Abstract
Telomerase reverse transcriptase (TERT) is the reverse transcriptase component of the telomeric complex, which synthesizes terminal DNA to protect chromosomal ends and to maintain genomic integrity. In melanoma, mutation in TERT promoter region is a common event and theses promoter variants have been shown to be associated with increased gene expression, decreased telomere length and poorer outcome. In this study, we determined the frequency of TERT promoter mutation in 88 Korean primary melanoma patients and aimed to see the association of TERT promoter mutation status to other major molecular features, such as BRAF, NRAS, KIT mutations and correlate with clinicopathological features. In our study, acral melanoma (n=46, 52.3%) was the most common type. Overall, TERT promoter mutation was observed in 15 cases (17%) with ten c. -124C>T altertions and five c. -146C>T alterations. None of our samples showed CC>TT mutation which is considered pathognomonic of UV induction. Among the 46 acral melanoma patients, 5 patients (10.9%) harbored TERT promoter mutation. Tumors with TERT promoter mutation showed significantly greater Breslow thickness compared to WT tumors (P=0.039). A combined analysis for the presence of TERT promoter and BRAF mutations showed that patients with both TERT promoter and BRAF mutation showed decreased survival compared with those with only TERT promoter mutation, only BRAF mutation, or without mutations in either TERT promoter or BRAF (P=0.035). Our data provides additional evidence that UV-induced TERT promoter mutation frequencies vary depending on melanoma subtype, but preserves its prognostic value.
Keywords: TERT mutation, Korean, melanoma, survival, prognosis
Telomerase reverse transcriptase (TERT) is the reverse transcriptase component of the telomeric complex, which synthesizes terminal DNA to protect chromosomal ends and to maintain genomic integrity. Its upregulation has been demonstrated in several human cancers, and the promoter region of the gene is considered the critical regulatory element for telomerase expression. Mutually exclusive -124C>T and -146C>T mutations in TERT promoter region have been detected in more than 65% of melanomas [1]. These promoter variants have been shown to be associated with increased gene expression, decreased telomere length and poorer outcome [2,3].
Here we intend to determine the frequency of TERT promoter mutation in 88 Korean primary melanoma patients who were followed at Severance hospital in Seoul, Korea during the period from 2005 to 2012 (Supplementary Table 1). Furthermore, we aimed to see the association of TERT promoter mutation status to other major molecular features, such as BRAF, NRAS, KIT mutations and correlate with clinicopathological features. The study was approved by the Institutional Review Board of Yonsei University College of Medicine. Written informed consent was obtained from all participants or their legal guardians. DNA was extracted from formalin-fixed, paraffin-embedded tumor tissues. Genomic DNA was isolated using proteinase K digestion and boiling method. Polymerase chain reaction (PCR) amplification of the TERT promoter region was performed using primers 5’-CCCACGTGCGCAGCAGGAC-3’ (forward), 5’-Biotin-CTCCCAGTGGATTCGCGGGC-3‘ (reverse) and 5’-AGGGGCTGGGAGGGC (sequencing). PCR products were used as templates for pyrosequencing with PyroMark Gold Q24 reagent (Qiagen, Germantown, MD, USA) according to the manufacture’s protocol. Sequencing analysis was performed using PyroMark Q24 version 1.0.10 software in the allele quantification analysis mode. For statistical analysis, categorical data are described using frequencies and percentages, and continuous data are described using means ± standard deviations or median (range) for normally distributed data. Chi-squared (χ2) test or Fisher’s exact test was used to differentiate the rates of different groups, and differences in measurement data of 2 groups were evaluated by unpaired t-test or Mann-Whitney test. We used univariate logistic regression analyses to explore associations of TERT promoter mutation status with available clinical and pathologic variables, including age, sex, stage, oncogene mutation status, anatomical distribution of primary tumor, Breslow’s thickness, and ulceration.
We investigated association between clinico-pathologic factors, TERT promoter mutation status, and oncogene mutation status with overall survival, defined as the interval from time of diagnosis of primary melanoma to death. Cases in which the endpoint was not reached at the time of the last follow-up were censored. Univariate results were displayed by the Kaplan-Meier method and hazard ratio estimates and p-values were derived from Cox proportional hazard model. Multivariable analyses were performed on variables with a p-value of 0.20 or less in univariate analyses. Confidence intervals (CI) were calculated with coverage of 95%. All reported p-value are nominal and two-sided. We applied a significance level of 5%. All statistical analyses were performed using SPSS Statistics software (version 18.0; SPSS Chicago, IL) or R 3.1.1.
The median age at diagnosis was 59 years (range 28-87 years) with equal male and female patients (M:F=44:44). Extremities (n=60) was the most common location of the primary melanoma, followed by head and neck area (n=12), other including mucosa (n=8), and trunk (n=6). Acral melanoma (n=46) was the most common type, followed by non-CSD (chronic sun-damage) melanoma (n=18), CSD melanoma (n=12), mucosal melanoma (n=8), and melanoma of unknown primary (n=4). Breslow tumor thickness ranged from 0.3 to 27 mm, with a median of 2.5 mm. BRAF mutations were detected in 14 tumors (15.9%), NRAS mutations in 10 cases (11.4%), and KIT mutations in 9 specimens (10.2%). TERT promoter variants were identified in 15 cases (17%) with ten c. -124C>T altertions and five c. -146C>T alterations. Among ther 46 acral melanoma patients, 5 patients (10.9%) harbored TERT promoter mutation. Three cases were c. -124C>T altertions and two cases were c. -146C>T alterations (Figure 1A). The clinical and pathologic characteristics of tumors with regard to TERT promoter mutation status are detailed in Table 1. There were no significant differences in age, gender, stage at diagnosis, subtype, oncogene mutation status, or anatomic site of tumor. However, tumors with TERT promoter mutation showed significantly greater Breslow thickness compared to WT tumors (P=0.039). TERT promoter mutations were observed in 33.3% of CSD melanoma, 22.2% of non-CSD melanoma, 12.5% of mucosal melanoma and 10.9% of acral melanoma. Four patients were identified to have both BRAF and TERT promoter mutation. Survival analyses were performed for all patients (Supplementary Table 2). Univariate predictors of survival were gender (P=0.019), Breslow thickness (P=0.003), stage at diagnosis (P<0.001), and BRAF mutation (P=0.001). Kaplan-Meier survival analysis showed no association of TERT promoter mutation status with patient survival (P=0.928, Figure 1B). Multivariable analysis indicated that stage at diagnosis (HR=4.328, 95% CI: 2.5-7.5; P<0.001) was the only independent factor associated with survival in our cohort. A combined data analysis for the presence of TERT promoter and BRAF mutations showed that the patients with both TERT promoter and BRAF mutation showed decreased survival compared with those with only TERT promoter mutation , only BRAF mutation, or without mutations in either TERT promoter or BRAF (P=0.035, Figure 1C).
Figure 1.
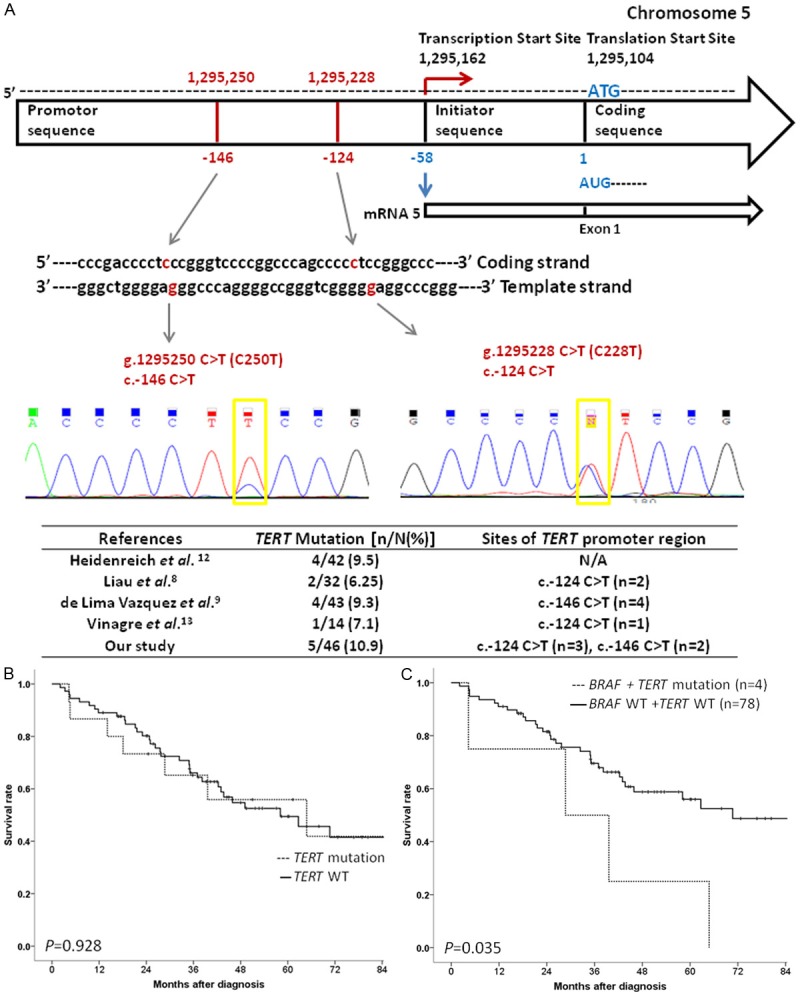
A. TERT promoter mutation variants identified in acral melanoma. B. Kaplan-Meier curves for overall survival according to TERT promoter mutation status. Kaplan-Meier survival analysis showed no association of TERT promoter mutation status with patient survival (P=0.928). C. Kaplan-Meier curves for overall survival in patients with both TERT promoter and BRAF mutation. Patients with both TERT promoter and BRAF mutation showed decreased survival compared with those with only TERT promoter mutation , only BRAF mutation, or without mutations in either TERT promoter or BRAF (P=0.035).
Table 1.
Associations of TERT mutation with clinical and pathological variables in 88 melanoma patients
| Clinicopathologic features | TERT genotype | ||
|---|---|---|---|
|
| |||
| Wild type (n=73) | Mutation (n=15) | p-value | |
| Age (year) | |||
| Mean | 60.2 | 57 | 0.415 |
| Gender | |||
| Male | 34 (46.6) | 10 (66.7) | 0.156 |
| Female | 39 (53.4) | 5 (33.3) | |
| Stage at diagnosis (%)* | |||
| 0/I/II | 43 (58.9) | 9 (60) | 0.937 |
| III/IV | 30 (41.1) | 6 (40) | |
| Subtype (%) | |||
| Acral | 41 (89.1) | 5 (10.9) | 0.389 |
| Mucosal | 7 (87.5) | 1 (12.5) | |
| CSD | 8 (66.7) | 4 (33.3) | |
| Non-CSD | 14 (77.8) | 4 (22.2) | |
| UP | 3 (75) | 1 (25) | |
| Mutant Oncogene | |||
| BRAF V600E | 10/67 (14.9) | 4/14 (28.5) | 0.219 |
| NRAS | 6/68 (8.8) | 1/14 (7.1) | 0.526 |
| KIT | 6/50 (12) | 3/11 (27.3) | 0.39 |
| Breslow thickness (mm) | |||
| Median | 2.35 | 5 | 0.039 |
| Range | 0.6-15 | 0.4-27 | |
| 0.01-1.00 | 11 (19.6) | 2 (18.2) | |
| 1.01-2.00 | 12 (21.4) | 2 (18.2) | |
| 2.01-4.00 | 11 (19.6) | 0 (0) | |
| >4.00 | 22 (39.3) | 7 (63.6) | |
| Anatomic sites of tumors | |||
| Trunk | 5 (83.3) | 1 (16.7) | 0.477 |
| Extremities | 51 (85) | 9 (15) | |
| Head and neck | 8 (66.7) | 4 (33.3) | |
| Other | 7 (87. 5) | 1 (12.5) | |
Staging according to the American Joint Committee on Cancer (AJCC) Melanoma Staging System 2009.
In this study carried out on patients with primary melanoma, the simultaneous occurrence of TERT promoter and BRAF mutations was associated with decreased survival in Korean melanoma patients. TERT promoter mutation has been reported to be associated with older patients, increased Breslow thickness, and worse prognosis in simultaneous occurrence with BRAF mutation which was in line with our study [4]. Overall, TERT promoter mutation was observed in 17% of the patients, which is much lower than previous reports showing up to 65% of the patients [1,3,4]. Huang et al. screened whole-genome sequencing data of melanoma and found that, apart from mutations in BRAF and NRAS, recurrent TERT promoter mutations were the most frequent genomic alteration [2]. The possible explanation of low incidence of TERT promoter mutation in our study may be due to the difference in the subtypes of melanoma in our cohort. It is well-known that acral melanoma is the most frequent type of melanoma in Asian patients [5]. In our study, acral and mucosal melanoma consisted of 61.4% of our patients. In acral melanoma, TERT promoter mutation was present in only 10.9% which is in line with previous studies reporting that TERT promoter mutation is uncommon in acral melanoma compared to non-acral melanoma [6]. Second possible reason is that our cohort of samples was all primary melanomas. Horn et al. detected TERT promoter mutations in 33% of primary melanomas and at considerably higher frequencies in melanoma cell lines (74%) and corresponding tissue from metastasis (85%) [2]. Dipyrimidine CC>TT mutations are considered pathognomonic of UV induction [7]. In our study, none of our samples showed CC>TT mutation, and TERT promoter mutations were considerably more frequent in nonacral cutaneous melanomas than acral melanomas. In the literature, there were only 16 cases (including 5 cases in our study) [6,8-10] of acral melanoma which harbor TERT promoter mutations and none of the cases had shown CC>TT mutation (Figure 1A). This finding is consistent with a role for UV-induction in the pathogenesis, as acral sites are rarely sun-exposed and further supported by the absence of UV signature mutations, particularly CC>TT. Our data provides additional evidence that UV-induced TERT promoter mutation frequencies vary depending on melanoma subtype, but preserves its prognostic value.
Acknowledgements
This work was made possible by a grant from the NIH (K24 CA149202 to HT), a grant from the Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Education, Science, and Technology (2011-0022376 to MRR), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI13C2096 to SYR) and the generous donors to the MGH Millennium Melanoma Fund and Innovations in Melanoma Care Fund.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 3.Nagore E, Heidenreich B, Rachakonda S, Garcia-Casado Z, Requena C, Soriano V, Frank C, Traves V, Quecedo E, Sanjuan-Gimenez J, Hemminki K, Teresa Landi M, Kumar R. TERT promoter mutations in melanoma survival. Int J Cancer. 2016;139:75–84. doi: 10.1002/ijc.30042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griewank KG, Murali R, Puig-Butille JA, Schilling B, Livingstone E, Potrony M, Carrera C, Schimming T, Moller I, Schwamborn M, Sucker A, Hillen U, Badenas C, Malvehy J, Zimmer L, Scherag A, Puig S, Schadendorf D. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JW. Acral melanoma: a unique disease in Asia. JAMA Dermatol. 2013;149:1272–1273. doi: 10.1001/jamadermatol.2013.5941. [DOI] [PubMed] [Google Scholar]
- 6.Liau JY, Tsai JH, Jeng YM, Chu CY, Kuo KT, Liang CW. TERT promoter mutation is uncommon in acral lentiginous melanoma. J Cutan Pathol. 2014;41:504–508. doi: 10.1111/cup.12323. [DOI] [PubMed] [Google Scholar]
- 7.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GR, Ye K, Alipaz J, Bauer MJ, Beare D, Butler A, Carter RJ, Chen L, Cox AJ, Edkins S, Kokko-Gonzales PI, Gormley NA, Grocock RJ, Haudenschild CD, Hims MM, James T, Jia M, Kingsbury Z, Leroy C, Marshall J, Menzies A, Mudie LJ, Ning Z, Royce T, Schulz-Trieglaff OB, Spiridou A, Stebbings LA, Szajkowski L, Teague J, Williamson D, Chin L, Ross MT, Campbell PJ, Bentley DR, Futreal PA, Stratton MR. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lima Vazquez V, Vicente AL, Carloni A, Berardinelli G, Soares P, Scapulatempo C, Martinho O, Reis RM. Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res. 2016;26:93–99. doi: 10.1097/CMR.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich B, Nagore E, Rachakonda PS, Garcia-Casado Z, Requena C, Traves V, Becker J, Soufir N, Hemminki K, Kumar R. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat Commun. 2014;5:3401. doi: 10.1038/ncomms4401. [DOI] [PubMed] [Google Scholar]
- 10.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, Melo M, da Rocha AG, Preto A, Castro P, Castro L, Pardal F, Lopes JM, Santos LL, Reis RM, Cameselle-Teijeiro J, Sobrinho-Simoes M, Lima J, Maximo V, Soares P. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


