Abstract
Last few decades have witnessed remarkable progress in our understanding of cancer initiation and progression leading to refinement of prevention and treatment approaches. Although these advances have improved the survival of cancer patients in general, certain racial/ethnic groups have benefited only partially. Footprints of cancer-associated racial disparities are very much evident in cancers of the prostate, breast, cervical, colorectal, endometrium, liver and lung. These health inequalities are mostly attributed to socioeconomic differences among races, but there is a growing realization that these may actually be due to inherent biological differences as well. Indeed, significant data now exist to support the biological basis of racial disparities in cancer, warranting basic research investigations, using appropriate tools and model systems. In this article, we have aimed to succinctly review the literature supporting the biological bases of racial disparities in cancer, along with available resources, databases and model systems that will be of interest to researchers. Moreover, we have highlighted the specific areas that need attention in terms of development of resources and/or tools, and discuss the opportunities and challenges in basic biological research in cancer health disparities.
Keywords: Racial disparity, cell line model, PDX, biobanks, web-based databanks
Cancer health disparities: an overview
Cancer is the second leading cause of deaths in the United States, and is soon expected to surpass heart diseases to become the number one killer disease [1]. A closer look at cancer statistics indicates that certain racial groups are affected disproportionately with regard to cancer incidence, age of onset, aggressiveness and mortality. Although cancer health disparities are observed among several races and ethnic groups, these are most thoroughly investigated between African American (AA) and Caucasian Americans/European American (CA/EA) populations (Table 1). Increase in the efforts to generate cancer awareness and provide access to healthcare to enable cancer screening, and thus timely diagnosis and treatment, has not proven enough to reduce the widening gaps in cancer incidence and mortality among racial groups [2,3]. This has supported a notion that there is more to underlying causes of cancer health disparities besides just the socioeconomic factors including poverty, limited access to healthcare, lack of health insurance, lack of transportation, poor health literacy, under-use of screening, inconsistency in follow-up and differences in behavior and culture [2,4]. Indeed, emerging data now suggest that biological factors such as differences at the genetic and epigenetic levels can be crucial for racial inequalities in cancer incidence and clinical outcomes [5,6]. This recognition has pushed basic research to establish and precisely understand the involved biological mechanisms, and characterize associated factors, so that appropriate and more effective strategies can be planned to deal with. As a result, some new knowledge has evolved along with development of research tools and resources to help with the development of novel research hypotheses and execution of investigations. However, such information is staggered and not readily visible for someone wanting to initiate or expand basic research in cancer health disparities. In this review, we have summarized and discussed the evidence that supports the biological basis of racial disparities in cancer. We have also compiled a list of available resources and tools, which we believe will be appreciated by researchers.
Table 1.
Age-adjusted cancer-associated mortality rate of African Americans (AA) vs. Caucasian Americans (CA) population in the US from the surveillance, epidemiology and end results-9 (SEER-9) database for 2013. Rates are represented per 100,000 and age-adjusted to the 2000 US population
| Cancer Site | Female | Male | Both | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| AA | CA | Disparity Ratio (%) | AA | CA | Disparity Ratio (%) | AA | CA | Disparity Ratio (%) | |
| All sites | 158.42 | 139.68 | 13.41 | 240.0 | 195.69 | 18.46 | 189.54 | 163.34 | 13.87 |
| Breast | 28.19 | 20.26 | 39.14 | ||||||
| Colon and Rectum | 15.97 | 11.86 | 34.65 | 24.78 | 16.86 | 46.97 | 19.28 | 14.12 | 36.54 |
| Corpus and Uterus NOS | 8.29 | 4.30 | 92.7 | ||||||
| Liver and Intrahepatic Bile Duct | 4.26 | 3.63 | 17.35 | 13.44 | 8.78 | 53.07 | 8.38 | 6.02 | 39.20 |
| Lung and Bronchus | 34.10 | 36.61 | -12.04 | 65.93 | 53.84 | 22.45 | 46.92 | 44.09 | 6.41 |
| Prostate | 39.05 | 17.87 | 118.52 | ||||||
Molecular basis of cancer health disparities
Results from epidemiological studies suggest that a number of factors are responsible for cancer-associated health inequalities. While the most emphasis is placed on socioeconomic factors, recent findings strongly argue in favor of the significant participation of biological factors [5,7]. In this section, we discuss research findings that have indicated involvement of various biological factors in cancer health disparities.
Prostate cancer disparity
Prostate cancer (PC) is the second leading cause of cancer-related deaths in American men; it also exhibits the most prominent racial disparity [8]. The data from various studies suggest that hormones and their receptors may be involved in the manifestation of racial disparity, more specifically in cancers that are hormone-dependent, including PC [9,10]. Elevated levels of circulating active form of estrogen, 17β-estradiol, implicated in increased risk of prostate cancer [11], have been reported in AA than CA men [12]. Moreover, the data from immunohistochemical (IHC) staining of PC tissues have also revealed higher expression of ERβ in AA men relative to their CA counterparts [13]. While studying the correlation of prostate-specific antigen (PSA) with tumor size and volume in AA and CA patients, Moul JW et al. observed elevated PSA levels across AA patients of all ages, stages and grades of tumor [14]. Of note, AA men with newly diagnosed localized PC also exhibited elevated levels of PSA in serum [15]. In another study, IHC analysis of androgen receptor (AR) in benign and malignant prostate tissues demonstrated 22% higher AR expression in benign samples and 81% higher expression in malignant tissues obtained from AA patients, compared to tissues from CA patients [16]. Correlative epidemiological studies have implicated selenium deficiency in a number of chronic diseases, including cancer [17]. Selenium is documented to have an inverse relation with PC risk [18]. Therefore, observed higher serum selenium content in CA, compared to AA, may possibly explain the reduced PC in CA. In addition to the individual factors studied for their role in PC racial disparity, several investigations have also looked at the molecular signatures for the biological basis of disparity. For example, gene expression profiles of primary prostate tumors resected from AA and CA patients revealed differential expression of genes associated with growth, epithelial-mesenchymal transition (EMT) and metastasis.
Breast cancer disparity
Breast cancer (BC) is the second leading cause of cancer-related deaths in women in the United States [8]. Elevated levels of inflammatory cytokines have been proposed as prognostic factors for BC [19]. These cytokines have also been implicated in BC racial disparity, as the expression of pro-inflammatory cytokines such as resistin and interleukin 6 (IL-6) was found to be significantly high in serum sample of AA, compared to CA BC patients, and the level of resistin was found to correlate with the expression of IL-6 [20]. Interestingly, cell line derived from AA BC patient (MDA-MB-468) also exhibited higher growth and aggressiveness compared to CA cell line (MDA-MB-231) after resistin treatment, likely due to greater expression of its receptor adenylyl cyclase-associated protein 1 (CAP1) [20]. This was the first report implicating resistin and IL-6 in BC racial disparity, even though their role in BC risk has been reported previously [21-23]. Several studies have shown differential level of polymorphisms in IFN-γ and IL-6 in CA vs AA women [24,25]. Pro-inflammatory cytokines, IFN-γ and IL-6 are crucial in nursing chronic inflammation and have been implicated in tumor growth and metastasis [26]. Interestingly, significant differences were noted in IL-6 and IFN-γ levels between healthy CA and AA women after controlling relevant covariates [27]. Further, DNA methylation and gene expression profiling performed in histologically normal breast tissues of healthy women indicated 282 hypermethylated CpG islands in AAs, compared to 203 hypermethylated CpGs in healthy CA women. These differentially-methylated CpGs were associated with cell death, survival and cell-to-cell signaling [28], indicating a contribution of differentially-expressed cytokines and genetic factors in BC racial disparities. Similar to the studies in PC discussed above that looked at molecular signatures for the biological basis of racial disparity, a number of such studies have been conducted in BC as well. For instance, a comprehensive gene-expression analysis identified a number of differentially-expressed genes in AA vs CA primary BC tumors.
Colorectal cancer disparity
Colorectal cancer (CRC) is the third most common cause of cancer-related deaths in the United States, with more than 102,000 new diagnoses every year [29]. Analysis of total genome methylation and dysregulation of gene expression from AA malignant colon tissues detected 1,588 hypermethylated and 100 hypomethylated regions. Whereas, DNA from the tumor of CA CRC patients showed 109 hypermethylated and 4 hypomethylated regions, indicating 14.6 and 25 fold changes relative to AA [30]. In this analysis, anti-inflammatory genes such as NELL1, GDF1, ARHGEF4, and ITGA4, and miRNAs including miR-9-3p and miR-124-3p (known for their significant role in CRC) were found to be hypermethylated in tumors from AA patients [30]. RNAseq data of this study further revealed upregulation of 34 genes, including Cytochrome P450 1B1 (CYP1B1) in AA CRC patients, compared to CA patients. CYP1B1 metabolizes the exogenous and endogenous compounds into carcinogenic derivatives [31]. Further, it is shown to promote the metabolism of several chemo-therapeutic drugs, including 5-fluorouracil, vincristine, vinblastine, etoposide and cyclophosphamide [32]. The gene expression profiling of CRC samples identified differential expression of genes in AA vs. CA patients.
Endometrial cancer disparity
Endometrial cancer (EC) is the fourth most commonly diagnosed cancer among women in the United States [8]. It is also referred to as the cancer of the uterine corpus as majority of tumors develop in the endometrium, which is the lining of the uterus. Several studies have identified distinct molecular signatures in AA vs CA EC. Gene mutation analysis of EC frozen tissues suggested an association of mutated p53 overexpression with poor clinical outcome, which was a more frequent occurrence in AA, as compared to CA, in both early- (34% vs 11%, respectively) and advanced-stage (55% vs 25%, respectively) patients [33-35]. Specific haplotype (PTEN/10q), found to be significantly different in CA and AA women diagnosed with EC, has been linked with unfavorable outcome [34]. A study on uterine serous papillary endometrial cancer, a highly aggressive variant of EC, identified more frequent overexpression of HER2/neu oncogene in AA patients, compared to their CA counterparts.
Other cancers
Liver cancer (LC) is the fifth most common cause of cancer-related deaths in males, and eighth in females, in the United States [8]. Hepatitis C virus (HCV)-induced hepatocellular carcinoma (HCC) is believed to be a major contributor to higher incidence and mortality of LC in AA than other ethnic groups in the United States [36]. A proteomic analysis of liver tissues from HCV+/HCC+ patients identified 7-fold differential expression of Apolipoprotein A1 (APOA1) in AA HCC, as compared to CA HCC. Further, hepatocyte nuclear factor4α (HNF4α) protein was found to be downregulated in AA, as compared to CA [37], and lower levels of HNF4α were detected in metastatic HCCs [38]. Reduced HNF4α expression correlates with aggressive clinico-pathological characteristics of HCC, and predicts poor prognosis [39]. Furthermore, AA non-small cell lung cancer (NSCLC) patients have higher circulating levels of IL-1β, interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α), as compared to CA patients [40]. Moreover, differential expression of these cytokines is found to be associated with lung cancer in AA [40]. Finally, a population-based case-control study of ovarian cancers (OC) linked short CAG repeat length with increased OC risk in AA patients [41].
Available resources to investigate molecular basis of cancer health disparities
The preceding section provided evidence for potential biological basis of cancer racial disparities in different human cancers. While these evidences strongly support biological basis of cancer racial disparity, this knowledge is just emerging. A challenge that the researchers face is the availability of resources to perform innovative research connecting cancer health disparity with their biological causes. Clearly, available resources from published studies as well as clinical resources from racially diverse groups can be immensely helpful in furthering innovative research in cancer health disparities towards identifying novel molecular distinctions among racially diverse groups of patients.
Repositories of biospecimens
Biobanks are repositories of biological specimens, usually derived from humans, and collected under specified guidelines for patient consent and enrollment, specimen collection, processing, annotation, storage and distribution. Many different kinds of biobanks are available these days that range from federally-supported to University-based to even ones that are purely commercial. Biorepositories have contributed to cancer research by lowering the cost and increasing the pace of research. Specimens can be shared by many projects and hence minimize the concerns about cohort size. The type of biospecimens include frozen tissues, formalin-fixed paraffin-embedded tissues, fresh tissues, blood or blood products, ascites/peritoneal fluid, urine, saliva, isolated cells, DNA and protein from both normal and tumor patients. These samples are of great significance in medical research and support studies related to genomics, personalized medicine and racial disparity. Nearly all major cancer institutes in the United States maintain their own biobanks, from which samples are available to researchers upon request, with some associated costs. It is beyond the scope of this article to list the hundreds of available biobank/biorepositories; however we have listed some prominent university/institutional based repositories of specimens in Table 2. Specimens belonging to different ethnicities can usually be obtained from these biobanks helping researchers unveil the mechanism of racial disparity in different types of cancers. Furthermore, the development and organization of such biobanks has facilitated extensive collaborative efforts between research groups from around the globe [42].
Table 2.
Repositories of ethnically diverse biospecimens*
| S. No | Repositories of biospecimens | |
|---|---|---|
|
| ||
| Name | Website | |
| 1 | Cooperative Human Tissue Network | http://www.chtn.org |
| 2 | Trans-Hit Biomarker | http://www.trans-hit.com |
| 3 | Tissue For Research | http://www.tissue4research.com |
| 4 | Gundersen Foundation Biobank | http://www.gundersenhealth.org/biobank |
| 5 | Cureline | http://www.cureline.com |
| 6 | Cell & Co. Biorepository | http://www.cell-and-co.com |
| 7 | American Type Culture Collection | http://www.atcc.org |
This non-exhaustive table lists representative repositories for biospecimens, which the researchers can contact as the starting points for their research needs.
Web-based databanks
The advancement of experimental technologies and integration of computational sciences in biology has aided in the significant strides being made to understand the molecular basis of diseases, particularly cancer. Furthermore, with the development of modern sequencing platforms, the cost of sequencing has considerably decreased, generating enormous data and minute details to quantify gene expression, miRNA expression and identification of alternative splicing events. Mass Spectrometry has also been employed to generate significant proteomics data comparing CA and AA tumors and several proteomics repositories have been setup to store and share the information generated. On similar lines, differences in the metabolome of CA and AA patients are also being explored to understand the altered onco-metabolic pathways. However, a publicly available repository for metabolomics studies is still lacking. A non-exhaustive list of databanks is presented in Table 3.
Table 3.
List of web-based databanks*
| S. No | Databank | |
|---|---|---|
|
| ||
| Name | Website | |
| 1 | The Cancer Genome Atlas | http://cancergenome.nih.gov |
| 2 | International Cancer Genome Consortium | http://icgc.org |
| 3 | Catalogue of Somatic Mutation in Cancer | http://cancer.sanger.ac.uk/cosmic |
| 4 | Collaborative Cancer Cell Line Encyclopedia | http://www.broadinstitute.org/ccle |
| 5 | Myriad | http://www.myriadtests.com |
| 6 | Peptide Atlas | http://www.peptideatlas.org |
| 7 | Global Proteome Machine Database | http://gpmdb.thegpm.org |
| 8 | Tranche | http://www.tranche.proteomecommons.org |
| 9 | PRIDE Proteomics Identification | http://www.ebi.ac.uk/pride/ |
| 10 | Human Proteinpedia | http://www.humanproteinpedia.org |
| 11 | Yeast Resource Center | http://depts.washington.edu/yeastrc |
This non-exhaustive list provides information on some of the prominent databanks that can serve as excellent starting points for interested researchers.
Novel and established models expediting cancer health disparities research
Suggested biological basis of racial disparity needs to be investigated further to establish cause-effect relationship. Moreover, involved molecular mechanisms need to be pinned down further to help in the planning of effective strategies to reduce the disparity gaps. The recruitment of patients for exploratory experimentation is restricted by ethical and safety issues. Hence, it is crucial to develop appropriate model systems to help understand molecular and biological basis of racial disparity, so that advancements could be made in cancer prevention and therapy. The routinely used laboratory models in cancer research include established cancer cell lines, xenograft-mouse models and genetically-engineered spontaneous progression mouse models. In this section, we discuss the importance of these model systems and provide information on commercially available cell lines from distinct ethnic backgrounds that can be utilized in disparity-focused research. We further advocate the need of new cell lines along with the generation of patient-derived xenografts or orthotropic mouse models.
Cancer cell lines for in vitro research
Patient-derived cancer cell lines have emerged as important models and invaluable tools in cancer research. Racial disparity in cancer is manifested in the form of incidences, aggressiveness, mortality as well as drug-resistance [43]. Cancer cell lines are routinely used as the first-line models to test drug potency [44-46]. Since the cell lines derived from different patients recapitulate the genetic background of the individual tumors, they serve as excellent tools to study the genetic and epigenetic differences. Further, the use of cell lines of diverse genetic background brings substantial weightage to the understanding of signaling pathways pertaining to a particular therapeutic response. In addition, cell lines facilitate the repetition of experiment on genetically identical cells, which cannot be substituted by primary cell lines obtained from multiple tissue donors. Another advantage of cell lines is that they provide a unique opportunity to manipulate the genetic content, which allows functional characterization of genes and their signaling network(s) pertaining to their roles in disease pathobiology. Although animal models, wherever available, are the most appropriate model systems for research, their use in certain studies including manipulations for the genetic and epigenetic analyses is difficult to practice. Under these circumstances, cell lines serve as a feasible alternative. They are easy to manipulate and molecularly characterize, and also address the potential ethical concerns [47]. The exceptional benefits of cell culture, such as, continuous supply of live cells and controllable experimental factors, have proven to be helpful in determining the major player(s) of differential tumor progression in diverse ethnic groups [47].
Considering the importance of in vitro model systems, significant efforts have been devoted for the establishment of cell lines from cancer patients. This endeavor resulted in development of several cell lines, although unintentionally, derived from racially disparate cancers (Table 4). These cell lines represent an excellent resource for continuing mechanistic studies on biological basis of cancer disparity, but there clearly is need for the development of more immortal cell lines for specific racial groups. Cell lines derived from different ethnic background can be helpful in understanding the biological factors that lead to adverse clinical outcome in a particular race.
Table 4.
Comprehensive list of cell lines derived from patients of different racial backgrounds
| Cancer | Race | Cell lines |
|---|---|---|
| Breast cancer | CA | AU565, BT-20±, BT-474, BT-549±, CAMA-1, DU4475±, HCC38±, HCC202, HCC1143±, HCC1187±, HCC1395±, HCC1428, HCC1599±, HCC1937, HCC2218, Hs 578T±, MCF7, MCF 10A, MCF-12A, MDA-kb2, MDA-MB-134-VI, MDA-MB-231±, MDA-MB-361, MDA-MB-436±, MDA-MB-453±, SK-BR-3, UACC-893, ZR-75-1 |
| AA | HCC70±, HCC1500, HCC1569, HCC1806±, HCC2157, MDA-MB-157±, MDA-MB-175-VII, MDA-MB-468±, ZR-75-30 | |
| Colorectal cancer | CA | SK-CO-1~, SW1116, SW948, LS123, SW837, SW48, COLO 205~, SW1417, LS411N, NCI-H508, HT-29, WiDr, LS 174T, DLD-1, LS1034, SW480, SW620~ |
| AA | NONE | |
| Cervical cancer | CA | Hs 588.T, C-4 I, C-4 II, C-33 A |
| AA | HeLa, HeLa 229, HeLa S3, H1HeLa, HeLaRC32 | |
| Asian | SiHa | |
| Ovarian cancer | CA | Caov-3, UACC-2727, OVCAR-3, TOV-112D, TOV-21G, SKOV-3, OV-90~, Caov-4, SW 626, PA-1 |
| AA | ES-2# | |
| Uterus and endometrial cancer | CA | SK-UT-1, SK-UT-1B, MES-SA#, MES-SA/MX2#, KLE, RL95-2, AN3 CA |
| AA | None | |
| Liver cancer | CA | C3A, HEP G2/2.2.1, Hep G2/HB-8065 |
| AA | Hep 3B2.1-7 | |
| Asian | SNU-449, SNU-475, SNU-387, SNU-398 | |
| Prostate cancer | CA | DU 145~, PC3~, LNCaP clone FGC*,~, CA-HPV-10, DuCaP*,~, RC-92a/hTERT, RC-170N/hTERT, RC-165N/hTERT+ |
| AA | E006AA-hT*, E006AA*, MDA PCa 2b+,~, S006AA*, RC-77N/E+, RC-77T/E+ | |
| Lung cancer | CA | NCI-H838~, HCC827, SK-LU-1, HCC2935, HCC4006~, NCI-H1819~, NCI-H676B~, Calu-3~, Hs 618.T#, HBE4-E6/E7, NCI-H1666~, NCI-H1568, NCI-H2126~, NCI-H596, SW 1573, MSTO-211H#,~, Hs 573.T#, Hs 573.Lu#, NCI-H727, A549, A-427, A549, NCI-H1688~, NCI-H187~, NCI-H661~, NCI-H1299~, NCI-H1155~, DMS 114, NCI-H69, DMS 79, DMS 53, SW 1271, SHP-77, NCI-H209~, NCI-H146~, NCI-H345~, DMS 153, NCI-H82~, NCI-H446~, NCI-H510A~, H69AR, Calu-1~, SW 900, NCI-H1703, SK-MES-1~, NCI-H2347, NCI-H2087~, NCI-H1437~, NCI-H2066, DMS 153, H2286, H1395, H522, H2073, H2342, H1993, H2023, H647, H1623, H1944, H1650, H1781, H1693, H1792, H2009, H1355, H1573, H2405, H1755, H920, H2122, H1770, H1581, H2106, H1869, H1915~, H889, H1417, H1694, H719, H1092, H740, H1105, H1436, H1876, H378, H2081, H774, H711, H2227, H211, H1238, H1618, H1882, H2195, H2196, H2029, H2141, H2198, H2171, H735~, H64, H526, H196, H1672, H1836, H1930, H865, H847, H1963, H1184, H2330, H524, H841 |
| AA | NCI-H23, NCI-H835, CCD-13Lu#, NCI-H128~, HLF-a#, NCI-H292, H810, H1648, H1385, H250, H748, H220, H2107, H2108 |
(androgen dependent);
(androgen sensitive);
(fibroblast);
(triple negative);
(derived from metastatic site);
S006AA is prostate cancer associated stromal cells.
Although the section above on racial disparity in individual cancers briefly describes a few studies that have employed cell line models to understand cancer racial disparity, additionally, it has been demonstrated that the racial differences in BC might even exist at the level of cancer stem cells (CSCs). Breast CSCs isolated from AA cell lines, CRL-2335 and MDA-MB-468, showed significantly longer survival and greater ability to develop mammospheres, compared to those from CA triple-negative breast cancer (TNBC) cell lines, BT549 and MDA-MB-231. Further, treatment with cisplatin plus TNF-related apoptosis-inducing ligand (TRAIL) decreased expression of miR-23b and miR-100 and significantly increased FZD8, a Wnt receptor crucial for CSC renewal, more profoundly in AA cell lines, as compared to CA cell lines [48,49]. The CSCs are believed to be involved in the initiation and progression of many malignancies, including CRC [50-52]. Another independent study suggested that the number of adenomas, which is significantly higher (48%) in AA, is associated with 50-80% higher CSC signature, CD44+ CD166-, in the colonic effluent and colonic mucosa from AA, compared to CA patients [53].
The studies described above are good examples of how investigations using cell lines have led the way in understanding the biological basis of cancer racial disparity. These studies have played a key role in re-enforcing the biological basis of racial disparity. A pubmed search in October 2016 for literature with the keywords ‘cancer cell line’ returned 1,83,004 articles, highlighting the importance of cancer cell lines in cancer research. However, when searched for ‘racial disparity and cancer cell line’ only 6 publications were retrieved, suggesting that this system has not been exploited much in the study of the racial disparity. This may be partly due to unavailability of cancer cell lines derived from different ethnic backgrounds. In order to study racial disparity in cancers such as prostate and breast, we do have available cell lines of AA vs CA origin, however, as is obvious from the list of cell lines (Table 4), there remains an urgent need to develop and establish cell lines for colorectal, uterus and endometrial cancers of AA background. Racial disparity is prominent in these cancers with a disparity ratio of 36.54% for both sexes in CRC and 92.7% for females in uterus and EC (Table 1). The availability of cell lines from different ethnic backgrounds will help us elucidate the mechanisms and signaling pathways behind the disparity. Therefore, in such circumstances it is practically difficult to determine the molecular differences between CA and AA ethnic background in vitro.
Translational model with clinical implications: patients-derived xenografts
Tumor heterogeneity poses one of the biggest challenges in development of effective cancer therapeutics and has led to use of a newer type of tumor model i.e. Patients-Derived Xenograft (PDX). PDX models are created by transplanting tissues from patients’ primary tumors directly into immunodeficient mice and thus reflect the biological characteristics of human tumors more accurately than cell line-derived xenografts. PDX models are being used in pre-clinical research to test the efficacy of newer drugs due to their closer similarity with tumor in terms of tumor heterogeneity, tumor architecture as well as tumor microenvironment [54,55]. Since the TME is implicated in tumor aggressiveness, drug resistance as well as in response to therapy, PDX models are now widely employed to study tumor heterogeneity, therapeutic target validation, and sensitivity and resistance to therapy [56]. They also mimic human clinical trials and are useful tools for testing the drugs or combinations of drugs prior to their actual testing in human patients [56,57].
To study the drug responses in racially disparate BC, AA and CA PDX models have been generated [52,58]. Azizi et al. generated PDX models utilizing tissues from various ethnic groups. Gene expression was analyzed in single cells and, interestingly, significant differences were observed in the expression of cancer-associated genes between each ethic group of BC PDX samples [59]. These PDX models revealed differential expression of genes such as Vimentin, EpCAM, HER2, CDH1, CDH2, TGFβ1, cytokeratins, GATA3 and MKI67, which are associated with EMT phenotype. In addition, differences in the expression of genes such as YAP1, TM4SF1, TSPAN6, AMOTL2, STAP2 and ANXA3 were also detected in the PDX samples. Furthermore, CSC markers ALDH1a1, ALDH1a3, CD44, CD24, and CD133 were found to be differentially-expressed in different PDX single cell samples. In pancreatic carcinoma, the orthotopic implantation more accurately predicted a patient’s response to chemotherapy than those models where implantation was done at a heterologous site [60]. Considering the advantages of PDX models in clinical trials, a Public Repository of Xenografts has been generated by orthotopic implantation of well-characterized leukemia and lymphoma (PRoXe, http://www.proxe.org).
Perspectives and future direction
Although the non-biological factors have long been held responsible for the observed racial disparities in cancer incidence and/or clinical outcome, recent years have witnessed a growing realization for the biological basis of cancer racial disparity. This has led to hunt for appropriate resources and models for a meaningful research. Here, we have provided a comprehensive overview and assessment of the available resources and tools that can potentially be utilized to explore the biological basis of health disparities in cancer (Figure 1). More than anywhere else, research on cancer health disparity relies heavily on available specimens from patients representing distinct racial backgro-unds. As pointed out, a number of biobanks and repositories are available, almost at every major institution, and several that are either developed through federal funds or for commercial purposes. With each repository being maintained individually, it warrants a major ground work to identify and procure specimens of interest. An integration of all such repositories or the possibility of a search engine with filtering capabilities would be a dream-come-true for researchers in the field. The endless possibilities that these specimens can be subjected to, from arrays to systemic profiling, has helped generate enormous data which, even when aided by numerous analyses software/platforms, is still overwhelming at times.
Figure 1.
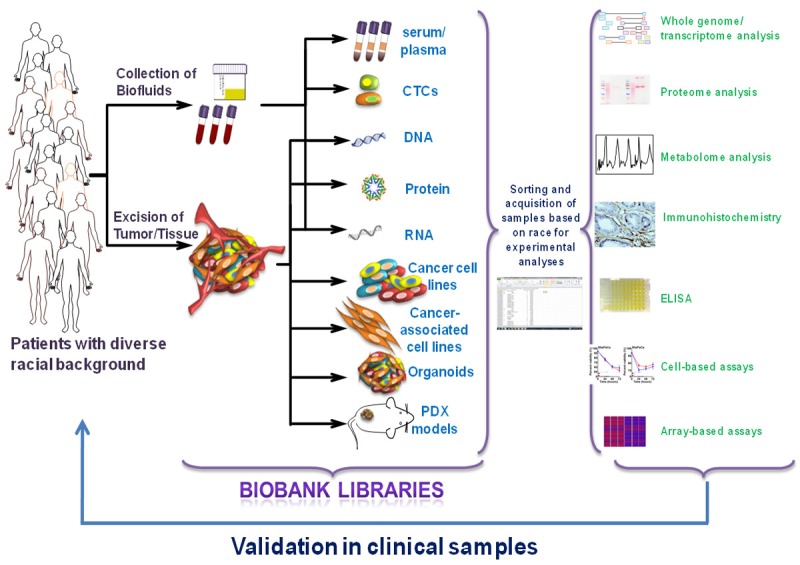
Tools to study cancer racial disparity. Patient derived samples; such as body fluids, excised tumor or biopsy tissue, from cohorts of diverse ethnic backgrounds are collected and maintained by biobanks/biorepositories. Biofluids can be used to isolate circulating tumor cells (CTCs), DNA, RNA, protein, which can be processed further in vitro to identify potential biological factors associated with disparity. Excised tumor or biopsy tissue can be the source of DNA, RNA, protein, cancer cell lines, cancer-associated tumor microenvironment cells, organoid culture and patient-derived xenografts (PDX) engraftment. The samples available from different Institutional/commercial biobanks can be sorted and acquired for detailed investigations. Samples can be subjected to detailed molecular/functional profiling and/or arrays in the search for novel factors underlining the biological basis of cancer racial disparity. As a final step that completes the loop, any novel findings can be validated in clinical samples from biobanks.
Both in vitro and in vivo models have their utility in cancer racial disparity research. With their versatility, ease of handling and possibility of genetic/molecular modifications, cell line-based models have become an important tool in the validation and mechanistic studies. Appropriate cell line(s) can be selected to improve current understanding of racial disparity, which can further lead to the development and testing of novel therapeutics to ultimately reduce the disparity gaps in clinical outcomes. However, as identified above, there is need for the development of AA cell lines representing individual cancers. With the increase in Hispanic and Asian populations in the US, and the realization that these populations might also have disparate cancer incidence and mortality, at least for a few specific cancers, the need for cell lines representing these ‘new’ population groups has also suddenly sprung. Understanding gained by the use of several resources; primary tumors, xenografts, paraffin-embedded samples, genetically-engineered mice and cancer cell lines, has opened uncharted territory of therapeutics to counter cancer. The same resources are being utilized for cancer racial disparity research, which has necessitated adaptation of these resources to fit the unique needs and requirements of research focused on cancer racial disparity. Last several years of efforts have resulted in development and/or adaptation of several resources to serve the needs of racial disparity research and this is predicted to continue, given the general increased interest in the research topic and the increased federal funding supporting such investigations.
Acknowledgements
This work was supported by the National Institute of Health/National Cancer Institute (CA185490 to APS and CA204801 to SS) and the University of South Alabama Mitchell Cancer Institute.
Disclosure of conflict of interest
APS and SS are co-founders, and serve on executive management team of Tatva Biosciences LLC, which is involved in the development of tools and models for cancer health disparity research. SKS serves as the Director of Cell Biology and Genetics at Tatva Biosciences LLC.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sankin A, Cohen J, Wang H, Macchia RJ, Karanikolas N. Rate of renal cell carcinoma subtypes in different races. Int Braz J Urol. 2011;37:29–32. doi: 10.1590/s1677-55382011000100004. [DOI] [PubMed] [Google Scholar]
- 3.Hayanga AJ, Zeliadt SB, Backhus LM. Residential segregation and lung cancer mortality in the United States. JAMA Surg. 2013;148:37–42. doi: 10.1001/jamasurgery.2013.408. [DOI] [PubMed] [Google Scholar]
- 4.Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff. 2005;24:325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- 5.Wallace TA, Martin DN, Ambs S. Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis. 2011;32:1107–1121. doi: 10.1093/carcin/bgr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65:221–238. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 7.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 9.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura T, Takahashi S, Kume H, Urano T, Takayama K, Yamada Y, Suzuki M, Fukuhara H, Nakagawa T, Inoue S, Homma Y. Toremifene, a selective estrogen receptor modulator, significantly improved biochemical recurrence in bone metastatic prostate cancer: a randomized controlled phase II a trial. BMC Cancer. 2015;15:015–1871. doi: 10.1186/s12885-015-1871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, Yager JD, Platz EA. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92:2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Christenson P, Swerdloff R. Editorial: clinical relevance of racial and ethnic differences in sex steroids. J Clin Endocrinol Metab. 2007;92:2433–2435. doi: 10.1210/jc.2007-1085. [DOI] [PubMed] [Google Scholar]
- 13.Abd Elmageed ZY, Moroz K, Srivastav SK, Fang Z, Crawford BE, Moparty K, Thomas R, Abdel-Mageed AB. High circulating estrogens and selective expression of ERbeta in prostate tumors of Americans: implications for racial disparity of prostate cancer. Carcinogenesis. 2013;34:2017–2023. doi: 10.1093/carcin/bgt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moul JW, Sesterhenn IA, Connelly RR, Douglas T, Srivastava S, Mostofi FK, McLeod DG. Prostate-specific antigen values at the time of prostate cancer diagnosis in African-American men. JAMA. 1995;274:1277–1281. [PubMed] [Google Scholar]
- 15.Vijayakumar S, Quadri SF, Dong L, Ignacio L, Kathuria IN, Sutton H, Halpern H. Results of a study to correlate serum prostate specific antigen and reproductive hormone levels in patients with localized prostate cancer. J Natl Med Assoc. 1995;87:813–819. [PMC free article] [PubMed] [Google Scholar]
- 16.Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Transl Res. 2009;1:235–248. [PMC free article] [PubMed] [Google Scholar]
- 17.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–1854. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 18.Vogt TM, Ziegler RG, Graubard BI, Swanson CA, Greenberg RS, Schoenberg JB, Swanson GM, Hayes RB, Mayne ST. Serum selenium and risk of prostate cancer in U. S. blacks and whites. Int J Cancer. 2003;103:664–670. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- 19.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, Dyess DL, Dal Zotto V, Carter JE, Singh S. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6:11231–11241. doi: 10.18632/oncotarget.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007;22:117–121. doi: 10.3346/jkms.2007.22.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun CA, Wu MH, Chu CH, Chou YC, Hsu GC, Yang T, Chou WY, Yu CP, Yu JC. Adipocytokine resistin and breast cancer risk. Breast Cancer Res Treat. 2010;123:869–876. doi: 10.1007/s10549-010-0792-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. [PubMed] [Google Scholar]
- 24.Govan VA, Carrara HR, Sachs JA, Hoffman M, Stanczuk GA, Williamson AL. Ethnic differences in allelic distribution of IFN-g in South African women but no link with cervical cancer. J Carcinog. 2003;2:3. doi: 10.1186/1477-3163-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and white Americans. Am J Epidemiol. 2004;160:1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 26.Cole SW. Chronic inflammation and breast cancer recurrence. J. Clin. Oncol. 2009;27:3418–3419. doi: 10.1200/JCO.2009.21.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park NJ, Kang DH. Inflammatory cytokine levels and breast cancer risk factors: racial differences of healthy caucasian and African American women. Oncol Nurs Forum. 2013;40:490–500. doi: 10.1188/13.ONF.40-05AP. [DOI] [PubMed] [Google Scholar]
- 28.Song MA, Brasky TM, Marian C, Weng DY, Taslim C, Dumitrescu RG, Llanos AA, Freudenheim JL, Shields PG. Racial differences in genome-wide methylation profiling and gene expression in breast tissues from healthy women. Epigenetics. 2015;10:1177–1187. doi: 10.1080/15592294.2015.1121362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howlader N, Ries LA, Stinchcomb DG, Edwards BK. The impact of underreported Veterans Affairs data on national cancer statistics: analysis using population-based SEER registries. J Natl Cancer Inst. 2009;101:533–536. doi: 10.1093/jnci/djn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Ji P, Zhang Y, LaComb JF, Tian X, Li E, Williams JL. Aberrant DNA methylation: implications in racial health disparity. PLoS One. 2016;11:0153125. doi: 10.1371/journal.pone.0153125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgoda R, Badal S. CYP1B1: friend or foe? A critical review. OA Biochemistry. 2013;1:8. [Google Scholar]
- 32.Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL. Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther. 2001;296:537–541. [PubMed] [Google Scholar]
- 33.Clifford SL, Kaminetsky CP, Cirisano FD, Dodge R, Soper JT, Clarke-Pearson DL, Berchuck A. Racial disparity in overexpression of the p53 tumor suppressor gene in stage I. Am J Obstet Gynecol. 1997;176:S229–232. doi: 10.1016/s0002-9378(97)70380-6. [DOI] [PubMed] [Google Scholar]
- 34.Sutton J, Orloff MS, Michener C, Chiesa-Vottero A, Prayson R, Nowacki AS, Eng C. Association of specific PTEN/10q haplotypes with endometrial cancer phenotypes in African-American and European American women. Gynecol Oncol. 2015;138:434–440. doi: 10.1016/j.ygyno.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell GL, Risinger JI, Hayes KA, Alvarez AA, Dodge RK, Barrett JC, Berchuck A. Racial disparity in the frequency of PTEN mutations, but not microsatellite. Clin Cancer Res. 2000;6:2999–3005. [PubMed] [Google Scholar]
- 36.De Oliveria Andrade LJ, D’ Oliveira A, Melo RC, De Souza EC, Costa Silva CA, Parana R. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1:33–37. doi: 10.4103/0974-777X.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon ST, Bhasin MK, Feng X, Koh DW, Daoud SS. Quantitative proteomic analysis in HCV-induced HCC reveals sets of proteins with potential significance for racial disparity. J Transl Med. 2013;11:1479–5876. doi: 10.1186/1479-5876-11-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Ning BF, Xu WP, Yin C, Zhang X, Xie WF. HNF4α inhibits liver cancer metastasis via suppression of NF-кB activity. [abstract] Proceedings of the 105th Annual Meeting of the American Association for Cancer Res. 2014:74. [Google Scholar]
- 39.Chellappa K, Robertson GR, Sladek FM. HNF4alpha: a new biomarker in colon cancer? Biomark Med. 2012;6:297–300. doi: 10.2217/bmm.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pine SR, Mechanic LE, Enewold L, Bowman ED, Ryan BM, Cote ML, Wenzlaff AS, Loffredo CA, Olivo-Marston S, Chaturvedi A, Caporaso NE, Schwartz AG, Harris CC. Differential serum cytokine levels and risk of lung cancer between African and European Americans. Cancer Epidemiol Biomarkers Prev. 2016;25:488–497. doi: 10.1158/1055-9965.EPI-15-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schildkraut JM, Murphy SK, Palmieri RT, Iversen E, Moorman PG, Huang Z, Halabi S, Calingaert B, Gusberg A, Marks JR, Berchuck A. Trinucleotide repeat polymorphisms in the androgen receptor gene and risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:473–480. doi: 10.1158/1055-9965.EPI-06-0868. [DOI] [PubMed] [Google Scholar]
- 42.Brankovic I, Malogajski J, Morre SA. Biobanking and translation of human genetics and genomics for infectious diseases. Appl Transl Genom. 2014;3:30–35. doi: 10.1016/j.atg.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balan V, Nangia-Makker P, Schwartz AG, Jung YS, Tait L, Hogan V, Raz T, Wang Y, Yang ZQ, Wu GS, Guo Y, Li H, Abrams J, Couch FJ, Lingle WL, Lloyd RV, Ethier SP, Tainsky MA, Raz A. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res. 2008;68:10045–10050. doi: 10.1158/0008-5472.CAN-08-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishiwata T. Cancer stem cells and epithelial-mesenchymal transition: novel therapeutic targets for cancer. Pathol Int. 2016;10:12447. doi: 10.1111/pin.12447. [DOI] [PubMed] [Google Scholar]
- 45.Tyagi N, Marimuthu S, Bhardwaj A, Deshmukh SK, Srivastava SK, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 (PAK4) maintains stem cell-like phenotypes in pancreatic cancer cells through activation of STAT3 signaling. Cancer Lett. 2016;370:260–267. doi: 10.1016/j.canlet.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Staveren WC, Solis DY, Hebrant A, Detours V, Dumont JE, Maenhaut C. Human cancer cell lines: experimental models for cancer cells in situ? for cancer stem cells? Biochim Biophys Acta. 2009;2:92–103. doi: 10.1016/j.bbcan.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Yin S, Xu L, Bandyopadhyay S, Sethi S, Reddy KB. Cisplatin and TRAIL enhance breast cancer stem cell death. Int J Oncol. 2011;39:891–898. doi: 10.3892/ijo.2011.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L, Yin S, Banerjee S, Sarkar F, Reddy KB. Enhanced anticancer effect of the combination of cisplatin and TRAIL in triple-negative breast tumor cells. Mol Cancer Ther. 2011;10:550–557. doi: 10.1158/1535-7163.MCT-10-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–2265. [PubMed] [Google Scholar]
- 51.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med. 2009;87:1097–1104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 52.Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 2015;17:015–0523. doi: 10.1186/s13058-015-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farhana L, Antaki F, Anees MR, Nangia-Makker P, Judd S, Hadden T, Levi E, Murshed F, Yu Y, Van Buren E, Ahmed K, Dyson G, Majumdar AP. Role of cancer stem cells in racial disparity in colorectal cancer. Cancer Med. 2016;14:690. doi: 10.1002/cam4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassidy JW, Caldas C, Bruna A. Maintaining tumor heterogeneity in patient-derived tumor xenografts. Cancer Res. 2015;75:2963–2968. doi: 10.1158/0008-5472.CAN-15-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sia D, Moeini A, Labgaa I, Villanueva A. The future of patient-derived tumor xenografts in cancer treatment. Pharmacogenomics. 2015;16:1671–1683. doi: 10.2217/pgs.15.102. [DOI] [PubMed] [Google Scholar]
- 56.Scott CL, Becker MA, Haluska P, Samimi G. Patient-derived xenograft models to improve targeted therapy in epithelial ovarian cancer treatment. Front Oncol. 2013;3:00295. doi: 10.3389/fonc.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ter Brugge P, Kristel P, van der Burg E, Boon U, de Maaker M, Lips E, Mulder L, de Ruiter J, Moutinho C, Gevensleben H, Marangoni E, Majewski I, Jozwiak K, Kloosterman W, van Roosmalen M, Duran K, Hogervorst F, Turner N, Esteller M, Cuppen E, Wesseling J, Jonkers J. Mechanisms of therapy resistance in patient-derived xenograft models of BRCA1-deficient breast cancer. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw148. [DOI] [PubMed] [Google Scholar]
- 58.Luther TK, Jiagge E, Lewis MT, Monsma D, Webb C, Liu S, Korkaya H, McDermott S, Clouthier SG, Newman L, Thomas D, Wicha MS. (November 9-12, 2014; San Antonio, TX) Creating a comprehensive patient-derived xenograft (PDX) bank to represent racial disparities in triple-negative breast cancer (TNBC) In Proceedings of The Seventh AACR Conference on The Science of Health Disparities in Racial/Ethnic Minorities and The Medically Underserved. Vol. 24 [Google Scholar]
- 59.Azizi E, Jiagge EM, Fouladdel S, Wong S, Dziubinski ML, Sehl M, Kyani A, Li J, Jiang H, Luther TK, Clouthier SG, McDermott SP, Carpten J, Newman LA, Merajver SD, Wicha MS. (April 18-22, 2015; Philadelphia, PA) Single cell multiplex gene expression analysis to unravel heterogeneity of PDX samples established from tumors of breast cancer patients with different ethnicity. In Proceedings of The 106th Annual Meeting of The American Association for Cancer Research. Vol. 75 [Google Scholar]
- 60.Garrido-Laguna I, Uson M, Rajeshkumar NV, Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor G, Sharma R, Hruban RH, Maitra A, Laheru D, Rubio-Viqueira B, Jimeno A, Hidalgo M. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res. 2011;17:5793–5800. doi: 10.1158/1078-0432.CCR-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]


