Abstract
Phosphodiesterase 5 (PDE-5) is a major isoform of cGMP phosphodiesterase in diverse tissues and plays a critical role in regulating intracellular cGMP concentrations. However, the distribution and expression of PDE-5 in colitis-related colon cancer was still unclear, not even the function and mechanism. Western blotting and ELISA were performed to detect colonic PDE-5 expression in AOM/DSS-induced tumorigenesis model. Sildenafil, a specific PDE-5 inhibitor, was used to treat AOM/DSS-induced and AOM-induced colonic tumorigenesis model and DSS-induced colitis model. The leukocyte infiltration in colonic tissue was examined by flow cytometry and immunofluorescence staining. Further matrigel-based invasion assay was employed to determine the effects of Sildenafil on myeloid-derived suppressor cell (MDSC) in vitro. We first demonstrated the upregulation of colonic PDE-5 expression and the prevention role of PDE-5 inhibition in AOM/DSS-induced tumorigenesis model. More importantly, PDE-5 inhibitor Sildenafil inhibited colonic tumorigenesis dependent on inflammation and suppressed DSS-induced colitis. Molecular mechanism investigation indicated that Sildenafil regulated inflammation microenvironment via directly inhibiting MDSC infiltration in colonic tissue. The study provides solid evidence for the use of PDE-5 inhibitor in preventing and treating colonic inflammation-related tumorigenesis.
Keywords: Colon cancer, colitis, PDE-5, Sildenafil, MDSC
Introduction
Primary tumors are initiated as a result of the stepwise acquisition of genetic alterations within the epithelial compartment, and defined as out control of cell proliferation, survival, and differentiation, which are critical to maintain tissue homeostasis [1]. However, increasing evidence has demonstrated the critical role of the tumor microenvironment in cancer progression in many types of neoplasia, including colon cancer [2-4]. It is well established that there was tight connection between colonic inflammation and cancer [5-7]. Clinical studies have mentioned that patients with inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis, have higher risk of colorectal cancer development than the healthy population [8]. Under inflamed microenvironment, continuous tissue destruction, renewal and persistent oxidative damage can trigger mutagenic processes that serve as cancer-initiating events [9,10]. Furthermore, the continuous presence of inflammatory cytokines may augment tumor progression through promoting excessive cell proliferation and survival [9,10]. Thus, targeting inflammation would be an effective strategy for colon cancer prevention and therapy.
Cyclic nucleotide PDEs are an important enzyme superfamily, including 11 PDE families with different substrate specificity, regulatory properties, tissue localization, and inhibitor sensitivity [11]. They are responsible for regulating second messenger signaling by hydrolyzing the phosphodiesterase bond in the cyclic nucleotides cGMP and/or cAMP [11]. The enzyme phosphodiesterase-5 (PDE-5) is known to be abundant in various tissues where it hydrolyses cyclic guanosine monophosphate (cGMP), a second messenger of NO [12-14]. PDE-5 inhibitors, Sildenafil and Tadalafil, are used to reduce oxidative stress during pulmonary hypertension [15], treat erectile dysfunction and testicular torsion [16,17], improve therapeutic efficacy of adipose-derived stem cells following myocardial infarction [13], and restrain ventricular and vascular function in heart failure [18]. Moreover, solid evidences have demonstrated the prevention role of PDE-5 inhibition in trinitrobenzenesulfonic acid-induced colitis [14,19], acetic acid-induced colitis [20], and ischemic colitis [21,22] in rat. However, the distribution and expression of PDE-5 in colitis-related colon cancer was still unclear, not even the function.
In the AOM/DSS-induced colitis-related colon cancer model, we found PDE-5 expression was upregulated accompanying with the processing of tumor development. Thus, we hypothesized that PDE-5 blockade may reverse colitis-related tumorigenesis. Sildenafil, a specific PDE-5 inhibitor, was used to treat mice and inhibited colon inflammation development and inflammation-related tumorigenesis through blocking the infiltration of MDSC in colonic tissue. The study provides solid evidence for the use of PDE-5 inhibitor in preventing and treating colon inflammation-related tumorigenesis.
Materials and methods
Animal model and treatment
Male BALB/c mice (20-22 g weight) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China) and allowed to acclimate for 1 week before use. AOM and DSS were purchased from Sigma and MP Biomedicals, separately. To establish colonic tumorigenesis model, 10 mg AOM/Kg mouse body was injected into the peritoneal of mice on the first day. After 7 days, 2% DSS was added into the drinking water for the mice and maintained for 7 days, and then removed for 14 days intervals. Three cycles of DSS are needed to establish this AOM/DSS-induced colonic tumorigenesis model. 10 mg AOM/Kg mouse body was injected for six times to establish AOM-induced colonic tumorigenesis model. After 10 weeks, the mice were killed and used for subsequent experimentation. The tumor numbers per colon were counted and the average tumor diameter was measured with the help of a sliding caliper as previously described. The total surface area of tumors per colon was analyzed among the different groups. Meanwhile, three cycles of 2% DSS are performed to establish DSS-induced colitis model. The hemoccult and stool consistency was observed and recorded according to the rules by previous study [23]. Nine weeks later, the mice were killed and the length of colon was measured.
25 mg/kg mice body weight Sildenafil was intraperitoneally injected after AOM or DSS treatment. In one cycle treatment, Sildenafil was injected for five times and with a 16 days interval. Normal saline (NS) was injected as the solvent comparison.
Cell culture and treatment
All of the colon cancer cells and human colon epithelial cell (HCoEpiC) were purchased from ATCC (USA) and cultured according to the instructions supplied by ATCC. Murine colon epithelial cells were isolated from male BALB/c mice (20-22 g weight) as the previous study [24]. Without culture, the cells were treated with LPS for 2 h, and the supernatant were collected for further study.
Western blotting
The cells and colon tissues were collected and lysed on ice for 30 min with RIPA Lysis Buffer (Beyotime, Beijing, China), containing protease inhibitor cocktail (Merck Millipore, Bedford, USA). The proteins (20 μg) were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electronically transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blocking, the membranes were incubated with PDE-5 and GAPDH, followed by incubation with peroxidase conjugated secondary antibodies (Zs bio, Beijing, China). Peroxidase-labeled bands were visualized using an ECL kit (Millipore, Bedford, MA).
Enzyme-linked immunosorbent assay
The colon tissues were collected and lysed on ice for 30 min with RIPA Lysis Buffer (Beyotime, Beijing, China), containing protease inhibitor cocktail (Merck Millipore, Bedford, USA). The protein concertation was measured by BCA assay (Beyotime, Beijing, China). After adjusting the concertation, the protein was used to measure the total levels of several cytokines using the mouse PDE-5 ELISA kit, mouse IL-6 ELISA kit, mouse TNF-α ELISA kit, IFN-γ ELISA kit and IL-1β ELISA kit according to the manufacturer’s instructions.
H&E staining and immunohistochemistry
Paraffin sections were used for H&E staining and immunohistochemical analysis. After deparaffinized in xylene, the sections were treated with a graded series of alcohol [100%, 95%, and 80% ethanol/double-distilled H2O (v/v)], and rehydrated in PBS (pH 7.4). The H&E staining kit were performed to stain cell nucleic and cytoplasm. PCNA staining was performed by staining with anti-mouse PCNA antibody, followed with goat anti-rabbit IgG detection kit (Zsbio, Beijing, China), according to the instructions. The immunoreaction was visualized by using diaminobenzidine (DAB) peroxide solution and cellular nuclei were counterstained with hematoxylin. MDSC imaging was performed by staining with a rabbit anti-mouse Ly6G antibody and mouse anti-mouse iNOS antibody. For fluorescent visualization of antibody reactions, secondary antibodies were labelled with the FITC-Texas Red, while the nuclei were detected with DAPI. All specimens were evaluated using Olympus B×600 microscope and Spot Fiex camera.
Terminal deoxynucleotidyl transferase-deoxyuridine triphosphate nick-end labeling (TUNEL) assay
To detect apoptotic cells in colonic tissues, TUNEL assay using a DeadEndTM Fluorometric TUNEL System (Promega, Madison, Wisc, USA) was performed following the manufacturer’s protocol. Cell nuclei with dark green fluorescent staining were defined as apoptosis cell. To quantify TUNEL-positive cells, the number of green fluorescence-positive cells was counted in random fields at ×100 magnification. Cell nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI).
Flow cytometry
Flow cytometry in colon tissue was performed according to the previous study [24]. Briefly speaking, colon tissue were collected and digested in PBS containing 0.2% Collagenase D at 37°C for 30 min. Then, isolated cells were stained with labeled antibodies in PBS with 2% fetal calfserum and analyzed on a BD FACSCalibur. Data were analyzed using FlowJo software.
Matrigel-based invasion assay
For the invasion assay, diluted Matrigel (1:5 dilution with PBS) was added into the cell culture inserts (8 μm, 24-well format, Millipore). And 30 min later, MDSC or macrophage (5,000) in 100 μl of conditioned supernatant was added to the upper chamber, while the lower chamber was filled with 0.5 ml of conditioned supernatant. The culture was maintained for 24 h. Then, the cell culture inserts were collected and the cells on the upper side of the filters were removed with cotton-tipped swabs. The cells on the underside of the filters were fixed with 4% formaldehyde for 10 min and stained with crystal violet for 10 min. The stained cells were counted in five randomly chosen fields. And the results were presented as the number of invasive cells in each field.
Statistical analysis
All experiments were repeated three to six times, and the data were expressed as the mean ± s.d. Statistical analysis was performed by the Student’s t-tests for comparing two groups by GraphPad Prism; P<0.05 was considered statistically significant.
Results
PDE-5 is overexpressed in colonic tumors
To investigate the role of PDE-5 in colonic tumorigenesis, 8 pairs of colonic tumor from patients, including cancer, adjacent and forward tissues, were collected for PDE-5 expression detection by ELISA. As shown in Figure 1A, PDE-5 is overexpressed in colonic tumors (625.0 ± 51.41 pg/ml), compared with adjacent tissues (254.1 ± 28.43 pg/ml) and forward tissues (83.0 ± 21.16 pg/ml). Further results indicated that PDE-5 was upregulated in all colon tumor cells except for SW480 cell, and the normal colonic epithelium cell HCoEpiC was used as the control (Figure 1B). Next, we investigated the expression of PDE-5 in AOM/DSS-induced colonic tumors by western blotting and ELISA. We found that PDE-5 was increased in AOM/DSS-induced colonic tumors from 2 weeks to 10 weeks post AOM injection, and keep at a high level from 4 weeks post AOM injection (Figure 1C, 1D). The above data indicated that PDE-5 may act as a promoting role in colonic tumorigenesis.
Figure 1.
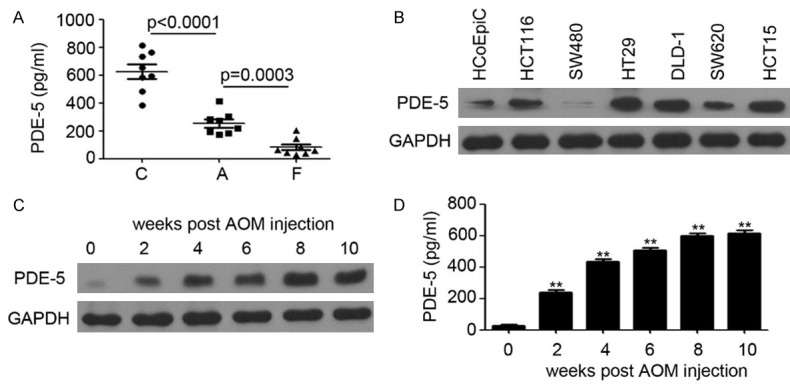
PDE-5 is overexpressed in colonic tumors. A: ELISA analysis of PDE-5 expression in colonic tissues of 8 pairs of patients (C, cancer; A, adjacent; F, forward). B: Western blotting analysis of PDE-5 expression in HCoEpiC cells and colon cancer cells. GAPDH was used as a loading control. C: Western blotting analysis of PDE-5 expression in colonic tissues from AOM/DSS-treated mice. GAPDH was used as a loading control. D: ELISA analysis of PDE-5 expression in colonic tissues from AOM/DSS-treated mice.
Inhibition of PDE-5 suppresses colonic inflammation-induced tumorigenesis
Next, to test the role of PDE-5 in colonic tumorigenesis, we employed Sildenafil, a specific inhibitor of PDE-5, to treat AOM/DSS-induced colonic tumor model. Sildenafil was intraperitoneally injected with a dose of 25 mg/kg, lasted 5 days and with a 16 days interval (Figure 2A). At the ending of treatment, the colonic tumors were collected and used for PDE-5 detection by ELISA. The results indicated that Sildenafil significantly inhibited colonic PDE-5 expression (NS group: 535.3 ± 30.61 pg/ml versus Sildenafil group: 86.67 ± 14.66 pg/ml; P=0.0002; Figure 2B). Despite that colonic tumors could be detected in both NS and Sildenafil treatment mice (Figure 2C), inhibition of PDE-5 by Sildenafil injection significantly inhibited the multiplicity of colonic tumors (P=0.0005; Figure 2D) and tumor burden (P=0.0010; Figure 2E). Moreover, the colon length was also recovered in Sildenafil injection group (6.00 ± 0.97 cm), compared with the NS injection group (4.93 ± 0.15 cm; P=0.0001; Figure 2F). Haematoxylinand eosin (H&E) staining also indicated that more tumor area was found in the colonic tissue of NS injection mice (P=0.0058; Figure 2G, 2H). PCNA staining also indicated that less proliferative cells were found in the colonic tissue of Sildenafil injection mice (P=0.0062; Figure 2I, 2J). Furthermore, more apoptotic cells were observed in the mice after PDE-5 inhibition (P=0.0175; Figure 2K, 2L). Collectively, inhibition of PDE-5 by Sildenafil injection dramatically restrained the colonic tumorigenesis in AOM/DSS model.
Figure 2.
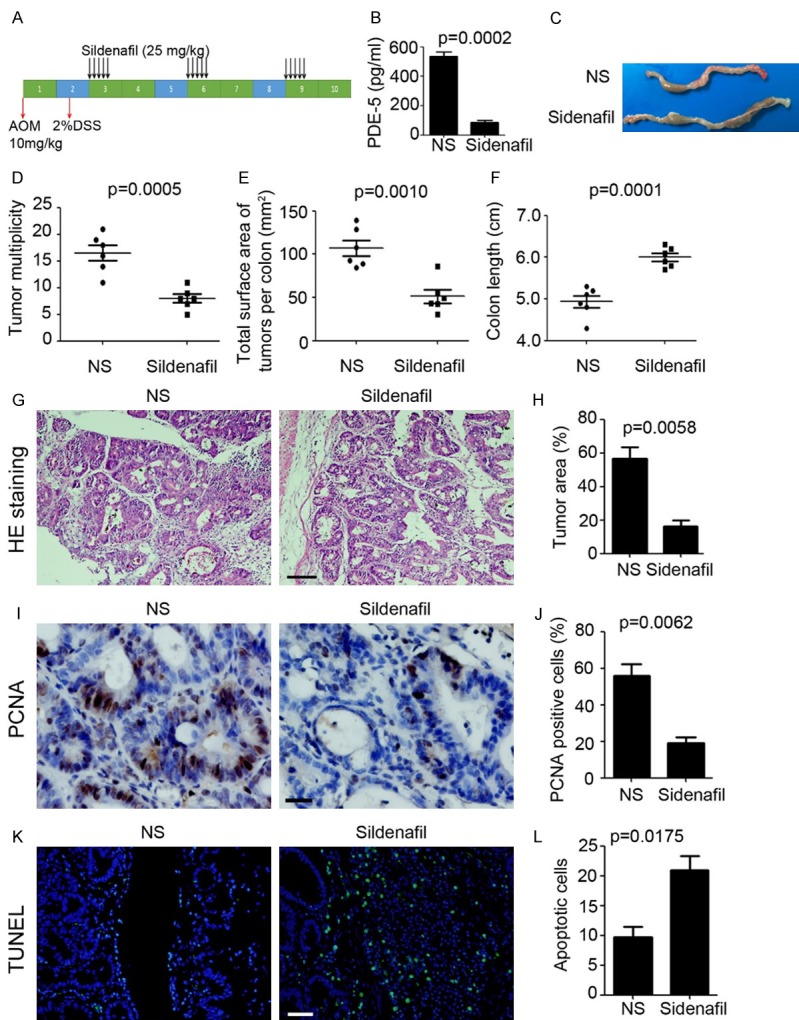
Inhibition of PDE-5 suppresses colonic inflammation-induced tumorigenesis. (A) Schema of AOM/DSS treatment and Sildenafil injection. (B) ELISA analysis of PDE-5 expression in colonic tissues from Sildenafil and normal saline (NS) treatment mice (n=5). (C) Representative macroscopic findings of colon tumors induced by AOM/DSS in Sildenafil and normal saline (NS) treatment mice. (D, E) Number of macroscopic tumors (D) and tumor burden of AOM/DSS-treated mice (E) per colon (n=6). (F) Colon length of AOM/DSS-treated mice per colon (n=6). (G, H) H&E staining of colonic tissue after AOM/DSS treatment (G). Scale bar=200 μm. Tumor area (%, tumor area/total area) was calculated (H, n=3). (I, J) PCNA staining of colonic tissue after AOM/DSS treatment (I). Scale bar=50 μm. PCNA positive cell (%, positive cell/total cell) was calculated (J, n=3). (K, L) TUNEL analysis of colonic tissue after AOM/DSS treatment (K). Scale bar=100 μm. Apoptotic cell per frame was calculated (L, n=3).
Sildenafil inhibits colonic tumorigenesis dependent on inflammation
Next, we investigated the role of Sildenafil in AOM-induced colonic tumorigenesis, without inflammation. As shown in Figure 3A, 10 mg/kg AOM was injected every week and last for six weeks to establish AOM-induced colonic tumorigenesis model. ELISA results indicated that Sildenafil injection significantly inhibited colonic PDE-5 expression (Sildenafil group: 21.0 ± 3.46 pg/ml versus NS group: 60.33 ± 6.94 pg/ml; P=0.0071; Figure 3B). Colonic tumors could be detected in both NS and Sildenafil treatment mice (Figure 3C), but there was no significant difference on multiplicity of colonic tumors (P=0.8284; Figure 3D) and tumor burden (P=0.8360; Figure 3E) between Sildenafil injection mice and NS injection mice. Moreover, the colon length was also not recovered in Sildenafil injection mice (P=0.5326; Figure 3F). Haematoxylin and eosin (H&E) staining also indicated that Sildenafil had no effect on colonic tumorigenesis in AOM-induced colonic tumorigenesis model (P=0.8330; Figure 3G, 3H). The above results indicated that Sildenafil inhibits colonic tumorigenesis dependent on inflammation.
Figure 3.
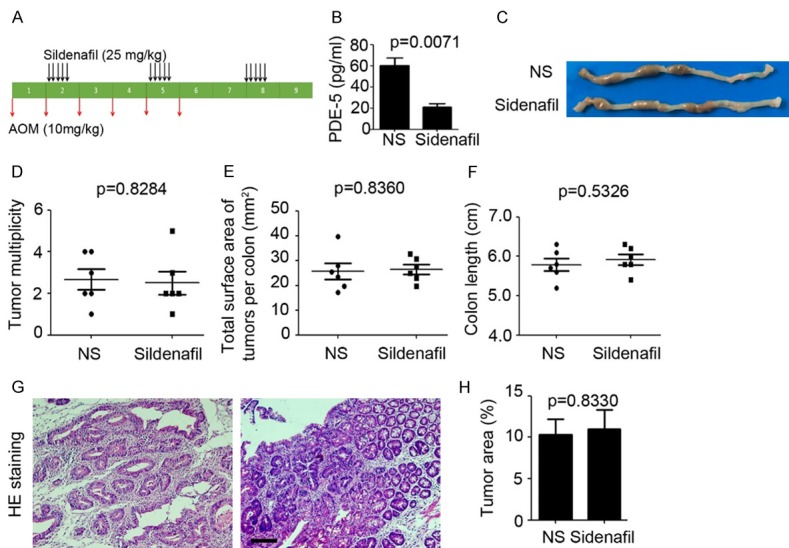
Sildenafil inhibits colonic tumorigenesis dependent on inflammation existence. (A) Schema of AOM treatment and Sildenafil injection. (B) ELISA analysis of PDE-5 expression in colonic tissues from Sildenafil and normal saline (NS) treatment mice (n=5). (C) Representative macroscopic findings of colon tumors induced by AOM in Sildenafil and normal saline (NS) treatment mice. (D, E) Number of macroscopic tumors (D) and tumor burden of AOM-treated mice (E) per colon (n=6). (F) Colon length of AOM/DSS-treated mice per colon (n=6). (G, H) H&E staining of colonic tissue after AOM treatment (G). Scale bar=200 μm. Tumor area (%, tumor area/total area) was calculated (H, n=3).
Inhibition of PDE-5 prevents colon from DSS-induced inflammation
According to the previous results, we next determined the role of PDE-5 in DSS-induced inflammation. 2% DSS was added into drinking water and maintained for 7 days, with 14 days interval (Figure 4A). Colonic PDE-5 expression was decreased in Sildenafil injection mice (Sildenafil group: 107.0 ± 15.37 pg/ml versus NS group: 710.3 ± 25.76 pg/ml; P<0.0001; Figure 4B). Colon length were observed recovered after PDE-5 inhibition in mice (Sildenafil group: 7.25 ± 0.22 cm versus NS group: 5.87 ± 0.22 cm; P=0.0012; Figure 4C, 4D). Furthermore, inflammation-associated rectal bleeding was determined by examination of blood in the stool, and rectal bleeding was found in the NS injection mice, whereas only positive hemoccult was found in the Sildenafil injection mice (Figure 4E). Meanwhile, less soft stool was observed in the Sildenafil injection mice (Figure 4F). HE staining was performed to measure the histological changes in the colon and indicated that less leukocyte infiltration and loss of goblet cells were found in the mice after PDE-5 inhibition (Figure 4G, 4H). Finally, colonic tissues were used for inflammation-related cytokine detection by ELISA. The results demonstrated that Sildenafil significantly inhibited the pro-inflammation cytokine expression, including IL-6 (P=0.0030), TNF-α (P=0.0006), IFN-1γ (P=0.0191) and IL-1β (P=0.0003; Figure 4I). These data demonstrated the inhibition role of Sildenafil in DSS-induced inflammation.
Figure 4.
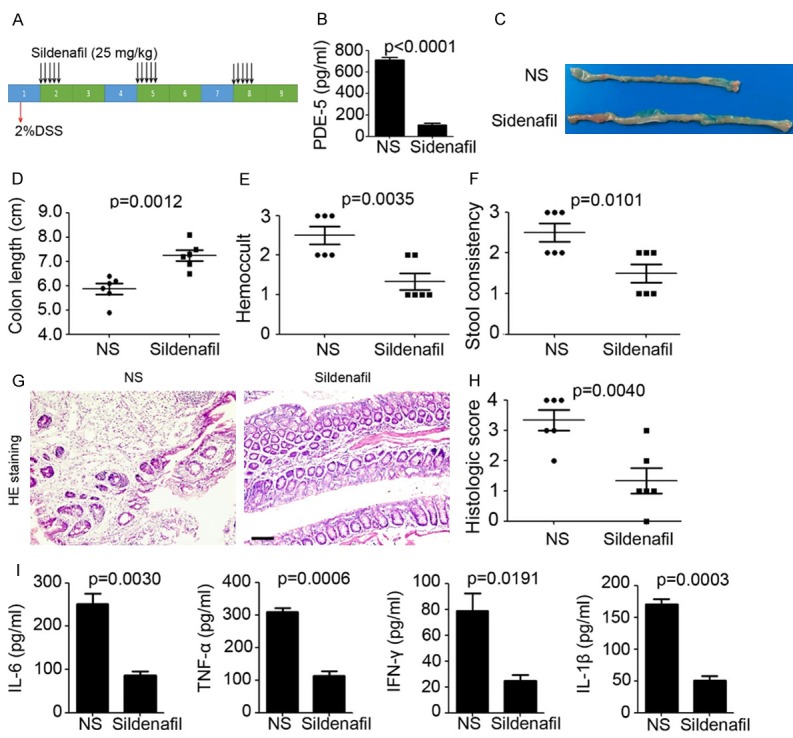
Inhibition of PDE-5 prevents colon from DSS-induced inflammation. (A) Schema of DSS treatment and Sildenafil injection. (B) ELISA analysis of PDE-5 expression in colonic tissues from Sildenafil and normal saline (NS) treatment mice (n=5). (C) Representative macroscopic findings ofcolon tumors induced by DSS in Sildenafil and normal saline (NS) treatment mice. (D) Colon length of DSS-treated mice per colon (n=6). (E, F) Hemoccult (E) and stool consistency analysis of DSS-treated mice (F) per colon (n=6). (G, H) H&E staining ofcolonic tissue after DSS treatment (G). Scale bar=200 μm. Histologic score was calculated (H, n=3). (I) ELISA analysis of inflammation-related cytokine expression in colonic tissue from DSS-treated mice (n=3).
Inhibition of PDE-5 blocks the recruitment of MDSC in colon tissue
To evaluate the changes of the inflammatory microenvironment, we examined leukocyte infiltration by flow cytometry. We found significantly decreased absolute numbers of MDSC (Ly6G+) and macrophages (F4/80+) and moderately decreased CD4+ cells and CD8+ cells in the colon of Sildenafil-treated mice compared with NS-treated mice both in AOM/DSS-induced tumorigenesis model (Figure 5A) and DSS-induced inflammation model (Figure 5B). IF staining showed extensive MDSC infiltration into colonic tissues and elevated expression of inducible nitric oxide synthase (iNOS) both in AOM/DSS-induced tumorigenesis model (Figure 5C) and DSS-induced inflammation model (Figure 5D). But, less iNOS+ MDSC were found in the colon of Sildenafil-treated mice (Figure 5C, 5D). The above data indicated that Sildenafil prevents colon from inflammation and inflammation-related tumorigenesis through blocking the recruitment of MDSC in colon tissue.
Figure 5.
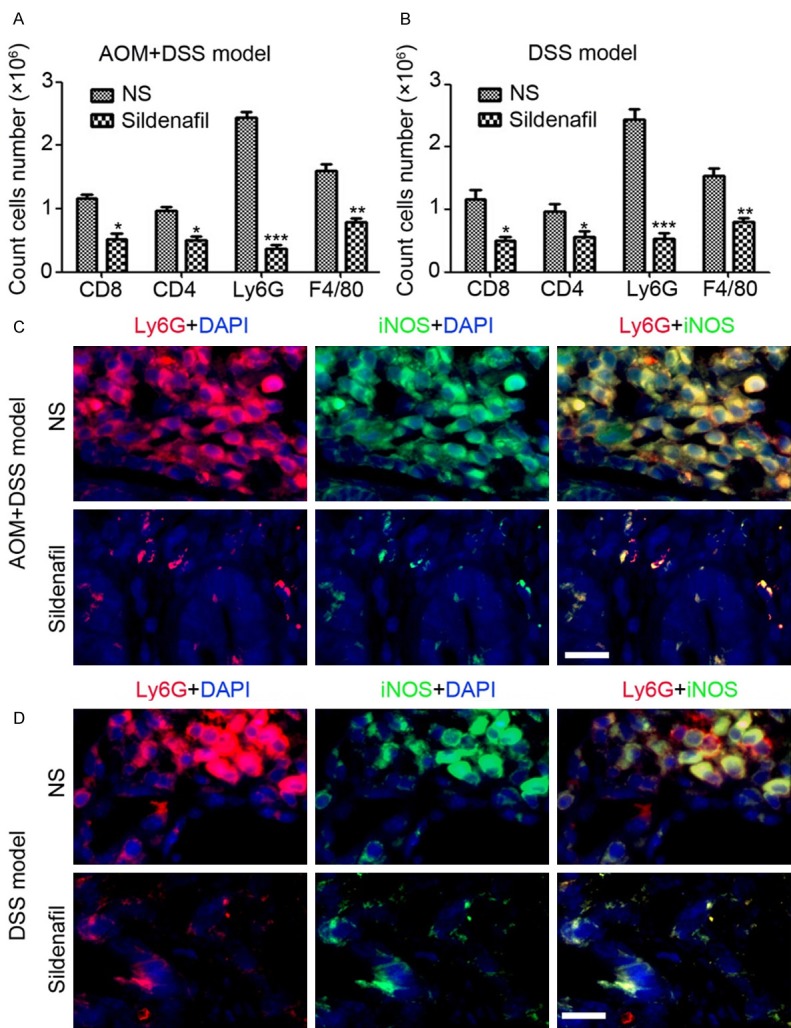
Inhibition of PDE-5 blocks the recruitment of MDSC in colon tissue. A: Flowcytometry analysis of leukocytes infiltration in colonic tissue of AOM/DSS-treated mice (n=4). B: Flowcytometry analysis of leukocytes infiltration in colonic tissue of DSS-treated mice (n=4). C: Immunofluorescent staining analysis of iNOS and Ly6G expression in colonic tissue of AOM/DSS-treated mice. D: Immunofluorescent staining analysis of iNOS and Ly6G expression in colonic tissue of DSS-treated mice.
Sildenafil inhibits MDSC invasion in vitro
To further determine the effect of Sildenafil on MDSC infiltration, colon epithelial cell and MDSC were isolated and cultured in vitro. LPS was employed to treat colon epithelial cell and the conditional medium was collected for PDE-5 detection by ELISA. The results indicated that LPS significantly induced PDE-5 expression in colon epithelial cell (P=0.0002), meanwhile Sildenafil dramatically inhibited PDE-5 expression (P=0.0013; Figure 6A). Next, matrigel-based invasion assay was performed to determine the effect of Sildenafil on MDSC and macrophage invasion. Our results indicated that the conditional medium from LPS-treated cell significantly promoted invasion of MDSC and macrophage (Figure 6B, 6C). Notably, inhibition of PDE-5 by Sildenafil only restrained the invasion of MDSC, but has no observed effect on macrophage invasion (Figure 6B, 6C). These data demonstrated that Sildenafil directly inhibits MDSC invasion in vitro and infiltration in vivo through decreasing PDE-5 expression, and the inhibition of macrophage infiltration in colon tissue is the downstream effect.
Figure 6.
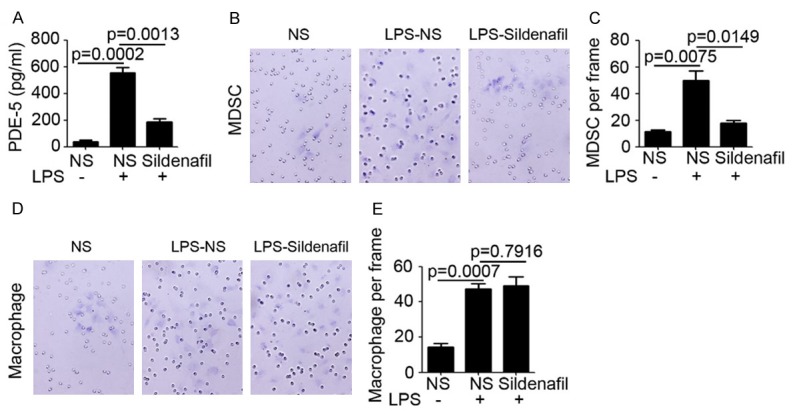
Sildenafil inhibits MDSC invasion in vitro. (A) ELISA analysis of PDE-5 expression in conditional medium of colon epithelium cell (n=3). (B, C) Matrigel-based invasion assay analysis of MDSC invasion under conditional medium stimulating (B). The number of MDSC per frame was calculated (C, n=3). (D, E) Matrigel-based invasion assay analysis of macrophage invasion under conditional medium stimulating (D). The number of macrophage per frame was calculated (E, n=3).
Discussion
Establishing a primary model that mimic the tumorigenesis in patients is necessary for cancer research and therapy. The AOM/DSS-induced colonic tumorigenesis model is a standard and widely used model for simulating colonic inflammation and tumor formation and progression [23,25,26]. AOM was used as the inducer of cell mutation that injected at the beginning, meanwhile DSS was performed to cause colonic damage and followed inflammation [23]. Furthermore, only injection of AOM with six times (one week interval between two injections) also stimulated observed colonic tumorigenesis without inflammation formation [23]. In the present study, we established the AOM/DSS-induced colonic tumorigenesis model and determined the expression of PDE-5 in the colonic tissue. The results indicated that PDE-5 was upregulated accompanying with the development of colonic tumorigenesis (Figure 1). Inhibition of PDE-5 by Sildenafil injection significantly inhibited colonic tumor formation and progression (Figure 2). The previous study by Zhu et al has demonstrated that knockdown of PDE-5 in colon cancer cell (HT-29) by siRNA could efficiently promotes apoptosis and proliferation delayed through regulating p21WAF/CIP1 signaling [27]. Activating of cGMP/PKG pathway, the direct target of PDE-5, by sulindac selectively inhibits colon tumor growth through suppressing Wnt/β-catenin pathway [12,28]. Furthermore, PDE-5 inhibitor, Tadalafil also augmented tumor specific immunity in patients with head and neck squamous cell carcinoma [29]. Thus, in our study, to investigate the mechanism under the effects of PDE-5 inhibition on colonic tumorigenesis, we employed Sildenafil to treat AOM-induced colonic tumorigenesis model. The results indicated that Sildenafil could significantly suppress colonic PDE-5 expression in mice, but has no observed effects on colonic tumor formation and progression in AOM-induced model (Figure 3). It demonstrated that Sildenafil inhibits colonic tumorigenesis dependent on inflammation.
Increasing evidences have demonstrated the tight connection between colonic inflammation and cancer [5-7]. Various molecule and chemistry was performed to establish colitis in rat and mice, including including trinitrobenzenesulfonic acid and acetic acid [14,19,20]. Although solid evidences have demonstrated the prevention role of PDE-5 inhibition in trinitrobenzenesulfonic acid-induced colitis [14,19], acetic acid-induced colitis [20] and ischemic colitis [21,22] in rat, but the role of PDE-5 in DSS-induced colitis in mice was still confused. Our findings first investigated the role of PDE-5 inhibition in DSS-induced colitis and indicated that Sildenafil suppressed the secretion of several pro-inflammation cytokines and immune cell infiltration, which was the biomarker of inflammation severity (Figure 4). It demonstrated that PDE-5 inhibition played an efficient preventing role in colonic inflammation formation. These results provide further evidence for the inhibition of colonic tumorigenesis by Sildenafil in AOM/DSS-induced tumorigenesis model.
MDSCs suppress various T cell responses and to promote Treg expansion during cancer and pathogenic conditions [30]. These cells contribute to the down-regulation of immune responses, constituting a unique component of the immune system in health and disease [30]. Thus, MDSCs have been considered a therapeutic target for the treatment of cancer and other diseases. During the colonic tumorigenesis, MDSC was expansion and infiltration into colonic tissue and promoted inflammation and inflammation-related tumor formation [31-33]. In our study, we found Sildenafil could inhibited the infiltration of functional MDSC (iNOS positive MDSC) into colonic tissue both in AOM/DSS-induced colonic tumor model and DSS-induced colitis model (Figure 5). Meanwhile, macrophage (F4/80 positive) was also suppressed by Sildenafil. Further studies are needed to demonstrate the direct target of PDE-5 inhibition. And matrigel-based invasion assay further demonstrated MDSC was the direct target of PDE-5 inhibition in colon epithelium cell, and the macrophage inhibition might be the effect of MDSC suppression (Figure 6). Collectively, PDE-5 inhibition by Sildenafil efficiently inhibits AOM/DSS-induced colonic tumorigenesis through blocking the recruitment of MDSC in colon tissue.
Taken together, we first demonstrated the upregulation of colonic PDE-5 expression and the prevention role of PDE-5 inhibition in AOM/DSS-induced tumorigenesis model. More importantly, PDE-5 inhibitor Sildenafil inhibited colonic tumorigenesis dependent on inflammation and suppressed DSS-induced colitis. Molecular mechanism investigation indicated that Sildenafil regulated inflammation microenvironment through directly inhibiting MDSC infiltration in colonic tissue. The study provides solid evidence for the use of PDE-5 inhibitor in prevention and treatment of colon inflammation-related tumorigenesis.
Acknowledgements
The present study was supported by Project of Natural Science Foundation of Guangdong Province (No. 2014A030310464). Guangzhou Pilot Project of Clinical and Translational Research Center (early gastrointestinal cancers, No. 7415696196402) and Guangdong Provincial Bio-engineering Research Center for Gastroenterology Diseases also supported this study.
Disclosure of conflict of interest
None.
References
- 1.Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, Duran A, Castilla EA, Metallo CM, Diaz-Meco MT, Moscat J. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26:121–135. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Hsu DS, Wang HJ, Tai SK, Chou CH, Hsieh CH, Chiu PH, Chen NJ, Yang MH. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell. 2014;26:534–548. doi: 10.1016/j.ccell.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Gupta J, del Barco Barrantes I, Igea A, Sakellariou S, Pateras IS, Gorgoulis VG, Nebreda AR. Dual function of p38alpha MAPK in colon cancer: suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell. 2014;25:484–500. doi: 10.1016/j.ccr.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N, Harpaz N, Itzkowitz S, Harris CC, Rotter V, Gorgoulis VG, Oren M. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–646. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonecchi R, Locati M, Mantovani A. Chemokines and cancer: a fatal attraction. Cancer Cell. 2011;19:434–435. doi: 10.1016/j.ccr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satpathy SR, Jala VR, Bodduluri SR, Krishnan E, Hegde B, Hoyle GW, Fraig M, Luster AD, Haribabu B. Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nat Commun. 2015;6:7064. doi: 10.1038/ncomms8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur JC, Gharaibeh RZ, Muhlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, Jobin C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun. 2014;5:4724. doi: 10.1038/ncomms5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, Piazza GA. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, Moyer MP, Grizzle WE, Chang WC, Clapper ML, Piazza GA. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/beta-catenin signaling. Mol Cancer Ther. 2013;12:1848–1859. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoke NN, Salloum FN, Kass DA, Das A, Kukreja RC. Preconditioning by phosphodiesterase-5 inhibition improves therapeutic efficacy of adipose-derived stem cells following myocardial infarction in mice. Stem Cells. 2012;30:326–335. doi: 10.1002/stem.789. [DOI] [PubMed] [Google Scholar]
- 14.Fakhfouri G, Rahimian R, Hashemi S, Rasouli MR, Bahremand A, Mehr SE, Khorramizadeh MR, Dehpour AR. Sildenafil attenuates TNBS-induced colitis in rats: possible involvement of cGMP and KATP channels. Fundam Clin Pharmacol. 2012;26:190–193. doi: 10.1111/j.1472-8206.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- 15.Fan YF, Zhang R, Jiang X, Wen L, Wu DC, Liu D, Yuan P, Wang YL, Jing ZC. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res. 2013;99:395–403. doi: 10.1093/cvr/cvt109. [DOI] [PubMed] [Google Scholar]
- 16.Garrido Abad P, Sinues Ojas B, Martinez Blazquez L, Conde Caturla P, Fernandez Arjona M. Safety and efficacy of intraurethral alprostadil in patients with erectile dysfunction refractory to treatment using phosphodiesterase-5 inhibitors. Actas Urol Esp. 2015;39:635–640. doi: 10.1016/j.acuro.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y, An R. Resveratrol and sildenafil synergistically improve diabetes-associated erectile dysfunction in streptozotocin-induced diabetic rats. Life Sci. 2015;135:43–48. doi: 10.1016/j.lfs.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Borlaug BA, Lewis GD, McNulty SE, Semigran MJ, LeWinter M, Chen H, Lin G, Deswal A, Margulies KB, Redfield MM. Effects of sildenafil on ventricular and vascular function in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:533–541. doi: 10.1161/CIRCHEARTFAILURE.114.001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margonis GA, Christoloukas N, Antoniou E, Arkadopoulos N, Theodoropoulos G, Agrogiannis G, Pikoulis E, Patsouris ES, Zografos GC, Papalois AE. Effectiveness of sildenafil and U-74389G in a rat model of colitis. J Surg Res. 2015;193:667–674. doi: 10.1016/j.jss.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Iseri SO, Ersoy Y, Ercan F, Yuksel M, Atukeren P, Gumustas K, Alican I. The effect of sildenafil, a phosphodiesterase-5 inhibitor, on acetic acid-induced colonic inflammation in the rat. J Gastroenterol Hepatol. 2009;24:1142–1148. doi: 10.1111/j.1440-1746.2009.05797.x. [DOI] [PubMed] [Google Scholar]
- 21.Aziret M, Irkorucu O, Reyhan E, Erdem H, Das K, Ozkara S, Surmelioglu A, Sozen S, Bali I, Cetinkunar S, Deger KC. The effects of vardenafil and pentoxifylline administration in an animal model of ischemic colitis. Clinics (Sao Paulo) 2014;69:763–769. doi: 10.6061/clinics/2014(11)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reyhan E, Irkorucu O, Surmelioglu A, Ozkara S, Deger KC, Aziret M, Erdem H, Cetinkunar S, Demirturk P, Sehirli AO. Efficacy of pentoxifylline and tadalafil in rat model of ischemic colitis. J Invest Surg. 2014;27:349–353. doi: 10.3109/08941939.2014.971204. [DOI] [PubMed] [Google Scholar]
- 23.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 24.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 25.Chen T, Yang M, Yu Z, Tang S, Wang C, Zhu X, Guo J, Li N, Zhang W, Hou J, Liu H, Han C, Liu Q, Gu Y, Qian C, Wan T, Cui L, Zhu M, Zheng W, Cao X. Small GTPase RBJ mediates nuclear entrapment of MEK1/MEK2 in tumor progression. Cancer Cell. 2014;25:682–696. doi: 10.1016/j.ccr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Dai L, Cui X, Zhang X, Cheng L, Liu Y, Yang Y, Fan P, Wang Q, Lin Y, Zhang J, Li C, Mao Y, Wang Q, Su X, Zhang S, Peng Y, Yang H, Hu X, Yang J, Huang M, Xiang R, Yu D, Zhou Z, Wei Y, Deng H. SARI inhibits angiogenesis and tumour growth of human colon cancer through directly targeting ceruloplasmin. Nat Commun. 2016;7:11996. doi: 10.1038/ncomms11996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhu B, Vemavarapu L, Thompson WJ, Strada SJ. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem. 2005;94:336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]
- 28.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, Gary BD, Keeton AB, Xi Y, Abadi AH, Grizzle WE, Piazza GA. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer Prev Res (Phila) 2012;5:822–833. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, Goodman S, Gourin CG, Ha PK, Fakhry C, Saunders J, Levine M, Tang M, Neuner G, Richmon JD, Blanco R, Agrawal N, Koch WM, Marur S, Weed DT, Serafini P, Borrello I. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–38. doi: 10.1158/1078-0432.CCR-14-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng Q, Wang Y, Yuan W, Ma J. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell. 2016;7:130–140. doi: 10.1007/s13238-015-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostanin DV, Bhattacharya D. Myeloid-derived suppressor cells in the inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:2468–2477. doi: 10.1097/MIB.0b013e3182902b11. [DOI] [PubMed] [Google Scholar]
- 33.Guan Q, Moreno S, Qing G, Weiss CR, Lu L, Bernstein CN, Warrington RJ, Ma Y, Peng Z. The role and potential therapeutic application of myeloid-derived suppressor cells in TNBS-induced colitis. J Leukoc Biol. 2013;94:803–811. doi: 10.1189/jlb.0113050. [DOI] [PubMed] [Google Scholar]


