Abstract
MicroRNA-338-3p (miR-338-3p) has recently been reported to have anti-cancer efficacy in several types of cancers. However, its biological function and underlying mechanism involved in modulation of human non-small cell lung cancer (NSCLC) remain largely unknown. The present study was designed to investigate the function and underlying mechanism of miR-338-3p in human NSCLC tissues and cell lines. We demonstrated that miR-338-3p was significantly decreased in NSCLC tissues and cell lines, and negatively correlated with advanced and tumor-node-metastasis (TNM) stage and lymph node metastasis (both P<0.01). Transient overexpression of miR-338-3p by transfecting with miR-338-3p mimic significantly suppressed NSCLC cell proliferation, migration, invasion and induced apoptosis and cell cycle at G1 phase. Additionally, insulin receptor substrate 2 (IRS2), a known oncogene, was identified as a potential target gene of miR-338-3p. Subsequent investigations found a negative correlation between the expression of miR-338-3p and IRS2 in NSCLC tissues. Furthermore, overexpression of IRS2 reversed the effects of miR-338-3p in NSCLC cells on cell proliferation, cycle, apoptosis, migration, invasion. These findings suggested that miR-338-3p might act as a tumor suppressor by directly targeting IRS2 in NSCLC.
Keywords: Non-small cell lung cancer, miR-338-3p, IRS2, proliferation, migration, invasion
Introduction
Lung cancer is a leading cause of death both in men and women around the world. Non-small cell lung cancer (NSCLC) represents the most frequent type of lung cancer, accounting for approximately 80-85% of all lung cancer cases [1,2]. Although great progress in surgical technique, diagnostic method, and new chemotherapy regimens, the 5-year overall survival (OS) rate is just 16% for all stages [3]. Therefore, further investigation of the molecular mechanisms involved in carcinogenesis and progression of NSCLC is essential for developing new effective therapeutic targets of NSCLC.
MicroRNAs (miRNAs) are a class of small, endogenous, non-coding RNA molecules (approximately 18-25 nucleotides in length) that control gene expression via partial base pairing to the 3’ untranslated region (UTR) of their targeting mRNAs for cleavage or repression of translation [4,5]. MiRNAs have been reported to get involved in various biological processes, such as differentiation, apoptosis, morphogenesis and tumorigenesis [6]. Accumulating evidences have shown that dysregulation of miRNAs played crucial roles in the initiation and progression of cancer by regulating cancer cell proliferation, cell cycle, apoptosis, migration, invasion or epithelial-mesenchymal transition (ETM) [7,8]. Up to now, a large number of miRNAs involved in cell proliferation, migration and invasion of NSCLC have been identified [9-11].
MiR-338-3p, located on chromosome 17q25, has been shown to play critical roles in promoting cell death, neuronal differentiation and neurite extension [12]. Recent several studies showed that miR-338-3p expression was downregulated and functioned as a tumor suppressor in several types cancers, such as hepatocellular carcinoma [13,14], neuroblastoma [15], ovarian cancer [16], malignant melanoma [17], gastric cancer [18,19], breast cancer [20], and colorectal cancer [21,22]. Although a report has demonstrated that miR-338-3p could regulate the survival of NSCLC cells partially through the downregulation of Ras-related protein 14 (RAB14) [23], its biological roles, especially with regard to migration and invasion, have not yet been thoroughly validated in NSCLC. Therefore, the aim of the present study was to investigate the role of miR-338-3p on the carcinogenesis of NSCLC.
Materials and methods
Tissue samples
Forty pairs of histologically confirmed NSCLC tissues (T) and adjacent non-tumor tissues (ANT) were obtained from patients who underwent routine curative surgery at Department of Thoracic Surgery, The First Hospital of Jilin University (Changchun, China) between July 2011 and September 2015. All tissue samples were snap-frozen in liquid nitrogen immediately after resection and stored at -80°C until use. None of these patients enrolled received local or systemic treatment before the surgery. Relevant clinical data of NSCLC patients were collected and listed in Table 1. We obtained informed written consent from all participants involved in our study and this study was approved by the ethics committee of the First of hospital of Jilin University (Changchun, China).
Table 1.
Correlation between clinicopathological features and miR-338-3p expression in NSCLCtissues
| Variables | No. of cases | miR-338-3p expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low (n %) | High (n %) | |||
| Age (years) | P>0.05 | |||
| <55 | 16 | 9 (56.3) | 7 (43.7) | |
| ≥55 | 24 | 13 (54.2) | 11 (45.8) | |
| Gender | P>0.05 | |||
| Man | 23 | 12 (52.2) | 11 (47.8) | |
| Woman | 17 | 10 (58.9) | 7 (41.1) | |
| TNM stage | P<0.01 | |||
| I-II | 31 | 14 (45.2) | 17 (54.8) | |
| III-IV | 9 | 8 (88.9) | 1 (11.1) | |
| Tumor size | P>0.05 | |||
| <5 cm | 28 | 15 (53.4) | 13 (46.6) | |
| ≥5 cm | 12 | 7 (58.3) | 5 (41.7) | |
| Lymph node metastasis | P<0.01 | |||
| No | 26 | 10 (38.5) | 16 (61.5) | |
| Yes | 14 | 12 (85.7) | 2 (14.3) | |
Cell lines and transfection
Four human NSCLC cell lines (A549, H1299, SPCA1 and H358) and normal lung cell (BEAS-2B) were purchased from Institute of Cell Biology of Chinese Academy of Science (Shanghai, China). All cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, USA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% non-essential amino acids (Invitrogen, Carlsbad, CA, USA) at 37°C in an incubator containing 5% CO2.
MiR-338-3p mimic (miR-338-3p), and corresponding miRNA negative control (miR-NC) were synthesized by GenePharma (Shanghai, China). Overexpressed IRS2 plasmid and blank vector were purchased from RiboBio (Guangzhou, China). Transfection was performed using lipofectamine 2000 (Invitrogen, USA) according to manufacturer’s instructions.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from clinical tissue samples and cultured cells using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The purity and concentration of RNA were determined by a dual-beam ultraviolet spectrophotometer (Eppendorf, Hamburg, Germany). For detection of miR-338-3p, the RNA was reversely transcribed into cDNA using One Step Prime script miRNA cDNA Synthesis Kit (Qiagen, Valencia, CA). Then miR-338-3p was quantified using TaqMan miRNA assay kits (Applied Biosystems, Foster City, CA, USA) under an ABI7900 real-time PCR system (Applied Biosystems) through the specific primers of miR-338-3p and U6 (Applied Biosystems). U6 was used as an endogenous control. To detection the mRNA levels of IRS2, cDNA was synthesized using the Primer Script RT reagent Kit (Takara, Dalian, China). Then IRS2 mRNA level was quantified using the Real-time PCR Mixture Reagent (Takara, Dalian, China) under an ABI7900 real-time PCR system through the specific primers of IRS2 and β-actin as described previously [24]. Β-actin was used as an endogenous control. The comparative 2-∆∆Ct method was used for relative quantification.
Cell proliferation
Cell proliferation was determined by Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan). Briefly, transfected cells (5×103 cells/well) were seeded into 24-well plates. At indicated time points (24 h, 48 h, 72 h), the cells were incubated in 10% CCK8 solution in normal culture medium at 37°C until visual color conversion occurred. The absorbance at 450 nm was measured with a microplate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
Cell cycle and apoptosis assay
Cells cycle and apoptosis assays were determined on A549 cells at 48 h after transfection. For cell cycle assay, the transfected cells (1×106 cells per well) were cultured in 12-well plates in triplicate for 24 h. Then the cells were collected by trypsinization, washed in PBS and fixed in ice-cold ethanol overnight at 4°C, followed by incubated in 1 ml staining solution containing 20 μg/ml propidium iodide (PI, Sigma, USA) and 10 U/mL RNaseA at room temperature for 30 min in the dark. The stained cells were determined using fluorescence-activated cell sorting (FACS) flow cytometry (FACSCalibur, Becton-Dickinson, Bedford, MA, USA).
Cell apoptosis was determined using Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences) under FACSCalibur flow cytometer (BD Biosciences) according to the manufacturer’s instructions.
Cell migration and invasion assays
To examine the migratory ability of cells in vitro, a wound-healing assay was performed. Briefly, 5×103 transfected cells were seeded into 24-well tissue culture plates and grown to a density of 70 to 80%. Thereafter, the linear wound of cellular monolayer was created by 200 μl of pipette tips. Migration of cells into the wound was observed and photographed at 0 and 24 h using an I×51 inverted light microscope (Olympus, Tokyo, Japan).
Cell invasion assay was carried out using 24-well Transwell chamber with 8 μM pores (Corning, Lowell, MA). Briefly, 2×104 transfected cells were suspended in serum-free medium and seeded into upper transwell chambers coated with Matrigel (BD Biosciences, Bedford, MA, USA). Bottom wells were filled with DMEM media containing 10% FBS. After incubation for 48 h in a humidified atmosphere of 5% CO2 at 37°C, non-invading cells were removed from the top well with a cotton swab, and invading cells present on the lower surface of the membrane were fixed in 70% ethanol for 30 min and stained with 2% crystal violet for 10 min, then photographed under an I×51 inverted light microscope (Olympus, Tokyo, Japan). The number of invading cells was counted at five randomly selected fields with a magnification of ×200.
Dual-luciferase reporter assay
3’-untranslated regions (3’-UTR) of IRS2 containing predicted miR-338-3p seed-matching sites and corresponding mutant sites were amplified by PCR, and inserted into downstream of the firefly luciferase gene in a pGL3-promoter vector (Ambion, Austin, TX, USA) at the NheI and XhoI sites, named as: Wt-IRS2-3’UTR and Mut-IRS2-3’UTR, respectively. For dual-luciferase assay, the luciferase reporter gene vector with wild-type IRS2 (Wt-IRS2-3’UTR) or mutate-type IRS2 (Mut-IRS2-3’UTR), together with miR-338-3p mimic or miR-NC were co-transfected into A549 cells. At 48 h after transfection, firefly and renilla luciferase activities were determined using the Dual Luciferase Reporter Assay System (Promega, WI, USA). Luciferase activities were normalized to Renilla luciferase.
Western blot analysis
Cultured cells and tissue samples were collected and lysed in RIPA lysis buffer (Heart Biological Technology, Xian, China). Concentrations of total protein were measured using a BCA assay kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. Equal amounts of protein (30 μg) were electrophoresed using 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Millipore, MA, USA), blocked in 5% non-fat milk dissolved in TBST (10 mM Tris-HCl and 0.05% Tween 20) for 2 h at room temperature. The membranes were then incubated at 4°C with primary antibodies of IRS2 (1:1000; Santa Cruz, CA, USA) and β-actin (1:5000; Santa Cruz, CA, USA) overnight, followed by incubation horseradish peroxidase (HRP)-conjugated goat-anti-mouse IgG (1:5000; Santa Cruz Biotechnology, CA, USA). The protein bands were observed on the X-ray film with a chemiluminescent detection system (ECL, Beyotime, Shanghai, China).
Statistical analysis
Quantitative data were presented as the mean ± SD (standard deviation) from at least three independent experiments. All data are analyzed using Statistical SPSS Version 19.0 (IBM, Chicago, USA). Student’s T-test or one-way ANOVA was used to assess the differences of different groups. The relationship between expression of miR-338-3p and clinical and pathological variables was analyzed using Pearson’s χ2 test. The correlations between expression of miR-338-3p and IRS2 were analyzed using Spearman’s rank test in NSCLC tissues. Statistical significance was accepted at P<0.05.
Results
MiR-338-3p is down-regulated in NSCLC cell lines and tissues
To examine the correlation between expression of miR-338-3p and NSCLC, we detected the expression of miR-338-3p in four human NSCLC cell lines and normal lung cell line by qRT-PCR. As shown in Figure 1A, miR-338-3p was significantly downregulated in all NSCLC cell lines including A549, H1299, SPCA1 and H358 cells, compared with normal lung cell line BEAS-2B. A549 cell line exhibited the lowest expression of miR-338-3p, and was therefore selected for following studies (Figure 1A). QRT-PCR assays were further carried out to quantify miR-338-3p levels in T and ANT from 40 patients with NSCLC. It was found that the expression levels of miR-338-3p in T tissues (0.37 ± 0.06) were significantly decreased compared to ANT tissues (1.04 ± 0.11) (P<0.01, Figure 1B). Based on the median (0.37) of miR-338-3p level in NSCLC tissues, all 40 NSCLC patients were divided into 2 subgroups: Low miR-338-3p group (<0.37, 22 cases) and high miR-338-3p group (>0.37, 18 cases). The association between miR-338-3p expression and the clinicopathological parameters of the patients were analyzed. As shown in Table 1, miR-338-3p was significantly associated with TNM stage (P<0.01) and lymph node metastasis (P<0.01), but not with age and gender, and tumor size (All P>0.05). These data indicated that miR-338-3p might get involved in NSCLC initiation and procession.
Figure 1.
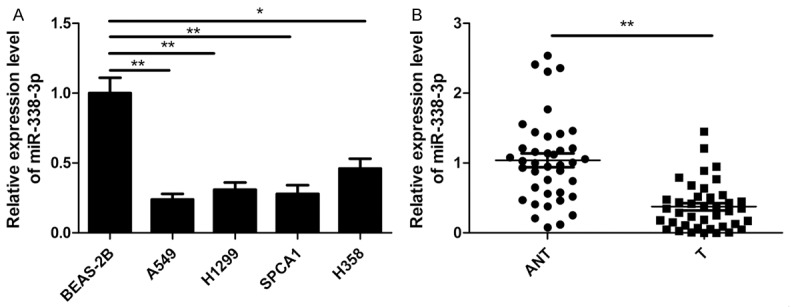
MiR-338-3p is down-regulated in NSCLC cell lines and tissues. A. MiR-338-3p expression in four human NSCLC cell lines (A549, H1299, SPCA1 and H358) and normal lung cell BEAS-2B were detected by qRT-PCR. *P<0.05; **P<0.01 versus BEAS-2B. B. MiR-338-3p expression in human NSCLC tissues samples (T) and their adjacent non-tumor tissues (ANT) from 40 patients with NSCLC were detected by qRT-PCR. **P<0.01 versus ANT.
MiR-338-3p overexpression inhibits NSCLC cell proliferation and induces apoptosis
To investigate the potential role of miR-338-3p in regulating NSCLC cell growth, we established miR-338-3p overexpressed cells by transfection of miR-338-3p mimic into A549 cells. The results from qRT-PCR assay confirmed that miR-338-3p mimic could significantly upregulate the expression level of miR-338-3p in A549 cells (Figure 2A). To testify the effects of miR-338-3p on proliferation of NSCLC cells, CCK8 assay was performed. As shown in Figure 2B, overexpression of miR-338-3p significantly inhibited cell proliferation of A549 cells. We also analyzed the cell cycle of miR-338-3p or miR-NC stably transfected-A549 cells to explain the cell growth suppression caused by miR-338-3p overexpression. As expected, the percentage of S phase cells decreased, while the percentage of G1 phase cells increased in A549 cells transfected with miR-338-3p compared to transfected with miR-NC (P<0.05, Figure 2C). To reveal the biological role of miR-338-3p on NSCLC cell apoptosis, cell apoptosis assay were performed in NSCLC cells. Our results showed that miR-338-3p overexpression significantly induced cell apoptosis (P<0.01, Figure 2D).
Figure 2.
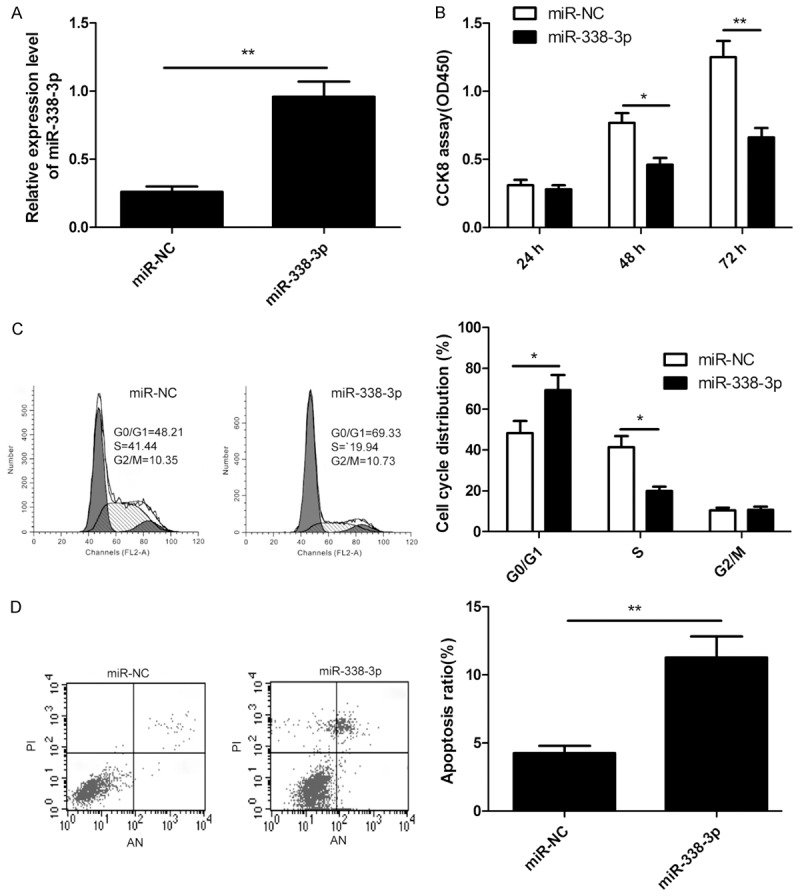
MiR-338-3p overexpression inhibits NSCLC cell proliferation and induces apoptosis. (A) QRT-PCR analysis of miR-338-3p expression in A549 cells after transfection of miR-338-3p or miR-NC. (B-D) Cell proliferation (B), cell cycle (C) and apoptosis (D) were determined in A549 cells after transfection of miR-338-3p or miR-NC. *P<0.05, **P<0.01 versus miR-NC.
MiR-338-3p overexpression inhibits NSCLC metastasis
To further investigate the role of miR-338-3p involved in NSCLC metastasis, migration and invasion assays were performed in A549 cells transfected with miR-338-3p mimic or miR-NC by wound healing and transwell invasion assays, respectively. It was found that miR-338-3p overexpression could remarkably suppress migration (Figure 3A) and invasion (Figure 3B) of A549 cells.
Figure 3.
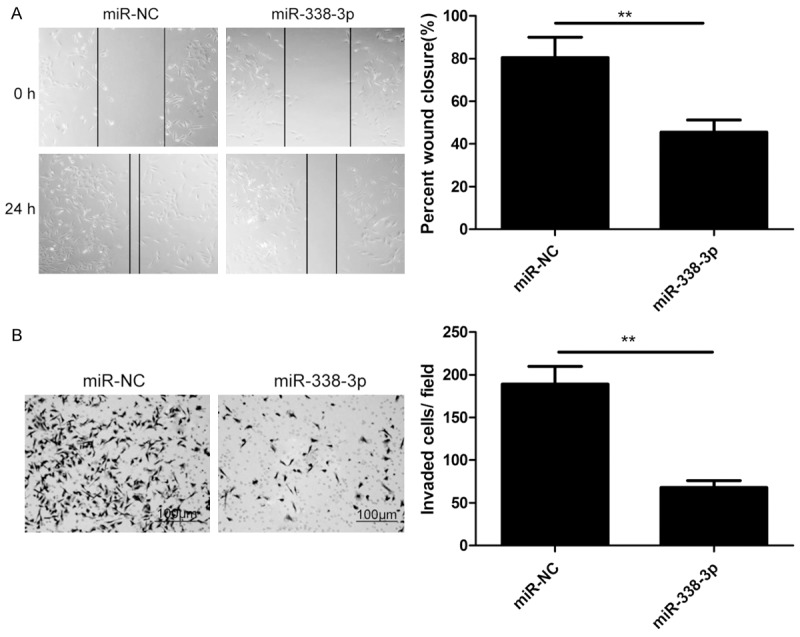
MiR-338-3p overexpression inhibits NSCLC cell metastasis. A. Cell migration was determined in A549 cells after transfection of miR-338-3p or miR-NC by wound healing assay. B. Cell invasion was determined in A549 cells after transfection of miR-338-3p or miR-NC by transwell invasion assay. *P<0.05, **P<0.01 versus miR-NC.
IRS2 is a direct target of miR-338-3p in NSCLC cells
To clarify the molecular mechanisms of miR-338-3p on growth and metastasis of NSCLC cells, we searched for the target mRNAs using TargetScan7.0 and miRanda. IRS2 was selected as one of the candidate targets of miR-338-3p, based on putative target sequences at 2349-2355 bp of IRS2 (Figure 4A). To further confirm targeting of IRS2 by miR-338-3p, luciferase activity assay was performed. Through the luciferase activity assay, we found that A549 cells transfected with miR-338-3p mimic significantly inhibited wild-type IRS2 3’UTR reporter activity (P<0.01; Figure 4B), while had no inhibitory effect on the mutant-type IRS2 3’UTR reporter activity (Figure 4B). In addition, the mRNA and protein levels of IRS2 were detected in NSCLC cells transfected miR-338-3p mimic or miR-NC. Our results showed that miR-338-3p overexpression significantly attenuated the mRNA and protein levels of IRS2 in A549 cells (Figure 4C, 4D). Above results implied that miR-338-3p suppressed IRS2 expression by binding to 3’UTR of IRS2 mRNA.
Figure 4.
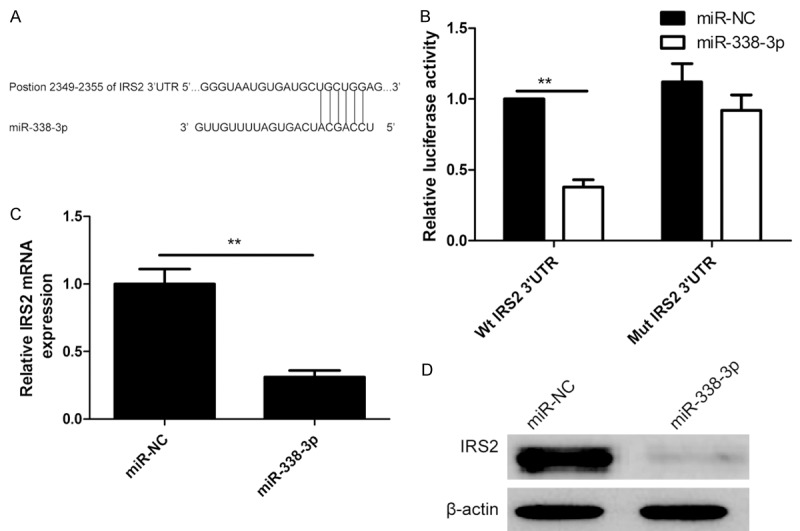
IRS2 is a direct target of miR-338-3p in NSCLC cells. A. Predicted miR-338-3p target sequence in IRS2-3’-UTR was shown. B. The luciferase activity was measured after co-transfection with luciferase reporter plasmids (Wt/Mut IRS2 3’UTR), and miR-338-3p mimic/miR-NC in A549 cells. Wt: Wide-type; Mut: Mutant-type. C. QRT-PCR analysis of IRS2 mRNA expression in A549 cells after transfection of miR-338-3p or miR-NC. D. Western blots analysis of IRS2 protein expression in A549 cells after transfection of miR-338-3p or miR-NC. *P<0.05, **P<0.01 versus miR-NC.
IRS2 was up-regulated and negatively correlated with miR-338-3p in NSCLC tissues
To further explore the relationship between miR-338-3p and IRS2, we examined the expression of IRS2 on mRNA level and protein levels in T and ANT tissues by qRT-PCR and western blot, respectively. We found that the expression of IRS2 on both mRNA and protein levels was significantly increased in T tissues compared to ANT tissues (P<0.01; Figure 5A, 5B). Through spearman’s correlation analysis, a statistically significant negative correlation was found between expression levels of miR-338-3p and IRS2 mRNA in T tissues. (r=-0712, P<0.001; Figure 5C), and ANT specimens (r=-0.643, P<0.001; Figure 5D). In addition, we also found that IRS2 expression both on mRNA level and protein levels was significantly upregulated in four NSCLC cell lines (A549, H1299, SPCA1 and H358), compared with normal lung cell line BEAS-2B (Figure 5E, 5F).
Figure 5.
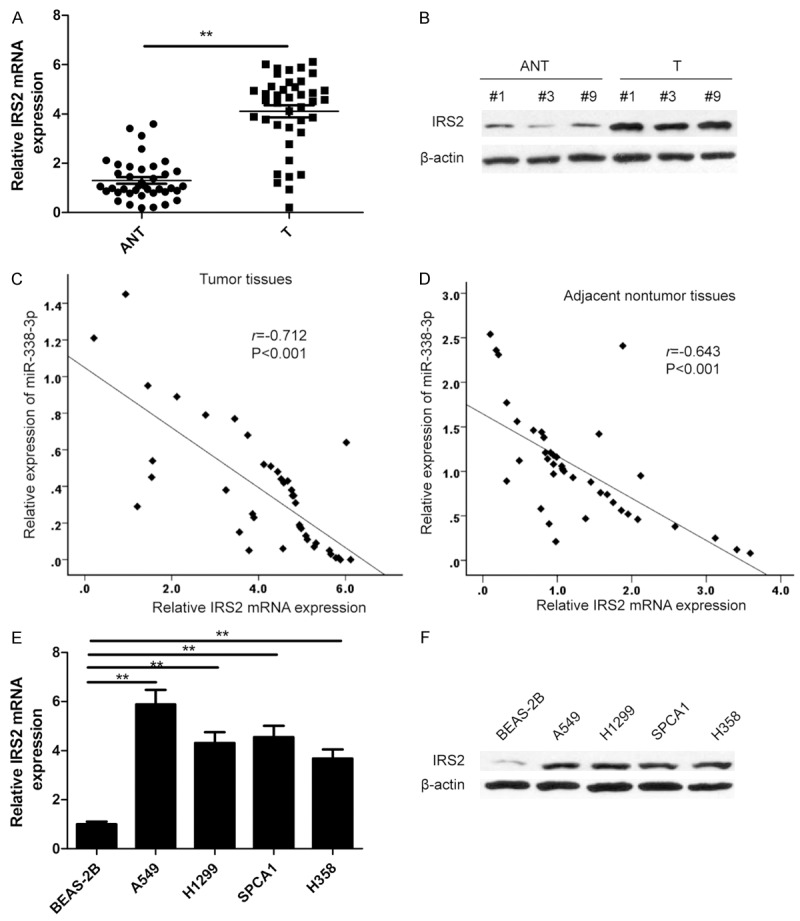
IRS2 was up-regulated and negatively correlated with miR-338-3p in NSCLC tissues. (A, B) IRS2 expression on mRNA level (A) and protein level (B) was determined in NSCLC tissues (T) and adjacent non-tumor tissues (ANT). **P<0.01 versus ANT. (C, D) Spearman’s correlation analysis was used to determine the correlations between the expression levels of IRS2 and miR-338-3p in NSCLC tissues (C) and adjacent non-tumor tissues (D). (E, F) IRS2 expression on mRNA level (E) and protein level (F) was determined in four NSCLC cell lines (A549, H1299, SPCA1 and H358) and normal lung cell line BEAS-2B. *P<0.05; **P<0.01 versus BEAS-2B.
Overexpression of IRS2 reverses the effects induced by miR-338-3p overexpression in A549 cells
To evaluate if IRS2 is responsible for the functional effect of miR-338-3p in NSCLC cells, some rescue experiments were performed. A549 cells were co-transduced with miR-338-3p mimic/miR-NC and blank vector or IRS2 overexpression vector without its 3’UTR. And then, cell proliferation, cycle, apoptosis, migration and invasion assays were performed in above cells. Western blot assay showed that A549 cells co-transduced with miR-338-3p mimic plus blank vector significantly decreased IRS2 protein expression, while cells co-transduced with miR-338-3p mimic plus IRS2 overexpression vectors could restore IRS2 protein expression (Figure 6A). In addition, we also found that IRS2 overexpression partially reverses the effects on cell proliferation, cycle, apoptosis, migration and invasion of A549 cells induced by miR-338-3p overexpression (Figure 6B-F), indicating that miR-338-3p exerts it biological roles in NSCLC by targeting IRS2.
Figure 6.

Overexpression of IRS2 reverses the effects induced by miR-338-3p overexpression in A549 cells. A. IRS2 protein expression was determined in A549 cells co-transfected with miR-338-3p mimic/miR-NC and blank vector or IRS2 overexpression vector. B-F. Cell proliferation, cell cycle, apoptosis, migration and invasion were determined in A549 cells co-transfected with miR-338-3p mimic/miR-NC and blank vector or IRS2 overexpression vector. *P<0.05; **P<0.01 versus miR-338-3p plus Vector.
Discussion
Accumulating evidences have been suggested that miRNAs function as either tumor suppressors or oncogenes by regulating various biological procession of cancer cells, such as cell proliferation, apoptosis, migration and invasion [7,8]. In NSCLC, some miRNAs, such as members of the let-7 family, miR-126, miR-145, miR-144 or miR-34a, have been identified as tumor suppressors [9,10], while miR-17-92, miR-21, miR-221, miR-222 and miR-31 were found to act as oncogenes in NSCLC [9,10], suggesting that miRNAs act as new direction in NSCLC diagnosis and treatment. In current study, we found that miR-338-3p is dramatically down-regulated in human NSCLC cell lines and tissues compared to normal lung cell and adjacent non-tumor tissues, which were consistent with previous results [24]. We also found that miR-338-3p overexpression suppresses NSCLC cell proliferation, migration, invasion, and induces cell apoptosis. In addition, we identified that miR-338-3p directly targeted the 3’UTR of IRS2 and suppressed its expression transcriptionally and post-transcriptionally. Of note, we also found that overexpression of IRS2 reversed the effects of miR-338-3p on cell proliferation, arrest, apoptosis, migration, invasion mediated by miR-338-3p mimics in NSCLC cells. These results provided new insights into NSCLC research and therapeutic strategies.
Down-regulation of miR-338-3p has been reported in several type cancers [13-23]. It has been showed that miR-338-3p could inhibit cancer cell proliferation, colony formation, migration and invasion, as well as induced cell apoptosis by targeting multiple genes, such as smoothened [13,22], CyclinD1 [14], PREX2a [15,19], SOX4 [20], SSX2IP [18], Runx2 [16], and Ras-related protein 14 [23]. Recently, a report showed that miR-338-3p expression was downregulated in NSCLC tissues and inhibited cell growth partially through the downregulation of Ras-related protein 14 (RAB14) [23]. However, its biological roles in NSCLC, especially with regard to migration and invasion, remained largely unknown. In the present study, we further investigated the precise biological role of miR-338-3p expression in NSCLC. It was found that the expression level of miR-338-3p was downregulated in NSCLC tissues and cell lines, and negatively associated with NSCLC metastasis and advanced TNM stages. Furthermore, we investigated the effects of miR-338-3p on NSCLC growth, and found that miR-338-3p overexpression inhibited NSCLC cells proliferation, induced cell cycle arrest at G1 phase and apoptosis. After that, we focused on the effects of miR-338-3p on NSCLC metastasis, and found that miR-338-3p overexpression inhibited NSCLC cells migration and invasion. These results indicated that miR-338-3p inhibited cell growth and metastasis of NSCLC. This study together with previous study suggested that miR-338-3p function as a tumor suppressor in NSCLC.
It has been shown that miRNAs exert biological functions via binding to the mRNA 3’-UTR of the target gene to block its expression [6]. To identify the target mRNAs of miR-338-3p, TargetScan and miRanda algorithm were used. IRS2 was postulated as a potential downstream target of miR-338-3p involved in tumor cell proliferation, migration and invasion. IRS2, located in the 13q34 region, is a member of the insulin receptor substrate (IRS) protein family that function as adaptor proteins for additional surface receptors, including the closely related insulin-like growth factor 1 receptor (IGF-1R) [25,26]. It has been shown that IRS2 can recruit and activate PI3K to promote Akt signaling when stimulated with IGF-1 [27]. Previous studies showed that IRS2 contributed to tumorigenesis by promoting cancer cell proliferation and inhibited apoptosis [28-30]. IRS2 expression has been reported to be upregulated in many types of cancers including NSCLC [31]. In addition, recently, a study showed that silencing IRS2 inhibited NSCLC cell invasion by regulating ETM [32]. These studies suggested that IRS2 function as an oncogene in NSCLC. Here, IRS2 was identified as a direct target of miR-338-3p. IRS2 expression level was upregulated, and negatively correlated with miR-338-3p expression levels in human T and ANT tissues. IRS2 overexpression reversed the effects on cell proliferation, cycle, apoptosis, migration and invasion mediated by miR-338-3p overexpression in NSCLC cells. These results suggests that miR-338-3p function as a tumor suppressor, at least in part, by repressing IRS2 expression.
In the present study, we verified that miR-338-3p is dramatically down-regulated in human NSCLC cell lines and tissues, and its expression was negatively associated with advanced and tumor-node-metastasis (TNM) stage and lymph node metastasis. Moreover, we also revealed that miR-338-3p overexpression suppresses NSCLC cell proliferation, migration, invasion, and promotes cell apoptosis and arrest at G1 phase, at least in part, through targeting IRS2. These data suggested that miR-338-3p might serve as a new therapy target for NSCLC.
Acknowledgements
The authors would like to thank all patients who provided tissues.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rivera MP. Multimodality therapy in the treatment of lung cancer. Semin Respir Crit Care Med. 2004;25(Suppl 1):3–10. doi: 10.1055/s-2004-829639. [DOI] [PubMed] [Google Scholar]
- 3.Laskin JJ, Sandler AB. State of the art in therapy for non-small cell lung cancer. Cancer Invest. 2005;23:427–442. doi: 10.1081/cnv-67172. [DOI] [PubMed] [Google Scholar]
- 4.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 5.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13:253–258. doi: 10.1016/s1044-579x(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 8.Farazi TA, Spitzer JI, Morozov P, Tuschl T. MiRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeri M, Sestini S, Fortunato O, Verri C, Suatoni P, Pastorino U, Sozzi G. Recent advances of microRNA-based molecular diagnostics to reduce false-positive lung cancer imaging. Expert Rev Mol Diagn. 2015;15:801–813. doi: 10.1586/14737159.2015.1041377. [DOI] [PubMed] [Google Scholar]
- 10.Skrzypski M, Dziadziuszko R, Jassem J. MicroRNA in lung cancer diagnostics and treatment. Mutat Res. 2011;717:25–31. doi: 10.1016/j.mrfmmm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Guan P, Yin Z, Li X, Wu W, Zhou B. Meta-analysis of human lung cancer microRNA expression profiling studies comparing cancer tissues with normal tissues. J Exp Clin Cancer Res. 2012;31:54. doi: 10.1186/1756-9966-31-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kos A, Olde Loohuis NF, Wieczorek ML, Glennon JC, Martens GJ, Kolk SM, Aschrafi A. A potential regulatory role for intronic microRNA-338-3p for its host gene encoding apoptosis-associated tyrosine kinase. PLoS One. 2012;7:e31022. doi: 10.1371/journal.pone.0031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang XH, Chen JS, Wang Q, Chen XL, Wen L, Chen LZ, Bi J, Zhang LJ, Su Q, Zeng WT. MiR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. J Pathol. 2011;225:463–472. doi: 10.1002/path.2877. [DOI] [PubMed] [Google Scholar]
- 14.Fu X, Tan D, Hou Z, Hu Z, Liu G, Ouyang Y, Liu F. The effect of miR-338-3p on HBx deletion-mutant (HBx-d382) mediated liver-cell proliferation through CyclinD1 regulation. PLoS One. 2012;7:e43204. doi: 10.1371/journal.pone.0043204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Chen X, Pan M, Han L, Lu H, Hao X, Dong Q. MiR-338-3p suppresses neuroblastoma proliferation, invasion and migration through targeting PREX2a. FEBS Lett. 2013;587:3729–3737. doi: 10.1016/j.febslet.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Wen C, Liu X, Ma H, Zhang W, Li H. MiR3383p suppresses tumor growth of ovarian epithelial carcinoma by targeting Runx2. Int J Oncol. 2015;46:2277–2285. doi: 10.3892/ijo.2015.2929. [DOI] [PubMed] [Google Scholar]
- 17.Caramuta S, Egyhazi S, Rodolfo M, Witten D, Hansson J, Larsson C, Lui WO. MicroRNA expression profiles associated with mutational status and survival in malignant melanoma. J Invest Dermatol. 2010;130:2062–2070. doi: 10.1038/jid.2010.63. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Chen X, Su L, Li C, Zhi Q, Yu B, Sheng H, Wang J, Feng R, Cai Q, Li J, Yu Y, Yan M, Liu B, Zhu Z. Epigenetic silencing of miR-338-3p contributes to tumorigenicity in gastric cancer by targeting SSX2IP. PLoS One. 2013;8:e66782. doi: 10.1371/journal.pone.0066782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo B, Liu L, Yao J, Ma R, Chang D, Li Z, Song T, Huang C. MiR-338-3p suppresses gastric cancer progression through a PTEN-AKT axis by targeting P-REX2a. Mol Cancer Res. 2014;12:313–321. doi: 10.1158/1541-7786.MCR-13-0507. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Zhao M, Xie Q, Zhang H, Wang Q, Ma Q. MicroRNA-338-3p functions as tumor suppressor in breast cancer by targeting SOX4. Int J Oncol. 2015;47:1594–1602. doi: 10.3892/ijo.2015.3114. [DOI] [PubMed] [Google Scholar]
- 21.Sun K, Su G, Deng H, Dong J, Lei S, Li G. Relationship between miRNA-338-3p expression and progression and prognosis of human colorectal carcinoma. Chin Med J (Engl) 2014;127:1884–1890. [PubMed] [Google Scholar]
- 22.Sun K, Deng HJ, Lei ST, Dong JQ, Li GX. MiRNA-338-3p suppresses cell growth of human colorectal carcinoma by targeting smoothened. World J Gastroenterol. 2013;19:2197–2207. doi: 10.3748/wjg.v19.i14.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Feng X, Gao S, Xiao Z. MicroRNA-338-3p functions as a tumor suppressor in human nonsmallcell lung carcinoma and targets Ras-related protein 14. Mol Med Rep. 2015;11:1400–1406. doi: 10.3892/mmr.2014.2880. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal P, Srivastava R, Srivastava AK, Ali S, Datta M. MiR-135a targets IRS2 and regulates insulin signaling and glucose uptake in the diabetic gastrocnemius skeletal muscle. Biochim Biophys Acta. 2013;1832:1294–1303. doi: 10.1016/j.bbadis.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 25.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 26.He W, Craparo A, Zhu Y, O’Neill TJ, Wang LM, Pierce JH, Gustafson TA. Interaction of insulin receptor substrate-2 (IRS-2) with the insulin and insulin-like growth factor I receptors. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J Biol Chem. 1996;271:11641–11645. doi: 10.1074/jbc.271.20.11641. [DOI] [PubMed] [Google Scholar]
- 27.Landis J, Shaw LM. Insulin receptor substrate 2-mediated phosphatidylinositol 3-kinase signaling selectively inhibits glycogen synthase kinase 3beta to regulate aerobic glycolysis. J Biol Chem. 2014;289:18603–18613. doi: 10.1074/jbc.M114.564070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day E, Poulogiannis G, McCaughan F, Mulholland S, Arends MJ, Ibrahim AE, Dear PH. IRS2 is a candidate driver oncogene on 13q34 in colorectal cancer. Int J Exp Pathol. 2013;94:203–211. doi: 10.1111/iep.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Melo Campos P, Machado-Neto JA, Eide CA, Savage SL, Scopim-Ribeiro R, da Silva Souza Duarte A, Favaro P, Lorand-Metze I, Costa FF, Tognon CE, Druker BJ, Olalla Saad ST, Traina F. IRS2 silencing increases apoptosis and potentiates the effects of ruxolitinib in JAK2V617F-positive myeloproliferative neoplasms. Oncotarget. 2016;7:6948–6959. doi: 10.18632/oncotarget.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, Divisova J, Britton OL, Mohsin S, Allred DC, Hadsell DL, Lee AV. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 2006;26:9302–9314. doi: 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusakabe M, Kutomi T, Watanabe K, Emoto N, Aki N, Kage H, Hamano E, Kitagawa H, Nagase T, Sano A, Yoshida Y, Fukami T, Murakawa T, Nakajima J, Takamoto S, Ota S, Fukayama M, Yatomi Y, Ohishi N, Takai D. Identification of G0S2 as a gene frequently methylated in squamous lung cancer by combination of in silico and experimental approaches. Int J Cancer. 2010;126:1895–1902. doi: 10.1002/ijc.24947. [DOI] [PubMed] [Google Scholar]
- 32.Park DH, Jeon HS, Lee SY, Choi YY, Lee HW, Yoon S, Lee JC, Yoon YS, Kim DS, Na MJ, Kwon SJ, Kim DS, Kang J, Park JY, Son JW. MicroRNA-146a inhibits epithelial mesenchymal transition in non-small cell lung cancer by targeting insulin receptor substrate 2. Int J Oncol. 2015;47:1545–1553. doi: 10.3892/ijo.2015.3111. [DOI] [PubMed] [Google Scholar]


