Abstract
MicroRNA-363-3p (miR-363-3p) reportedly plays crucial roles in tumor development and progression in many types of cancers. However, its role in papillary thyroid carcinoma (PTC) remain largely unclear. We therefore investigated the function and underlying mechanism of miR-363-3p in PTC. Here, we found that miR-363-3p was significantly downregulated in human PTC tissue samples and cell lines, and that miR-363-3p levels are negatively correlated with advanced clinical stage and lymph node metastasis. In addition to suppressing tumor growth in vivo, restoration of miR-363-3p in TPC-1 cells significantly inhibits proliferation, migration, and invasion and induced apoptosis in vitro. Mechanistically, miR-363-3p was verified to directly bind to 3’UTR of the phosphoinositide-3-kinase catalytic subunit alpha (PIK3CA) mRNA, and reduce its expression at both mRNA and protein levels, which further inhibits phosphatidylinositol 3-kinase/Akt signaling pathway. PIK3CA expression was also found to be increased in human PTC tissues, and were inversely correlated with miR-363-3p. Furthermore, restoration of PIK3CA partially rescued the miR-363-3p-induced inhibition effect on TPC-1 cell proliferation, migration and invasion. Taken together, these findings indicated for the first time that miR-363-3p functions as a tumor suppressor in PTC, and its suppressive effect is mediated by repressing PIK3CA.
Keywords: Papillary thyroid carcinoma, miR-363-3p, PIK3CA, proliferation
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignant tumor, accounting for approximately 90% of all thyroid cancers, and is one of the most rapidly growing in many countries over the last several decades [1]. In most cases, patients with PTC have an excellent prognosis after surgical resection combined with radioiodine and levothyroxine treatment, however, 10-15% of patients with PTC with relapses and distant metastases frequently have a poor response to standard treatments and have poor clinical outcome [2]. Therefore, there exists an urgent need to understand of the molecular mechanisms underlying carcinogenesis and progression in PTC, which contribute to finding novel diagnosis markers and novel therapeutic targets.
MicroRNAs (miRNAs), a class of endogenous, small (19-25 nucleotides), non-protein-coding RNAs, can posttranscriptionally modulate gene expression by binding to complementary sequences at the 3’-untranslated regions (3’-UTRs) of their target mRNAs, leading to target mRNAs degradation and/or translational inhibition [3]. MiRNAs has been shown to play a critical role in various biological processes, such as proliferation, migration, invasion, and tumorigenesis [4,5]. Several studies suggest that alterations in miRNA expression were involved in PTC development and progression, and they could potentially act as oncogenes or tumor suppressors in PTC [6,7].
Recently, the role of miR-363-3p in carcinogenesis and cancer treatment has been widely focused in many types of cancers, such as gallbladder cancer [8], colorectal cancer [9], hepatocellular carcinoma [10], gastric cancer [11], head and neck cancer [12], and breast cancer [13]. Despite a study showed that miR-363-3p expression was downregulated in PTC tissues using gene expression profiles in 499 PTC samples and 58 normal thyroid tissues [14], its role in PTC remain largely unclear. We therefore investigated the function and underlying mechanism of miR-363-3p in PTC in the present work.
Materials and methods
Tissue samples and cell lines
Thirty tissue samples pairs, each comprising PTC and matched adjacent normal tissue, were collected from patients having undergone surgery at the First Hospital of Jilin University (Changchun, China) between June, 2014 and June, 2015. All samples were immediately frozen in liquid nitrogen following surgery, and stored at -80°C until RNA extraction. All diagnoses were based on pathological and/or cytological evidence. Informed consent was obtained before using samples in all cases. The study protocol was approved by the Medical Ethics Committee of the First Hospital, Jilin University.
Human PTC cell lines (TPC-1, BCPAP, K1) and the human thyroid epithelial cell line Nthy-ori3-1 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), and 100 U/ml penicillin or 100 mg/ml streptomycin at 37°C in humidified air with 5% CO2.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from tissues and cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. MiRNAs were reverse-transcribed using the TaqMan MiRNA Reverse Transcript Kit (Applied Biosystems, Foster City, CA, USA) and quantified by using SYBR Premix Ex TaqTM (TaKaRa, Dalian, Chian) under ABI 7500 Fast Sequence Detection System (Applied Biosystems Prism) using primers for miR-363-3p and U6 (Applied Biosystems). The human PIK3CA cDNA were reverse-transcribed using PrimeScriptTM RT-PCR Kit (TaKaRa), and quantified using SYBR Premix Ex TaqTM (TaKaRa) under ABI 7500 Fast Sequence Detection System. The primes of PIK3CA and β-actin used in this study were described previously [15]. The relative expression of miR-363-3p and PIK3CA was normalized using the 2-ΔΔCT method relative to U6 or β-actin, respectively.
Cell transfection
An miR-363-3p mimic and a corresponding negative control (miR-NC) were purchased from GenePharma Co., Ltd. (Shanghai, China). The PIK3CA overexpression plasmid (pCDNA3.1-PIK3CA) were a gift from Dr Jun Zhang (Jilin University). Mimic or plasmids were transfected into cells using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instruction. At 24 h posttransfection, cells were harvested and subjected o gene expression analysis and cell proliferation, cycle, migration and invasion assays.
Cell proliferation assay
Cells were seeded at 5000 cells per well in 96-well plates (5000 cells per well) at 24 h after transfection. Cell proliferation was measured using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) at 24, 48, and 72 h after the cells were seeded. Absorbance was determined at 450 nm using a microplate spectrophotometer (Thermo Labsystems, Vantaa, Finland).
Cell cycle analysis
The cell cycle distribution was determined using a fluorescence-activated cell sorting (FACS) flow cytometer (FACSCalibur, Becton Dickinson). Briefly, cells were harvested at 48 h after transfection. The cells were digested and subsequently fixed in 70% ethanol at 4°C overnight. Then fixed cells were stained with 50 μg/mL propidium iodide (BD Biosciences) at room temperature for 30 min in the dark. The cell cycle distribution were analyzed by with a FACS Calibur system (BD Biosciences) and ModFit 3.0 software (BD Biosciences).
Cell migration and invasion assays
Cell migration was evaluated using a wound healing assay. In brief, transfected cells were cultured in six-well plates (5×104 cells per well). At 90-95% confluence, the monolayer of cells was scratched with using a sterile plastic micropipette tip, and then cell were cultured under standard conditions for 24 h. Following several washes, recovery of the wound was observed and photographed using an X71 inverted microscope (Olympus, Tokyo, Japan).
The transwell invasion assay was performed to determine cell invasion. 1×105 transfected cells were seeded into the upper chamber of Matrigel-coated inserts with free-serum medium. Medium with 10% FBS was added to the lower chamber as chemoattractant. The cells were allowed to invade for 48 h at 37°C with 5% CO2. Then cells invaded to the lower surface of filter were fixed in 70% ethanol for 30 min and stained with 0.1% crystal violet for 10 min. The number of cells that migrated to the lower side was counted in five randomly selected fields under an X71 inverted microscope (Olympus, Tokyo, Japan).
Dual luciferase report assay
The wild-type (Wt) and mutant (Mut) 3’-untranscriptional region (3’-UTR) of PIK3CA were designed and prepared by GenePharma Co., Ltd, and were cloned into the pMIR-Report plasmid (Ambion, Austin, TX, USA) at the Spe I and Mlu I site, referred to Wt-PIK3CA and Mut-PIK3CA. For the luciferase assay, 1×105 TPC-1 cells were plated and cultured in 12-well plates to reach ~70% confluency. Cells were co-transfected with miR-363-3p mimic or miR-NC and Wt/Mut PIK3CA-3’-UTR reporter plasmid. Luciferase assays were performed 48 h post-transfection using a using a dual luciferase reporter gene assay kit (BioVision, Milpitas, CA, USA). Renilla luciferase activity was normalized to firefly luciferase activity.
Western blot
Cells or tissues lysed in RIPA lysis buffer (Beyotime, Shanghai, China) for 30 min on ice. After centrifugation at 12000 g, 4°C for 10 min, the supernatants were harvested and the protein concentration was quantified using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). A volume of the extract equivalent to 100 mg of total protein was separated on a 10% using SDS-PAGE, followed by transfer to a PVDF membrane (Millipore, Billerica Ma, USA) for immunoblotting. The membranes were blocked in 5% non-fat milk diluted in TBST and incubated with the indicated primary antibody: Anti-PIK3CA (1:1000, Abcam, Shanghai, China), anti-AKT (1:1000, Cell Signaling, Danvers, MA, USA), anti-p-AKT (1:1000, Cell Signaling), anti-PI3K (1:2000, Cell Signaling), anti-p-PI3K (1:1000, Cell Signaling) and anti-β-actin (1:3000, Cell Signaling, which was used as the internal reference. After incubation with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h at room temperature, proteins were detected using a ChemiDoc XRS imaging system and Quantity One analysis software (Bio-Rad Laboratories, San Francisco, CA, USA).
In vivo tumorigenicity
Animal studies were approved by the Institutional Animal Care and Use Committees of Jilin University. TPC-1 cells stably transformed with miR-363-3p or negative control miRNA (miR-NC, 2×106 cells per mouse) were inoculated subcutaneously into the flanks of 5-week-old, female nude mice (Laboratory Animal Research Center of Jilin University, Changchun, China). Each experimental group included 5 mice. Tumor volume was measured using a caliper every seven days for 35 days, and calculated by the formula: V=1/2× Length × Width2 (V, volume; L, length; W, width of tumor). Tumor growth curves were plotted. At 35 days after implantation, mice were sacrificed, and tumor weights were assessed. Tumor tissues were snap-frozen in liquid nitrogen immediately and stored at -80°C for detection miR-363-3p and PIK3CA expression.
Statistical analysis
Quantitative data were analyzed using SPSS version 19.0 (IBM, USA) and expressed as the mean ± SD (standard deviation) from at least three times independently experiment. Significant differences between groups were compared using ANOVAs or two-tailed t-tests. The correlations between miR-363-3p expression levels and PIK3CA mRNA levels were analyzed using Spearman’s rank test. The data were considered significant if the P value was <0.05 (indicated by*), <0.01 (indicated by**).
Results
MiR-363-3p is downregulated in PTC tissues and cell lines
First, we determined the expression patterns of miR-363-3p in 30 PTC specimens and adjacent normal tissues. The results of qPCR showed that miR-363-3p levels were significantly decreased in PTC tissues compared to adjacent normal tissues (Figure 1A). To further investigate the clinicopathological significance of miR-363-3p level in patients with PTC, 30 patients were divided into 2 subgroups based on mean (0.381) of all thyroid cancer samples: Low miR-363-3p group (<0.381, 17 cases) and a high miR-363-3p group (>0.381, 13 cases). As shown in Table 1, miR-363-3p levels in PTC tissues were negatively correlated with advanced clinical stages and lymph node metastasis, but not with age, gender, or tumor size. Consistently, miR-363-3p expression was also decreased in PTC cell lines (TPC-1, BCPAP, K1) compared with the human thyroid epithelial cell line Nthy-ori3-1 (Figure 1B). MiR-363-3p expression was lowest in TPC-1 cells among PTC cell lines (Figure 1B); Therefore, this line was used for subsequent experiments.
Figure 1.
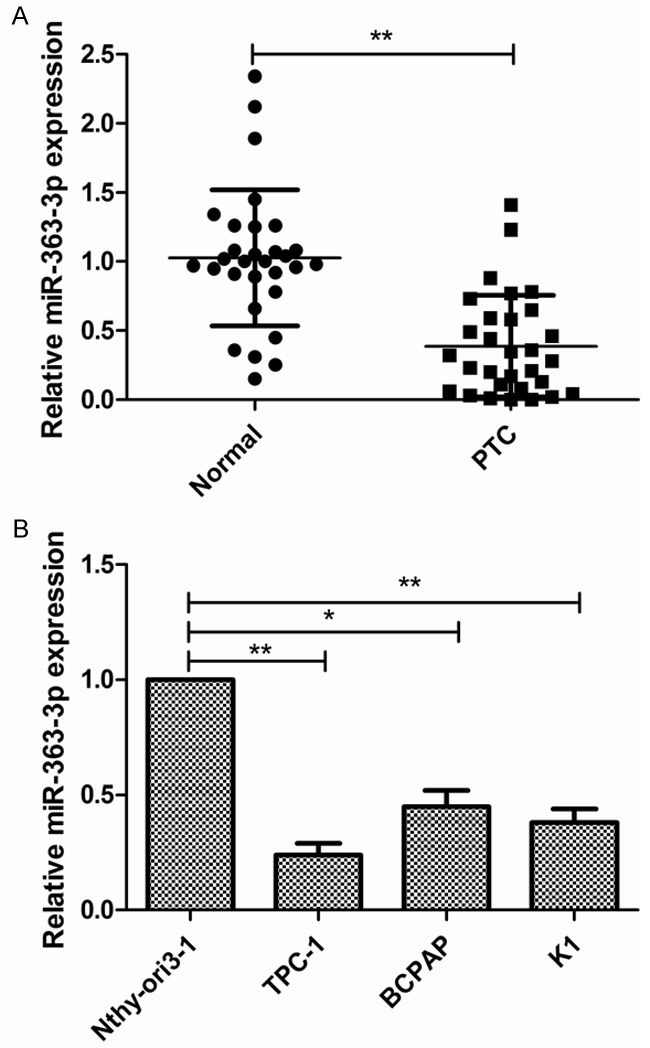
The levels of miR-363-3p expression are downregulated in PTC tissues and cell lines. A. The relative expression of miR-363-3p in 30 human PTC tissues and adjacent normal tissues was measured by qRT-PCR; B. The relative expression of miR-363-3p in PTC lines (TPC-1, BCPAP, K1) and the human thyroid epithelial cell line Nthy-ori3-1 was measured by qRT-PCR. *P<0.05, **P<0.01.
Table 1.
Correlation between clinicopathological features and miR-363-3p expression in 30 patients with PTC
| Variables | No. of cases | miR-363-3p expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low (n %) | High (n %) | |||
| Age (years) | P>0.05 | |||
| <60 | 14 | 8 (57.1) | 6 (42.9) | |
| ≥60 | 16 | 9 (56.3) | 7 (43.7) | |
| Gender | P>0.05 | |||
| Male | 12 | 7 (58.3) | 5 (41.7) | |
| Female | 18 | 10 (55.5) | 8 (44.5) | |
| TNM stage | P<0.01 | |||
| I-II | 21 | 9 (42.9) | 12 (57.1) | |
| III-IV | 9 | 8 (88.9) | 1 (11.1) | |
| Tumor size | P>0.05 | |||
| <5 cm | 19 | 10 (52.6) | 9 (47.4) | |
| ≥5 cm | 11 | 7 (63.6) | 4 (36.4) | |
| Lymph node metastasis | P<0.01 | |||
| No | 20 | 8 (40.0) | 12 (60.0) | |
| Yes | 10 | 9 (90.0) | 1 (10.0) | |
MiR-363-3p overexpression suppresses the proliferation of PTC cells
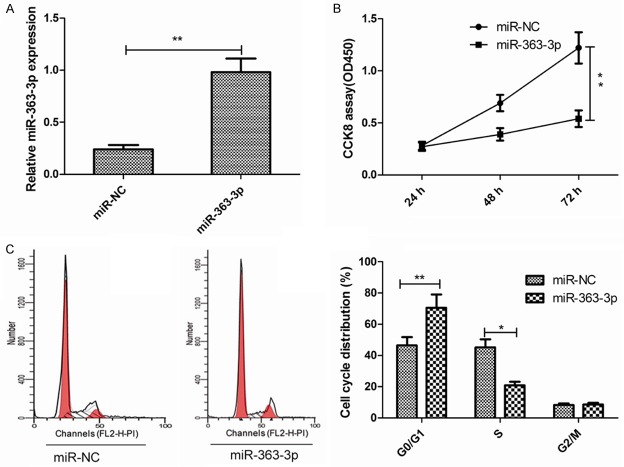
To determine whether miR-363-3p regulates PTC proliferation, we ectopically expressed miR-363-3p in the TPC-1 cells by transfecting cells with miR-363-3p mimic and then examined the effects of miR-363-3p on cell proliferation using CCK8 assay. The efficient overexpression of miR-363-3p in TPC-1 cells is shown in Figure 2A. The results of the CCK8 assay demonstrated that the upregulation of miR-363-3p significantly suppresses the proliferation of TPC-1 cells (Figure 2B). To further investigate the mechanism through which miR-363-3p inhibits cell proliferation, we determined the effects of miR-363-3p on cell cycle distribution. Flow cytometry assay showed that TPC-1 cells transfected with miR-363-3p mimic showed a promotion of cells in the G0/G1 stage, whereas the numbers of cells in the S phases decreased (Figure 2C), which suggested that miR-363-3p inhibits cell proliferation by regulating cell cycle.
Figure 2.
MiR-363-3p overexpression suppresses the proliferation of PTC cells. A. TPC-1 cells were transfected with miR-363-3p mimic, followed by qRT-PCR detection of miR-363-3p expression at 48 h after the treatment. B, C. Cell proliferation and cycle distribution were determined in TPC-1 cells transfected with miR-363 mimic or miR-NC. *P<0.05, **P<0.01.
MiR-363-3p overexpression suppresses the migration and invasion of PTC cells
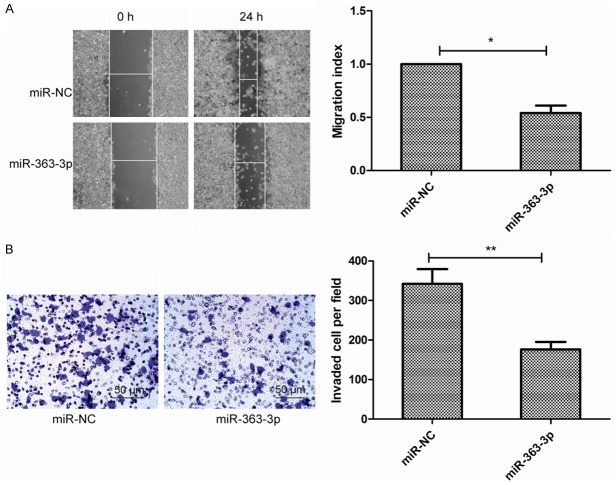
To further assess the effects of miR-363-3p on metastasis in vitro, cell migration and invasion were determined in TPC-1 cells tranfected with miR-363-3p or miR-NC. Wound healing assay showed that overexpression of miR-363-3p decreased cell motility (Figure 3A). Meanwhile, transwell invasion assay showed that miR-363-3p overexpression suppressed cell invasion (Figure 3B). These result suggested that miR-363-3p inhibited metastasis of PTC cells.
Figure 3.
MiR-363-3p overexpression suppresses the migration and invasion of PTC cells. A. Cell migration was determined by wound healing assay in TPC-1 cells transfected with miR-363-3p mimic or miR-NC. B. Cell invasion was determined by transwell invasion assay in TPC-1 cells transfected with miR-363-3p mimic or miR-NC. *P<0.05, **P<0.01.
PIK3CA is a target gene of miR-363-3p
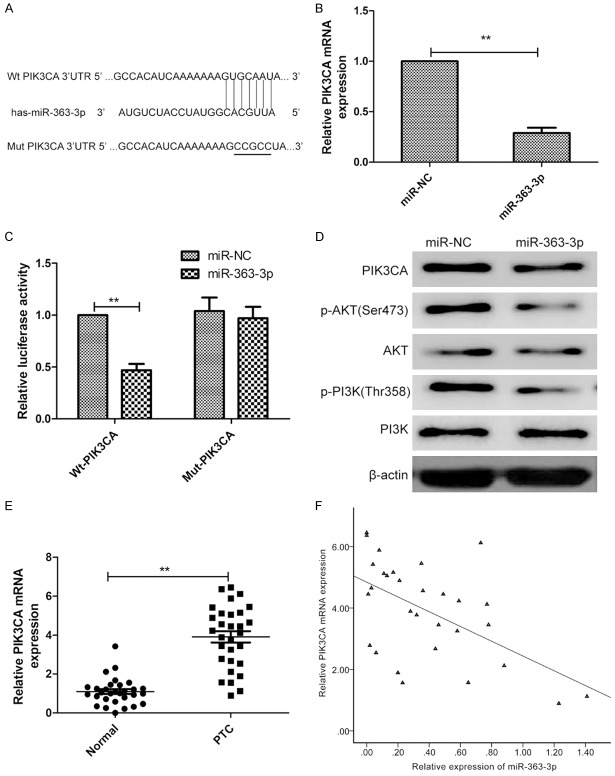
To explore the molecular mechanism by which miR-363-3p inhibit progression, three computational algorithms including TargetScan, miRanda and PicTar were used in combination to search for potential targets of miR-363-3p. Among the candidates, phosphoinositide-3-kinase catalytic subunit alpha (PIK3CA), a pro-oncogene gene that is frequently upregulated in various cancers, was predicted to be a miR-363-3p target by all three of the algorithms and was selected for further experimental verification. The predicted interaction between miR-363-3p and the target site in the PIK3CA 3’-UTR is illustrated in Figure 4A. To explore whether miR-363-3p targets PIK3CA by binding to its 3’-UTR region, a dual-luciferase reporter assay was performed and miR-363-3p overexpression was shown to significantly decrease the luciferase activity of Wt-PIK3CA-3’UTR in TPC-1 cells, while it failed to repress the Mut-PIK3CA-3’UTR (Figure 4B). The correlation between miR-363-3p and PIK3CA was further examined by evaluating PIK3CA expression levels in TPC-1 cells after overexpression of miR-363-3p. We found that overexpression of miR-363-3p inhibited PIK3CA expression on mRNA expression and protein expression (Figure 4C and 4D). Furthermore, miR-363-3p overxpression significantly lowered protein expression of PIK3CA downstream protein p-PI3K and p-AKT expression, which lead to inhibiting PI3K/AKT pathway (Figure 4D). Because miRNAs are generally thought to have expression patterns that are opposite to that of their targets, we next investigated whether miR-363-3p expression is inversely correlated with PIK3CA expression in PTC. We measured the PIK3CA mRNA expression in the same 30 pairs of PTC tissues and corresponding adjacent normal tissues, and found that PIK3CA mRNA levels were consistently higher in the PTC tissues compared to adjacent normal tissues (Figure 4E). In PTC tissues, PIK3CA transcript levels were also found to be inversely correlated with miR-363-3p expression by Spearman’s rank test (r=-0.554, P=0.001) (Figure 4F).
Figure 4.
PIK3CA is a target gene of miR-363-3p. A. Conservation of the miR-363-3p-targeting sites in the PIK3CA-3’UTR and its mutant sequence that abrogates miR-363-3p binding to target mRNA. B. Luciferase reporter assays were performed using TPC-1 cells co-transfected with the miR-363-3p mimic or miR-NC and PIK3CA-Wt-3’-UTR or PIK3CA-Mut-3’-UTR reporter plasmid. C. QRT-PCR was used to measure PIK3CA mRNA expression in TPC-1 cells transfected with miR-363-3p mimic or miR-NC. Β-actin was used as an internal control. D. Western blotting was used to measure PIK3CA, AKT, p-AKT, PIEK, p-PI3K expression in TPC-1 cells transfected with miR-363-3p mimic or miR-NC. Β-actin serving as a loading control. E. PIK3CA mRNA expression in 30 cases of PTC and adjacent normal tissues were detected by qRT-PCR. Β-actin was used as an internal control. F. Spearman’s correlation analysis was used to determine the correlations between the levels of PIK3CA and miR-363-3p in human thyroid cancer (n=30).
Restoration of PIK3CA reverses miR-363-3p suppressed cell proliferation, cycle, migration, and invasion of PTC cells
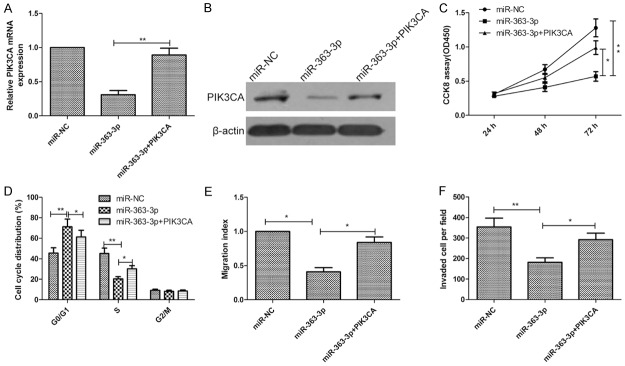
To investigate whether the regulation of cell proliferation, migration and invasion of PTC cells by miR-363-3p is executed through a PIK3CA-dependent manner, we co-transfected TPC-1 cells with miR-363-3p mimic and the PIK3CA-overexpression plasmid. Compared with cells transfected with miR-363-3p mimic, the cells transfected with both miR-363-3p mimic and the PIK3CA-overexpression plasmid exhibited a higher expression on mRNA level and protein level (Figure 5A and 5B), suggesting that miR-363-3p-resistant PIK3CA is sufficient to rescue the suppression. Overexpression of PIK3CA was furthermore shown to reverse the inhibitory effects of miR-363-3p on cell proliferation, cycle, migration, and invasion in TPC-1 cells (Figure 5C-F).
Figure 5.
Restoration of PIK3CA reverses miR-363-3p suppressed cell proliferation, cycle, migration, and invasion of PTC cells. A, B. PIK3CA expression on mRNA level and protein level was determined in TPC-1 cells transfected with miR-363-3p mimic with or without PIK3CA overexpression plasmid. Β-actin serving as a loading control. C-F. Cell proliferation, cycle, migration, and invasion were determined in TPC-1 cells transfected with miR-363-3p mimic with or without PIK3CA overexpression plasmid by CCK8, flow cytometry, wound-healing, and invasion assays, respectively. *P<0.05, **P<0.01.
MiR-363-3p suppressed tumor growth in nude mice by inhibiting PIK3CA
We next evaluated the biological effects of miR-363-3p on thyroid tumorigenesis in a PTC xenograft mouse model. TPC-1 cells were transfected with miR-363-3p or miR-NC and then implanted subcutaneously into nude mice. Tumor growth was evaluated at 7 days for 35 days. Tumor growth curves showed progressive expansion of TPC-1 cells transfected with miR-NC, while those transfected with the miR-363-3p mimic exhibited slower growth (Figure 6A). After 5 weeks after cell implantation, the nude mice was killed, and tumors were harvested and weighted. A significant decrease in the size and weight of the tumors was observed in the miR-363-3p-overexpressing group compared to control group (Figure 6B and 6C). Subsequently, the expression levels of miR-363-3p and PIK3CA were determined in tumor tissues by qRT-PCR and western blot, respectively. We found that tumors from the miR-363-3p-overexpressing group showed a significant increase in miR-363-3p expression (Figure 6D), and a decrease in PIK3CA expression compared to tumors from the miR-NC group (Figure 6E).
Figure 6.
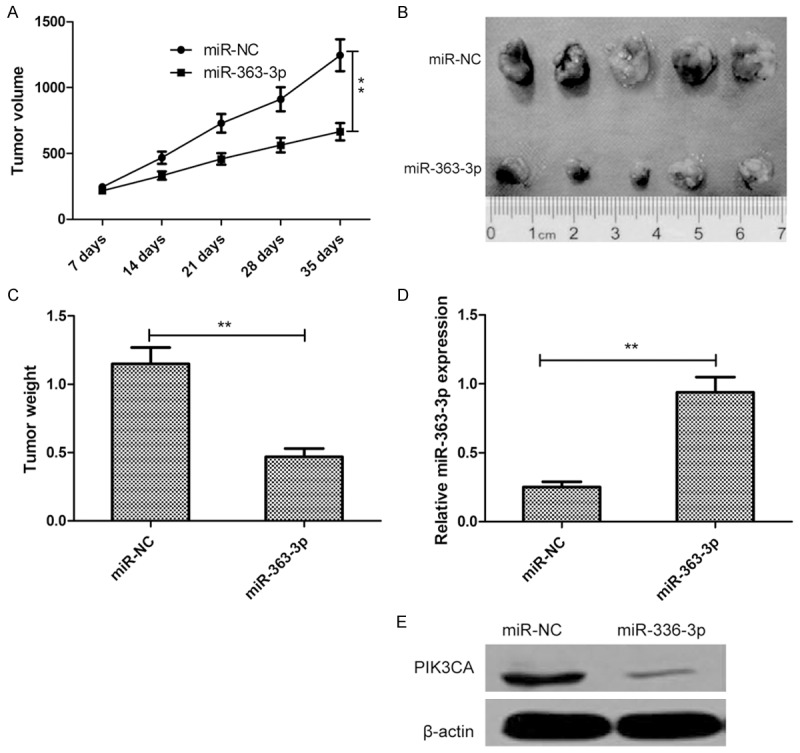
MiR-363-3p suppressed tumor growth in nude mice by inhibiting PIK3CA. A. Tumor growth curves were established by measuring tumor volume every 7 days for 35 days after injection. B. Photographs of xenograft tumors from nude mice in each treatment group on day 35 after injection. C. Tumor weight isolated from nude mice in each treatment group on day 35 after injection. D. Relative miR-363-3p expression in xenograft tumors was determined by qRT-PCR. E. PIK3CA protein expression in xenograft tumors was determined by western blot. Β-actin serving as a loading control. *P<0.05, **P<0.01.
Discussion
MicroRNAs (miRNAs), a novel class of regulatory molecules, have been documented to play crucial roles in thyroid cancer development by regulating many cellular events [6,7]. For example, Dong et al reported that miR-141 inhibited cell proliferation, induced cell apoptosis, and decreased migration, invasion in thyroid cancer cells, as well as tumor growth in nude mice by regulating insulin receptor substrate 2 (IRS2) [16]. Wen et al showed that miR-126 functioned as tumor suppressor of thyroid cancer by targeting LRP6 regulating Wnt/β-catenin signaling pathway [17]. Wang et al found that restoration of miR-497 expression significantly inhibited proliferation, colony formation, migration and invasion of thyroid cancer cells, and suppresses tumor growth in vivo by targeting BDNF [18]. Data from the current study provide evidence that miR-363-3p expression was downregulated in thyroid cancer and cell lines, and was negatively associated with clinical stage and lymph node metastasis. Restoration of expression miR-363-3p in PTC cells by transfection with miR-363-3p mimic significantly inhibited proliferation, migration and invasion, and suppresses tumor growth in vivo. These findings together provides a new insight into the mechanism of PTC progression.
MiR-363-3p, a discovery miRNA, has been reported to be downregulate in osteosarcoma [19], colorectal cancer [9], larynx cancer [20], head and neck cancer [12], breast cancer[13], hepatocellular carcinoma[10], renal cancer [21] and neuroblastoma [22], suggesting that it functions as a tumor suppressor in these malignancies. In contrast, several authors have reported that miR-363-3p is upregulated and functions as an oncogene in prostate cancer [23] and gastric cancer [11]. These findings highlight the conflicting roles of miR-363-3p in different cancers, which may depend on the specific tissue and cancer types. Recently a study showed that miR-363-3p expression was downregulated in thyroid cancer tissues by gene expression profiles assay [14]. However, the biological role and their underlying molecular mechanisms remain unclear. The present study further confirmed that the expression of miR-363-3p was downregulated in PTC cell lines and tissues by qPCR assay. Our results also showed that lower expression of miR-363-3p was negative associated with clinical stage and lymph node metastasis. Moreover, overexpression of miR-363-3p significantly suppressed PTC growth in vitro and in vivo. These results suggested that miR-363-3p functioned as a tumor suppressor in PTC.
To date, several targets of miR-363-3p have been identified, such as SOX4, FBW7, myosin 1B, Mcl-1 and S1PR1 [9-13]. On the basis of bioinformatics analysis, we further predicted another miR-363-3p target, PIK3CA, because of its known role in promoting tumorigenesis and cancer progression [24-26]. PIK3CA, located on chromosome 3q26.3 has been reported to be upregulated in many types of cancer including PTC [27]. Growing evidence has shown that PIK3CA overexpression promoted cell proliferation, invasion, and inhibited cell apoptosis by regulating PI3K/AKT pathway [28-30]. In the present study, using a dual luciferase reporter gene system, we confirmed the results from the bioinformatic tool, that miR-363-3p regulates PIK3CA expression at the translational and transcriptional levels by targeting its 3’-UTR. MiR-363-3p overexpression also inhibited p-PI3K and p-AKT expression, which lead to inhibiting PI3K/AKT signal pathway. Moreover, a significant inverse correlation was observed between PIK3CA and miR-363-3p expression in patients with PTC. Of note, overexpression of PIK3CA could abolish the inhibitive effect of miR-363-3p on proliferation, migration and invasion of PTC. These results suggested that miR-363-3p exerted suppressive role in PTC, at least in part, by repressing PIK3CA.
Taken together, the present study showed that the expression of miR-363-3p was downregulated in PTC cell lines and tissues, and was negative associated with clinical stage and lymph node metastasis. Moreover, we also found that miR-363-3p overexpression inhibits thyroid cancer cell proliferation, migration and invasion in vitro, as well as suppress tumor growth in vivo via directly targeting PIK3CA. These results provide new insights into the mechanism of thyroid cancer progression, and suggest that miR-363-3p might potentially serve as therapeutic target for PTC.
Disclosure of conflict of interest
None.
References
- 1.Liebner DA, Shah MH. Thyroid cancer: pathogenesis and targeted therapy. Ther Adv Endocrinol Metab. 2011;2:173–195. doi: 10.1177/2042018811419889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol) 2010;22:395–404. doi: 10.1016/j.clon.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Valinezhad Orang A, Safaralizadeh R, Kazemzadeh-Bavili M. Mechanisms of miRNA-mediated gene regulation from common downregulation to mRNA-Specific upregulation. Int J Genomics. 2014;2014:970607. doi: 10.1155/2014/970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bushati N, Cohen SM. MicroRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 5.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aragon Han P, Weng CH, Khawaja HT, Nagarajan N, Schneider EB, Umbricht CB, Witwer KW, Zeiger MA. MicroRNA expression and association with clinicopathologic features in papillary thyroid cancer: a systematic review. Thyroid. 2015;25:1322–1329. doi: 10.1089/thy.2015.0193. [DOI] [PubMed] [Google Scholar]
- 7.de la Chapelle A, Jazdzewski K. MicroRNAs in thyroid cancer. J Clin Endocrinol Metab. 2011;96:3326–3336. doi: 10.1210/jc.2011-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SH, Zhang WJ, Wu XC, Weng MZ, Zhang MD, Cai Q, Zhou D, Wang JD, Quan ZW. The lncRNA MALAT1 functions as a competing endogenous RNA to regulate MCL-1 expression by sponging miR-363-3p in gallbladder cancer. J Cell Mol Med. 2016;20:2299–2308. doi: 10.1111/jcmm.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu F, Min J, Cao X, Liu L, Ge Z, Hu J, Li X. MiR-363-3p inhibits the epithelial-to-mesenchymal transition and suppresses metastasis in colorectal cancer by targeting Sox4. Biochem Biophys Res Commun. 2016;474:35–42. doi: 10.1016/j.bbrc.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 10.Zhou P, Huang G, Zhao Y, Zhong D, Xu Z, Zeng Y, Zhang Y, Li S, He F. MicroRNA-363-mediated downregulation of S1PR1 suppresses the proliferation of hepatocellular carcinoma cells. Cell Signal. 2014;26:1347–1354. doi: 10.1016/j.cellsig.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Zhang PF, Sheng LL, Wang G, Tian M, Zhu LY, Zhang R, Zhang J, Zhu JS. MiR-363 promotes proliferation and chemo-resistance of human gastric cancer via targeting of FBW7 ubiquitin ligase expression. Oncotarget. 2016;7:35284–92. doi: 10.18632/oncotarget.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman BV, Wald AI, Akhtar P, Munko AC, Xu J, Gibson SP, Grandis JR, Ferris RL, Khan SA. MicroRNA-363 targets myosin 1B to reduce cellular migration in head and neck cancer. BMC Cancer. 2015;15:861. doi: 10.1186/s12885-015-1888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Li Y, Dong X, Peng L, Nie X. MiR-363 sensitizes cisplatin-induced apoptosis targeting in Mcl-1 in breast cancer. Med Oncol. 2014;31:347. doi: 10.1007/s12032-014-0347-3. [DOI] [PubMed] [Google Scholar]
- 14.Cong D, He M, Chen S, Liu X, Liu X, Sun H. Expression profiles of pivotal microRNAs and targets in thyroid papillary carcinoma: an analysis of the cancer genome Atlas. Onco Targets Ther. 2015;8:2271–2277. doi: 10.2147/OTT.S85753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu QQ, Wu H, Huang X, Shen H, Shu YQ, Zhang B, Xiang CC, Yu SM, Guo RH, Chen L. MiR-1 targets PIK3CA and inhibits tumorigenic properties of A549 cells. Biomed Pharmacother. 2014;68:155–161. doi: 10.1016/j.biopha.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Dong S, Meng X, Xue S, Yan Z, Ren P, Liu J. MicroRNA-141 inhibits thyroid cancer cell growth and metastasis by targeting insulin receptor substrate 2. Am J Transl Res. 2016;8:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Q, Zhao J, Bai L, Wang T, Zhang H, Ma Q. MiR-126 inhibits papillary thyroid carcinoma growth by targeting LRP6. Oncol Rep. 2015;34:2202–2210. doi: 10.3892/or.2015.4165. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Meng X, Huang Y, Lv Z, Liu J, Wang G, Meng W, Xue S, Zhang Q, Zhang P, Chen G. MicroRNA-497 inhibits thyroid cancer tumor growth and invasion by suppressing BDNF. Oncotarget. 2016 doi: 10.18632/oncotarget.13747. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Frezzetti D, Reale C, Calì G, Nitsch L, Fagman H, Nilsson O, Scarfò M, De Vita G, Di Lauro R. The microRNA-processing enzyme Dicer is essential for thyroid function. PLoS One. 2011;6:e27648. doi: 10.1371/journal.pone.0027648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karatas OF, Suer I, Yuceturk B, Yilmaz M, Oz B, Guven G, Cansiz H, Creighton CJ, Ittmann M, Ozen M. Identification of microRNA profile specific to cancer stem-like cells directly isolated from human larynx cancer specimens. BMC Cancer. 2016;16:853. doi: 10.1186/s12885-016-2863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Chen D, Li Y, Jin L, Liu J, Su Z, Qi Z, Shi M, Jiang Z, Ni L, Yang S, Gui Y, Mao X, Chen Y, Lai Y. Oncogenic cAMP responsive element binding protein 1 is overexpressed upon loss of tumor suppressive miR-10b-5p and miR-363-3p in renal cancer. Oncol Rep. 2016;35:1967–1978. doi: 10.3892/or.2016.4579. [DOI] [PubMed] [Google Scholar]
- 22.Qiao J, Lee S, Paul P, Theiss L, Tiao J, Qiao L, Kong A, Chung DH. MiR-335 and miR-363 regulation of neuroblastoma tumorigenesis and metastasis. Surgery. 2013;154:226–233. doi: 10.1016/j.surg.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Lu X, Wu B, Su Y, Li J, Wang H. MicroRNA 363 mediated positive regulation of c-myc translation affect prostate cancer development and progress. Neoplasma. 2015;62:191–198. doi: 10.4149/neo_2015_024. [DOI] [PubMed] [Google Scholar]
- 24.Mei ZB, Duan CY, Li CB, Cui L, Ogino S. Prognostic role of tumor PIK3CA mutation in colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:1836–1848. doi: 10.1093/annonc/mdw264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada K, Baba Y, Shigaki H, Ishimoto T, Miyake K, Kosumi K, Tokunaga R, Izumi D, Ohuchi M, Nakamura K, Kiyozumi Y, Kurashige J, Iwatsuki M, Miyamoto Y, Sakamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic and clinical impact of PIK3CA mutation in gastric cancer: pyrosequencing technology and literature review. BMC Cancer. 2016;16:400. doi: 10.1186/s12885-016-2422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JY, Cheng YN, Han L, Wei F, Yu WW, Zhang XW, Cao S, Yu JP. Predictive value of K-ras and PIK3CA in non-small cell lung cancer patients treated with EGFR-TKIs: a systemic review and meta-analysis. Cancer Biol Med. 2015;12:126–139. doi: 10.7497/j.issn.2095-3941.2015.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojciechowska-Durczynska K, Krawczyk-Rusiecka K, Cyniak-Magierska A, Zygmunt A, Galecka E, Lewinski A. Relative quantification of PIK3CA gene expression level in fine-needle aspiration biopsy thyroid specimens collected from patients with papillary thyroid carcinoma and non-toxic goitre by real-time RT-PCR. Thyroid Res. 2010;3:5. doi: 10.1186/1756-6614-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang H, Wang R, Jin Y, Li J, Zhang S. MiR-422a acts as a tumor suppressor in glioblastoma by targeting PIK3CA. Am J Cancer Res. 2016;6:1695–1707. [PMC free article] [PubMed] [Google Scholar]
- 29.Guerriero I, D’Angelo D, Pallante P, Santos M, Scrima M, Malanga D, De Marco C, Ravo M, Weisz A, Laudanna C, Ceccarelli M, Falco G, Rizzuto A, Viglietto G. Analysis of miRNA profiles identified miR-196a as a crucial mediator of aberrant PI3K/AKT signaling in lung cancer cells. Oncotarget. 2016 doi: 10.18632/oncotarget.13432. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asati V, Bharti SK, Mahapatra DK, Asati V, Budhwani AK. Triggering PIK3CA mutations in PI3K/AKT/mTOR axis: Exploration of newer inhibitors and rational preventive strategies. Curr Pharm Des. 2016;22:6039–6054. doi: 10.2174/1381612822666160614000053. [DOI] [PubMed] [Google Scholar]