Abstract
Recently, many reports have shown that Averrhoa carambola L. (Oxalidaceae) juice (EACJ) could reduce blood glucose in humans. However, its mechanisms have not been well explored; therefore, our study aimed to investigate the beneficial effects of EACJ on hyperglycemia, hyperlipidemia and renal injury in streptozotocin (STZ)-induced diabetic mice. Those mice were injected with STZ via the tail vein (120 mg/kg body weight) and were identified as diabetic mice when the level of blood glucose was ≥ 11.1 mmol/L. Those mice were intragastriced gavage with saline, EACJ (25, 50, 100 g/kg body weight/d) and metformin (320 mg/kg body weight/d) for 21 days. The fasting blood glucose (FBG), free fatty acids (FFA), total cholesterol (TC), triglycerides (TG), Scr (CREA) and blood urea nitrogen (BUN) were significantly decreased, while the sorbitol dehydrogenase (SDH), Cyclic Adenosine monophosphate (cAMP), malondialdehyde (MDA), superoxide dismutase (SOD), and insulin were elevated. Diabetes-dependent alterations in the kidney, such as glomerular hypertrophy, thicken and tubular basement membrane, were improved after 21 days of EACJ treatment. Hyperglycemia, renal formation and the expressions of related proteins such as connective tissue growth factor (CTGF) and transforming growth factor beta 1 (TGF-β1) were markedly decreased by EACJ. These results indicate that EACJ treatment decrease hyperglycemia, hyperlipidemia and inhibit the progression of diabetic nephropathy (DN), which may be linked to regulating several pharmacological targets for treating or preventing DN.
Keywords: Averrhoa carambola L. (Oxalidaceae) juice (EACJ), diabetic mice, blood glucose, kidney
Introduction
Diabetes is a disease characterized by chronic hyperglycemia as well as hyperlipidemia. Diabetic complications include atherosclerosis, cardiac dysfunction, hepatopathy, and nephropathy [1]. Diabetic nephropathy (DN) is characterized histologically by glomerular basement thickening and mesangial expansion related to the loss of renal function and clinically progressive albuminuria, followed by a gradual decline in renal function [2]. The main manifestations in the early stage of DN and glomerular hypertrophy, the gradual emergence of the extracellular matrix, renal interstitial fibrosis as the main performance, resulting in damage to the kidney, reducing the effective filtration rate, and finally leading to renal failure because of renal insufficiency [3].
Developing studies have indicated that diabetes in animals is accompanied by the elevation of triglycerides (TG), total cholesterol (TC), free fatty acids (FFAs), CREA (Scr) serum urea nitrogen, and blood urea nitrogen (BUN) [4-6]. Previous studies have demonstrated that FFAs are critical for the formation and development of DN [7]. FFAs are considered to inactivate the insulin receptor in target cells and to inhibit the combination between insulin and its receptor [8]. FFAs could also promote superoxide dismutase (SOD) activity, reduce the contents of malondialdehyde (MDA), cyclic adenosine phosphate (cAMP) and sorbitol dehydrogenase (SDH) [9-13]. Many studies have shown that both elevated TC and LDL-C in blood are powerful risk factors for coronary heart disease and high HDL-C. The LDL-C ratio may protect against coronary heart disease [14].
In the early prevention and treatment of DN, an inhibitor block can effectively block and delay the development of its onset and the course of the disease. Connective-tissue growth factor (CTGF) and transforming growth factor beta 1 (TGF-β1) play an important role in the development and the progression of DN. TGF-β1 takes part in the uptake of albumin by renal proximal tubule cells and in the development of albuminuria. TGF-β1 also stimulates the synthesis of matrix components, such as collagen IV. A renal CTGF gene expression is strongly upregulated in experimental DN [15-20].
Averrhoa carambola L. (Oxalidaceae) is also known as the star fruit, is a plant originally from Asia that has become acclimatized to many tropical countries. The root of this tree has been used as a traditional Chinese medicine for treating diabetes for a long time. Averrhoa carambola L. (Oxalidaceae) is divided into two categories: sweet and sour [21]. In this study, that an Averrhoa carambola L. (Oxalidaceae) juce (EACJ) has a therapeutic potential in diabetes. Further, we show that the diabetic mice were established by injecting them with STZ via the tail vein and by treating them with different concentrations of EACJ via intragastric administration. To investigate the effects of EACJ on hyperglycemia, hyperlipidemia, and regulatory protein expression in the injured kidneys of STZ-Induced diabetic mice were considered, and the intervention effect of EACJ on DN in diabetic model mice was explored. This would likely provide the theoretical basis for the prevention and treatment of diabetes and its complications. In this study, EACJ as the research object, the diabetic mice were established by injecting them with STZ via the tail vein and by treating them with different concentrations of EACJ via intragastric administration. To investigate the effects of EACJ on hyperglycemia, hyperlipidemia, and regulatory protein expression in the injured kidneys of STZ-Induced diabetic mice were considered, and the intervention effect of EACJ on DN in diabetic model mice was explored. This would likely provide the theoretical basis for the prevention and treatment of diabetes and its complications.
Materials and methods
Material and preparation of EACJ
Averrhoa carambola L. (Oxalidaceae) plants were obtained from Lingshan County, Guangxi Province, China, batch number: 20130909 (identified by Professor Maoxiang Lai at the Traditional Chinese Medicine Research Institute of Guangxi).
The fruit of Averrhoa carambola L. (Oxalidaceae) weighing 2.24 kg was made into juice with purified water. Then, the extracts were filtered and concentrated at 70 ± 5°C and ground into 900 mL of EACJ (concentration of 5 g/mL Averrhoa carambola L. [Oxalidaceae] fruit). It was then administered to the mice.
Animals and treatment
The experimental procedures and protocols were approved by the Ethical Committee of the Experimental Use of Animals at Guangxi Medical University (Guangxi, China), registration number SCXK 2009-0002.
Healthy male Kunming (KM) mice, which were each approximately 18-22 g, were housed in individual cages under controlled temperature (25 ± 1°C) and humidity (60 ± 5%) on a 12:12 hour light-dark cycle, were fed with standard rodent food (Beijing Vital River Laboratories, China), and were provided free access to water. The mice were injected with STZ via the tail vein (120 mg/kg body weight) after 12 hours of fasting [22-24]. Seventy-two hours later, the fasting blood glucose (FBG) testing was conducted, and the mice with an FBG ≥ 11.1 mmol/L were selected as the diabetic mice.
The mice were assigned to the following groups
Normal control group (Group I) (n = 10): healthy mice administered distilled water.
Model control group (Group II) (n = 10): diabetic mice administered distilled water.
Metformin control group (Group III) (n = 10): diabetic mice administered metformin (320 mg/kg body weight) by gastric perfusion once a day for 21 days. The concentration of the metformin was adjusted to 16 mg/ml before the administration to the mice.
High group (Group IV) (n = 10): diabetic mice administered EACJ (100 g/kg body weight) by gastric perfusion once a day for 21 days.
Moderate group (Group V) (n = 10): diabetic mice administered EACJ (50 g/kg body weight) by gastric perfusion once a day for 21 days.
Low group (Group VI) (n = 10): diabetic mice administered EACJ (25 g/kg body weight) by gastric perfusion once a day for 21 days.
FBG assay and body weight
During the experiment, FBG was measured from the tail vein and body weight on day 0, day 7, and day 21 according to the Roche ACCU-CHEK® Performa operation instructions, and the following formula to calculate the FBG descent rate (%).
Collection of blood and tissues
After 21 days of treatment with EACJ, the blood and tissue biochemical indexes were collected and analyzed. The whole blood was collected from the intraocular canthal, and the mice were sacrificed by cervical dislocation. The blood was centrifuged at 3000 rpm, 4°C for 10 minutes, and the serum was transferred into new tubes that were stored at -20°C until further analysis. From each mouse, a kidney and the liver were harvested, immediately, fixed in 10% formaldehyde solution, embedded in paraffin, and sectioned at 5 μm for further analysis. Then, the other kidney and liver tissues were removed and washed with cold saline; the serum and tissues were stored at -80°C for further determination.
Biochemical index assays
The serum levels of TC (CHOL), TG, HDL-C, LDL-C, CREA (Scr), and BUN were measured using an automatic biochemical analyzer (Hitachi Model 7100 Automatic Analyzer). The concentration of serum fasting blood insulin (FIN) and the insulin sensitivity index (ISI) was analyzed using an Iodine [125I] Insulin Radioimmunoassay Kit (Beijing North Institute of Biotechnology, Beijing, China, number: 140102) according to the manufacturer’s instructions. The ISI was calculated using the following formula [13]: ISI = ln (FBG × FINS)-1.
Lipid peroxidation assays
The liver tissue samples weighing 0.5 g were made 10% liver tissue with normal saline. The liver tissue was prepared with a high-speed homogeneous mechanism, at 3000 r.min.-1 for 15 minutes. The supernatant was diluted with normal saline for certain times, then liver tissue protein of SOD and MDA were analyzed using an assay kit (Nanjing Institute of Biological Engineering); another liver tissue protein of SDH and cAMP were analyzed using an ELISA assay kit (Beijing Chenglin Biotechnology Co., LTD., number: 201411).
Histopathological examination
The kidney and pancreatic samples were fixed with 10% formaldehyde for over 24 hours and then embedded in paraffin. The slices were prepared using regular hematoxylin-eosin (HE) staining and were observed under a light microscope magnification of 400 × by a pathologist who was blinded to the experimental profile.
Immunohistochemical analysis
The expression levels of CTGF and TGF-β1 were analyzed by immunohistochemistry. All of the renal samples were treated following the manufacturers’ procedures noted in the descriptions of the commercial kits [25,26].
RNA extraction and quantitative real-time PCR
Total RNA of the kidney tissue was extracted by the Trizol method (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s recommendations. Each sample was reverse-transcribed into cDNA using Takara, PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time). The gene expression was quantified by means of the comparative Ct method (ΔΔCt), and the relative quantification (RQ) was calculated as 2-ΔΔCt [27-30]. The relative mRNA levels of CTGF and TGF-β1 were examined and normalized to β-actin mRNA. All real-time PCRs were performed in triplicate, and the data are presented as mean ± SD. The primers of TGF-β1 and CTGF are asfollows: CTGF forward primer, 5’-GACCCAACTATGATGCGAGCC-3’, and reverse primer, 5’-CCCATCCCACAGGTCTTAGAAC-3’ (77 bp); and TGF-β1 forward primer, 5’-CTTCAATACGTCAGACATTCGGG-3’, and reverse primer, 5’-GTAACGCCAGGAATTGTTGCTA-3’ (142 bp) with annealing temperature 60°C.
Statistical analysis
All of the experimental data were statistically processed using SPSS 19.0 software (SPSS Inc., USA). The values were represented as the mean ± standard error (SE) after testing the homogeneity of variance; the significance of the data was analyzed using a one-way analysis of variance (ANOVA). The differences between the groups were considered statistically significant at P < 0.05.
Results
Body weight, blood glucose, and insulin sensitivity
The effects of EACJ on the body weight and blood glucose levels in the animals are shown in Figure 1A, 1B. In the behavioristic observations, the mice in the normal control group exhibited a good mental condition, activity, smooth fur, and a steady increase in body weight. On the contrary, the mice in the model control group exhibited a poor mental condition, rare fur, and a significant decrease in weight gain. After 21 days of treatment, both the metformin- and the EACJ-administered groups had a beneficial effect on mitigating the loss of body weight in the model control group (P < 0.01).
Figure 1.
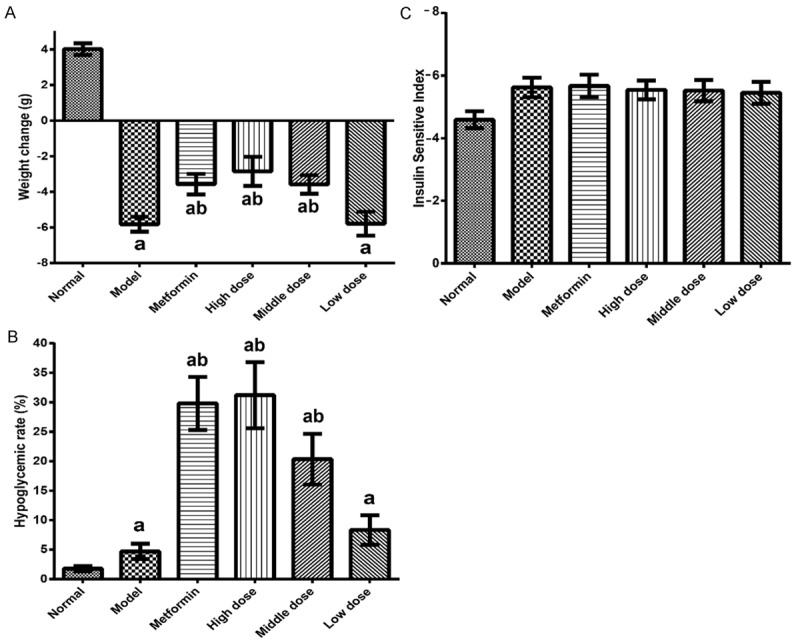
The effects of EACJ on the body weight, blood glucose levels, and insulin sensitivity in mouse. A: Effect of EACJ on body weight; B: Hypoglycemic effect of EACJ; C: Effect of EACJ on insulin sensitivity index (ISI): Normal control: administered distilled water; Model control: administered distilled water; Metformin: 320 mg/kg of metformin; High dose: 100 g/kg of EACJ; Middle dose: 50 g/kg of EACJ; Low dose: 25 g/kg of EACJ. The results are presented as the means ± SE for 10 animals in each group. aP < 0.01 compared with the normal control group; bP < 0.01, cP < 0.05 compared with the model group.
As demonstrated after 21 days of treatment, the high-dose, moderate-dose, and low-dose EACJ rates were 31.20%, 20.33%, and 8.34%, respectively. The metformin hypoglycemic rate was 29.80%. Compared with the model control group, high dose, moderate dose, and low dose of EACJ with FBG were statistically significant (P < 0.05).
Compared with the model control group, high dose and moderate dose of EACJ’s blood glucose were significantly decreased (P < 0.01). However, the dose compared with the model control group, the drug groups’ fasting insulin was not statistically different (P > 0.05). EACJ is high and the middle-dose group can effectively promote the secretion of insulin in diabetic mice, reduce blood sugar, but did not improve the body’s insulin sensitivity (Figure 1C).
Histopathological findings
Under a light microscope, it was observed that the blank control group of the pancreatic acinar and insulin morphology have a complete structure, without exception. The model control group’s pancreatic acinar significantly decreased. Several insulin cells were atrophied, the cytoplasm decreased significantly, and the cell nuclei were crowded together. The metformin control group, high dose, and moderate dose of EACJ group of the pancreatic acinar and insulin morphology have a complete structure, without exception, but cell numbers were less. The low dose of the EACJ group of the pancreatic and insulin tissue demonstrated a slight atrophy, mild acinar number decreased, and only some insulin structure was damaged, with some of insulin cells’ nuclei appearing dense (Figure 2).
Figure 2.
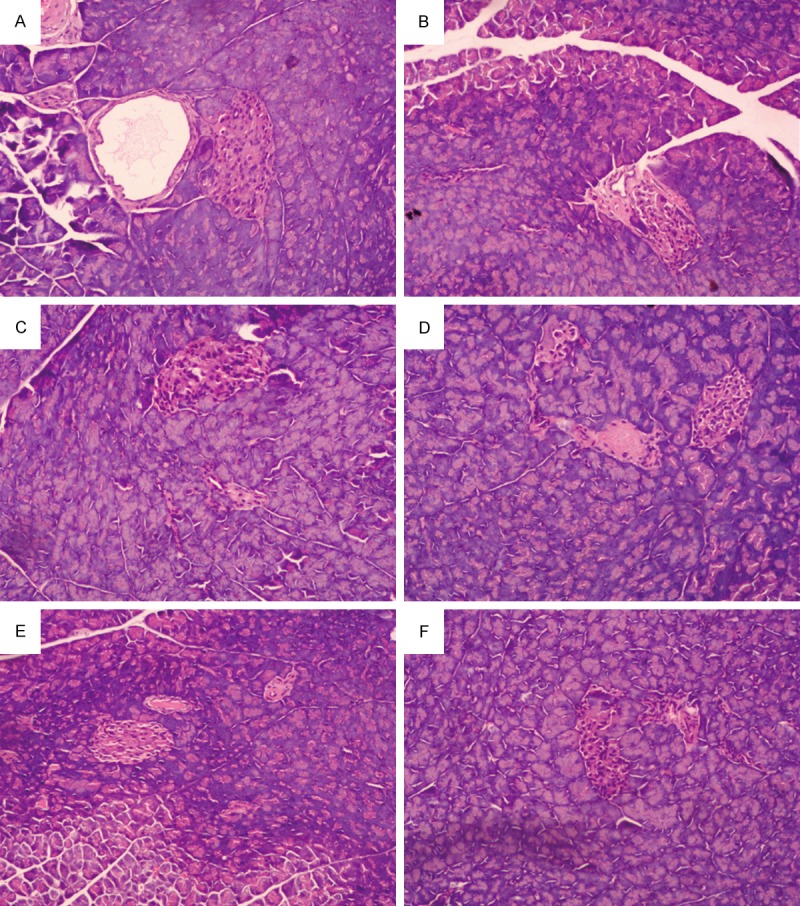
Histological observations of pancreas tissue. A: Normal control; B: Model control; C: 320 mg/kg of metformin; D: 100 g.kg-1.d-1 of EACJ; E: 50 g.kg-1.d-1 of EACJ; F: 25 g.kg-1.d-1 of EACJ. Note: The arrows point to the islet containing certain pancreatic β cells.
The normal mice showed an integrated cell structure in the kidney tissue. The kidneys of the model control group revealed renal tubule hypertrophy, glomerulus hypertrophy, vacuolar degeneration, slight inflammatory cell infiltration, and apparent glomerular collapse. After 21 days of treatment with EACJ and metformin, the kidney with lesions can relieve the degree of pathological changes, and smaller than the kidney of the model control group. Moreover, the histological studies of the high-dose-treated animal kidneys revealed less renal tubule expansion and glomerular basement membrane thickening compared with the STZ-induced mice (Figure 3).
Figure 3.
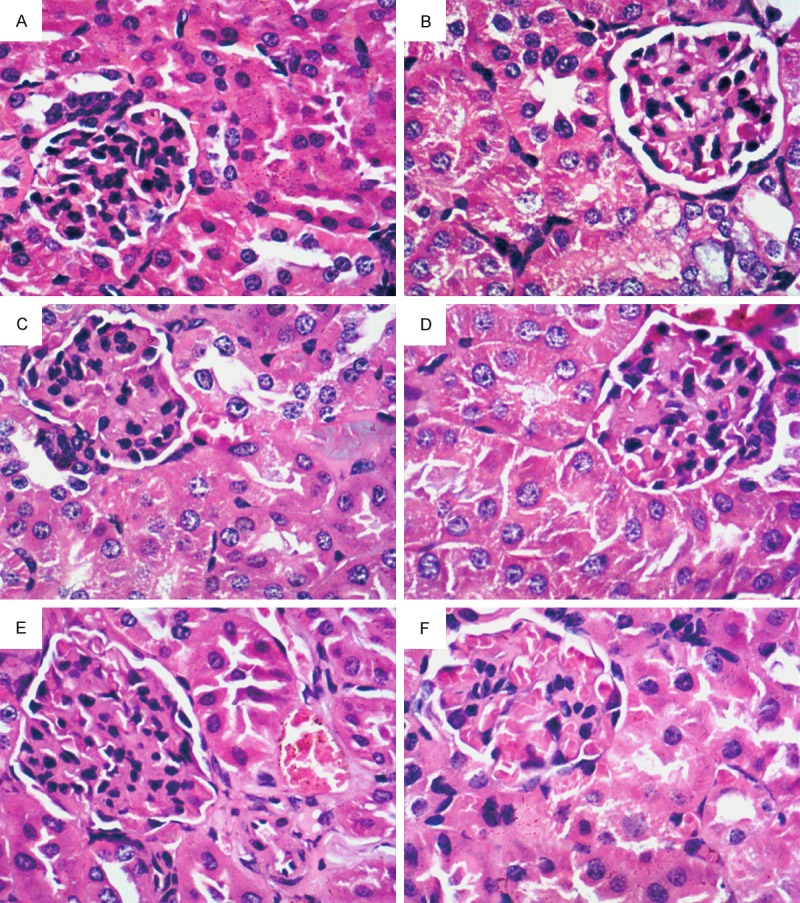
Renal sections of the mice. Histology of kidneys was characterized by staining with hematoxylin-eosin staining (magnification: 400 ×). A: Normal control; B: Model control; C: 320 mg/kg of metformin; D: 25 g.kg-1.d-1 of EACJ; E: 50 g.kg-1.d-1 of EACJ; F: 100 g.kg-1.d-1 of EACJ. Note: The arrows point to glomerulus hypertrophy.
Effect of EACJ on lipid metabolism
The serum contents of TC, TG, HDL-C, LDL-C, CREA (Scr), and BUN of the EACJ-treated mice were clearly lower when compared with those values in the model control group. Remarkably, the abnormal changes in the normal mice were lower than those changes in the STZ-diabetic mice (Figures 4, 5).
Figure 4.

Effect of EACJ on serum contents of TC, TG, LDL-C, and HDL-C. Normal control: administered distilled water; Model control: administered distilled water; Metformin: 320 mg/kg of metformin; High dose: 100 g/kg of EACJ; Middle dose: 50 g/kg of EACJ; Low dose: 25 g/kg of EACJ. The results are presented as the means ± SE for 10 animals in each group. aP < 0.01 compared with the normal control group; bP < 0.05 compared with the model group.
Figure 5.

Effect of EACJ on serum contents of BUN and Scr. Normal control: administered distilled water; Model control: administered distilled water; Metformin: 320 mg/kg of metformin; High dose: 100 g/kg of EACJ; Middle dose: 50 g/kg of EACJ; Low dose: 25 g/kg of EACJ. The results are presented as the means ± SE for 10 animals in each group. aP < 0.01 compared with the normal control group; bP < 0.01, cP < 0.05 compared with the model group.
Lipid histology assay
The antioxidant capacity was decreased in the diabetic mice, as there was significant reduction in MDA and increase in SOD in all values of the EACJ-treated groups. The contents of SDH significantly increased, while the contents of cAMP significantly decreased in the EACJ-treated groups, so EACJ could be used to protect liver tissues against oxidative damage in DN (Figure 6).
Figure 6.
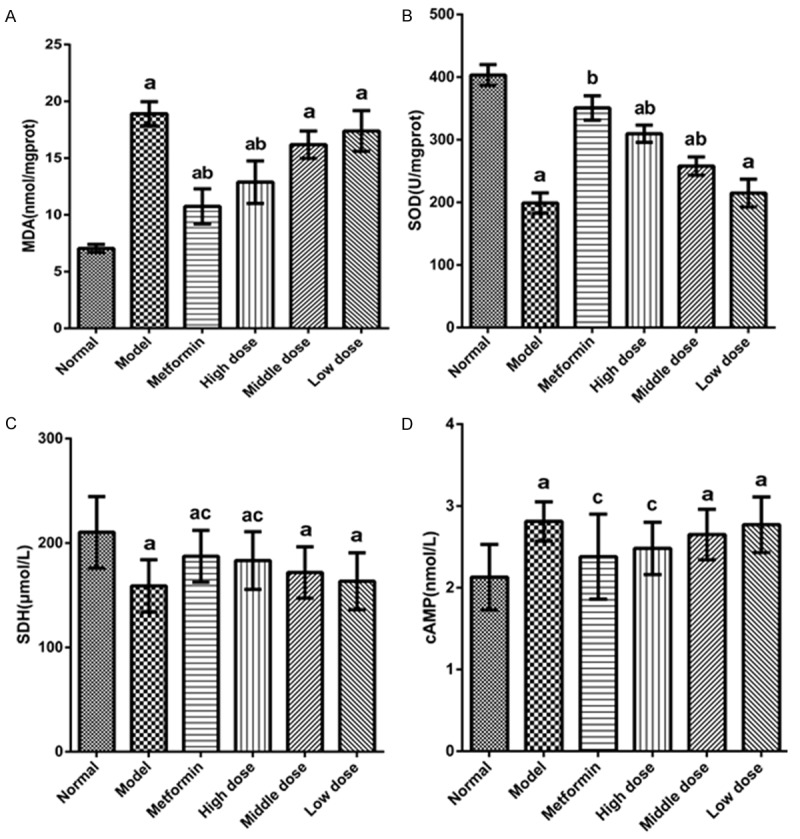
Effect of EACJ treatment on oxidant/antioxidant enzyme activities in liver cortex. A: MDA; B: SOD; C: SHD; D: cAMP. Normal control: administered distilled water; Model control: administered distilled water; Metformin: 320 mg/kg of metformin; High dose: 100 g/kg of EACJ; Middle dose: 50 g/kg of EACJ; Low dose: 25 g/kg of EACJ. The results are presented as the means ± SE for 10 animals in each group. aP < 0.01 compared with the normal control group; bP < 0.01, cP < 0.05 compared with the model group.
Immunohistochemistry
The immunohistochemistry assay results showed that the cytoplasm and nuclei were dyed yellow and brown as the positive expression of CTGF protein and TGF-β1 protein. Compared with the blank control group, the model control group’s positive expression range increased. The high-dose EACJ groups were compared with the model control group for the positive expression range decrease, and in the moderate dose and low-dose EACJ groups they were not decreased obviously. That high-dose EACJ could protect mice kidneys (Figures 7, 8).
Figure 7.
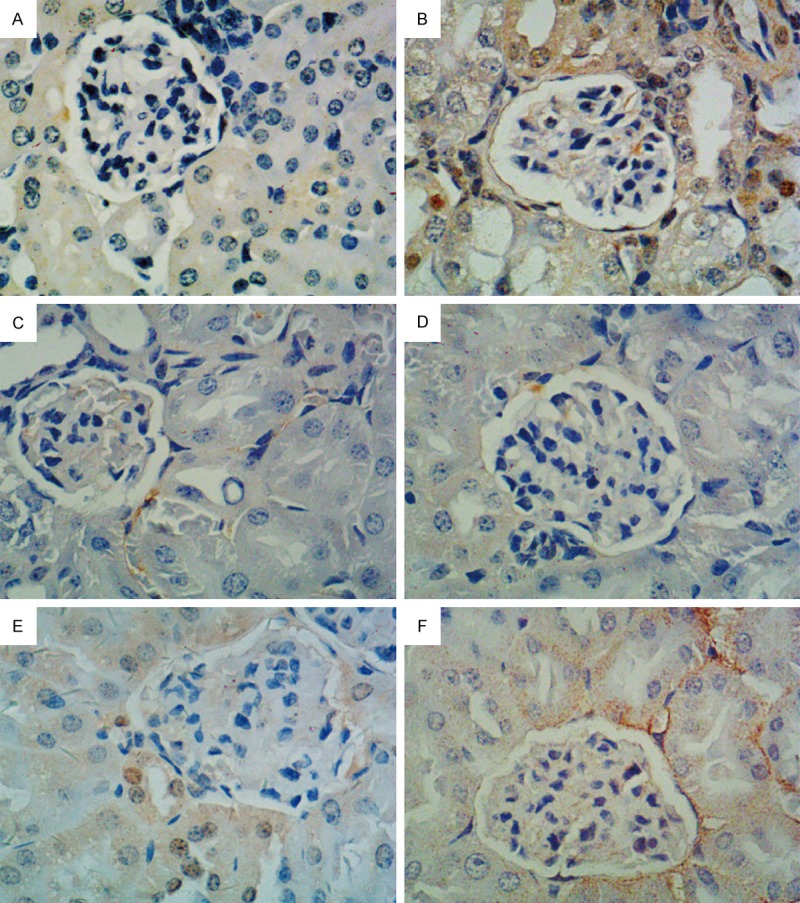
Effect of EACJ on the expression of CTGF in mouse kidney tissue (immunohistochemical analysis, scale bar: 400 μm). A: Normal control: administered distilled water; B: Model control: administered distilled water; C: Metformin: 320 mg/kg of metformin; D: High dose: 100 g/kg of EACJ; E: Middle dose: 50 g/kg of EACJ; F: Low dose: 25 g/kg of EACJ. Note: The arrows represent the glomerulus-included immunity-positive CTGF protein (brown).
Figure 8.
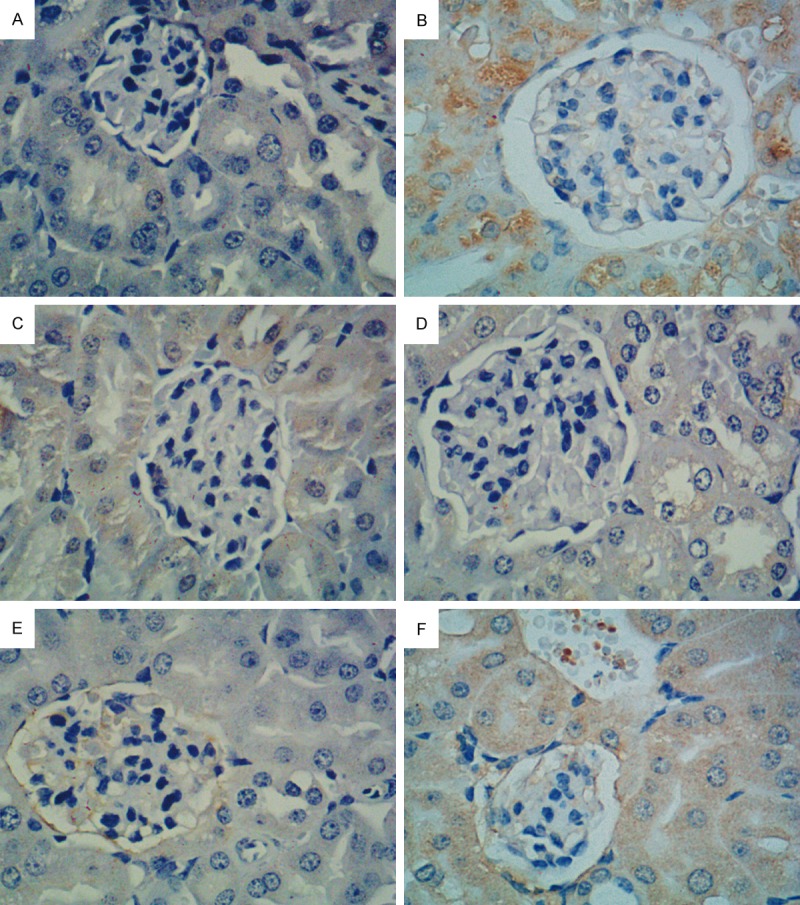
Effect of EACJ on the expression of TGF-β1 in mouse kidney tissue (Immunohistochemical analysis, scale bar: 400 μm). A: Normal control: administered distilled water; B: Model control: administered distilled water; C: Metformin: 320 mg/kg of metformin; D: High dose: 100 g/kg of EACJ; E: Middle dose: 50 g/kg of EACJ; F: Low dose: 25 g/kg of EACJ. Note: The arrows represent the glomerulus-included immunity-positive TGF-β1 protein (brown).
Real-time PCR
The CTGF and TGF-β1 gene relative expression results are as follows: Compared with the blank control group, the model control group CTGF and TGF-β1 gene expression quantity increased (P < 0.01). Compared with the model control group, the high-dose EACJ groups of CTGF and TGF-β1 gene expression quantity reduced, whereas the mRNA levels in the EACJ-treated healthy mice were not significantly different from the normal controls (Figure 9).
Figure 9.
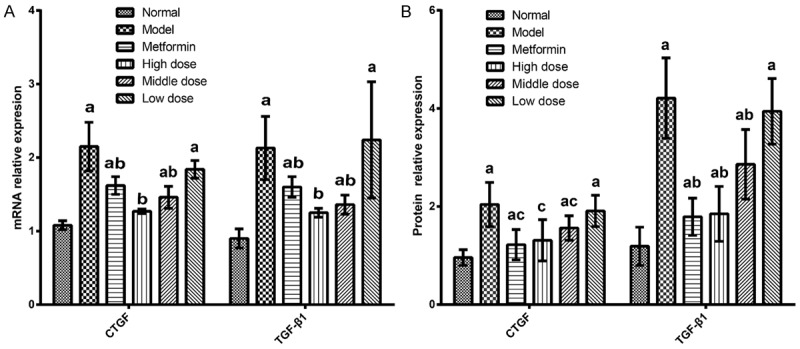
Effect of EACJ on the expressions of CTGF and TGF-β1 mRNA in mouse kidney tissue (n = 10). Normal control: administered distilled water; Model control: administered distilled water; Metformin: 320 mg/kg of metformin; High dose: 100 g/kg of EACJ; Middle dose: 50 g/kg of EACJ; Low dose: 25 g/kg of EACJ. The results were presented as the means ± S E. aP < 0.01 compared with the normal control group; bP <0.01, cP < 0.05 compared with the model group.
Discussion
For the treatment of diabetes as well as the prevention and treatment of diabetic complications caused by various drugs, the most critical in pharmacological research is to establish a high stability, reliability, and animal model of good suitability. A century ago, Minkowski and Vonmehring resected a dog’s pancreas, thus creating a diabetic animal model. The experimental basis is a continuous process and development; scientists have made a variety of attempts. So far, there have been many types of methods that can be used for the establishment of the diabetic animal model. The following four types of models are widely used at present: (1) the pancreatectomy, (2) induced by chemical drugs, (3) spontaneous diabetes model, and (4) transgenic animals [31-33]. This experiment is induced by chemical drugs, such as STZ. STZ is a broad-spectrum antibiotic that selectively destroys certain properties of animal, induces diabetes in many animals, relatively lowers tissue toxicity, increases animal survival rate, and can be used as a method for preparing a diabetes animal model [34-37]. This experiment used STZ through fasting injection to induce diabetes in mice for scientific research [38].
In this experiment, we found that the extracts from the fruits of Averrhoa carambola L. (Oxalidaceae) clearly decreased the level of FBG and weight gain in the diabetic mice after 21 days of treatment. In addition, the physical and chemical test results show that the serum of TC, TG, HDL-C, LDL-C (Figure 4), CREA (Scr), and BUN (Figure 5) of the EACJ-treated (25 g/kg, 50 g/kg, and 100 g/kg body weight/d) groups were lower than those concentrations in the STZ-diabetic mice. These beneficial phenomenon illustrated that the EACJ-mediated hypoglycemic function was related to attenuating lipotoxicity or to reducing lipogenesis. Clinically, metformin, acting as one of the antidiabetic drugs, has been shown to effectively guard against diabetes and related complications. The pharmacological action of metformin is primarily involved in enhancing insulin sensitivity and increasing peripheral glucose uptake, thereby reducing hyperglycemia and metabolic disturbance [39]. Therefore, this study indicated that for diabetes patients, metformin treatment and an EACJ complement can contribute to diabetes management and can output additional benefits through combined administration.
More and more studies have demonstrated that oxidative stress is a key pathogenic factor in the development of diabetic complications, including nephropathy [40,41]. In this study, in the experimental group EACJ in different doses and tissue MDA values were lower, but the SOD activity in the high-dose group was improved. Therefore, the experiment proved that EACJ on the SOD activity has an enhancing effect and strengthens the anti-oxidation ability in the mice, reducing the in vivo accumulation of free radicals, preventing damage to the body, and improving the function of the diabetic mice [42]. SDH is a sensitive index to detect the degree of mitochondrial damage. The proof that EACJ is a high-dose group could significantly increase the activity of SDH in liver mitochondria, indicating the high-dose group of EACJ to improve one of the pathways of glucose metabolism in diabetic mice is by improving the tricarboxylic acid cycle activity of SDH, oxidation accelerated succinic acid, enhanced the tricarboxylic acid cycle operation, thereby increasing the sugar utilization. An important substance that regulates the metabolism and biological function of a substance is cAMP. It is the second messenger of life information transmission, regulating the physiological activities and metabolism of cells. Metabolism plays an important role in the metabolism of sugar. The experimental results show that the EACJ high-dose group can significantly reduce the cAMP level in the liver tissue of mice and plays a certain role in the glucose-lowering effect, but the specific mechanism still needs further research.
At the early stage of DN, mainly in glomerular hypertrophy as the main performance, the gradual emergence of an extracellular matrix increased, the latter mainly to glomerular sclerosis, renal interstitial fibrosis particularly, resulting in nephron damage, decrease in the effective filtration rate, and finally by the kidney function not complete transition to renal failure. TGF-β1 and CTGF play an important role in the occurrence and progression of DN. The results of this study were similar to those reported by Wolf, and the expression of CTGF and TGF-β1 was increased in the model group compared with the normal group. CTGF can regulate its downstream gene TGF-β1, CTGF upregulation-induced TGF-β1 high expression, and mediate renal injury [43]. In addition, there is a study of CTGF to regulating TGF-β1-induced renal cell damage, it can also directly regulate other genes, leading to hardening and causing renal fibrosis and sclerosis [44]. Therefore, blocking TGF-β1, CTGF mediated renal injury can play a role in protecting the kidneys. The experimental results show that with the model group compared, and high-dose EACJ can down regulate the expression of CTGF and TGF-β1 mRNA and protein (P < 0.05), so in the case of EACJ on diabetic mouse model of early DN with renal protective effect, the protective effect might be related to the downregulation of CTGF and TGF-β1 mRNA and protein expression, but the specific molecular regulation mechanism and the target pathway need further study.
Acknowledgements
This research is supported by the National Natural Science Foundation of China (No. 81160533, 81360129, 81460205); the Guangxi Natural Science Foundation (No. 2012GXNSFAA053106); the Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (Guangxi Normal University), the Ministry of Education of China (No. CMEMR2014-B01); the Guangxi Key Laboratory for Metabolic Disease Research (No. 2012-181h-02); Nanning Science and Technology Research and Production of New Products (No. 201102084C); and the Scientific Research and Technology Development Plan of Qingxiu District, Nanning (2013S12).
Disclosure of conflict of interest
None.
References
- 1.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004;17:31S–38S. doi: 10.1016/j.amjhyper.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 3.LeRoith D, Rayfield EJ. The benefits of tight glycemic control in type 2 diabetes mellitus. Clin Cornerstone. 2007;8(Suppl 7):S19–29. doi: 10.1016/s1098-3597(07)80018-4. [DOI] [PubMed] [Google Scholar]
- 4.Saravanan G, Ponmurugan P. Ameliorative potential of S-allylcysteine: effect on lipid profile and changes in tissue fatty acid composition in experimental diabetes. Exp Toxicol Pathol. 2012;64:639–644. doi: 10.1016/j.etp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Steve O, Joy O, Veronica E. Evaluation of hypoglycaemic and hypolipidaemic effects of aqueous ethanolic extracts of Treculia africana Decne and Bryophyllum pinnatum Lam. Afr J Biotechnol. 2008;7:2535–2539. [Google Scholar]
- 6.Zhang J, Meng G, Zhang C, Lin L, Xu N, Liu M, Cui F, Jia L. The antioxidative effects of acidic-, alkalic-, and enzymatic-extractable mycelium zinc polysaccharides by on Pleurotus djamor liver and kidney of streptozocin-induced diabetic mice. BMC Complement Altern Med. 2015;15:440. doi: 10.1186/s12906-015-0964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian S, Chait A. Hypertriglyceridemia secondary to obesity and diabetes. BBA-Mol Cell Biol L. 2012;1821:819–825. doi: 10.1016/j.bbalip.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing L, Huang QF, Zhang SJ, Ming L, Huang RB. Influence of water extract from helicteres angustifolia on lipid peroxidation induced by carbon tetrachlorile in mice. Chinese Journal of Experimental Traditional Medical Formulae. 2010 [Google Scholar]
- 10.Lamb J, Schlis K, Agarwal J, Stam RW. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Qu XA, Rajpal DK. Applications of Connectivity Map in drug discovery and development. Drug Discov Today. 2012;17:1289–1298. doi: 10.1016/j.drudis.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 13.Holmes RS, Duley JA, Hilgers J. Sorbitol dehydrogenase genetics in the mouse: a ‘null’ mutant in a ‘European’ C57BL strain. Anim Blood Groups Biochem Genet. 1982;13:263–272. doi: 10.1111/j.1365-2052.1982.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 14.Ezhumalai M, Ashokkumar N, Pugalendi KV. Combination of carvacrol and rosiglitazone ameliorates high fat diet induced changes in lipids and inflammatory markers in C57BL/6J mice. Biochimie. 2015;110:129–136. doi: 10.1016/j.biochi.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Ott KM. Unraveling the role of connective tissue growth factor in diabetic nephropathy. Kidney Int. 2008;73:375–376. doi: 10.1038/sj.ki.5002661. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TQ, Tarnow L, Andersen S, Hovind P, Parving HH, Goldschmeding R, van Nieuwenhoven FA. Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:83–88. doi: 10.2337/diacare.29.1.83. [DOI] [PubMed] [Google Scholar]
- 17.Pantsulaia T. Role of TGF-beta in pathogenesis of diabetic nephropathy. Georgian Med News. 2006;131:13–18. [PubMed] [Google Scholar]
- 18.Di CN, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, Picard JY, Whitty A, Josso N, Pepinsky RB, Cate RL. Processing of anti-mullerian hormone regulates receptor activation by a mechanism distinct from TGF-beta. Mol Endocrinol. 2010;24:2193–2206. doi: 10.1210/me.2010-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo LM, Del RE, Brown D, Lin HY. Evidence for a role of transforming growth factor (TGF)-beta1 in the induction of postglomerular albuminuria in diabetic nephropathy: amelioration by soluble TGF-beta type II receptor. Diabetes. 2007;56:380–388. doi: 10.2337/db06-1018. [DOI] [PubMed] [Google Scholar]
- 20.Tahara A, Tsukada J, Tomura Y, Yatsu T, Shibasaki M. Vasopressin increases type IV collagen production through the induction of transforming growth factor-beta secretion in rat mesangial cells. Pharmacol Res. 2008;57:142–150. doi: 10.1016/j.phrs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Abeysekera RA, Wijetunge S, Nanayakkara N, Wazil AW, Ratnatunga NV, Jayalath T, Medagama A. Star fruit toxicity: a cause of both acute kidney injury and chronic kidney disease: a report of two cases. BMC Res Notes. 2015;8:1–4. doi: 10.1186/s13104-015-1640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:1339–1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita H, Nagai Y, Takamura T, Nohara E, Kobayashi K. Thiazolidinedione derivatives ameliorate albuminuria in streptozotocin-induced diabetic spontaneous hypertensive rat. Metabolism. 2002;51:403–408. doi: 10.1053/meta.2002.30953. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2009;2008:704045–704045. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Candido R, Jandeleit-Dahm KA, Cao Z, Nesteroff SP, Burns WC, Twigg SM, Dilley RJ, Cooper ME, Allen TJ. Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation. 2002;106:246–253. doi: 10.1161/01.cir.0000021122.63813.32. [DOI] [PubMed] [Google Scholar]
- 26.Wen Q, Liang T, Qin F, Wei J, He Q, Luo X, Chen X, Zheng N, Huang R. Lyoniresinol 3α-O-β-D-glucopyranoside-mediated hypoglycaemia and its influence on apoptosis-regulatory protein expression in the injured kidneys of streptozotocin-induced mice. PLoS One. 2013;8:e81772–e81772. doi: 10.1371/journal.pone.0081772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borghetti G, Yamazaki RK, Coelho I, Pequito DC, Schiessel DL, Kryczyk M, Mamus R, Naliwaiko K, Fernandes LC. Tumor growth reduction is regulated at the gene level in Walker 256 tumor-bearing rats supplemented with fish oil rich in EPA and DHA. Braz J Med Bio Res. 2013;46:696–699. doi: 10.1590/1414-431X20132970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YY, Lin YC, Hung HC, Tien WY, Shieh TY. Polymorphisms in Kallikrein7 and 10 genes and oral cancer risks in Taiwan betel quid chewers and smokers. Oral Dis. 2013;19:824–832. doi: 10.1111/odi.12072. [DOI] [PubMed] [Google Scholar]
- 29.Costamatos L, Batista P, Monteiro N, Simões M, Egas C, Pereira J, Pinho H, Santos N, Ribeiro J, Cipriano MA. Liver hepcidin mRNA expression is inappropriately low in alcoholic patients compared with healthy controls. Eur J Gastroenterol Hepatol. 2012;24:1158–1165. doi: 10.1097/MEG.0b013e328355cfd0. [DOI] [PubMed] [Google Scholar]
- 30.Zhang N, Gao Y, Zou D, Wang J, Li J, Zhou S, Zhu Z, Zhao X, Xu L, Zhang H. Effects of Chinese medicine Tong xinluo on diabetic nephropathy via inhibiting TGF-β1-induced epithelial-to-mesenchymal transition. Evid Based Complement Alternat Med. 2014;2014:123497–123497. doi: 10.1155/2014/123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, José PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 32.Mauvaisjarvis F, Kahn CR. Understanding the pathogenesis and treatment of insulin resistance and type 2 diabetes mellitus: what can we learn from transgenic and knockout mice? Diabetes Metab. 2000;26:433–448. [PubMed] [Google Scholar]
- 33.Terauchi Y, Iwamoto K, Tamemoto H, Komeda K, Ishii C, Kanazawa Y, Asanuma N, Aizawa T, Akanuma Y, Yasuda K. Development of non-insulin-dependent diabetes mellitus in the double knockout mice with disruption of insulin receptor substrate-1 and β-cell glucokinase genes. Genetic reconstruction of diabetes as a polygenic disease. J Clin Invest. 1997;99:861–866. doi: 10.1172/JCI119250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dufrane D, Van SM, Guiot Y, Goebbels RM, Saliez A, Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation. 2006;81:36–45. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 35.Gajdosík A, Gajdosíková A, Stefek M, Navarová J, Hozová R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999 18 spec no: 54-62. [PubMed] [Google Scholar]
- 36.Harlan DM, Barnett MA, Abe R, Pechhold K, Patterson NB, Gray GS, June CH. Very-low-dose streptozotocin induces diabetes in insulin promoter-mB7-1 transgenic mice. Diabetes. 1995;44:816–823. doi: 10.2337/diab.44.7.816. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi S, Ohba Y, Oka T. Involvement of epidermal growth factor deficiency in pathogenesis of oligozoospermia in streptozotocin-induced diabetic mice. Endocrinology. 1990;127:2136–2140. doi: 10.1210/endo-127-5-2136. [DOI] [PubMed] [Google Scholar]
- 38.Daneshgari F, Leiter EH, Liu G, Reeder J. Animal models of diabetic uropathy. J Urology. 2009;182:S8–13. doi: 10.1016/j.juro.2009.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruckbauer A, Zemel MB. Synergistic effects of metformin, resveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes Metab Syndr Obes. 2013;6:93–102. doi: 10.2147/DMSO.S40840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010;40:742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 41.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. The effect of verapamil on the antioxidant defence system in diabetic kidney. Clin Chim Acta. 2002;322:105–112. doi: 10.1016/s0009-8981(02)00167-5. [DOI] [PubMed] [Google Scholar]
- 43.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med. 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 44.Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallasbonke V, Thomas MC, Cooper ME, Kantharidis P. Connective Tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol. 2006;17:2484–2494. doi: 10.1681/ASN.2006050525. [DOI] [PubMed] [Google Scholar]


