Abstract
Osteosarcoma is a rare malignant bone tumor in adolescents, with high degree of malignancy, and highly incidence of recurrence and metastasis. Our study aimed to explore the role of miR-101 in osteosarcoma cells by targeting ROCK1. In the present study, reverse transcription-quantitative polymerase chain reaction data revealed that miR-101 was down-regulated in the tissue samples of 20 patients with osteosarcoma compared with their matched adjacent non-tumor tissues (P < 0.01). Furthermore, miR-101 was significantly down-regulated in three common OS cell lines, MG63, U2OS, and OS732 compared with the human osteoblast cell line, hFOB1.19 (P < 0.01). MiR-101 was shown to target the ROCK1 3’-UTR in dual-luciferase reporter assays in MG63 cells. Overexpression of miR-101 significantly suppressed the protein expression levels of ROCK1, while knockdown of miR-101 significantly enhanced the formers’ expression levels in MG63 cells (P < 0.05). Overexpression of miR-101 inhibited cell viability, migration, and invasion while promoted apoptosis. Independent inhibition of ROCK1 and knockdown of miR-101 expression levels significantly promoted MG63 cell proliferation, migration and invasion while inhibited apoptosis (P < 0.01). Moreover, knockdown of ROCK1 reversed the promotion effect of miR-101 knockdown on proliferation, migration, and invasion while promoted apoptosis of MG63 cells, suggesting that miR-101 acts as a tumor suppressor in osteosarcoma cells via targeting ROCK1. Furthermore, overexpression of miR-101 inhibited tumor growth and motion by inactivating PI3K/AKT and JAK/STAT signaling pathways via downregulation of ROCK1. To conclude, miR-101/ROCK1 may be a potential therapeutic target for osteosarcoma therapy.
Keywords: Osteosarcoma, microRNA-101, ROCK1, cell apoptosis, cell migration
Introduction
Osteosarcoma is a rare malignant bone tumor in adolescents, with high degree of malignancy, and highly incidence of recurrence and metastasis [1,2]. It is characterized by the direct formation of immature bone or osteoid tissue by the tumor cells [1]. The most common symptoms include pain and swelling in the affected bone and is more commonly observed in young adults and adolescents [1,3]. The relative 5-year survival rate of young-onset osteosarcoma is 61.6% globally [4]. Therapeutic strategies for treating osteosarcoma include surgical resection combined with radiotherapy and chemotherapy [3]. The overall survival still remains poor despite this treatment [3]. Few studies have demonstrated that certain oncogenes or tumor suppressors were deregulated in the osteosarcoma disease condition. Therefore, continued investigations for discovering potential therapeutic targets in the above mentioned areas may improve the overall survival rate associated with osteosarcoma [5].
MicroRNAs (miRNAs) are a class of highly conserved small molecule RNA, which is widely expressed in eukaryotic cells [6]. Recent studies have confirmed that the expression of miRNAs in normal tissues is different from the expression in tumor tissues, and many miRNAs have been reported to play an important role in the growth and metastasis of tumor cells [7]. For example, miR-199a-3p is downregulated in osteosarcoma and regulates the cell proliferation and migration [8]. Another example include miR-218 which revealed inhibition of osteosarcoma cell migration and invasion by targeting T-cell lymphoma invasion and metastasis 1, and matrix metalloproteinases 2 and 9 [8].
MiR-101 deregulation has been demonstrated to be involved in several types of human malignances [9,10]. MiR-101 is considered to be a tumor suppressor, which is down-regulated in gastric cancer, non-small cell lung cancer, colon cancer, and hepatocellular carcinoma cells [9,10]. The downregulation of miR-101 in gastric cancer is associated with cyclooxygenase-2 (Cox-2) overexpression and tumor growth [11]. Recently, miR-101 has been implicated in osteosarcoma. For example, Chang et al reported that miR-101 blocked the autophagy of osteosarcoma cells and thus enhanced osteosarcoma cell chemosensitivity [12]. Overexpression of miR-101 can inhibit tumor cell proliferation, migration and invasion. However, the effects of miR-101 on human osteosarcoma cells are still unclear. Another study has revealed that miR-101 inhibited the metastasis of OS cells by targeting enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) [2]. In addition, miR-101 was revealed to inhibit proliferation and induce the apoptosis of osteosarcoma cells by targeting mechanistic target of rapamycin (mTOR) [13]. The above discussed findings suggest that miR-101 has a tumor suppressive role in osteosarcoma. The underlying mechanism of miR-101 in regulating the proliferation, migration, invasion and apoptosis of osteosarcoma cells still remains unclear. In addition, as miR has various target genes [14], other targets of miR-101 may also be involved in the effect of miR-101 on the malignant phenotypes of osteosarcoma cells.
Hence, the present study aimed to explore the molecular mechanism involving miR-101 in regulating the proliferation, migration, invasion and apoptosis of osteosarcoma cells.
Materials and methods
Clinical specimens
Clinical human osteosarcoma tissues and the corresponding normal osteoblast (n = 20) were attained from China-Japan Union Hospital of Jilin University (Changchun, China). None of the patients received any therapies before surgery. Informed consents from every patient were obtained, and the present study was approved by the Medical Ethics Committee of the China-Japan Hospital of Jilin University.
Cell culture
The human osteosarcoma cell lines MG63, U2OS, OS732 and human osteoblast cell lines hFOB1.19 were obtained from Shanghai Institutes for Biological Sciences Cell Resource Center and were cultured in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). All the cells were incubated at 37°C in a humidified incubator with 5% CO2.
MiRNAs transfection
MiR-101 mimic, si-miR-101 si-ROCK1 and the NC controls were synthesized by GenePharma Co. (Shanghai, China). Cell transfections were conducted using Lipofectamine 3000 reagent (Invitrogen) following the manufacturer’s protocol.
CCK-8 assay
Cells were seeded in 96-well plate with 5000 cells/well. Cell proliferation was assessed by a Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD). Briefly, after stimulation, the CCK-8 solution was added to the culture medium, and the cultures were incubated for 1 hour at 37°C in humidified 95% air and 5% CO2. The absorbance was measured at 450 nm using a Microplate Reader (Bio-Rad, Hercules, CA, USA).
Migration and invasion assay
Cell migration was determined by using a modified two-chamber migration assay with a pore size of 8 mm. For migration assay, cells suspended in 200 ml of serum-free medium were seeded on the upper compartment of 24-well Transwell culture chamber, and 600 ml of complete medium was added to the lower compartment. After incubation at 37°C, cells were fixed with methanol. Non-traversed cells were removed from the upper surface of the filter carefully with a cotton swab. Traversed cells on the lower side of the filter were stained with crystal violet and counted.
The invasion behavior of was determined using 24-well Millicell Hanging Cell Culture inserts with 8 mm PET membranes (Millipore, Bedford, Massachusetts, USA). Briefly, after the cells were treated for indicated condition, 5.0 × 104 cells in 200 μl serum-free DMEM medium were plated onto BD BioCoatTMMatrigel TM Invasion Chambers (8 μM pore size polycarbonate filters; BD Biosciences), while complete medium containing 10% FBS was added to the lower chamber. After processing the invasion chambers for 48 hours (37°C, 5% CO2) in accordance with the manufacturer’s protocol, the non-invading cells were removed with a cotton swab; the invading cells were fixed in 100% methanol and then stained with crystal violet solution and counted microscopically. The data are presented as the average number of cells attached to the bottom surface from five randomly chosen fields.
Apoptosis assay
Cell apoptosis analysis was performed using propidium iodide (PI) and fluorescein isothiocynate (FITC)-conjugated Annexin V staining. Briefly, cells were washed in phosphatebuffered saline (PBS) and fixed in 70% ethanol. Fixed cells were then washed twice in PBS and stained in PI/FITC-Annexin V in the presence of 50 μg/ml RNase A (Sigma-Aldrich), and then incubated for 1 h at room temperature in the dark. Flow cytometry analysis was done by using a FACS can (Beckman Coulter, Fullerton, CA, USA). The data were analyzed by using FlowJo software.
QRT-PCR
Total RNA was extracted from cells and tissues using Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions. The Taqman MicroRNA Reverse Transcription Kit and Taqman Universal Master Mix II with the TaqMan MicroRNA Assay of miR-101 and U6 (Applied Biosystems, Foster City, CA, USA) were used for testing the expression levels of miR-101 in cells.
Dual luciferase activity assay
The 3’UTR target site was generated by PCR and the luciferase reporter constructs with the ROCK1 3’UTR carrying a putative miR-101-binding site into pMiR-report vector were amplified by PCR. Cells were co-transfected with the reporter construct, control vector and miR-101 or scramble using Lipofectamine 3000 (Life Technologies, USA). Reporter assays were done using the dual-luciferase assay system (Promega) following to the manufacturer’s information.
Western blot
The protein used for western blotting was extracted using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) supplemented with protease inhibitors (Roche, Guangzhou, China). The proteins were quantified using the BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). The western blot system was established using a Bio-Rad Bis-Tris Gel system according to the manufacturer’s instructions. Primary antibodies were prepared in 5% blocking buffer at a dilution of 1:1,000. Primary antibody was incubated with the membrane at 4°C overnight, followed by wash and incubation with secondary antibody marked by horseradish peroxidase for 1 hour at room temperature. After rinsing, the Polyvinylidene Difluoride (PVDF) membrane carried blots and antibodies were transferred into the Bio-Rad ChemiDoc™ XRS system, and then 200 μl Immobilon Western Chemiluminescent HRP Substrate (Millipore, MA, USA) was added to cover the membrane surface. The signals were captured and the intensity of the bands was quantified using Image Lab™ Software (Bio-Rad, Shanghai, China).
Statistical analysis
All experiments were repeated three times. The results of multiple experiments are presented as mean ± SD. Statistical analyses were performed using SPSS 19.0 software. P-values were calculated using one-way analysis of variance (ANOVA). P-value of < 0.05 was considered to be statistically significant.
Results
MiR-101 is downregulated in osteosarcoma tissues and cell lines
To reveal the role of miR-101 in osteosarcoma, RT-qPCR was performed to examine the expression levels of miR-101 in the tissue samples of 20 patients with osteosarcoma, with adjacent normal tissues from the respective patients used as controls. As revealed in Figure 1A, miR-101 was frequently downregulated in osteosarcoma tissues compared with their adjacent non-tumor tissues (P < 0.01). Following this, the expression levels of miR-101 in several common osteosarcoma cell lines, MG63, U2OS, and OS732 were investigated. The human osteoblast cell line hFOB1.19 was used as a control. The data of the present study revealed that miR-101 was significantly downregulated in osteosarcoma cell lines compared with the human osteoblast cell line, hFOB1.19 (Figure 1B, P < 0.01). In addition, MG63 cells displayed the significant decrease in miR-101 expression levels. Therefore, MG63 cells were used in the subsequent investigations in the present study.
Figure 1.
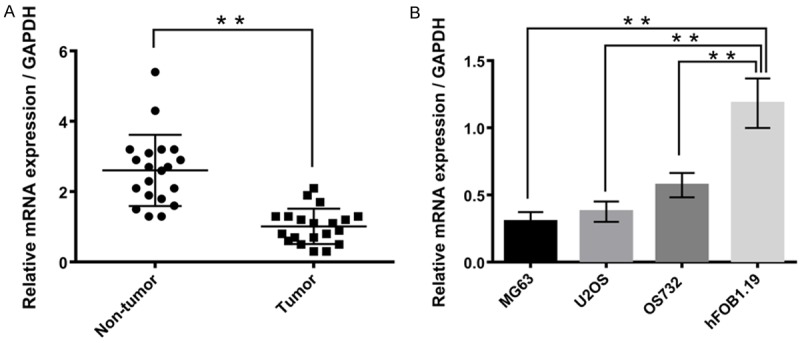
MiR-101 was downregulated in osteosarcoma tissues and cell lines. A. MiR-101 was downregulated in osteosarcoma tissues. B. MiR-101 was downregulated in osteosarcoma cell lines. Data represent the mean ± SD of three independent experiments. **P < 0.01.
Abnormal expression of miR-101 on cell viability, migration, invasion, and apoptosis
MG63 cells were separately transfected with miR-101 mimics. qRT-PCR analysis was performed to detect the expression level of miR-101 after transfection. The expression level of miR-101 was significantly upregulated in the MG63 cells after treatment with miR-101 mimics, compared with the scramble (P < 0.001, Figure 2A). Meanwhile, there was a significant difference in the miR-101 expression between the si-NC and si-miR-101 (P < 0.01, Figure 2A). This suggested that the expression of miR-101 was either knock down or overexpressed in the MG63 cells.
Figure 2.
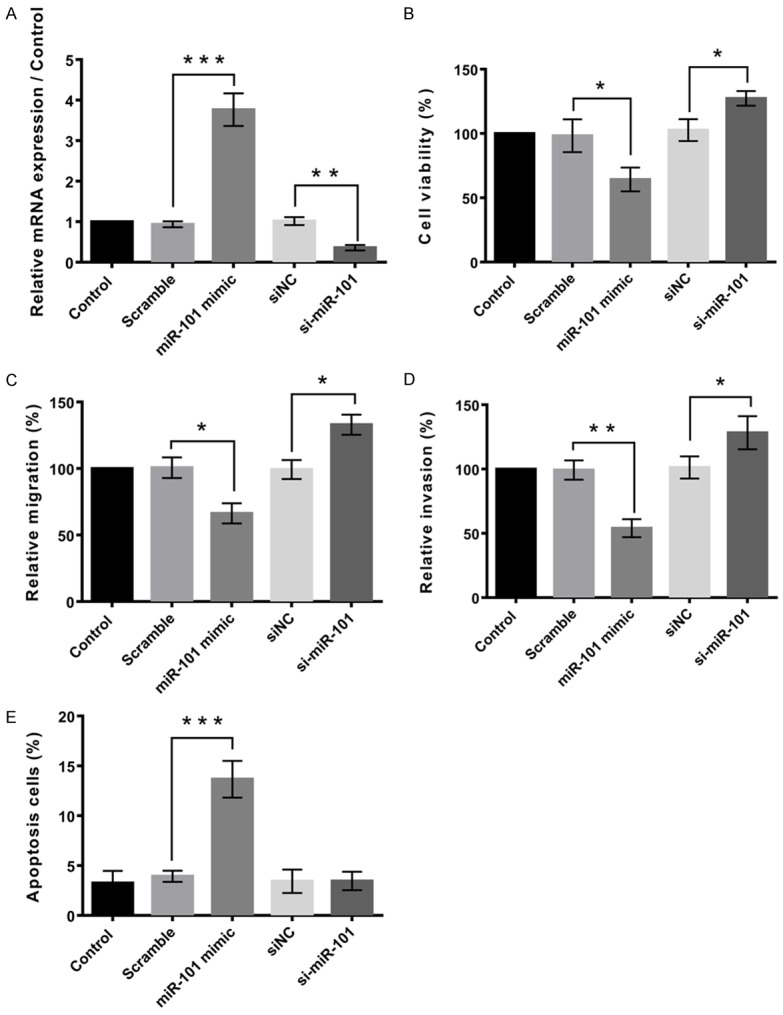
Abnormal expression of miR-101 on cell viability, migration, invasion, and apoptosis. A. Expression of miR-101 was knock down or overexpressed. B. Overexpression of miR-101 inhibited cell viability, and knockdown of miR-101 promoted the cell viability. C. Overexpression of miR-101 inhibited cell migration, and knockdown of miR-101 promoted the cell migration. D. Overexpression of miR-101 inhibited cell invasion, and knockdown of miR-101 promoted the cell invasion. E. Overexpression of miR-101 promoted cell apoptosis, and knockdown of miR-101 inhibited the cell apoptosis. Data represent the mean ± SD of three independent experiments. *P < 0.05; **P < 0.01.
Cell viability of MG63 cells was determined by the influence of miR-101 on the MG63 cells, by performing CCK8 assay. Figure 2B demonstrated that miR-101 significantly reduced the viability of MG63 cells, compared to the scramble (P < 0.05). Figure 2B also showed that there was a significant reduction in the si-NC compared to the si-miR-101 group (P < 0.05). This suggested that overexpression of miR-101 inhibited cell viability, while knockdown of miR-101 promoted the cell viability.
We used the Transwell assay to measure the migratory and invasive capacities of MG63 cells. The results showed that osteosarcoma cells treated with the miR-101 mimics displayed significantly lower transwell migration capacity, compared with the cells untreated or treated with the NC mimics (P < 0.05, Figure 2C and 2D). In the invasion assay, ectopic expression of miR-101 led to significantly decreased invasion of the osteosarcoma cells (P < 0.05, Figure 2C and 2D). These results indicate a functional role for miR-101 in downregulating the migration and invasion of osteosarcoma cells.
Apoptosis assay was performed to determine the apoptotic rate of cells. MiR-101 mimics treatment resulted in a significant increase in osteosarcoma cell apoptosis. (P < 0.001, Figure 2E). This suggested that overexpression of miR-101 promoted cell apoptosis, and knockdown of miR-101 inhibited the cell apoptosis.
ROCK1 was a target of miR-101
ROCK1 was hypothesized to be a potential target of miR-101. Figure 3A demonstrated that miR-101 negatively regulated the expression of ROCK1. To verify whether miR-101 was able to directly bind to its seed sequences in the c-ROCK1 3’-UTR in MG63 cells, ROCK1-wt and ROCK1-mt containing the wild-type and mutant binding sequences of miR-101 within the 3’-UTR of ROCK1 mRNA were generated, respectively (Figure 3B). A luciferase reporter assay revealed that the luciferase activity was significantly reduced in MG63 cells when co-transfected with ROCK1-wt in miR-101 mimics compared with the control group (P < 0.01). However, the luciferase activity revealed no significant difference in MG63 cells co-transfected with ROCK1-mt and miR-101 mimics when compared with the control group (Figure 3B). The data indicates that miR-101 is able to directly bind to its seed sequences in the ROCK1 3’-UTR in U2OS cells. Therefore, ROCK1 was identified as a target of miR-101 in MG63 cells.
Figure 3.
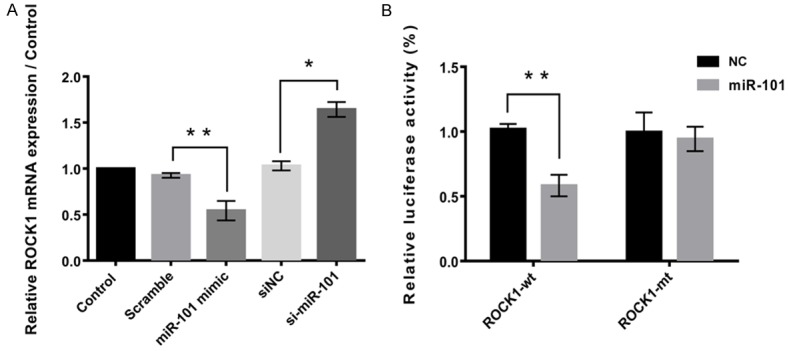
ROCK1 was a target of miR-101. A. MiR-101 negatively regulated the expression of ROCK1. B. Dual luciferase activity assay showed that ROCK1 was a target of miR-101. Data represent the mean ± SD of three independent experiments. *P < 0.05; **P < 0.01.
MiR-101 inhibits proliferation, migration and invasion and promotes apoptosis in osteosarcoma cells by inhibiting ROCK1
The effects of miR-101 and ROCK1 on the proliferation, migration, invasion and apoptosis of MG63 cells were investigated. ROCK1 expression was significantly downregulated by si-miR-101 and si-ROCK1 treatment (Figure 4A). Results identified that miR-101 knockdown or ROCK1 knockdown significantly inhibited MG63 cell proliferation (P < 0.01, Figure 4B), migration and invasion (P < 0.001, Figure 4C and 4D, respectively). Apoptosis results showed that knockdown of miR-101 significantly inhibited cell apoptosis by upregulation of ROCK1 (P < 0.0001, Figure 4E). However, the suppressive effect of miR-101 overexpression on MG63 cell proliferation, migration and invasion was reversed by the upregulation of ROCK1. The aforementioned data suggest that miR-101 inhibits MG63 cell proliferation, migration and invasion, to a certain extent, via targeting ROCK1.
Figure 4.
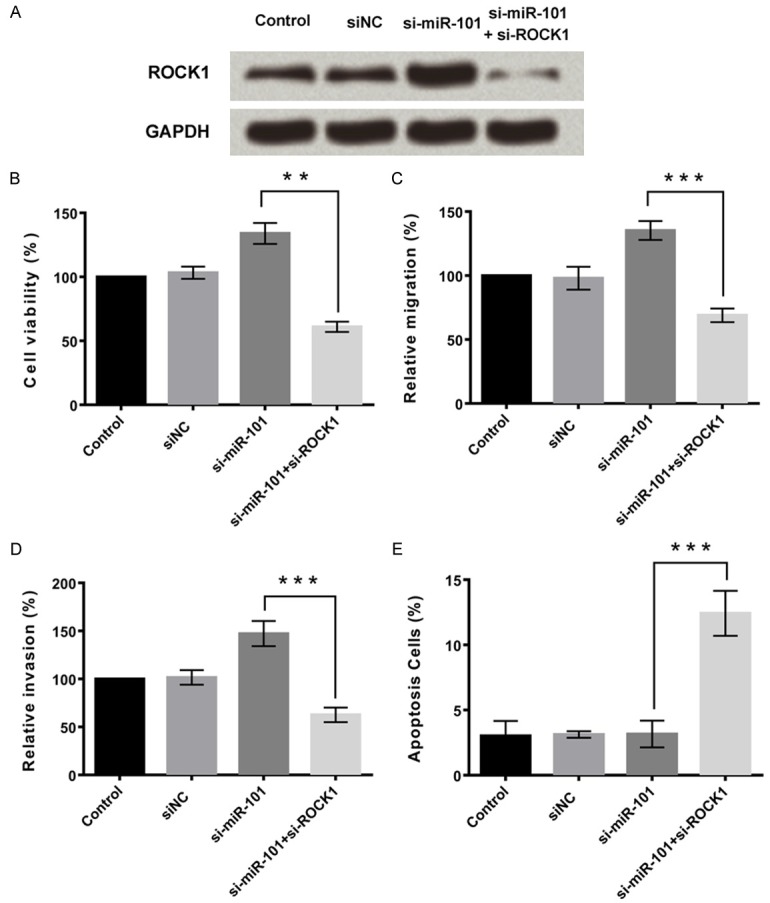
MiR-101 inhibits proliferation, migration and invasion and promotes apoptosis in osteosarcoma cells by inhibiting ROCK1. A. Effects of knockdown of miR-101 and/or ROCK1 on expression of ROCK1. B. Knockdown of miR-101 promoted cell viability by upregulation of ROCK1, and the effect was reversed by knockdown of ROCK1. C. Knockdown of miR-101 promoted cell migration by upregulation of ROCK1, and the effect was reversed by knockdown of ROCK1. D. Knockdown of miR-101 promoted cell invasion by upregulation of ROCK1, and the effect was reversed by knockdown of ROCK1. E. Knockdown of miR-101 inhibited cell apoptosis by upregulation of ROCK1, and the effect was reversed by knockdown of ROCK1. Data represent the mean ± SD of three independent experiments. **P < 0.01; ***P < 0.001.
PI3K/AKT and JAK/STAT pathways
To clarify the signaling pathways involved in the functional effects of MG63 cells mediated by the interaction between miR-101 and ROCK1, we investigated the expression of components of the pathways by PI3K/AKT and JAK/STAT pathways. As shown in Figure 5A, miR-101 overexpression resulted in downregulated ROCK1 expression, which was associated with downregulated expression of p-PI3K and p-AKT. Knockdown of miR-101 alone resulted in upregulated expression of ROCK1, while expression of both p-PI3K and p-AKT increased. As shown in Figure 5B, miR-101 overexpression resulted in downregulated ROCK1 expression, which was associated with downregulated expression of p-JAK1 and p-STAT3. Knockdown of miR-101 alone resulted in upregulated expression of ROCK1, while expression of both p-JAK1 and p-STAT3 increased. These effects were abolished by concurrent knockdown of both miR-101 and ROCK1.
Figure 5.
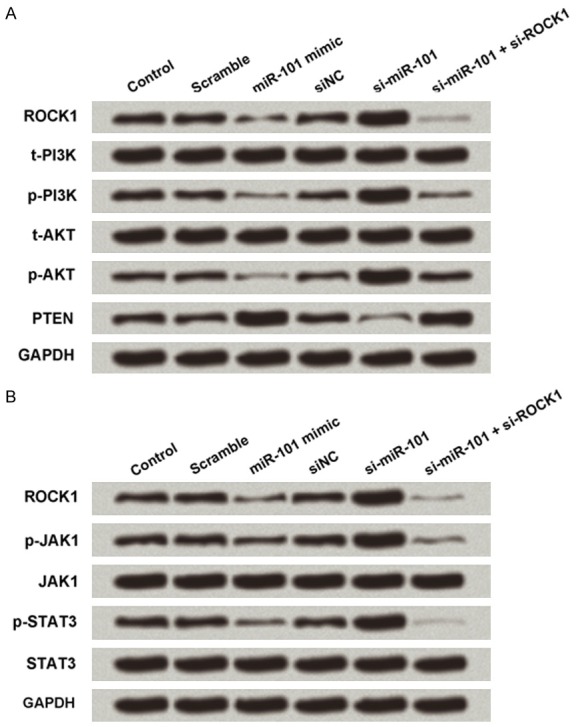
PI3K/AKT and JAK/STAT pathways. A. Overexpression of miR-101 inhibited PI3K/AKT pathway by ROCK1 downregulation. B. Overexpression of miR-101 inhibited JAK/STAT pathway by ROCK1 downregulation.
Discussion
Several studies in the recent years have demonstrated significant roles of miRNAs in the etiology of cancer and are implicated in numerous biological processes in a wide range of cancers, including metastasis. During cancer progression, aggressive tumor cell metastasis is an important step, and it also contributes to the secondary tumor formation at distant sites. Studies have reported that several miRNAs, such as miR-126, miR-335 and miR-145, are metastasis suppressors [15,16]. These data bring new insights into novel therapeutic strategies in highly-metastatic tumors which includes osteosarcoma, in which pulmonary metastasis is the leading cause of death [17].
The effects and mechanisms of miR-101 on the growth and movement of human osteosarcoma cells were studied. Studies have shown that the expression of miR-101 was downregulated in many tumors. Similarly, the expression of miR-101 was also downregulated in osteosarcoma tissue and osteosarcoma cell lines, indicating that miR-101 may also serve as a tumor suppressor in osteosarcoma. Overexpression of miR-101 could inhibit the proliferation, migration, and invasion of osteosarcoma cells, and promote cell apoptosis. MiR-101 knockdown showed contrary results. ROCK1 is a serine/threonine kinase that belongs to the Rho family of GTPase proteins that facilitate the reorganization of the actin cytoskeleton, a pivotal event during cell motion and invasion. We found that miR-101 negatively regulated the expression of ROCK1, and ROCK1 was a target of miR-101. Overexpression of miR-101 inhibited cell proliferation, migration and invasion and promoted cell apoptosis by downregulating the expression of ROCK1. Further studies showed that overexpression of miR-101 inhibited tumor growth and motion by inactivating PI3K/AKT and JAK/STAT signaling pathways via downregulation of ROCK1.
The tumor suppressive role of miR-101 has been demonstrated in several types of human cancer, including osteosarcoma. Zhang et al [18] in their study revealed that the expression levels of miR-101 were significantly decreased in hepatocellular carcinoma (HCC), while its downregulation was associated with tumor aggressiveness and poor prognosis. The study by Zhang et al also demonstrated that overexpression of miR-101 significantly inhibited the proliferation and tumorigenicity of HCC cells by targeting SRY-box 9 [19]. Another study by Lei et al [20] reported that miR-101 inhibited migration and invasion, and enhanced the cisplatin chemosensitivity, of bladder cancer cells via vascular endothelial growth factor C inhibition. Other study by Guo et al [19] identified that miR-101 suppressed the epithelial-to-mesenchymal transition in the ovarian carcinoma cells by targeting zinc finger E-Box binding homeobox 1 and 2. Several studies have demonstrated the role of miR-101 in osteosarcoma [2,12,13]. Also, one of the study by Lin et al [13] demonstrated that miR-101 overexpression inhibited the proliferation and promoted the apoptosis of Saos-2 cells in an mTOR-dependent manner.
To our knowledge this is the first study that demonstrates the association between miR-101 and ROCK1 which has also been identified to be beneficial in osteosarcoma. Previous studies have demonstrated the screening of human protein kinases by using a lentiviral short hairpin RNA (shRNA) library in osteosarcoma cell lines [21,22]. Rho-associated coiled-coil containing protein kinase 1 (ROCK1) is one of the four kinases, when inhibited by shRNA that resulted in the decreased cell viability and increased apoptosis in osteosarcoma [23]. Since ROCK1 is a unique kinase with no previously described role in osteosarcoma, and hence was selected for further study. Lie et al study have demonstrated that knockdown of ROCK1 decreased cell proliferation and induced cell death in both osteosarcoma cell lines [23]. In addition, ROCK1 overexpression is associated with poor prognosis in osteosarcoma patients, indicating that ROCK1 might be a prognosis marker and therapeutic target for the treatment of osteosarcoma [24]. Several other recent study reports have demonstrated the cell survival effects that were mediated by ROCK1 in epithelial, endothelial, and cancer cells [25].
Overexpression of miR-101 inhibited tumor growth and motion by inactivating PI3K/AKT and JAK/STAT signaling pathways via downregulation of ROCK1. The PI3K/Akt pathway is the most frequently activated signal transduction pathways associated with osteosarcoma [26]. This in turn contributes to the disease initiation and development, uncontrolled cell proliferation, tumor cell invasion and metastasis, cell-cycle progression, inhibition of apoptosis, angiogenesis, and chemoresistance. The PI3K/Akt pathway is activated by the binding of ligands to the respective RTKs (including IGF-1R, c-Met, and EGFR). Several studies have demonstrated the role of PI3K/AKT in osteosarcoma [27,28]. Downstream signals activate targets involved in cell survival and inactivate pro-apoptotic proteins. Similar to this pathway JAK/STAT also was inactivated by miR-101.
In conclusion, the present study suggests that miR-101 has a suppressive role in the regulation of osteosarcoma cell proliferation, migration, invasion, and apoptosis to a certain extent, via targeting ROCK1. The inactivation of PI3K/AKT and JAK/STAT pathways may be also involved. These findings provide an improved understanding of the mechanism by which miR-101 is involved in the pathogenesis of osteosarcoma. This information is critical for the development of novel therapies for osteosarcoma.
Disclosure of conflict of interest
None.
Abbreviations
- ROCK1
rho-associated
- coiled
coil-containing protein kinase 1
- PI3K
Phosphoinositide 3-kinase
- JAK
Janus kinase
- STAT
Signal Transducer and Activator of Transcription
References
- 1.Wang Z, He R, Xia H, Wei YU, Wu S. MicroRNA-101 has a suppressive role in osteosarcoma cells through the targeting of c-FOS. Exp Ther Med. 2016;11:1293–1299. doi: 10.3892/etm.2016.3085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Zhang K, Zhang Y, Ren K, Zhao G, Yan K, Ma B. MicroRNA-101 inhibits the metastasis of osteosarcoma cells by downregulation of EZH2 expression. Oncol Rep. 2014;32:2143–2149. doi: 10.3892/or.2014.3459. [DOI] [PubMed] [Google Scholar]
- 3.Thompson LD. Osteosarcoma. Ear Nose Throat J. 2013;92:288, 290. doi: 10.1177/014556131309200704. [DOI] [PubMed] [Google Scholar]
- 4.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013;25:398–406. doi: 10.1097/CCO.0b013e3283622c1b. [DOI] [PubMed] [Google Scholar]
- 6.Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, Hornicek F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10:1337–1345. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin J, Cai L, Liu ZM, Zhou XS. MiRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev. 2013;14:3681–3684. doi: 10.7314/apjcp.2013.14.6.3681. [DOI] [PubMed] [Google Scholar]
- 9.Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT, Xia YJ, Ye ZY, Tao HQ. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur J Cancer. 2010;46:2295–2303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JG, Guo JF, Liu DL, Liu Q, Wang JJ. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J Thorac Oncol. 2011;6:671–678. doi: 10.1097/JTO.0b013e318208eb35. [DOI] [PubMed] [Google Scholar]
- 11.He XP, Shao Y, Li XL, Xu W, Chen GS, Sun HH, Xu HC, Xu X, Tang D, Zheng XF, Xue YP, Huang GC, Sun WH. Downregulation of miR-101 in gastrc cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J. 2012;279:4201–4212. doi: 10.1111/febs.12013. [DOI] [PubMed] [Google Scholar]
- 12.Chang Z, Huo L, Li K, Wu Y, Hu Z. Blocked autophagy by miR-101 enhances osteosarcoma cell chemosensitivity in vitro. ScientificWorldJournal. 2014;2014:794756. doi: 10.1155/2014/794756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S, Shao NN, Fan L, Ma XC, Pu FF, Shao ZW. Effect of microRNA-101 on proliferation and apoptosis of human osteosarcoma cells by targeting mTOR. J Huazhong Univ Sci Technolog Med Sci. 2014;34:889–895. doi: 10.1007/s11596-014-1369-y. [DOI] [PubMed] [Google Scholar]
- 14.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negrini M, Calin GA. Breast cancer metastasis: a microRNA story. Breast Cancer Res. 2008;10:203. doi: 10.1186/bcr1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watahiki A, Wang Y, Morris J, Dennis K, O’Dwyer HM, Gleave M, Gout PW, Wang Y. MicroRNAs associated with metastatic prostate cancer. PLoS One. 2011;6:e24950. doi: 10.1371/journal.pone.0024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada T, Isu K, Takeda N, Usui M, Ishii S, Yamawaki S. A preliminary report of neoadjuvant chemotherapy NSH-7 study in osteosarcoma: preoperative salvage chemotherapy based on clinical tumor response and the use of granulocyte colony-stimulating factor. Oncology. 1996;53:221–227. doi: 10.1159/000227564. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Guo X, Xiong L, Kong X, Xu Y, Liu C, Zou L, Li Z, Zhao J, Lin N. MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 2012;586:4362–4370. doi: 10.1016/j.febslet.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Guo F, Cogdell D, Hu L, Yang D, Sood AK, Xue F, Zhang W. MiR-101 suppresses the epithelial-to-mesenchymal transition by targeting ZEB1 and ZEB2 in ovarian carcinoma. Oncol Rep. 2014;31:2021–2028. doi: 10.3892/or.2014.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei Y, Li B, Tong S, Qi L, Hu X, Cui Y, Li Z, He W, Zu X, Wang Z, Chen M. MiR-101 suppresses vascular endothelial growth factor C that inhibits migration and invasion and enhances cisplatin chemosensitivity of bladder cancer cells. PLoS One. 2015;10:e0117809. doi: 10.1371/journal.pone.0117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan Z, Ji D, Weinstein EJ, Liu X, Susa M, Choy E, Yang C, Mankin H, Hornicek FJ. Lentiviral shRNA screen of human kinases identifies PLK1 as a potential therapeutic target for osteosarcoma. Cancer Lett. 2010;293:220–229. doi: 10.1016/j.canlet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Yang C, Ji D, Weinstein EJ, Choy E, Hornicek FJ, Wood KB, Liu X, Mankin H, Duan Z. The kinase Mirk is a potential therapeutic target in osteosarcoma. Carcinogenesis. 2010;31:552–558. doi: 10.1093/carcin/bgp330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Choy E, Hornicek FJ, Yang S, Yang C, Harmon D, Mankin H, Duan Z. ROCK1 as a potential therapeutic target in osteosarcoma. J Orthop Res. 2011;29:1259–1266. doi: 10.1002/jor.21403. [DOI] [PubMed] [Google Scholar]
- 24.Villalonga P, Fernandez de Mattos S, Ridley AJ. RhoE inhibits 4E-BP1 phosphorylation and eIF4E function impairing cap-dependent translation. J Biol Chem. 2009;284:35287–35296. doi: 10.1074/jbc.M109.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Li G, Li R, Liu Q, He Q, Zhang J. Rho-kinase inhibitor, fasudil, suppresses glioblastoma cell line progression in vitro and in vivo. Cancer Biol Ther. 2010;9:875–884. doi: 10.4161/cbt.9.11.11634. [DOI] [PubMed] [Google Scholar]
- 26.Sampson VB, Yoo S, Kumar A, Vetter NS, Kolb EA. MicroRNAs and potential targets in osteosarcoma: review. Front Pediatr. 2015;3:69. doi: 10.3389/fped.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao G, Cai C, Yang T, Qiu X, Liao B, Li W, Ji Z, Zhao J, Zhao H, Guo M, Ma Q, Xiao C, Fan Q, Ma B. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLoS One. 2013;8:e53906. doi: 10.1371/journal.pone.0053906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y, Luo LH, Li S, Yang C. MiR-17 inhibitor suppressed osteosarcoma tumor growth and metastasis via increasing PTEN expression. Biochem Biophys Res Commun. 2014;444:230–234. doi: 10.1016/j.bbrc.2014.01.061. [DOI] [PubMed] [Google Scholar]


