-targeted amplicon sequencing data, “Ca. Brocadia fulgida” remained the dominant anammox candidate species throughout the experiment.
In R2, only the relative abundance of AOB decreased exponentially during Phase I, while AMX increased from 17% on day 17 to 51% on day 66 (Fig. 1(d)). In contrast to R1, the biomass aggregates in the inoculation sludge of R2 displayed a more compact architecture and kept their structure during Phase I (Fig. 3(e–h)). AOB were mainly present in small aggregates in the inoculum (Fig. 3(e)), possibly explaining their apparent selective washout. Once aeration was stopped, AMX became the dominant population in the system with relative abundances reaching 80%. From day 207, the relative abundance of AMX matched the trend observed in the anammox activity, notably with a slight decrease right after the beginning of Phase III. Analogously to R1, no shift in the predominant phylotype was detected inside the AMX guild using amplicon sequencing analysis, and “Ca. Brocadia fulgida” remained the main candidate species throughout the experimental period.
During Phase II and Phase III, in both reactors the biomass evolved from a suspended growth inoculum to a hybrid system with suspended flocs and wall-attached biofilm. The two fractions were regularly sampled from day 240 on and featured comparable relative abundances of AMX as estimated by qFISH and confirmed by the 16S rRNA gene-targeted amplicon sequencing data (5 time points for R6 and 4 for R4; (Weissbrodt et al. 2015)). Total solids (suspended + wall-attached biofilm) were estimated at the end of the experiment to be 0.66 (R1) and 0.45 (R2) gTSS∙L−1 (Fig. 1(b) and (d), x).
3.5. Consumption of easily degradable organic compounds
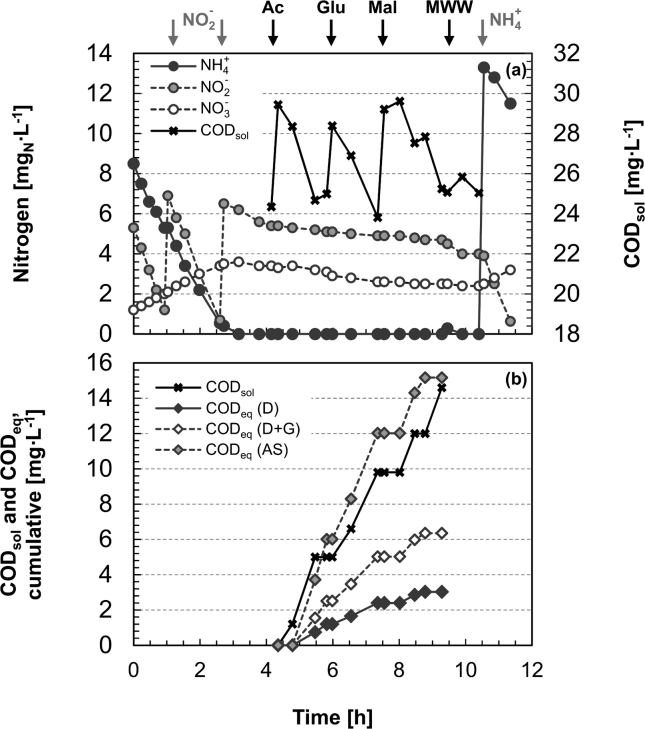
The presence of heterotrophic activity was evaluated three times in R1 and once in R2 by supplying acetate, glucose or maleate carbon sources as well as pre-settled MWW in order to (i) assess if competition for NO2− by heterotrophs could explain the observed decrease in anammox activity in R1 after the change to MWWpre-treated (Fig. 1(a)), and (ii) quantitatively compare heterotrophic and anammox activities to better understand their competition in MWWpre-treated. Both reactors yielded comparable results and therefore only data for R1 are shown (Fig. 4). Nitrite and ammonium were consumed in a ratio of 1.32 gNO2-N/gNH4-N and NO3− production was 0.11 gNO3-N/g(NH4+NO2)-N, in perfect agreement with the anammox stoichiometry (Strous et al. 1999). After NH4+ depletion, the three organic compounds were added in subsequent pulses in the presence of non-limiting concentrations of NO2− and NO3− (4–10 mgN/L). Although all added organics were consumed within 2 h (as measured in solution, CODsol), only limited NO3− and NO2− consumption was observed. Overall, only 20% of the COD consumption could be explained by heterotrophic denitrification if biomass growth was not considered (yields of 2.86 gCODsol/gNO3-N and 1.71 gCODsol/gNO2-N) and 41% if the growth on nitrite and nitrate was accounted for according to the parameters provided in the Activated Sludge Model No. 3 (ASM3) (Gujer et al., 1999, Muller et al., 2003) (Fig. 4(b); Table S2). The marked change in the NO2− depletion rate after the addition of NH4+ further confirmed that anammox was the dominant N-consumption route in the mixed culture, while heterotrophic denitrification played a minor role. After 9.3 h of batch experiment, fresh MWW was added as an additional source of organics. However, the amount of CODsol was too small for any conclusions to be drawn (note: the slight NO2− consumption was due to the ammonium contained in the fresh MWW).
Fig. 4.
Heterotrophic activity test conducted by spiking different carbon sources to assess the potential heterotrophic activity in R1. (a) Time profiles of ammonium (NH4+), nitrite (NO2−), nitrate (NO3−), and soluble organic carbon (CODsol; corrected by considering the contribution of the NO2− concentration to COD with the theoretical factor of 1.143 mgCODsol/mgNO2-N). (b) Cumulative curves of the observed consumption of CODsol (black crosses) and the amount of COD equivalents required for denitrification only (D, black diamonds), denitrification and growth on NO2− and NO3− (D + G, white diamonds) and for anoxic storage (AS, gray diamonds) with the consumed NO3− and NO2−. The stoichiometry of these reactions is described in Table S2 in the Supporting Information.
3.6. Incorporation of easily degradable organic compounds
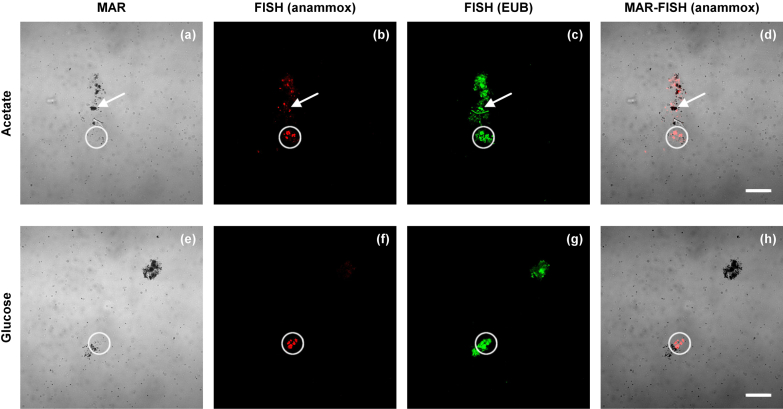
A sludge sample from R1 collected on day 322 was incubated with radio-labeled acetate and glucose for microautoradiography (MAR) analysis in order to test whether anammox populations did directly incorporate or store the supplied CODsol (Fig. 4). Representative digital images of combined MAR-FISH analysis are shown in Fig. 5. The activity of the sludge in consuming the organics was confirmed by the significant number of MAR-positive cells: cells that were active in the substrate uptake are covered with silver-grains and visualized in black in the bright-field images (Fig. 5(a, e)). However, no MAR-positive anammox cells were detected by the FISH probes used (circles in Fig. 5), suggesting that these organisms did not incorporate the studied organics. Anammox activity was maintained during the MAR incubations as estimated from the transformation of ammonia and nitrite.
Fig. 5.
Single and combined microautoradiography (MAR) and FISH digital images of mechanically homogenized biomass from R1, after anoxic incubation with [3H]acetate (a–d) and [3H]glucose (e–h). (a,e) Microautoradiographic image with black silver grains revealing cells that were active in the incorporation of radioactive labeled substrate. (b,f) Fluorescence in situ hybridization of the microscopic field in panels (a) and (e) with Cy3-labeled Amx820+Bfu613 oligonucleotide probes targeting for anammox populations (AMX, red color allocation). (c,g) Fluorescence in situ hybridization of the microscopic field in panels (a) and (e), respectively, with the FLUOS-labeled EUB-mix oligonucleotide probes (green). (d,h) Overlays of microautoradiographic and FISH digital images, namely (a+b) and (e + f). White arrows indicate the localization of cells that actively incorporated the radiolabeled substrates. White circles indicate the localization of probe-defined anammox cells (scale bars: 20 μm). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
4.1. Anammox grow on MWWpre-treated, even at low temperatures
Anammox growth on MWWpre-treated amended with NO2− was shown in three parallel reactors at total N concentrations in the range 5–20 mgN∙L−1. The observed maximum anammox activities ranged between 205 and 465 mgN∙L−1∙d−1 at 29 °C, with estimated doubling times of anammox populations between 18 and 46 days (Fig. 1). A temperature decrease to 12.5 °C resulted in a decline in anammox activity from over 400 to about 40 mgN∙L−1∙d−1, associated with an increase in the estimated anammox doubling time from 24 to 79 days (Fig. 2). This unfavorable temperature effect was particularly relevant between 15 and 12.5 °C, as recently also observed by Gilbert et al. (2014) and Lotti et al. (2015). Our results are in good agreement with Hendrickx et al. (2014), who have enriched anammox bacteria at 10 °C from conventional activated sludge with a mixture of synthetic media and 10% (v/v) filtered anaerobic effluent (61 mg(NH4+NO2)-N∙L−1). The authors reported activities of 27 mgN∙L−1∙d−1 and a growth rate of 0.011 d−1 (doubling time of 71 days) at solids concentrations of 0.5 gVSS∙L−1, which are comparable to those of the present study. Significantly higher activities have been reported under anoxic conditions for systems with higher biomass concentrations. Ma et al. (2013) maintained anammox activities up to 2280 mgN∙L−1∙d−1 at 16 °C in an anammox UASB reactor operated with the addition of powdered activated carbon and fed with the effluent from a secondary clarifier (17 and 20.5 mgN∙L−1 of NH4+ and NO2−, respectively). In line with these findings, Lotti et al. (2014) obtained activities of up to 430 mgN∙L−1∙d−1 at 10 °C with a continuous upflow fluidized granular sludge reactor (6.7 gVSS∙L−1) fed with MWWpre-treated (60 mg(NH4+NO2)-N∙L−1). The authors estimated growth rates of anammox populations of 0.020 d−1 (doubling time of 35 days) and 0.005 d−1 (132 days) at 20 and 10 °C respectively.
Accordingly, the results presented here strongly support the capability of the anammox guild to grow on MWW at conditions relevant for mainstream applications and reach turnovers comparable to those of conventional systems, i.e. 50 mgN∙L−1∙d−1 (Metcalf & Eddy et al. 2013). Future research efforts would need to prove the long-term stability at low temperatures and identify proper treatment configurations to provide the nitrite for the anammox reaction (e.g. separate or combined partial nitritation and anammox). Meeting the required discharge limits will constitute an additional challenge.
4.2. From synthetic media to MWWpre-treated: significant impacts on anammox growth rates
No direct impact of MWWpre-treated on the activity and growth of anammox populations has yet been reported. In the present study, a two to three-fold increase in the estimated doubling times was observed in R1 and R2 respectively when moving from synthetic media to MWWpre-treated. This unfavorable effect on the anammox performance was further supported by the initial decrease in anammox activity when the feed was changed to MWWpre-treated over days 203–268 in R1 and 241–256 in R2 (Fig. 1).
Competition between anammox and heterotrophic populations for NO2− was initially hypothesized as the cause of the observed behavior. Specifically, in the studied system where no O2 was supplied and NH4+ and NO2− were spiked in the influent (Phase III), AMX and heterotrophs were expected to be the two main microbial guilds competing for NO2− as growth-limiting substrate. The latter could potentially grow on soluble microbial products (Kindaichi et al. 2004) or be accidentally carried over from the COD removal step, whereas the influent itself was assumed to have only slowly biodegradable COD. Nevertheless, after several months of continuous anoxic feeding with MWWpre-treated, anammox remained the dominant N-consumption route in both reactors, as supported by (i) the stoichiometric ratio of NO2− to NH4+ consumptions and the NO3− production, as well as (ii) the marked increase in NO2− depletion after NH4+ addition (Fig. 4(a)).
Furthermore, despite significant changes in anammox activity, no shift in the predominant anammox populations was observed throughout the experiment. According to the 16S rRNA gene-targeted amplicon sequencing data, “Ca. Brocadia fulgida” was the dominant anammox candidate strain. Interestingly, the same strain has been identified by FISH in the study of Lotti et al. (2014). These results confirm the versatility of “Ca. Brocadia fulgida” and its competitive advantage over other anammox species in the presence of complex substrates (Jenni et al., 2014, Kartal et al., 2008, Winkler et al., 2012).
In conclusion, these results indicate the appropriateness of the applied aerobic MWW pre-treatment as the residual organic content was insufficient to sustain a heterotrophic biomass able to outcompete the anammox population. High-rate activated sludge can thus be suggested as an appropriate pre-treatment for future implementations. In addition, anammox bacteria affiliating with the “Candidatus Brocadia fulgida” strain dominated throughout the experiments, even after prolonged exposure to MWWpre-treated. Neither heterotrophic competition for NO2− nor a shift in the dominant anammox population could explain the adverse effects on anammox performance observed after the shift from synthetic media to MWWpre-treated. The delay in anammox activity growth could therefore be interpreted by the acclimatization of the initial anammox population (e.g. to the composition or salinity of MWWpre-treated) or/and the development of a side population beneficial to it (e.g. organisms metabolizing and decreasing the fraction of compounds affecting the anammox bacteria in MWWpre-treated).
4.3. Heterotrophic substrates: depletion of soluble organics and the role of anammox
Heterotrophic denitrification and growth on NO2− and NO3− could explain less than half of the observed CODsol removal in batch tests (Fig. 4(b)). Yields of anoxic heterotrophic growth on soluble organic substrates in the range 0.9–0.97 gCODbiom/gCODsol should be postulated to explain all the COD consumption via heterotrophic denitrification. These values are, however, significantly higher than the ranges between 0.53 and 0.54 gCODbiom/gCODsol proposed in the literature (Gujer et al., 1999, Muller et al., 2003).
A stoichiometric model including anoxic intracellular storage of soluble organic matter was developed on the basis of ASM3 parameters (see Table S2, (Gujer et al., 1999, Muller et al., 2003)), and the observed CODsol consumption seemed to occur at an overall stoichiometry comparable to the modeled storage (Fig. 4(b)). Thus, anoxic storage of organics could have played an important role in the described system. The ability to store organics may in fact have represented a competitive advantage in the present system, as electron acceptors (NO2− and/or NO3−) were always present whereas the availability of electron donors (CODsol) was the limiting factor (Shimada et al. 2007). However, the storage products were not measured nor was their subsequent utilization characterized.
Nevertheless, to better understand the CODsol depletion in the studied sludge and to elucidate the role of anammox populations, sludge from R1 was incubated with radio-labeled acetate and glucose for microautoradiography analysis. Anammox are known to be able to oxidize acetate using NO3− as the electron acceptor (Kartal et al. 2008) while, apparently, they do not oxidize glucose directly (Jenni et al. 2014). Moreover, Winkler et al. (2012) have shown that anammox bacteria can outcompete heterotrophic denitrifiers in catabolizing acetate at ambient temperatures (18 °C) on a synthetic cultivation medium. In all conducted incubations, the probe-defined anammox bacteria were MAR-negative, proving that anammox did not directly incorporate or store the amended acetate and glucose in the present system (Fig. 5). A heterotrophic catabolic activity of anammox populations can however not be excluded.
These results constitute an independent confirmation that, under the tested conditions, anammox (“Ca. Brocadia fulgida”) do not incorporate organics as previously discussed for propionate (Güven et al. 2005) and acetate (Kartal et al. 2008).
4.4. Initial activity loss, sludge wash out and biofilm formation
Two distinct suspended sludges originating from sidestream nitritation/anammox reactors were used as inoculum sources and initially operated under micro-aerobic conditions for partial nitritation/anammox. The two biomasses featured comparable biomass compositions but substantially different morphologies (Fig. 3(a, e)) that apparently led to distinct responses to MWWpre-treated in Phase I (Fig. 1(b, d)). The large aggregates present in the R1 inoculum became progressively looser and broke down into smaller flocs with probably poorer settling properties. This possibly resulted in the washout of both AMX and AOB (Fig. 3(a–d)). It is speculated here that the change to lower operating oxygen and nitrogen concentrations, as compared to side-stream treatment, and the differences in rheological characteristics from a full- to lab-scale reactor resulted in the disruption of the floc structure. Conversely, the dense anammox aggregates in the R2 inoculum maintained their structure throughout Phase I, and only the small AOB micro-colonies were selectively washed out (Fig. 3(e–h)). The low oxygen concentrations applied to avoid the risk of oxygen inhibition of the anammox organisms probably did not sustain the growth of AOB. As a result of the washout of AOB, we suppose that the anammox were progressively exposed to less nitrite and higher oxygen concentrations. This could explain the marked loss of anammox activity despite the limited solids loss and the increase in anammox relative abundance (Fig. 1(c, d)).
These observations underline how particular attention should be paid to the treatment conditions under reactor start-up in order to maintain a balanced combined partial nitritation/anammox, especially when moving from side-to main-stream applications. The governing mechanisms for the observed sludge disintegration differences in Phase I remain unclear. However, (i) the greater stability of the dense aggregates and the reduced sludge loss in R2 together with (ii) the observed transition to a hybrid system in both reactors, with most biomass as biofilm on the walls, strongly indicate the potential advantages of using granular and biofilm biomasses for anammox-based MWW applications. This is expected to allow for better biomass retention finally resulting in higher volumetric activity.
5. Conclusions
The feasibility of mainstream anammox application was studied in three parallel reactors both on synthetic media and real municipal wastewater. The process performance investigations at reactor level were efficiently complemented by metabolic and molecular analyses and led to the following key conclusions of significant relevance for MWW treatment applications:
-
•
the active growth of anammox guild on aerobically pre-treated MWW (MWWpre-treated) was proved over the temperature range 12.5–29 °C with volumetric nitrogen removal rates in the same order of magnitude as in current municipal wastewater treatment systems;
-
•
direct unfavorable impacts of MWWpre-treated on anammox performance were revealed by a two to three-fold slower anammox growth compared to synthetic MWW;
-
•
“Ca. Brocadia fulgida” remained the dominant anammox candidate species throughout the experiment, as revealed by high-throughput 16S rRNA gene-targeted amplicon sequencing analyses;
-
•
direct incorporation or storage of organics (acetate and glucose) by the anammox organisms involved in the process could be excluded by means of combined microautoradiography and FISH analysis;
-
•
appropriate MWW pre-treatment, here with a high-rate aerobic activated sludge process, allowed potentially competing heterotrophic activity in the anoxic anammox stage to be significantly limited.
Acknowledgments
This study was funded by the European Research Council ERC via the ATHENE project (grant agreement 267897). We sincerely thank Dr. Per Falås for inspiring discussions and critically reviewing the manuscript, Sarina Jenni for useful inputs on anoxic storage modelling, Marco Kipf for his support in the laboratory, and Claudia Baenninger-Werffeli and Karin Rottermann at Eawag for their assistance with the analytical methods and the analysis of all our samples.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.watres.2015.04.026.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- American Public Health Association, E.A.D.A.W.W.A.W.E.F . APHA-AWWA-WEF; Washington, D.C.: 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Bürgmann H., Jenni S., Vazquez F., Udert K.M. Regime shift and microbial dynamics in a sequencing batch reactor for nitrification and anammox treatment of urine. Appl. Environ. Microbiol. 2011;77(17):5897–5907. doi: 10.1128/AEM.02986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daims H., Lücker S., Wagner M. daime, a novel image analysis program for microbial ecology and biofilm research. Environ. Microbiol. 2006;8(2):200–213. doi: 10.1111/j.1462-2920.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- De Clippeleir H., Vlaeminck S.E., De Wilde F., Daeninck K., Mosquera M., Boeckx P., Verstraete W., Boon N. One-stage partial nitritation/anammox at 15°C on pretreated sewage: feasibility demonstration at lab-scale. Appl. Microbiol. Biotechnol. 2013;97(23):10199–10210. doi: 10.1007/s00253-013-4744-x. [DOI] [PubMed] [Google Scholar]
- Galloway J.N., Aber J.D., Erisman J.W., Seitzinger S.P., Howarth R.W., Cowling E.B., Cosby B.J. The nitrogen cascade. BioScience. 2003;53(4):341–356. [Google Scholar]
- Gilbert E.M., Agrawal S., Karst S.M., Horn H., Nielsen P.H., Lackner S. Low temperature partial nitritation/anammox in a moving bed biofilm reactor treating low strength wastewater. Environ. Sci. Technol. 2014;48(15):8784–8792. doi: 10.1021/es501649m. [DOI] [PubMed] [Google Scholar]
- Gujer W., Henze M., Mino T., van Loosdrecht M.C.M. Activated sludge model no. 3. Water Sci. Technol. 1999;39(1):183–193. [Google Scholar]
- Güven D., Dapena A., Kartal B., Schmid M.C., Maas B., van de Pas-Schoonen K.T., Sozen S., Mendez R., Op den Camp H.J.M., Jetten M.S.M., Strous M., Schmidt I. Propionate oxidation by and methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl. Environ. Microbiol. 2005;71(2):1066–1071. doi: 10.1128/AEM.71.2.1066-1071.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx T.L.G., Kampman C., Zeeman G., Temmink H., Hu Z., Kartal B., Buisman C.J.N. High specific activity for anammox bacteria enriched from activated sludge at 10°C. Bioresour. Technol. 2014;163(0):214–221. doi: 10.1016/j.biortech.2014.04.025. [DOI] [PubMed] [Google Scholar]
- Hu Z., Lotti T., de Kreuk M., Kleerebezem R., van Loosdrecht M.C.M., Kruit J., Jetten M.S.M., Kartal B. Nitrogen removal by a nitritation-anammox bioreactor at low temperature. Appl. Environ. Microbiol. 2013;79(8):2807–2812. doi: 10.1128/AEM.03987-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka K., Date Y., Kimura Y., Sumino T., Tsuneda S. Nitrogen removal performance using anaerobic ammonium oxidation at low temperatures. FEMS Microbiol. Lett. 2008;282(1):32–38. doi: 10.1111/j.1574-6968.2008.01095.x. [DOI] [PubMed] [Google Scholar]
- Jenni S., Vlaeminck S.E., Morgenroth E., Udert K.M. Successful application of nitritation/anammox to wastewater with elevated organic carbon to ammonia ratios. Water Res. 2014;49:316–326. doi: 10.1016/j.watres.2013.10.073. [DOI] [PubMed] [Google Scholar]
- Joss A., Derlon N., Cyprien C., Burger S., Szivák I., Traber J., Siegrist H., Morgenroth E. Combined nitritation–anammox: advances in understanding process stability. Environ. Sci. Technol. 2011;45(22):9735–9742. doi: 10.1021/es202181v. [DOI] [PubMed] [Google Scholar]
- Kartal B., van Niftrik L., Rattray J., van de Vossenberg J.L.C.M., Schmid M.C., Sinninghe Damsté J., Jetten M.S.M., Strous M. Candidatus ‘Brocadia fulgida’: an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiol. Ecol. 2008;63(1):46–55. doi: 10.1111/j.1574-6941.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- Kindaichi T., Ito T., Okabe S. Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl. Environ. Microbiol. 2004;70(3):1641–1650. doi: 10.1128/AEM.70.3.1641-1650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner S., Gilbert E.M., Vlaeminck S.E., Joss A., Horn H., van Loosdrecht M.C.M. Full-scale partial nitritation/anammox experiences – an application survey. Water Res. 2014;55:292–303. doi: 10.1016/j.watres.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Lackner S., Terada A., Smets B.F. Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms: results of a modeling study. Water Res. 2008;42(4–5):1102–1112. doi: 10.1016/j.watres.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Lotti T., Kleerebezem R., van Erp Taalman Kip C., Hendrickx T.L.G., Kruit J., Hoekstra M., van Loosdrecht M.C.M. Anammox growth on pretreated municipal wastewater. Environ. Sci. Technol. 2014;48(14):7874–7880. doi: 10.1021/es500632k. [DOI] [PubMed] [Google Scholar]
- Lotti T., Kleerebezem R., van Loosdrecht M.C.M. Effect of temperature change on anammox activity. Biotechnol. Bioeng. 2015;112(1):98–103. doi: 10.1002/bit.25333. [DOI] [PubMed] [Google Scholar]
- Ma B., Peng Y., Zhang S., Wang J., Gan Y., Chang J., Wang S., Wang S., Zhu G. Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour. Technol. 2013;129:606–611. doi: 10.1016/j.biortech.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Metcalf & Eddy, I, Tchobanoglous G., Stensel H.D., Tsuchihashi R., Burton F. McGraw-Hill Education; 2013. Wastewater Engineering: Treatment and Resource Recovery. [Google Scholar]
- Mulder A., van de Graaf A.A., Robertson L.A., Kuenen J.G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 1995;16(3):177–184. [Google Scholar]
- Muller A.W., Wentzel M.C., Loewenthal R.E., Ekama G.A. Heterotroph anoxic yield in anoxic aerobic activated sludge systems treating municipal wastewater. Water Res. 2003;37(10):2435–2441. doi: 10.1016/S0043-1354(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Nielsen J.L., Nielsen P.H. Advances in microscopy: microautoradiography of single cells. Methods Enzym. 2005;397:237–256. doi: 10.1016/S0076-6879(05)97014-6. [DOI] [PubMed] [Google Scholar]
- Nielsen P.H., Daims H., Lemmer H. IWA Publishing; London: 2009. FISH Handbook for Biological Wastewater Treatment : Identification and Quantification of Microorganisms in Activated Sludge and Biofilms by FISH. [Google Scholar]
- Shimada T., Zilles J., Raskin L., Morgenroth E. Carbohydrate storage in anaerobic sequencing batch reactors. Water Res. 2007;41(20):4721–4729. doi: 10.1016/j.watres.2007.06.052. [DOI] [PubMed] [Google Scholar]
- Siegrist H., Salzgeber D., Eugster J., Joss A. Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci. Technol. 2008;57(3):383–388. doi: 10.2166/wst.2008.048. [DOI] [PubMed] [Google Scholar]
- Strous M., Fuerst J.A., Kramer E.H.M., Logemann S., Muyzer G., van de Pas-Schoonen K.T., Webb R., Kuenen J.G., Jetten M.S.M. Missing lithotroph identified as new planctomycete. Nature. 1999;400(6743):446–449. doi: 10.1038/22749. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M.C.M., Brdjanovic D. Water treatment. Anticipating the next century of wastewater treatment. Science. 2014;344(6191):1452–1453. doi: 10.1126/science.1255183. [DOI] [PubMed] [Google Scholar]
- Versprille A.I., Zuurveen B., Stein T. The A–B process: a novel two stage wastewater treatment system. Water Sci. Technol. 1985;17(2–3):235–246. [Google Scholar]
- Weissbrodt D.G., Wells G.F., Goel R.K., Laureni M., Bürgmann H., Johnson D.R., Men Y., Fischer S., Minder A., Aluri S., Harhangi H.R., Kipf M., Joss A., Christensson M., Nielsen J.L., Morgenroth E. A process engineering vista in the ecogenomics of aerobic-anaerobic ammonium oxidation. In: Oleszkiewicz, editor. IWA Nutrient Removal and Recovery 2015: Moving Innovation into Practice, May 18–21, 2015, Gdańsk, Poland. 2015. (accepted) [Google Scholar]
- Winkler M.K.H., Kleerebezem R., van Loosdrecht M.C.M. Integration of anammox into the aerobic granular sludge process for main stream wastewater treatment at ambient temperatures. Water Res. 2012;46(1):136–144. doi: 10.1016/j.watres.2011.10.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.