Abstract
Cisplatin is a common chemotherapeutic drug for cancer treatment, but its effect is limited because of its cytotoxicity and chemoresistance. The combinational use of cisplatin with some natural compounds has provided a potential option to improve its effect and lower its side effects in cancer treatment. Here, we investigated the role of melatonin in the regulation of cisplatin-mediated antitumor activity in hepatocellular carcinoma cells. The combined treatment of cisplatin with melatonin significantly inhibited cell proliferation and resulted in a corresponding decrease of the IC50 values of cisplatin in four hepatocellular carcinoma cell lines. Cotreatment with melatonin also increased the cisplatin-induced apoptosis in hepatocellular carcinoma cells compared with cisplatin treatment alone. Further mechanism studies showed that the combined treatment of melatonin and cisplatin enhanced the cleavage of caspase-3, caspase-9 and poly-(ADP-ribose) polymerase (PARP), decreased the expression of Bcl-2 and p-IKKα/β, suppressed the nuclear translocation of NF-κB p50/p65 proteins, and abrogated the binding of p65 to COX-2 promoter, thereby inhibiting COX-2 expression. Furthermore, melatonin was found to synergistically enhance cisplatin-mediated inhibition of AP-2β and hTERT expression. Overexpression of AP-2β reversely rescued this inhibition mediated by the combined treatment of these two drugs. Collectively, our results demonstrated that melatonin sensitizes the cisplatin-mediated growth suppression of cells via the inactivation of NF-κB/COX-2 and AP-2β/hTERT signaling in hepatocellular carcinoma cells.
Keywords: Cisplatin, melatonin, hepatocellular carcinoma, NF-κB, AP-2β, COX-2, hTERT
Introduction
Hepatocellular carcinoma is the fifth most common cancer in the world with high morbidity and fatality rate, and has already become a major health burden in present years, especially as the cell resistance to chemotherapy and radio therapy develops. Its sharp increase and aggressive recurrence in clinical scenario have placed great pressure of a pandemic [1,2]. Cisplatin is one of the agents developed in platinum-based compounds (PBC), which is constitutively applied in many cancers with clinical activity against a wide spectrum of cancers as the first-line chemotherapeutic treatments, including liver cancer, ovarian and small cell lung cancer [3]. However, the undesirable side effects of these drugs, including severe cytotoxicity against normal cells and tissues, and high incidence of chemoresistance, limited their clinical usefulness as anticancer drugs [4]. Therefore, the new therapeutic strategies, which could attenuate its cytotoxicity to normal tissues and avoid or antagonize the development of chemoresistance, is required for cancer therapy. The combinational use of chemotherapeutic drug with natural products provides such a beneficial therapeutic strategy that may overcome these problems of PBC agents [5].
Melatonin, the chemical name of which is N-acetyl-5-methoxytryptamine, is an indoleamine compound produced in the pineal gland and also in plants [6,7]. As a pleiotropic and multitasking molecule, it has shown numerous biological activities, including antioxidant, anti-aging, seasonal reproduction, anti-obesity, regulation of immune systems, and anti-cancer [8-10]. Melatonin has been shown to inhibit growth of different tumors in experimental preclinical models in vitro and in vivo, including liver cancer [11]. It is found to be capable of modulating the related signaling pathways associated with anti-proliferation, pro-apoptosis, and pro-oxidant actions in cancer cells. It is also reported to be able to decrease angiogenesis in cancer cells to contribute to the blockage of blood supply to the tumor, resulting in tumor suppression [10]. Furthermore, in the previous study, melatonin has been shown to potentiate flavone-induced apoptosis in human cancer cells by increasing the level of glycolytic end products [12]. Therefore, more attempts deserve to be made to develop melatonin as a potential combinational treatment agent to enhance the efficacy of conventional anticancer agents and meanwhile reduce their side effects.
Cyclooxygenase 2 (COX-2) is an important inflammation factor and inducible in response to certain stimuli such as growth factors and cytokines and is involved in many pathological processes, including carcinogenesis [13,14]. More than 15% of malignant tumors have been shown to correlate with infection and can be promoted by inflammation [15], indicating the key role of COX-2 in cancer progression. NF-κB is a family of important transcriptional factors, and has been identified to be the potential regulator of COX-2 expression by binding to the corresponding sites of its promoters [16]. Meanwhile, as the key upstream molecules of NF-κB signaling pathway, IKKα and IKKβ could be activated by many stimuli leading to the inducible phosphorylation and degradation of IκB proteins and final activation of NF-κB, implying the key role of NF-κB signaling pathway in cancer survival [17], and also providing the possibility that the anti-cancer effect of drugs may be concomitant with the inactivation of NF-κB signaling and down-regulation of COX-2 expression.
As an important component of human telomerase, human telomerase reverse transcriptase (hTERT) has been shown to be not expressed in most human somatic cells, but commonly overexpressed in a variety range of human cancers, including gastric, breast, and head and neck cancer [18]. Both in vivo and in vitro models, hTERT has been variously reported to enhance carcinogenesis, accelerate tumor progression, stimulate angiogenesis, and promote metastasis [19,20]. The activating enhancer-binding protein-2β (AP-2β), one of retinoic acid inducible gene of AP-2 transcription factor family [21], has been shown to be involved in the major mechanism for cancer-specific activation of telomerase, which transcriptionally regulates the expression of hTERT. Therefore, the suppression of the AP-2β/hTERT signaling might contribute to the antitumor activity of the anti-cancer agents.
In the current study, we explored the synergizing function of melatonin in cisplatin-mediated antitumor effect in hepatocellular carcinoma cells and further investigated the detailed underlying mechanisms of such combination in anti-cancer treatment. A potential mechanism model in which melatonin could be used as a potential combinational agent to sensitize the antitumor effect of cisplatin was suggested. Such sensitization was very possibly realized through suppression of the NF-κB/COX-2 and AP-2β/hTERT signaling pathways and activation of the mitochondria-related apoptosis pathway.
Materials and methods
Cell culture
The human hepatocellular carcinoma cell lines Bel-7402 were obtained from China Center for Type Culture Collection (CCTCC, Wuhan, China) and SNU-449, HepG2, Hep3B 2.1-7 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). All the cells were cultured as monolayer by RPMI or MEM culture media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco), and maintained in an environment with a humidified incubator with 5% CO2 at 37°C.
Reagents and antibodies
Melatonin was purchased from J&K, Chemical Ltd. Cis-dichlorodiamineplatinum (CDDP) were purchased from Sigma (St. Louis, USA) and dissolved in dimethyl sulphoxide (DMSO) before addition to the complete cell culture medium. For the experiment, the solution of melatonin (1.0 mM) and cisplatin (CDDP) (5.0 μM) in DMSO was prepared and kept at -40°C for further dilution in culture medium to maintain stability of used drugs. The primary antibodies for COX-2, IKKα, IKKβ, p-IKKα/β, IκB-α, p-IκB-α, cleaved caspase-3, cleaved caspase-9, cleaved PARP, Bcl-2, NF-κB p50 and p65 were purchased from Cell Signaling Technology (Cell Signaling Technology, Inc, USA). The antibodies for AP-2β and cytochrome c were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies for β-actin, GAPDH, TFIIB were purchased from Proteintech group (CA). Trypsin, MEM, RPMI 1640 and fetal bovine serum (FBS) were purchased from HyClone Laboratories (HyClone Laboratories Inc.). Unless what specified noted, other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Plasmid vectors
The transfection was performed using Lipofectamine 2000 reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Recombinant plasmid vectors pGL3-hTERT-438 expressing luciferase driven by hTERT promoter (-378 to +60) were constructed in our lab.
Cell viability assay
Cell viability was determined using the MTT assay (Roche Diagnosis, Indianapolis, IN). Briefly, four different cell lines were seeded into 96 - well plates. Cells were cultured for 36 h, and then the cell medium was changed to fresh medium containing various concentrations of CDDP and melatonin, and five replicated wells were put up for each group, or Bel-7402 cells seeded in 96 - well plates (2000 cells/well) were transfected with plasmid of hTERT, AP-2β or control vector Lac Z for 36 h after being treated with different drugs (5.0 μM CDDP, 1.0 mM MT). After 48 h, MTT assay was done. The effect for cell viability was assessed as cell viability compared with untreated vehicle-treated control group. The drug concentration required to cause 50% cell growth inhibition (IC50) was determined by interpolation from dose-response curves.
Colony formation assay
Briefly, Bel-7402 cells or SNU-449 cells were seeded into six - well plate (2 × 103 per well) and were incubated for 24 h. Then, the medium was removed and cells were exposed to various drugs (CDDP: 2.5 μM, MT: 1.0 mM). After 24 h, cells were changed into fresh medium containing 10% FBS and incubated in a 37°C, 5% CO2 incubator for 2 weeks until cells grew into macroscopic colonies. Finally the medium was removed, and the colonies were stained by 0.1% crystal violet and counted.
Scratch assay
Scratch assay was performed to detect wound healing ability of cells (bel-7402 and snu-449). The cells were seeded into six-well plates and incubated in the medium with 5% FBS until grown to full confluency. Cell monolayers were wounded by a sterile 200 μl pipette tip, and washed with PBS for three times to remove detached cells. Cells were treated with indicated doses of cisplatin or melatonin in full medium and kept in a CO2 incubator. After 36 h, medium was replaced with PBS buffer and the wound gap was photographed using a Leica DM 14000B microscope fixed with digital camera.
Apoptosis assay
Apoptosis was measured by flow cytometry using Annexin-V FITC staining-based -fluorescence-activated cell sorter (FACS). In brief, cells seeded in 6 - well plates were treated with indicated doses of cisplatin or melatonin. After 24 h, cells were collected and washed once with cold PBS, and subsequently stained simultaneously with FITC-labeled annexin V and PI. Stained cells were analyzed using FACS Accuri C6 (Genetimes Technology Inc.).
Western blot
Proteins from cell lysate were separated by electrophoresis in a 10% polyacrylamide gelelectrophoresis(SDS-PAGE) and electrophoretically transferred to a polyvinylidene fluoride (Millipore, USA). Western blots were probed with the specific antibodies. The protein bands were detected by enhanced chemiluminescence. The total protein concentration was determined using a BCA protein assay kit.
Pulldown assay
The biotin-labeled double-stranded oligonucleotide probe, which corresponds to -892/+9 fragments of COX-2 promoter sequence were synthesized by PCR using biotin-labeled primers from TAKARA Company (sense, 5’-ACGTGACTTCCTCG -ACCCTC-3’; antisense, 5’-AAGACTGAAAACCAAGCCCA-3’). The nuclear proteins (400 μg) were mixed with double-strand biotinylated COX-2 promoter probe (4 ug), streptavidin agarose beads (50 ml) in 500 ml PBSI buffer containing 0.5 mM PMSF, 10 mM NaF, 25 mM, β-glycerophosphate and rotated for 4 h at RT. The beads were centrifuged, washed with PBSI buffer for two times, then the beads were resuspended by loading buffer and cooked at 100°C for 10 min. The supernatant was analyzed by western blot.
Confocal immunofluorescence
For confocal microscopy analysis, cells grow on chamber slides were washed by PBS and fixed for 10 min at room temperature (RT) with 4% paraformaldehyde. The samples were permeabilized with 0.2% TritonX-100 for 5 min. And then blocked with 10% bovine serum albumin (BSA) in PBS for 30 min. Antibodies against Cytochrome -c, p65 and p50 in the 1% blocking solution were added to the sample and incubated for overnight at 4°C. Following 10-min washes for three times with PBS, fluorescein isothiocyanate and rhodamine conjugated secondary antibodies were added in 1% blocking solutions and incubated for 90 min. Subsequently, the stained samples were mounted with 4’6-diamidino-2-phenylindole (DAPI)-containing Vectashield solution (Vector Laboratories Inc.) to stain cell nuclei. After five additional 10-min washes, samples were examined with a Leica DM 14000B confocal microscope.
Acridine orange/ethidium bromide fluorescence staining
Bel-7402 cells were grown on chamber slides and treated with indicated doses of cisplatin with or without melatonin, After 24 h, cells were washed by PBS to remove detached cells ,and then fixed by 95% ethanol for 15 min. After slightly drying cells, 5 ul AO/EB (50 ug/ml) were added with gently pipetting to mix before photographing by Leica DM 14000B microscope fitted with digital camera.
Densitometric analysis
Scion Image Software (Frederick, MD) was used to determine the density of the protein bands from Western blot analysis. The data are expressed as an arbitrary unit.
Statistical analysis
Data are represented as mean ± standard deviation (SD). Analysis of variance and Student’s t test were used to compare the values of the test and control samples. P<0.05 was considered to be a statistically significant difference. SPSS17.0 software was used for statistical analysis, and all the experiments were done three times.
Results
Melatonin potentiated the cisplatin-mediated inhibition of cell proliferation and change of cell morphology
To investigate whether melatonin affects the cell proliferation inhibition mediated by cisplatin, we first observed the morphology change of Bel-7402 and SNU-449 cells under co-treatment with these two compounds (cisplatin: 2.5 μM and melatonin: 1.0 mM). We found that the cells with combined treatment exhibited highly reduced cell-to-cell contact and was mostly individualized as compared with the cells treated with cisplatin or melatonin alone. By contrast, the cells treated with cisplatin or melatonin alone formed a cell layer, and more spread and filopodia were observed. Similarly, SNU-449 cells under the combined treatment with cisplatin and melatonin displayed lower spreading with fewer formation of filopodia (Figure 1A).
Figure 1.
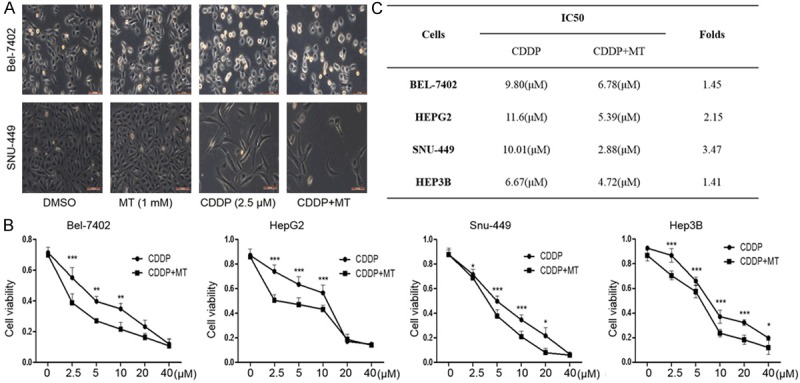
Melatonin potentiated cisplatin-mediated cell morphology change and cell proliferation inhibition. A. The changes in cell morphology and spreading in Bel-7402 cells treated with cisplatin (2.5 μM) alone or combined with MT (1.0 mM) for 48 h were observed and cells were photographed using a microscope fitted with digital camera. B. Human liver cancer Bel-7402, HepG-2, SNU-449, Hep-3B cells were treated with the indicated doses of cisplatin (CDDP) alone or combined with melatonin (MT) (1.0 mM) for 48 h, and the cell viability was examined by MTT assay. C. The IC50 values of cisplatin for cell viability inhibition in cells treated with or without melatonin (MT) were determined. The data is presented as mean ± SD of three separate experiments, *P < 0.05, **P < 0.01, significant differences compared to the control groups.
Next, MTT assay was performed in four different hepatocellular carcinoma cell lines to further evaluate the synergistic inhibitory effects of cisplatin and melatonin on cancer cell growth. Cells were co-treated with melatonin (1.0 mM) and various concentrations of cisplatin (0, 2.5, 5, 10, 20 or 40 μM) for 48 h, and cell viability was determined. As shown in Figure 1B, melatonin markedly increased the suppression of cisplatin on cell viability. Moreover, such combinational treatment resulted in the dose-dependent growth inhibition of hepatocellular carcinoma cells. To evaluate the degree of cisplatin and melatonin combination-mediated the enhancement of anti-proliferative effect, we analyzed the IC50 values of cisplatin in these four cells treated with or without melatonin. As shown in Figure 1C, IC50 value of cisplatin was significantly decreased in the combination group (cisplatin and melatonin) compared to the single drug group (cisplatin alone). These results demonstrated that the combinational use of cisplatin and melatonin resulted in improved anti-proliferative effects in hepatocellular carcinoma cells compared with cisplatin treatment alone.
Melatonin enhanced cisplatin-mediated inhibition of colony formation and cell migration
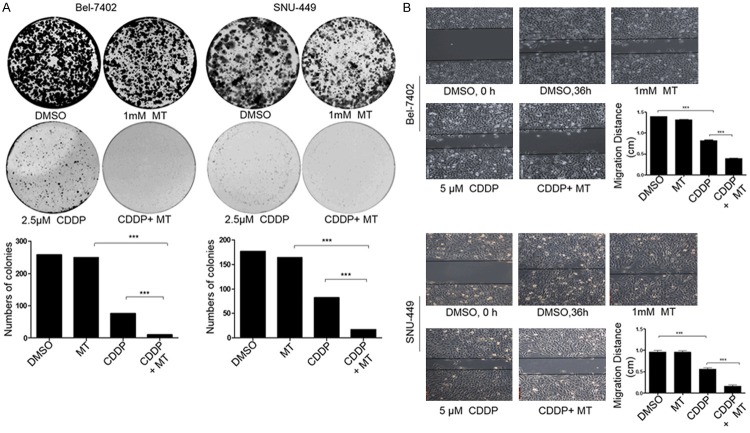
We also evaluated the influence of the combined treatment with melatonin and cisplatin on the clonogenic ability in cancer cells. The clonogenic cell survival assay was employed in Bel-7402 and SNU-449 cells. Being consistent with cell proliferation inhibition, the combinational use of cisplatin and melatonin significantly inhibited colony formation and resulted in a notable decrease at colony formation ratio compared with the single compound treatment (Figure 2A).
Figure 2.
Melatonin enhanced cisplatin-mediated inhibition of colony formation and cell migration in hepatocellular carcinoma cells. A. Clone formation in BEL-7402 cells and SNU-449 cells treated with cisplatin (CDDP) (2.5 μM) and melatonin (MT) (1.0 mM) for 48 h were observed. B. Cell migration was analyzed by a scratch assay. BEL-7402 cells and SNU-449 cells were grown to full confluency. The cell monolayers were wounded with a sterile pipette tip, and washed with medium to remove detached cells from the plates. Then the cells were left either untreated or treated with cisplatin (CDDP) (2.5 μM) or melatonin (MT) (1.0 mM). After 36 h, the wound gap was observed and photographed. *P < 0.05, significant differences between the CDDP+MT-treated groups and the CDDP-treated groups.
Further, the scratch assay was employed to determine the combined effect of cisplatin with melatonin on cell migration in Bel-7402 and SNU-449 cells. We found that the part of gap or wounding space between cell layers was occupied almost or completely by the migrating cells after 48 h in the control group and the group with melatonin treatment alone. The treatment with cisplatin (2.5 μM) alone inhibited cell migration. However, its combined treatment with melatonin (1.0 mM) markedly enhanced the cisplatin-mediated inhibition of cell migration in hepatocellular carcinoma cells (Figure 2B). These results suggested that the combinational use of cisplatin and melatonin showed more potential effect in suppressing cell colony formation and migration in hepatocellular carcinoma cells.
Melatonin increased cisplatin-induced apoptosis by modulating cytochrome c and caspase signaling pathway
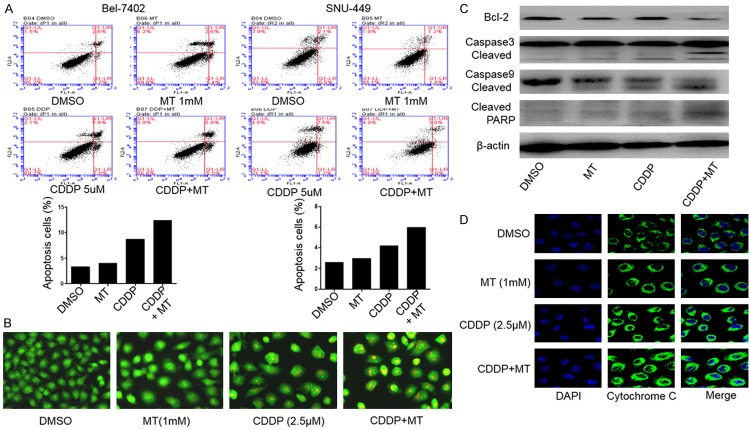
To assess the effect of the combinational use of cisplatin and melatonin on apoptosis, we confirmed the pro-apoptotic function of such combinational use by FACS analysis. As shown in Figure 3A, treatment with cisplatin alone at the doses of 5 μM induced 8.7% and 4.2% apoptotic cells in Bel-7402 and SNU-449 cells respectively. However, the addition of melatonin (1.0 mM) greatly increased the cisplatin-induced apoptosis, resulting in a 12.4% and 6.0% induction of apoptosis in Bel-7402 and SNU-449 cells respectively at 24 h after treatment.
Figure 3.
Melatonin increased cisplatin-induced apoptosis by activating cytochrome-c/caspase signaling pathway. Human Bel-7402 cells were treated with CDDP (5.0 μM) and melatonin (MT) (1.0 mM) for 24 h. A. The apoptosis was then determined by a FACS analysis. B. Acridine orange/ethidium bromide fluorescence staining was performed in Bel-7402 cells. C. The levels of the cleaved Bcl-2, caspase-3, 9 and PARP proteins were analyzed by Western blotting. D. The release of cytochrome c (cyto-c) was monitored by immunofluorescence imaging analysis from the inter-mitochondrial space into the cytosol. The apoptosis are represented by relative percentages of apoptotic cells versus that in DMSO-treated cells.
We further compared the damage extent of cells co-treated with cisplatin and melatonin by AO/EB staining. The phenomenon that more EB passed through damaged cell membrane, embedded in nuclear DNA and much brighter orange red fluorescence accumulated in the nuclear was observed in the group treated with combined compounds when compared to the control group or single drug treatment group (Figure 3B), indicating that more apoptosis was induced in hepatocellular carcinoma cells when they were exposed to cisplatin and melatonin simultaneously but not to single compound alone.
Then we detected the expression of some key proteins (Bcl-2, caspase-3, caspase-9, PARP) involved in early apoptosis induced by compound treatment in Bel-7402 cells by western blot analysis. As shown in Figure 3C, the level of Bcl-2 was obviously attenuated in the combinational treatment group than the single drug treatment group. By contrast, the expressions of cleaved caspase-3, cleaved caspase-9 and cleaved PARP were improved in the combinational treatment group with melatonin and cisplatin. We also performed immunofluorescence (IF) analysis to monitor the subcellular localization of cytochrome c, which is an upstream molecule of the caspase cascade-dependent apoptotic signaling pathway. Treatment with cisplatin (5.0 μM) or melatonin (1.0 mM) alone for 24 h triggered the release of cytochrome c from the inter-mitochondrial space into the cytosol in Bel-7402 cells, but the combined treatment with these two drugs markedly elevated the release of cytochrome c (Figure 3D).
Melatonin enhanced cisplatin-induced inhibition of COX-2 expression by inhibiting NF-κB signaling pathway
COX-2 expression has been demonstrated to be involved in cell proliferation, migration, and angiogenesis in cancer cells [22]. In order to identify the involvement of COX-2 expression and the further mechanistic basis in melatonin-sensitized proliferative inhibition mediated by cisplatin, we analyzed the effect of combinational treatment on COX-2 expression and found that cisplatin (5.0 μM) alone lowered the expression of COX-2. However, co-treatment with cisplatin (5.0 μM) and melatonin (1.0 mM) almost diminished the expression of COX-2 compared with cisplatin itself (Figure 4A). To further confirm the involvement of COX-2 signaling in the combinational treatment of melatonin and cisplatin in hepatocellular carcinoma cells, Bel-7402 cells were treated with melatonin (1.0 mM) and cisplatin (5.0 μM) at 8 h after pretreatment with COX-2-selective inhibitor celecoxib (CB, 25.0 μM). After continuous incubation of 48 h, the cell viability was analyzed by a MTT assay. As shown in Figure 4B, treatment with CB alone showed inhibitory effect on cell viability, however, the combined treatment with melatonin, cisplatin and CB markedly increase such inhibition. These results suggested that the elevated proliferative inhibition by co-treatment with melatonin and cisplatin might partially be realized through inhibiting the activation of COX-2 signaling.
Figure 4.
Melatonin enhanced cisplatin-induced inhibition of COX-2 expression by inhibiting NF-κB signaling pathway. A. The expression level of COX-2 and P50/65 protein were analyzed by Western blot in human Bel-7402 cells treated with the indicated doses of cisplatin (CDDP) (5.0 μM) and melatonin (MT) (1.0 mM) for 24 h. B. Cell viability was analyzed by MTT assay in Bel-7402 cells treated with CDDP (5.0 μM) combined with or without MT (1.0 mM) for 48 h after pretreatment with the COX-2 selective inhibitor celecoxib (CB) (25 μM) for 24 h. C. The streptavidin-biotin pulldown assay was performed to analyze the binding of P65 protein to COX-2 promoter. Nuclear extracts prepared from human liver cancer cell (Bel-7402) were incubated with biotin-labeled COX-2 promoter probe (-508 to +30) and streptavidin-agarose beads. The DNA-protein complexes were separated by SDS-PAGE, and the P65 protein bands were determined by western blot. D. The expression of p50/p65 was determined from nuclear extracts prepared from Bel-7402 by Western blotting. E. The subcellular localization of p50 and p65 and their co-localization in human Bel-7402 cells treated with 5.0 μM cisplatin (CDDP) and 1.0 mM melatonin (MT) for 48 h were examined by confocal microscopy. Cells with typical morphology were presented from more than 100 cells at each experiment. F. Human Bel-7402 cells were treated with 5.0 μM cisplatin (CDDP) and 1.0 mM melatonin (MT). At 48 h after treatment, the IKKβ, p-IKKβ, IκBα and p-IκBα proteins were analyzed by Western blotting. Each data point was calculated from three triplicate groups and the data is presented as the mean ± SD. *P < 0.05, significant difference between treatment group and control group.
As COX-2 expression is known to be transcriptionally regulated by NF-κB [23,24], we first determined the level of p50/p65 in the whole cell lysate, and found that they were not changed (Figure 4A). Further, western blot assay and immunofluorescence assay were performed to examine the expression and nuclear localization of p50/p65 in Bel-7402 cells. The results showed, cisplatin (5.0 μM), but not melatonin (1.0 mM), reduced the expression of p50/p65 in the nuclei of bel-7402 cells (Figure 4C), and the treatment with melatonin and cisplatin markedly inhibited translocation of the NF-κB p65/p50 proteins from cell cytoplasm to nucleus by comparison with the DMSO control or single drug treatment group (Figure 4C, 4D). Furthermore, we determined the effect of the combinational treatment on the binding of p65 at COX-2 promoter region. Pull down assay indicated that the binding of p65 to the COX-2 promoter was almost diminished after co-treatment with melatonin and cisplatin compared to the single compound treatment alone (Figure 4E). These results indicate that the inhibition of COX-2 expression in Bel-7402 cells by combinational use of melatonin and cisplatin might be mediated by inhibiting the translocation of NF-κB p50/p65 from cytoplasm to cell nuclei and further inhibiting their binding at COX-2 promoter.
Moreover, to better understand the effect of the combinational use of melatonin and cisplatin on the NF-κB signaling pathway, we then investigated their influence on IKK protein activity, the upstream signaling molecules of NF-κB pathway. As shown in Figure 4F, treatment with combined drugs not only significantly suppressed the phosphorylation of IKKβ in Bel-7402 cells without affecting its overall expression, but also decreased the expression level of phosphorylated IκBα. All of these results supported that NF-κB signaling pathway was a potential target of co-treatment with melatonin and cisplatin in hepatocellular carcinoma cells to finally suppress COX-2 expression.
Melatonin enhanced cisplatin-mediated suppression of AP-2β/hTERT signaling
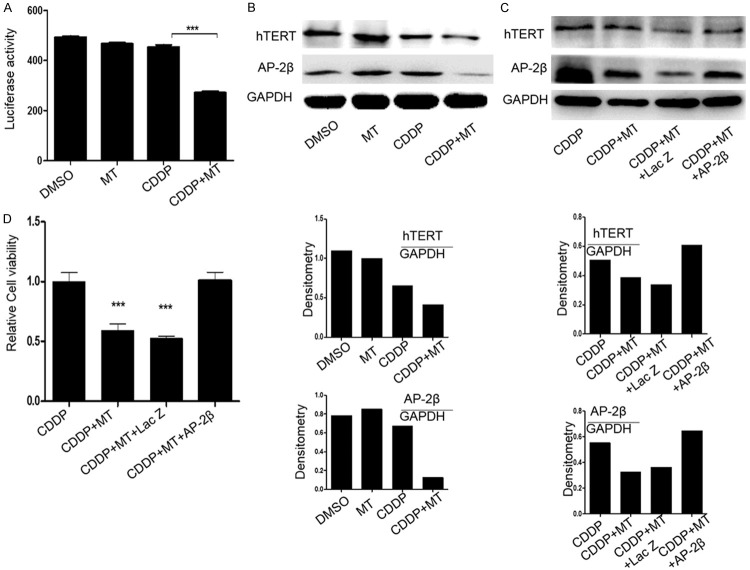
We next explored the other molecular events possibly participating in the proliferation inhibition of hepatocellular carcinoma cells mediated by the combined treatment with melatonin and cisplatin. hTERT, a main component of telomerase, has been shown to contribute to transforming normal human cells into cancer cells. A number of factors have been identified to be the upstream targets that regulate the expression of hTERT directly or indirectly [25-27]. To detect the effect of the combinational treatment of melatonin and cisplatin on hTERT expression, we transfected Bel-7402 cells with the hTERT promoter driven-luciferase plasmid after pretreatment with the two agents. At 48 h after transfection, we found that co-treatment of melatonin and cisplatin markedly attenuated the expression of hTERT promoter driven luciferase as compared with control group or single drug treatment group (Figure 5A). Further, we found that the expression of hTERT was apparently down-regulated in the group treated with both melatonin (1.0 mM) and cisplatin (5.0 μM) in contrast to the control group (Figure 5B). Meanwhile, as a key known transcriptional factor to control hTERT transcription, AP-2β was also dramatically decreased under combinational treatment of melatonin (1.0 mM) and cisplatin (5.0 μM) in Bel-7402 cells, while melatonin (1.0 mM) or cisplatin (5.0 μM) treatment alone showed nearly no effect on AP-2β expression (Figure 5B). Finally, we performed a rescue experiment to examine whether hTERT expression is involved in the enhancement of melatonin and cisplatin-mediated tumor cell growth inhibition. We transfected Bel-7402 cells with AP-2β overexpressing plasmids after pretreatment with these two compounds. At 48 h later, we checked the level of hTERT and AP-2β (Figure 5C), and also analyzed cell viability using MTT assay. As shown in Figure 5D, the cell proliferation ability was dramatically rescued when AP-2β was overexpressed after the treatment with melatonin and cisplatin, suggesting that the melatonin-sensitized proliferative inhibition caused by cisplatin might also be partially realized via inactivating AP-2β/hTERT signaling.
Figure 5.
Melatonin enhanced cisplatin-mediated suppression of AP-2β/hTERT signaling. A. Bel-7402 cells were co-treated with the plasmids of hTERT promoter driven-luciferase and cisplatin (5.0 μM) with or without melatonin (1.0 mM) for 48 h followed by a dual-luciferase assay. The relative luciferase intensity per mg protein was calculated in the treated cells. B. The expression level of hTERT protein and AP-2β protein were analyzed by Western blot in human Bel-7402 cells treated with the indicated doses of cisplatin (CDDP) (5.0 μM) and melatonin (MT) (1.0 mM) for 48 h. C. The rescue of hTERT protein expression mediated by the overexpression of AP-2β in Bel-7402 cells. Cells were treated with cisplatin (5.0 μM) combined with or without melatonin (1.0 mM) for 24 h followed by transfecting with AP-2β plasmid for 48 h, and the expression of AP-2β itself and hTERT protein in the cytoplasm were analyzed by Western blot. D. Bel-7402 cells were treated with the cisplatin (5.0 μM) combined with or without melatonin (1.0 mM) for 24 h followed by transfection with AP-2β plasmid for 48 h, and the cell viability was determined by MTT analysis. The data are presented as the mean ± SD of three separate experiments. *P < 0.05, significant differences between treatment groups and DMSO control groups.
Discussion
In this study, we demonstrated that melatonin sensitized cisplatin-mediated anti-tumor growth in hepatocellular carcinoma cells, which was represented by the improved cell viability suppression, clonogenicity and migration inhibition, and apoptosis induction. The IC50 value of cisplatin was dramatically decreased under co-treatment with melatonin in comparison with cisplatin treatment alone. Although many previous studies have described the antitumor activity of melatonin [9-11], the potential synergized action and the associated regulatory mechanisms remain unclear. Our present work not only demonstrates the role of melatonin in improving cisplatin’s antitumor effect and attenuating its cytotoxicity in hepatocellular carcinoma therapy, but also more importantly clarifies the possible mechanisms of such combinational treatment in enhancing anti-cancer effects.
In our study, we functionally examined the potential of melatonin as a synergistic natural product of chemotherapeutic reagent [28]. Although cisplatin itself caused significant apoptosis of tumor cells, such apoptosis induction was largely augmented under its co-treatment with melatonin. Furthermore, the combined treatment of melatonin and cisplatin resulted in more activation of caspase-dependent apoptotic signaling pathway [12,29], which includes inhibiting the expression of Bcl-2, promoting the expression of cleaved caspase-9, caspase-3 and PARP, and stimulating the release of cytochrome c from mitochondria to cytoplasm, as compared with cisplatin treatment alone. All these data collectively demonstrated that the elevated antitumor effect of cisplatin by melatonin was in part established based on the enhanced induction of mitochondria-mediated apoptosis pathway, which is consistent with the previous studies that described the inhibitory effect of melatonin on cell growth by inducing apoptosis [12].
Our further molecular mechanism studies indicated that two pathways might also contribute to the improved antitumor function of combined treatment with melatonin and cisplatin. One is NF-κB/COX-2 signaling pathway and the other is AP-2β/hTERT signaling pathway. We found that melatonin could increase the inhibition of COX-2 expression mediated by cisplatin when the cells were co-treated with the two agents through suppressing the phosphorylation of IKKα/β and IκBα, thereby inhibiting the translocation of NF-κB p65/p50 proteins from cell cytoplasm to nucleus and abrogating p65’s binding to COX-2 promoter to finally suppress COX-2 expression. Based on the critical role of COX-2 in multiple pathophysiological processes, including tumorigenesis, and the causal linkage of deregulated activation of NF-κB to tumor chemoresistance, the elevated inactivation of NF-κB signaling pathway and inhibition of COX-2 expression mediated by the co-treatment with melatonin and cisplatin showed important significance in the aspect of improving sensitivity of chemotherapy.
Overexpression of hTERT is a hallmark of carcinogenesis, and the inhibition of hTERT expression was found to contribute to prevent proliferation and angiogenesis and induce apoptosis of cancer cells [30]. Melatonin was also reported to display its anti-tumor effect by affecting hTERT expression and telomerase activity [31]. Being consistent with the previous study, we also found that melatonin-mediated enhancement of cisplatin on tumor cell proliferation inhibition was partially realized through inhibiting AP-2β/hTERT signaling. When hepatocellular carcinoma cells were treated with cisplatin and melatonin together, we found that the activity of luciferase reporter driven by hTERT promoter was lower compared with that in the cells treated with single drug melatonin or cisplatin, and the same result happened to the expression level of hTERT. The overexpression of AP-2β, which resulted in the rescued expression of hTERT, reversed the inhibition of hTERT expression and cell viability caused by co-treatment with melatonin and cisplatin, indicating the key role of hTERT in the sensitization of antitumor effect of cisplatin mediated by melatonin. Based on our present study, although we indicated and confirmed the participation of NF-κB/COX-2 and AP-2β/hTERT signaling pathway in sensitizing cisplatin-mediated anti-cancer effect by melatonin, it is difficult to tell which pathway takes more responsibilities during this treatment process, or they happened in parallel or successively. All of these questions deserve better studies in the near future.
In summary, we found that melatonin enhanced the anti-tumor activity mediated by cisplatin in hepatocellular carcinoma cells. Furthermore, we elucidated the underlying molecular mechanisms of such enhanced action. The combined effects of cisplatin and melatonin might be achieved through activating cytochrome c and caspase-dependent apoptotic signaling and inhibiting NF-κB/COX-2 and AP-2β/hTERT signaling pathways (Figure 6). All these data indicated the great potential of melatonin, not only in antagonizing the toxicity exerted by chemotherapeutic agents, such as cisplatin, in cancer patients, but also in augmenting their chemosensitivities [30]. Together with other notions, our study might serve as a basis and direction for the application of the combinational treatment of melatonin with cisplatin or other chemotherapeutic agents in improving therapeutic efficiency for cancers, especially for hepatocellular carcinoma [11].
Figure 6.
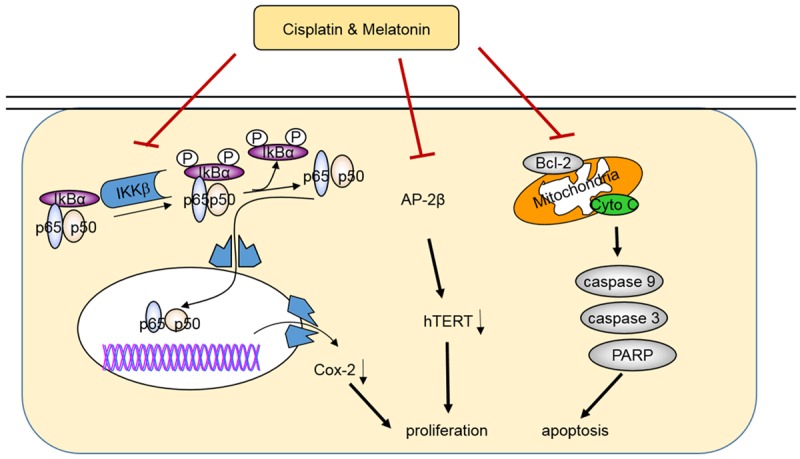
The schematic diagram of the molecular mechanisms involved in the melatonin-mediated enhancement of antitumor activity in hepatocellular carcinoma. Red symbol (⊦) indicates negative regulation. The black colored arrow (→) indicates direct or indirect positive regulation. Such combined effects of cisplatin and melatonin might be achieved through activating the cytochrome c/caspase-dependent apoptotic signaling and inhibiting the NF-κB/COX-2 and AP-2β/hTERT signaling.
Acknowledgements
This work was supported by the funds from the National Natural Science Foundation of China (81572706 WG, 81301721 WG, 81470337 YC, 81472178 WD, 81272195 WD), the Education Department of Liaoning Province, China (“the Program for Pan-Deng Scholars”), the scientific research project from the Education Department of Liaoning Province, China (L2015142).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Giannelli G, Rani B, Dituri F, Cao Y, Palasciano G. Moving towards personalised therapy in patients with hepatocellular carcinoma: the role of the microenvironment. Gut. 2014;63:1668–1676. doi: 10.1136/gutjnl-2014-307323. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa T, Higuchi K, Kubota T, Seki K, Honma T, Yoshida T, Kamimura T. Prevention of intrahepatic distant recurrence by transcatheter arterial infusion chemotherapy with platinum agents for stage I/II hepatocellular carcinoma. Cancer. 2011;117:4018–4025. doi: 10.1002/cncr.25989. [DOI] [PubMed] [Google Scholar]
- 4.Huang TH, Chiu YH, Chan YL, Wang H, Li TL, Liu CY, Yang CT, Lee TY, You JS, Hsu KH, Wu CJ. Antrodia cinnamomea alleviates cisplatin-induced hepatotoxicity and enhances chemo-sensitivity of line-1 lung carcinoma xenografted in BALB/cByJ mice. Oncotarget. 2015;6:25741–25754. doi: 10.18632/oncotarget.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamseddine AI, Farhat FS. Platinum-based compounds for the treatment of metastatic breast cancer. Chemotherapy. 2011;57:468–487. doi: 10.1159/000334093. [DOI] [PubMed] [Google Scholar]
- 6.Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, Garcia-Corzo L, Lopez LC, Reiter RJ, Acuna-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56:371–381. doi: 10.1111/jpi.12137. [DOI] [PubMed] [Google Scholar]
- 10.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DT, Rollag MD, Zalatan F. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–11184. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 11.Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez-Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol. 2002;54:1299–1321. doi: 10.1211/002235702760345374. [DOI] [PubMed] [Google Scholar]
- 12.Bizzarri M, Proietti S, Cucina A, Reiter RJ. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets. 2013;17:1483–1496. doi: 10.1517/14728222.2013.834890. [DOI] [PubMed] [Google Scholar]
- 13.Zuo C, Qiu X, Liu N, Yang D, Xia M, Liu J, Wang X, Zhu H, Xie H, Dan H, Li Q, Wu Q, Burns M, Liu C. Interferon-alpha and cyclooxygenase-2 inhibitor cooperatively mediates TRAIL-induced apoptosis in hepatocellular carcinoma. Exp Cell Res. 2015;333:316–326. doi: 10.1016/j.yexcr.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Liu YT, Xiao L, Zhu L, Wang Q, Yan T. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation. 2014;37:2085–2090. doi: 10.1007/s10753-014-9942-x. [DOI] [PubMed] [Google Scholar]
- 15.Gomes M, Teixeira AL, Coelho A, Araujo A, Medeiros R. The role of inflammation in lung cancer. Adv Exp Med Biol. 2014;816:1–23. doi: 10.1007/978-3-0348-0837-8_1. [DOI] [PubMed] [Google Scholar]
- 16.Ueda Y, Richmond A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi D, Xiao X, Wang J, Liu L, Chen W, Fu L, Xie F, Huang W, Deng W. Melatonin suppresses proinflammatory mediators in lipopolysaccharide-stimulated CRL1999 cells via targeting MAPK, NF-kappaB, c/EBPbeta, and p300 signaling. J Pineal Res. 2012;53:154–165. doi: 10.1111/j.1600-079X.2012.00982.x. [DOI] [PubMed] [Google Scholar]
- 18.Tarocchi M, Polvani S, Peired AJ, Marroncini G, Calamante M, Ceni E, Rhodes D, Mello T, Pieraccini G, Quattrone A, Luchinat C, Galli A. Telomerase activated thymidine analogue pro-drug is a new molecule targeting hepatocellular carcinoma. J Hepatol. 2014;61:1064–1072. doi: 10.1016/j.jhep.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leon-Blanco MM, Guerrero JM, Reiter RJ, Calvo JR, Pozo D. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35:204–211. doi: 10.1034/j.1600-079x.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 20.Polvani S, Calamante M, Foresta V, Ceni E, Mordini A, Quattrone A, D’Amico M, Luchinat C, Bertini I, Galli A. Acycloguanosyl 5’-thymidyltriphosphate, a thymidine analogue prodrug activated by telomerase, reduces pancreatic tumor growth in mice. Gastroenterology. 2011;140:709–720. e709. doi: 10.1053/j.gastro.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Bar-Eli M. Gene regulation in melanoma progression by the AP-2 transcription factor. Pigment Cell Res. 2001;14:78–85. doi: 10.1034/j.1600-0749.2001.140202.x. [DOI] [PubMed] [Google Scholar]
- 22.van Nes JG, de Kruijf EM, Faratian D, van de Velde CJ, Putter H, Falconer C, Smit VT, Kay C, van de Vijver MJ, Kuppen PJ, Bartlett JM. COX2 expression in prognosis and in prediction to endocrine therapy in early breast cancer patients. Breast Cancer Res Treat. 2011;125:671–685. doi: 10.1007/s10549-010-0854-7. [DOI] [PubMed] [Google Scholar]
- 23.Abdullah M, Rani AA, Sudoyo AW, Makmun D, Handjari DR, Hernowo BS. Expression of NF-kB and COX2 in colorectal cancer among native Indonesians: the role of inflammation in colorectal carcinogenesis. Acta Med Indones. 2013;45:187–192. [PubMed] [Google Scholar]
- 24.Liou CJ, Len WB, Wu SJ, Lin CF, Wu XL, Huang WC. Casticin inhibits COX-2 and iNOS expression via suppression of NF-kappaB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J Ethnopharmacol. 2014;158 Pt A:310–316. doi: 10.1016/j.jep.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 25.Soreide K, Buter TC, Janssen EA, Gudlaugsson E, Skaland I, Korner H, Baak JP. Cell-cycle and apoptosis regulators (p16INK4A, p21CIP1, beta-catenin, survivin, and hTERT) and morphometry-defined MPECs predict metachronous cancer development in colorectal adenoma patients. Cell Oncol. 2007;29:301–313. doi: 10.1155/2007/457427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Zhu H, Lu J. PTEN and hTERT gene expression and the correlation with human hepatocellular carcinoma. Pathol Res Pract. 2015;211:316–319. doi: 10.1016/j.prp.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Wei YB, Martinsson L, Liu JJ, Forsell Y, Schalling M, Backlund L, Lavebratt C. hTERT genetic variation in depression. J Affect Disord. 2016;189:62–69. doi: 10.1016/j.jad.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A. Potential chemoprotective effect of melatonin in cyclophosphamide-and cisplatin-induced testicular damage in rats. Fertil Steril. 2009;92:1124–1132. doi: 10.1016/j.fertnstert.2008.07.1758. [DOI] [PubMed] [Google Scholar]
- 29.Su YJ, Cheng TT, Chen CJ, Chang WN, Tsai NW, Kung CT, Wang HC, Lin WC, Huang CC, Chang YT, Su CM, Chiang YF, Cheng BC, Lin YJ, Lu CH. Investigation of the caspase-dependent mitochondrial apoptotic pathway in mononuclear cells of patients with systemic lupus erythematosus. J Transl Med. 2014;12:303. doi: 10.1186/s12967-014-0303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang SK, Putnam L, Dufour J, Ylostalo J, Jung JS, Bunnell BA. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue-derived stromal cells. Stem Cells. 2004;22:1356–1372. doi: 10.1634/stemcells.2004-0023. [DOI] [PubMed] [Google Scholar]
- 31.Leon-Blanco MM, Guerrero JM, Reiter RJ, Pozo D. RNA expression of human telomerase subunits TR and TERT is differentially affected by melatonin receptor agonists in the MCF-7 tumor cell line. Cancer Lett. 2004;216:73–80. doi: 10.1016/j.canlet.2004.05.003. [DOI] [PubMed] [Google Scholar]