Abstract
The release of plastics into the environment has been identified as an important issue for some time. Recent publications have suggested that the degradation of plastic materials will result in the release of nano-sized plastic particles to the environment. Nanoparticle tracking analysis was applied to characterise the formation of nanoplastics during the degradation of a polystyrene (PS) disposable coffee cup lid. The results clearly show an increase in the formation of nanoplastics over time. After 56 days' exposure the concentration of nanoplastics in the PS sample was 1.26 × 108 particles/ml (average particles size 224 nm) compared to 0.41 × 108 particles/ml in the control.
Keywords: Nanoplastics, Microplastics, Polystyrene, Degradation, Environment, Nanoparticle tracking analysis
Graphical abstract
Highlights
-
•
Polystyrene degrades into nanoplastics.
-
•
The formation of nanoplastic particles increase over time.
-
•
Results suggest a continuous process of plastic surface erosion.
1. Introduction
The release of plastic materials into the environment is recognised as an important pollution related issue (Sutherland et al., 2010, UNEP, 2011). Once in the environment plastics undergo abiotic and biotic weathering processes that cause their degradation and fragmentation into increasingly smaller particles, commonly termed microplastics (MPs; often defined as fragments <5 mm). A number of environmental monitoring studies have quantified the environmental occurrence of MPs in surface waters (Faure et al., 2015), coastal sediments (Browne et al., 2011), beach sands (Liebezeit and Dubaish, 2012), freshwater sediments (Castañeda et al., 2014), and deep-sea environments (Woodall et al., 2014). MPs are also known to effectively sorb organic pollutants from surrounding water (Mato et al., 2001, Endo et al., 2005, Van et al., 2012). Therefore, internalized MPs might not only lead to direct physical injury, but also to a chemical exposure of the organism through the ingestion of pollutant loaded MPs (Ryan et al., 1988, Saal et al., 2008).
Recent publications have also suggested that MPs will subsequently degrade into nano-sized plastic particles (see Andrady, 2011, Lambert et al., 2013, Mattsson et al., 2015). The environmental impacts of nanoplastics will be different to those presented by microplastics, because of their smaller size makes tissue penetration and accumulation in organs a possibility (Mattsson et al., 2015). This is a potentially important issue given the current concerns regarding the environmental behaviour and ecotoxicity of engineered nano-materials (Lambert et al., 2014). Therefore, the aim of this work was to test the hypothesis that nanoplastic particles are formed during plastic degradation processes and that the concentrations will increase over time.
2. Methods
To investigate the potential formation of nanoplastics a time course study was performed using a polystyrene (PS) disposable coffee cup lid. The PS sample was cut into 1 cm squares (using scissors), placed in glass vials, immersed in 20 ml demineralized water, and placed in a weathering chamber (Binder GmbH). The exposure conditions were kept static with the temperature set to 30 °C and 24 h exposure to light in both the visible and ultra-violet (UV; 320–400 nm) range. Samples were taken after 7, 14, 28 and 56 days, for each time point individual samples were established in triplicate alongside control samples (i.e. water only). A laboratory blank was also created on the day of each sample collection to document any contamination that may result from the analytical process.
Nanoparticle tracking analysis (NTA) was used to determine the particle size distribution in the liquid samples. NTA is capable of analysing particles in the size range of 30–2000 nm. Analysis was performed using NanoSight LM 10 (NanoSight Ltd, Wiltshire, UK). To characterise each individual replicate PS sample, control, and blank in a representative manner three video images of each were taken. Video image length was set at 30 s and imaging was performed at room temperature. The video images were then processed using NTA 2.3 software, with the following parameters; detention threshold set to auto; blur set to 5 × 5; minimum track length and minimum expected particle size were both set to auto.
3. Results and discussion
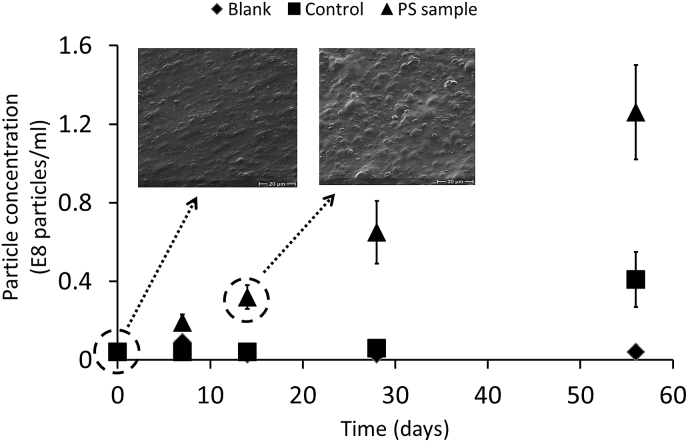
In this study the formation of nanoplastic particles was measured during the degradation of a disposable PS coffee cup lid. NTA allows individual particles to be analysed in liquid samples and its application on heterogeneous samples has previously been demonstrated (Filipe et al., 2010, Montes-Burgos et al., 2010, La Rocca et al., 2014). Fig. 1 shows an increase in the formation of nanoplastics over time and after 14 days a difference is observed between the PS samples (0.32 × 108 particles/ml) and the corresponding control sample (0.04 × 108 particles/ml). At day 56 the particle concentration in the PS solutions was 1.26 × 108 particles/ml compared to 0.41 × 108 particles/ml in the control. Weight loss of the PS samples up until day 56 was negligible. As observed by La Rocca et al. (2014) the main disadvantage of NTA is that it does not provide images of the visualized particles, and the structure of the particles cannot be evaluated. However, the SEM images depict bubbling on the PS material surface. This suggests that the degradation process begins with a steady process of surface erosion leading to the continuous release of nanoplastics into the surrounding solution.
Fig. 1.
Measured particle concentrations in the blank, control, and polystyrene samples. Particle concentrations were calculated by the NTA software and the ±values represent the standard error associated with the measurement, with scanning electron microscope images for sample taken at day 0 and 14. Further details are provided as supporting information.
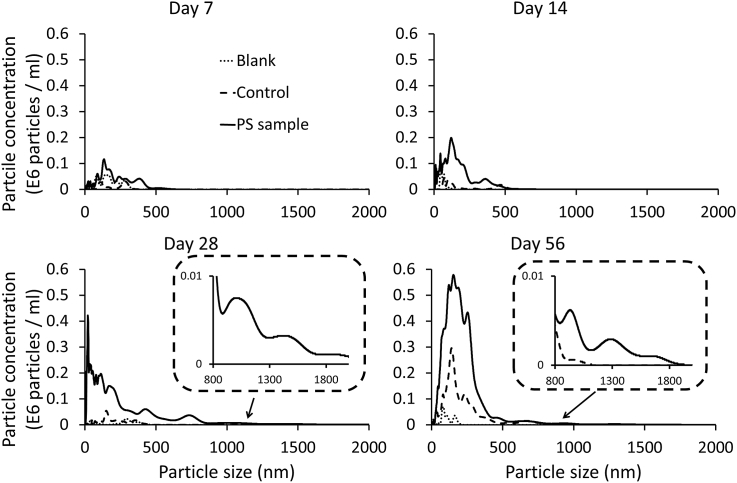
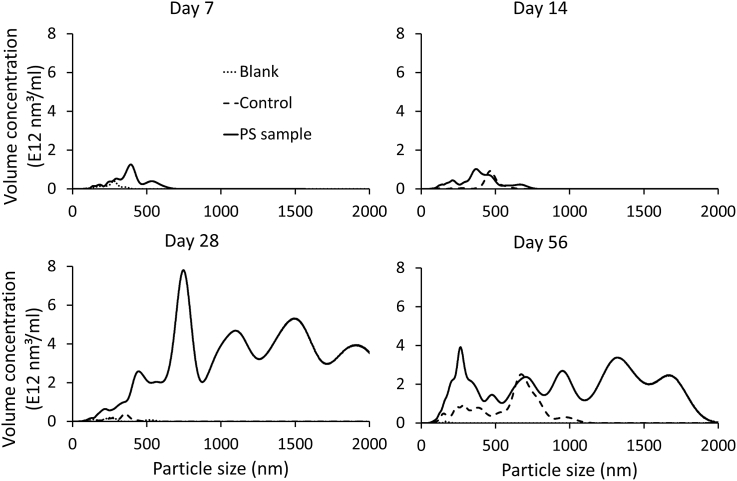
The increase in the formation of nanoplastics is further demonstrated by the particle size distributions (Fig. 2). The blank samples are used to represent background concentrations, while the control samples are used to define any naturally occurring particles that may enter the samples during the exposure period (e.g. atmospheric dust). The NanoSight platform also allows for the calculation of particle volume distribution (Fig. 3). As particle abundance increases exponentially with decreasing particle size (Fig. 2), this function can be used to represent the presence of larger particles and demonstrates the formation of particles up to 2000 nm. Particle size is an important property in determining the interaction of nano-particles with living systems (Montes-Burgos et al., 2010). A study by Browne et al. (2008) using the mussel Mytilus edulis indicated that small microspheres (3.0 μm) can be translocated from the gut to the circulatory system within 3 days and then persist in circulation for over 48 days. The short-term exposure used in this study did not find any significant biological effects (Browne et al., 2008). However, environmental exposure will occur over a longer time period and the formation of plastic particles below this size range during plastic degradation is highly likely.
Fig. 2.
Size distribution data of particles formed during the degradation of a polystyrene coffee cup lid measured by nanoparticle tracking analysis.
Fig. 3.
Volume concentration of particles formed during the degradation of a polystyrene coffee cup lid measured by nanoparticle tracking analysis.
4. Conclusion
The aim of this work was to test the hypothesis that nanoplastics are formed during the degradation of a disposable PS coffee cup lid. The findings show that nanoplastics are formed during the degradation process of plastic materials, and that their increase in concentration over time is measurable. This work is part of a larger ongoing study that aims to assess potential differences in particle formation between polymer types, and the extent to which microbial communities can utilise particles in this size range.
Acknowledgements
This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No 660306. We would also to thank Dr Robert Tampé at the Institute of Biochemistry, Goethe University Frankfurt for access to the NanoSight, and Manfred Ruppel at Goethe University Frankfurt for taking the SEM images.
Handling Editor: Tamara S. Galloway
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.chemosphere.2015.11.078.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Browne M.A., Crump P., Niven S.J., Teuten E., Tonkin A., Galloway T., Thompson R. Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ. Sci. Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Browne M.A., Dissanayake A., Galloway T.S., Lowe D.M., Thompson R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.) Environ. Sci. Technol. 2008;42:5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- Castañeda R.A., Avlijas S., Simard M.A., Ricciardi A. Microplastic pollution in St. Lawrence river sediments. Can. J. Fish. Aquat. Sci. 2014;71:1767–1771. [Google Scholar]
- Endo S., Takizawa R., Okuda K., Takada H., Chiba K., Kanehiro H., Ogi H., Yamashita R., Date T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar. Pollut. Bull. 2005;50:1103–1114. doi: 10.1016/j.marpolbul.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Faure F., Saini C., Potter G., Galgani F., de Alencastro L.F., Hagmann P. An evaluation of surface micro- and mesoplastic pollution in pelagic ecosystems of the Western Mediterranean Sea. Environ. Sci. Pollut. Res. Int. 2015;19:19. doi: 10.1007/s11356-015-4453-3. [DOI] [PubMed] [Google Scholar]
- Filipe V., Hawe A., Jiskoot W. Critical evaluation of nanoparticle tracking analysis (NTA) by NANOSIGHT for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010;27:796–810. doi: 10.1007/s11095-010-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rocca A., Di Liberto G., Shayler P.J., Parmenter C.D.J., Fay M.W. Application of nanoparticle tracking analysis platform for the measurement of soot-in-oil agglomerates from automotive engines. Tribol. Int. 2014;70:142–147. [Google Scholar]
- Lambert S., Sinclair C.J., Boxall A.B.A. Occurrence, degradation and effects of polymer-based materials in the environment. Rev. Environ. Contam. Toxicol. 2014;227:1–53. doi: 10.1007/978-3-319-01327-5_1. [DOI] [PubMed] [Google Scholar]
- Lambert S., Sinclair C.J., Bradley E.L., Boxall A.B.A. Effects of environmental conditions on latex dergadation in aquatic systems. Sci. Total Environ. 2013;447:225–234. doi: 10.1016/j.scitotenv.2012.12.067. [DOI] [PubMed] [Google Scholar]
- Liebezeit G., Dubaish F. Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull. Environ. Contam. Toxicol. 2012;89:213–217. doi: 10.1007/s00128-012-0642-7. [DOI] [PubMed] [Google Scholar]
- Mato Y., Isobe T., Takada H., Kanehiro H., Ohtake C., Kaminuma T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001;35:318–324. doi: 10.1021/es0010498. [DOI] [PubMed] [Google Scholar]
- Mattsson K., Hansson L.A., Cedervall T. Nano-plastics in the aquatic environment. Environ. Sci. Process. Impacts. 2015;17:1712–1721. doi: 10.1039/c5em00227c. [DOI] [PubMed] [Google Scholar]
- Montes-Burgos I., Walczyk D., Hole P., Smith J., Lynch I., Dawson K. Characterisation of nanoparticle size and state prior to nanotoxicological studies. J. Nanopart. Res. 2010;12:47–53. [Google Scholar]
- Ryan P.G., Connell A.D., Gardner B.D. Plastic ingestion and pcbs in seabirds – is there a relationship. Mar. Pollut. Bull. 1988;19:174–176. [Google Scholar]
- Saal F.S., Parmigiani S., Palanza P.L., Everett L.G., Ragaini R. The plastic world: sources, amounts, ecological impacts and effects on development, reproduction, brain and behavior in aquatic and terrestrial animals and humans introduction. Environ. Res. 2008;108:127–130. doi: 10.1016/j.envres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Sutherland W.J., Clout M., Cote I.M., Daszak P., Depledge M.H., Fellman L., Fleishman E., Garthwaite R., Gibbons D.W., De Lurio J., Impey A.J., Lickorish F., Lindenmayer D., Madgwick J., Margerison C., Maynard T., Peck L.S., Pretty J., Prior S., Redford K.H., Scharlemann J.P.W., Spalding M., Watkinson A.R. A horizon scan of global conservation issues for 2010. Trends Ecol. Evol. 2010;25:1–7. doi: 10.1016/j.tree.2009.10.003. [DOI] [PubMed] [Google Scholar]
- UNEP . 2011. UNEP Year Book 2011: Emerging Issues in Our Global Environment. [Google Scholar]
- Van A., Rochman C.M., Flores E.M., Hill K.L., Vargas E., Vargas S.A., Hoh E. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere. 2012;86:258–263. doi: 10.1016/j.chemosphere.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Woodall L.C., Sanchez-Vidal A., Canals M., Paterson G.L.J., Coppock R., Sleight V., Calafat A., Rogers A.D., Narayanaswamy B.E., Thompson R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014;1:140317. doi: 10.1098/rsos.140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.