Abstract
Candida albicans is an important opportunistic fungus causing both disseminated and local infections. The discovery of neutrophil extracellular traps (NETs) has presented a new strategy to kill microorganisms in host’s innate immune response. Although it has been reported that NETs can trap and kill both yeast and hyphal forms of C. albicans, the mechanism by which C. albicans escape from NETs has not been fully understood. In this study, the ability of two strains of C. albicans SC5314 and 3683 to escape NETs-mediated killing was compared. It was found that SC5314 induced higher levels of reactive oxygen species (ROS) and expressions of Rac1/2 and more NETs formation by neutrophils, and also generated more deoxyribonucleases (DNase) than 3683 did. However, resistance to neutrophils killing was greater in SC5314 than that of 3683. When extracellular traps were degraded by exogenous DNase I or catalase, and neutrophil phagocytic activity blocked by cytochalasin D, the killing capacity of neutrophils co-cultured with either C. albicans SC5314 or 3683 was significantly decreased. This study indicates that C. albicans can escape from the trapping and killing of NETs by secreting DNase, which offers further insights into the basis for differences in virulence of different strains of C. albicans.
Keywords: Candida albicans, deoxyribonucleases, neutrophil extracellular traps
Introduction
Candida albicans which can cause systemic fungal infections in humans is the predominant opportunistic yeast in oral cavity. Immunocompromised patients are particularly susceptible to the infection, although it can be detected in interdigital skin, oral cavity, and on the intestinal and vagina mucosa among healthy people. A wide usage of antibiotics and immunosuppressants, as well as the prevalence of HIV infection, has resulted in a steady rise in prevalence of both oral and systemic candidiasis [1-3].
The innate immune system is the first defense line against invasion of microorganisms, in which neutrophils is an essential component playing a significant role in host’s defense against candidiasis [4-7]. It is generally accepted that neutrophils eliminate invading microbes via phagocytic uptake, secretion of antimicrobials, and release of neutrophil extracellular traps (NETs) [8]. NET is a complex extracellular matrix primarily composed of chromatin (histones and DNA), and includes granule proteins such as neutrophil elastase, myeloperoxidase and calprotectin, which are bound to the DNA [8,9].
Urban et al. [10] reported that C. albicans can induce neutrophils forming extracellular traps that capture and kill hyphal as well as yeast becoming fungus. However, C. albicans blastospores captured by NETs can be released, and recovered in vitro by incubation with DNase in cell culture medium [11]. Neutrophils can kill invading microorganisms by producing high concentrations of superoxide, and studies on neutrophils from patients with chronic granulomatous disease (CGD) have provided evidence that reactive oxygen species (ROS) is essential for formation of NETs [12,13].
Our previous studies on virulence of C. albicans in inbred mice demonstrated that strain SC5314 could develop a more severe tissue damage and fungal burdens in infected organs than 3683 after challenging with live yeasts [14], and C. albicans 3683 was killed more efficiently by murine neutrophils in vitro than strain SC5314 [15]. In this study, it was proposed that differences in virulence of these two strains of C. albicans partially resulted from their different ability to escape from trapping and killing by NETs. The expressions of Rac1/2 and ROS generation by yeasts were also explored since neutrophils from CGD patients displayed a defect in ROS formation, which resulted in an inability to produce NETs, and Rac isoforms which are the key regulators of ROS generation via NADPH oxidase system [16-18].
Materials and methods
Strains and incubation
Candida albicans SC5314 and 3683 were a gift from Dr. C.S. Farah in School of Dentistry, University of Queensland. C. albicans 3683 was isolated from the oral cavity of a patient with cutaneous candidiasis, and C. albicans SC5314 from a patient with disseminated candidiasis [19]. Yeasts were stored at -70°C in 15% (V/V) glycerol in Sabouraud’s broth (OXOID, UK). Yeasts were grown for 18 h at 37°C with continuous agitation, and then washed three times in PBS, and resuspended in RPMI 1640 (Gibco, USA). The study was approved by the Ethics Committee of Sun Yat-Sen University (ERC-2011-14).
Isolation of neutrophils
Human neutrophils were isolated from peripheral blood of healthy donors by using PolymorphprepTM as described previously [20]. Whole blood was collected into a tube containing anticoagulant (EDTA). And 5 ml of anticoagulant whole blood was carefully layered over 5 ml of PolymorphprepTM in a 15 ml centrifuge tube. The samples were centrifuged at 450 g for 35 min in a swing-out rotor at 20°C. After centrifugation, the lower band containing PMNs was harvested, diluted with PBS, and centrifuged at 400 g for 10 min. The cells were resuspended in RPMI 1640 containing 2% Fetal bovine serum.
Confocal immunofluorescence observe net-formation
Immunofluorescence assay was performed as described by Urban et al. [10]. Briefly, 13 mm glass coverslips were treated with 0.001% polylysine to allowed neutrophils settlement and added to 24 well plates in triplicate. 4×105 neutrophils and 8×104 C. albicans (cells: yeasts = 5:1) were resuspended in RPMI 1640 containing 2% FBS in 24 well plates. Sample plates were centrifuged for 3 min at 700 g and incubated at 37°C for 30, 60 and 120 min respectively. The negative control was also incubated for 30, 60 and 120 min in the absence of C. albicans. After incubation, the samples were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature, and washed three times with PBS. The samples were blocked overnight with 10% normal goat serum, 1% bovine serum albumin and 0.05% Tween-20 in PBS and incubated with primary antibodies which were directed against human neutrophil elastase (rabbit polyclonal, Santa Cruz, USA). Then the samples were washed three times with PBS, and the secondary antibodies specific for rabbit IgG (FITC-labeled Goat polyclonal, Abcam, USA) were added into the samples for 1 h incubation. After samples were washed three times with PBS again, DAPI was added to detect DNA. Finally, the samples were observed with a LSM780 confocal microscope (Zeiss, Germany).
Detection of ROS generation
DCFH-DA assay was used to detect ROS generation as described by Yu CL et al. [21]. Briefly, ROS was measured by using OxiSelect™ Intracellular ROS Assay Kit (Cell Biolabs, San Diego, CA) according to the manufacturer’s instruction. DCFH-DA readily diffuses through the cell membrane and is hydrolyzed by intracellular esterases to non-fluorescent 2’, 7’-dichlorofluorescin. In the presence of intracellular ROS, it is rapidly oxidized to highly fluorescent. The DCF fluorescence intensity is proportional to the amount of ROS formed intracellularly. Neutrophils were co-cultured with SC5314 or 3683 at a ratio of 10:1 in 96-well plate (black) for 5 min, 10 min, 30 min, and 60 min respectively. After incubation, the samples were centrifuged, washed with PBS, and then 100 µL of 1X DCFH-DA/media solution was added to cells for incubation at 37°C for 30 min. Each sample was assayed in triplicate. The excess of DCFH-DA was removed and washed with PBS. The DCF fluorescence intensity was detected by Microplate Reader (SpectraMax M5, USA) at an excitation wavelength of 480 nm and at an emission wavelength of 530 nm.
SDS-PAGE and immunoblotting for Rac1/2
Neutrophils were incubated with SC5314 or 3683 at a ratio of 10:1 (cells: yeasts) in 24-well plates at 37°C for 30 min and 2 h respectively. Samples were then centrifuged at 1000 rpm for 5 min and washed with PBS before added to a lysis buffer. Lysates were subjected to EP tubule to centrifuge at 14000 rpm for 15 min and separated by SDS-PAGE followed by immunoblotting according to manufacturer’s instructions (Bio-Rad Laboratories) as described previously [14]. Blots were probed with mouse monoclonal anti-Rac1 Ab (Abcam, UK), Rabbit polyclonal anti-Rac2 Ab (Abcam, UK) and detected by using an HRP-labeled secondary Ab (Southern Biotech, USA).
NET killing activity
Killing assays were performed as described in our previous study [22]. Briefly, 2×104 neutrophils and 2×102 C. albicans (SC5314 or 3683) (cells:yeasts = 100:1), 2×103 (10:1), 2×104 (1:1) were co-cultured in RPMI 1640 containing 2% FBS at 37°C in 5% CO2 for 2 h. After incubation, cells were lysed by hypotonic shock (1 ml distilled water was added into the samples for 10 min). Yeasts were then serially diluted, overlaid with YPD, and incubated 48 h at 37°C. CFU were counted and percentage of killing was calculated as following: [1-(CFU/ml experiment group)/(CFU/ml control group)]×100%.
Neutrophils with C. albicans were designed as seven groups: a group with exogenous DNase (100 U/ml) (Sigma, USA); a group with 100 μg/ml G-actin (Worthington, USA) to inhibit DNase activity; a group with exogenous DNase and 10 μg/ml cytochalasin D (sigma, USA) to inhibit both phagocytotic uptake and extracellular killing; a group with exogenous catalase (100 U/ml) (Worthington, USA); a group with 3-Amino-1,2,4-triazole (AT) (1 mM) (Calbiochem, Germany) to inhibit catalase activity; a group with exogenous catalase and cytochalasin D; and a group without neutrophils as an internal control to determine baseline fungal counts.
DNase activity assay
DNase activity assays were conducted by using exogenous DNA and agarose gel electrophoresis as described by Buchanan et al. [23]. Briefly, C. albicans SC5314 and 3683 were cultured overnight in Sabouraud’s broth respectively, and the supernatants were collected after centrifugation. 2.5 μl supernatant was mixed with 7.5 μl calf thymus DNA (1 μg/μl) (Sigma, USA) in 40 μl DNase (Sigma, USA) buffer (3 mM MgCl2, 3 mM CaCl2, 300 mM Tris; pH7.4) and incubated for 1 h. Samples were added in triplicate. To halt DNase activity, 12.5 μl of 0.33 M EDTA was added. After incubation, electrophoresis in 1% agarose gel (Sigma, USA) was used to visualize and compare relative DNA degradation by the two strains supernatant.
Detection of DNase concentration
Radial enzyme diffusion assays were used to detect DNase concentration as described by Macanovic et al. [24]. Briefly, 1.5×103 neutrophils and 1.5×103 C. albicans (SC5314 or 3683) (cells: yeasts = 1:1), 3.0×103 (1:2), 6×103 (1:4) were co-cultured in RPMI 1640 containing 2% at 37°C in 5% CO2 for 2 h and 4 h. After incubation, the samples were centrifuged, and supernatants were collected and stored at -80°C. For preparation of agarose plates, 7.91 ml of 0.05 M Ca/Mg buffer (0.05 M Tris-HCl with pH of 7.2 containing 0.05 M MgCl2, 0.025 M CaCl2, 0.015 M NaN3), 0.93 ml of calf thymus DNA (Sigma, USA) at 5 mg/ml, 0.04 ml of ethidium bromide at 10 mg/ml and 10 ml of melted 2% agarose (Sigma, USA) were poured to 8×8 cm plates. The 3 mm wells (3×4 wells/plate) were gently punched on the plates, and then filled with 10 µl of samples and incubated at 37°C overnight. At the same time, Deoxyribonuclease I (DNase I) (Sigma, USA) was also added into wells following standard at concentrations of 0.25, 0.5, 1, 2 ug/ml respectively. After that, 0.05 M EDTA was overlaid onto the plates (to stop the reaction) and the plates were photographed by a UV gel imaging system (FluorChem Q, ALPHA, USA). The diameters of dark circles of hydrolyzed DNA were analyzed with Image-Pro Plus 6.0.
Statistical analysis
Experiments were repeated three times on separate days and all quantitative data were analyzed by using one-way analysis of variance (ANOVA) and student’s t-test as implemented in GraphPad Prism Version5.01 (GraphPad Inc, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
Candida albicans induces NETs
Human neutrophils were stimulated with either C. albicans SC5314 or 3683 to observe the formation of NET. NETs were visualized by immunofluorescent staining of extracellular DNA and neutrophil elastase. NET formation was rarely observed in neutrophils with PBS, or in C. albicans-infected neutrophils at 30 min, 60 min (data not shown). Significant NET formation was only generated after 120 min of infection (Figure 1). In addition, more neutrophil-released NETs were reproducibly visualized after challenge with SC5314 when compared with 3683.
Figure 1.

Immunostaining of NETs-formation induced by C. albicans. The isolated human neutrophils were incubated with C. albicans SC5314 (B1-3) or 3683 (C1-3) or PBS (A1-3) at a ratio of 5:1 (cells: yeasts) for 120 min. Then the samples were fixed, and stained with antibodies against human neutrophil elastase (green) and DAPI to detect DNA (blue). At last, the samples were observed and photographed with confocal microscope. White arrows point to neutrophils, red arrows point to NETs.
ROS production by neutrophils
As the generation of ROS via the NADPH oxidase complex was found to be essential in the production of NETs [25], ROS production of neutrophils induced by the two strains of C. albicans was compared at different periods of incubation. The DCF fluorescence intensity of neutrophils co-cultured with SC5314 was significantly higher than 3683 at 5 min, 10 min, 30 min, which suggested that SC5314 induced more ROS than 3683. Besides, only minimal levels of intercellular ROS were detected in neutrophils after 60 min of culture. However, ROS was rarely detected in neutrophils (as negative control) during the whole incubation (Figure 2).
Figure 2.
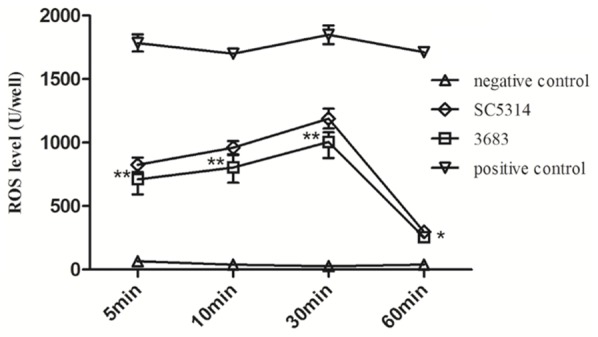
ROS generation by neutrophils co-cultured with C. albicans for 5 min, 10 min, 30 min and 60 min. After incubation, the sample were centrifuged and resuspended in DCFH-DA, and incubated at 37°C for 20 min. \Microplate Reader detected the DCF fluorescence. *P<0.05, **P<0.01.
Catalase, as one of the key enzymes found in C. albicans, leads to the breakdown of ROS, and is believed to affect the extracellular killing abilities of neutrophils [26]. When exogenous catalase was added to neutrophils incubated with SC5314 and 3683 for 2 h, the killing of both strains was significantly decreased (P<0.05). In the presence of AT, a catalase inhibitor, the killing capacity of the neutrophils was significantly enhanced (P<0.05). When neutrophil killing was blocked by cytochalasin D and ROS were simultaneously inhibited by catalase, resulting in NETs formation deficiency [27], a dramatic decrease of fungal killing of both stains of C. albicans was observed (Figure 3).
Figure 3.

The catalase effects on killing of yeast by neutrophils. Neutrophils were infected with C. albicans SC5314 or 3683 and incubated for 2 h, a group with exogenous catalase (100 U/ml) (Worthington, USA); a group with 3-Amino-1,2,4-triazole (AT) (1 mM) (Calbiochem, Germany) to inhibit catalase activity; a group with exogenous DNase and 10 μg/ml cytochalasin D (sigma, USA) to inhibit both phagocytotic uptake and extracellular killing; and a group without neutrophils as an internal control to determine baseline fungal counts. The percentage of neutrophils killing was [1-(CFU/ml experiment group)/(CFU/ml control group)]×100%. *P<0.05, **P<0.01.
Immunoblot analysis of Rac1 and Rac2
Rac1 and Rac2, which share over 90% identity in primary sequence, are critically important in regulating multiple signal transduction pathways. They have long been proven to be involved in NADPH oxidase activation [28], essential for the generation of NETs. The expression levels of Rac1 and Rac2 were compared in human neutrophils infected with SC5314 and 3683. After 30 min culture, the expression levels of both Rac1 and Rac2 in neutrophils infected by SC5314 were significantly higher than those induced by 3683 (Figure 4A, 4B). However, after 2 h, only Rac2 expression remained significantly higher in neutrophils infected by SC5314 than by 3683 (Figure 4C, 4D).
Figure 4.

Immunoblot analysis of Rac1 and Rac2 levels in human neutrophils induced by yeasts. After co-cultured with two strains of yeasts, the level of Rac1 and Rac2 expression by neutrophils at 30min (A and B) and 2 h (C and D) were detected. Lane 1, the uninfected controls were incubated in the absence of C. albicans; Lane 2, C. albicans SC5314 and human neutrophils were mixed and incubated; Lane 3 C. albicans 3683 and human neutrophils were mixed and incubated; Lane 4 uninfected neutrophils were stimulated by LPS as the positive control group. GAPDH was used as a loading control *P<0.05, **P<0.01.
Comparison of Candida killing by NETs
Firstly, the killing abilities of neutrophils were evaluated at 1 h and 2 h after co-cultured with C. albicans SC5314 and 3683 respectively. The higher killing percentages of 3683 by neutrophils were observed compared with those of SC5314 (Figure 5). The difference was most significant at a ratio of 100:1, after 2 h of culture. The data also demonstrated that the killing capacity of neutrophils increased with the ratio and duration of culture (Figure 5).
Figure 5.

The percentage of killing of yeasts by neutrophils. Neutrophils were infected with C. albicans SC5314 or 3683 and incubated for 1 h or 2 h. Yeasts were then diluted and overlaid with YPD, incubated 48 h at 37°C. The percentage of NETs killing was [1-(CFU/ml experiment group)/(CFU/ml control group)]×100%. *P<0.05, **P<0.01.
In order to evaluate the relative contributions of intracellular and extracellular killing, cytochalasin D was added to block neutrophils phagocytosis in the cultures. In contrast to our previous study [15], in which total killing by neutrophils was measured in the absence of cytochalasin D, the percentages of NETs killing were significant higher in the SC5314 group compared with 3683 group at 2 h incubation, whereas only at 100:1 (cells: yeasts), the killing of SC5314 was higher than that of 3683 at 1 h incubation (Figure 6) (P<0.05).
Figure 6.

The percentage of killing of yeasts by NETs. Neutrophils were infected with C. albicans SC5314 or 3683 and incubated for 1 h or 2 h respectively. In NETs killing assays, cytochalasin D was added to inhibit phagocytotic uptake. Yeasts were then diluted and overlaid with YPD, incubated 48 h at 37°C. The percentage of NETs killing was [1-(CFU/ml experiment group)/(CFU/ml control group)]×100%. *P<0.05, **P<0.01.
DNase production by C. albicans
DNase, which can damage DNA structure of NETs [8], has been found to help many microorganisms escape from NETs [23,24]. To study the role of DNase in the escape of C. albicans from trapping and killing by neutrophils, supernatants of the yeasts culture were collected for the detection of DNase activity. High DNase activity of both SC5314 and 3683 were observed. More importantly, when the radial enzyme diffusion assay was used to detect the DNase concentrations of supernatants from two strains of C. albicans co-cultured with neutrophils (cell: yeast = 1:1, 1:2, 1:4) for 2 h, 4 h, it was found that SC5314 significantly generated more DNase than 3683 (Figure 7).
Figure 7.

The concentrations of DNase of SC5314 and 3683 after co-cultured with neutrophils. Radial enzyme diffusion assays was used to detect the DNase concentration. After neutrophils co-cultured with C. albicans at ratio of 1:1, 1:2, 1:4 for 2 h, 4 h, supernatant were collected and added 10 ul/well to DNA agarose gel, and incubated overnight at 37°C. The second line was standard DNase I at 0.25, 0.5, 1 ug/ml (from left to light). The diameter of dark circles of hydrolyzed DNA was analyzed (A: 2 h, B: 4 h) according to making DNase I standard wave (X: DNase I concentration (ng/ml), Y: the diameter of dark circles). *P<0.05, **P<0.01.
Effect of DNase on neutrophils killing of C. albicans
When exogenous DNase (100 U/ml) was added into co-cultures of neutrophils with two strains of yeasts for 1 h, there was little difference in killing between control groups and experimental groups (Figure 8). This implied that DNase did not facilitate the escape of C. albicans in the early phase of fungicidal activity, since there was no NETs formation occurring at the moment (Figure 1).
Figure 8.

The percentages of killing neutrophils to yeasts with addition of DNase for 1 h. Neutrophils were co-cultured with DNase, infected with C. albicans SC5314 (A) or 3683 (B), and incubated for 1h. Controls were without DNase. Yeasts were then diluted and overlaid with YPD, incubated 48 h at 37°C. The percentage of neutrophils killing was [1-(CFU/ml experiment group)/(CFU/ml control group)]×100%.
To determine whether there was a later involvement of DNase in escape from NET-mediated killing, the fungicidal activity of neutrophils was measured after being co-cultured with SC5314 or 3683 in the presence of exogenous DNase for 2 h. This resulted in decreased killings of both strains. As expected, the samples in the presence of G-actin, a DNase inhibitor, showed significantly increased killing by the neutrophils. When neutrophils killing was blocked by cytochalasin D and extracellular traps degraded by DNase, fungal killing was decreased significantly in both stains of C. albicans, particularly SC5314 (Figure 9). It is indicated that the alteration in DNase activities had a greater impact on the killing of SC5314 than on 3683.
Figure 9.

The effects of DNase on killing of yeasts by neutrophils. Neutrophils were infected with C. albicans SC5314 (A) or 3683 (B) then incubated for 2 h, the percentage of killing of C. albicans SC5314 or 3683 by neutrophils was decreased after addition of external DNase, however, the killing capacity of neutrophils was increased after addition of G-actin, a DNase inhibitor. When neutrophil killing was blocked with cytochalasin D and simultaneously, the NETs were degraded by DNase, killing of both strains of C. albicans decreased significantly.*P<0.05, **P<0.01.
Discussion
NETs are anti-microbial structures released by activated neutrophils, and have opened a new path for the research on mechanisms of innate immunity. Nevertheless, different microorganisms and strains show considerable variations in resistance to NETs [29], and such mechanisms are poorly understood.
Our previous study demonstrated that the neutrophils from inbred mice could kill C. albicans 3683 more efficiently than SC5314 [15], which partially explained why C. albicans SC5314 was more virulent than strain 3683, and higher fungal burdens were established in the brain and kidney of susceptible (CBA/CaH) and resistant (BALB/c) mice after intravenous challenge [14]. The present work also demonstrated that human neutrophils could kill more strain 3683 than SC5314 in vitro (Figure 5), which again confirmed that C. albicans SC5314 was more virulent to host when compared with 3683. Taken together, how C. albicans such as SC5314 resist in antifungal activity of neutrophils becomes essential in understanding the interactions between yeasts and hosts.
There is no doubt that NETs can trap or/and kill many kinds of microorganisms including hyphal and yeast form fungus either through myeloperoxidase or calprotectin [8-10,30]. In this study, it was found that SC5314 could induce human neutrophils more formation of NETs than 3683 (Figure 1), and that the killing of SC5314 by NETs was significantly more efficient than that of 3683 (Figure 6), implying that the elimination of SC5314 by neutrophils relied on NETs more than the 3683.
The generation of ROS, mainly through the enzymatic reactions mediated by NADPH oxidase and myeloperoxidase (MPO), has been proven to be essential in the release of NETs [8]. Patients with chronic granulomatous disease (CGD), who cannot produce ROS, fail to generate NETs upon exposure to PMA [12], and it is the same for MPO-deficient patients [31]. In addition, the assessment on the ROS production of neutrophils treated with specific inhibitors also confirmed the function of NADPH oxidase and MPO in NET release [32]. In this study, neutrophils co-cultured with C. albicans SC5314 after 5 min, 10 min and 30 min generated more ROS than those with C. albicans 3683 (Figure 2). The results were consistent with the confocal immunofluorescence assay that SC5314-induced NETs exceed 3683-induced NETs (Figure 1), which implied the crucial role of ROS in determining the release of NETs. According to studies on catalase-deficient C. albicans hyphae that showed enhanced susceptibility to damage by human neutrophils [33], it was hypothesized that as an important antioxidant abundant in C. albicans, catalase might be responsible for a diverse virulence of C. albicans by degrading ROS to reduce NETs release in this study. The killing assays then demonstrated a decreased percentage of killing of neutrophils treated with exogenous catalase and an increased killing capacity of neutrophils treated with AT (a catalase inhibitor). Moreover, when phagocytotic uptake of neutrophils was blocked by cytochalasin D, the addition of catalase can significantly decrease the fungal killing of both stains of C. albicans (Figure 3). However, unlike the destructive case where neutrophil killing and extracellular traps was blocked by cytochalasin D and DNase, there still remained some killing activity at this time, especially in neutrophils infected with C. albicans SC5314. This result might be due to NETs formation rescued by compromised source of ROS such as the function of mitochondrial electron transport chain.
Rac1 and Rac2, Rho family GTPase members that used to be recognized as important constituents of the NADPH oxidase complex, have been proved essential in the formation of NETs [34]. In this study, we found that neutrophils infected with SC5314 expressed higher Rac1 and Rac2 than those infected with 3683 (Figure 4), which was consistent with our above results that SC5314 stimulated neutrophils producing more ROS and NETs formation. Moreover, the more distinct Rac2 expression of SC5314-infected neutrophils also confirmed the previous studies on Rac1null and Rac2null mice which suggested Rac2 was the major isoform involved in NETs formation [34]. Previous reports provided evidence that Rac2 was a critical regulator that activated the neutrophil NADPH oxidase through specific signaling pathways involving not only ERK2/1 (p42/44) MAP kinase but also JNK and p38 MAP kinase [35,36]. Nevertheless, the specific mechanism of NETs formation still remains unclear so far, which will lead us to put the efforts on studies of the interactions between neutrophils with C. albicans, particularly pathogen recognition receptors (PRRs) and pathogen-associated molecular patterns (PAMPs) such as enolase [22].
DNase activity plays an important role in promoting resistance of microorganisms to neutrophil killing, because of its potential to damage the DNA framework of NETs [8]. DNase expressed by Streptococcus, Pneumococcus and Staphylococcus was demonstrated to be both necessary and sufficient to promote neutrophil resistance and increase virulence, by promoting degradation of NETs [23,24]. It was reported that DNase could facilitate microbial evasion of neutrophil-derived NETs, not only by direct degradation of DNA structure, but also by attenuating neutrophil ROS production [37]. Another study on neutrophils infected with C. albicans showed weakened NETs-associated killing after the treatment with DNase I [10]. Moreover, C. albicans blastospores captured by NETs could be released, and recovered by incubation in cell medium with DNase [11]. Therefore, there was precedent for consideration that DNase had a key role in the escape from NETs of the isolates of C. albicans used in this study.
In this study, the existence of C. albicans-derived DNase was first confirmed by demonstrating the high DNA degradation ability of supernatants from cultures of C. albicans SC5314 and 3683. Furthermore, SC5314 could generate more DNase than 3683 after being stimulated with neutrophils (Figure 7). Then we found that the addition of exogenous DNase into co-cultures of neutrophils with two strains of yeasts for 1 h did not reduce the killing of yeasts (Figure 8), indicating that DNase rarely facilitated the evasion of C. albicans at early phase of the fungicidal activities or DNase only functioned after the formation of NETs. However, the killing of yeasts was expectedly reduced when the neutrophils was incubated with DNase for 2 h (Figure 9), and significant increase in killing effect of G-actin (a DNase inhibitor) on neutrophils was also found, which confirmed the impairment of DNase on NETs-derived neutrophil killing. Especially, when the phagocytotic uptake of neutrophils was blocked by cytochalasin D and the NET killing was inhibited with the addition of exogenous DNase simultaneously, the neutrophil defense was almost completely abrogated (Figure 9).
In conclusion, this present study demonstrates that the distinct virulence between C. albicans SC5314 and 3683 is associated with their different ability to escape from the trapping and killing of NETs, which might be attributed to the secretion of DNase by two strains of yeasts.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China, NSFC (81170969).
Disclosure of conflict of interest
None.
References
- 1.Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA, Phelan M, Morgan J, Lee-Yang W, Ciblak MA, Benjamin LE, Sanza LT, Huie S, Yeo SF, Brandt ME, Warnock DW. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–1527. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thanyasrisung P, Kesakomol P, Pipattanagovit P, Youngnak-Piboonratanakit P, Pitiphat W, Matangkasombut O. Oral Candida carriage and immune status in Thai human immunodeficiency virus-infected individuals. J Med Microbiol. 2014;63:753–759. doi: 10.1099/jmm.0.069773-0. [DOI] [PubMed] [Google Scholar]
- 3.Li YY, Chen WY, Li X, Li HB, Li HQ, Wang L, He L, Yang XP, Wang XC, Huang YL, Yao YG. Asymptomatic oral yeast carriage and antifungal susceptibility profile of HIV-infected patients in Kunming, Yunnan Province of China. BMC Infect Dis. 2013;13:46. doi: 10.1186/1471-2334-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 5.Geiszt M, Kapus A, Ligeti E. Chronic granulomatous disease: more than the lack of superoxide? J Leukoc Biol. 2001;69:191–196. [PubMed] [Google Scholar]
- 6.Mayer-Scholl A, Averhoff P, Zychlinsky A. How do neutrophils and pathogens interact? Curr Opin Microbiol. 2004;7:62–66. doi: 10.1016/j.mib.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002;417:91–94. doi: 10.1038/417091a. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 9.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 11.Menegazzi R, Decleva E, Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood. 2012;119:1214–1216. doi: 10.1182/blood-2011-07-364604. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Farah CS, Ashman RB. Isolates of Candida albicans that differ in virulence for mice elicit strain-specific antibody-mediated protective responses. Microbes Infect. 2006;8:612–620. doi: 10.1016/j.micinf.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Farah CS, Ashman RB. Effector function of leucocytes from susceptible and resistant mice against distinct isolates of Candida albicans. Immunol Cell Biol. 2006;84:455–460. doi: 10.1111/j.1440-1711.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- 16.Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 18.Sun CX, Magalhaes MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol. 2007;179:239–245. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y. Isolation of human and mouse neutrophils ex vivo and in vitro. Methods Mol Biol. 2012;844:101–113. doi: 10.1007/978-1-61779-527-5_7. [DOI] [PubMed] [Google Scholar]
- 21.Yu CL, Zhao XM, Niu YC. Ferulic acid protects against lead acetate-induced inhibition of neurite outgrowth by upregulating HO-1 in PC12 cells: involvement of ERK1/2-Nrf2 pathway. Mol Neurobiol. 2015;53:6489–6500. doi: 10.1007/s12035-015-9555-x. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Hu X, Zhang X, Ge Y, Zhao S, Hu Y, Ashman RB. Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine. 2011;29:5526–5533. doi: 10.1016/j.vaccine.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 24.Macanovic M, Lachmann PJ. Measurement of deoxyribonuclease I (DNase) in the serum and urine of systemic lupus erythematosus (SLE)-prone NZB/NZW mice by a new radial enzyme diffusion assay. Clin Exp Immunol. 1997;108:220–226. doi: 10.1046/j.1365-2249.1997.3571249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 26.Seger RA. Modern management of chronic granulomatous disease. Br J Haematol. 2008;140:255–266. doi: 10.1111/j.1365-2141.2007.06880.x. [DOI] [PubMed] [Google Scholar]
- 27.Bekeschus S, Winterbourn CC, Kolata J, Masur K, Hasse S, Broker BM, Parker HA. Neutrophil extracellular trap formation is elicited in response to cold physical plasma. J Leukoc Biol. 2016;100:791–799. doi: 10.1189/jlb.3A0415-165RR. [DOI] [PubMed] [Google Scholar]
- 28.Dusi S, Donini M, Rossi F. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem J. 1996;314:409–412. doi: 10.1042/bj3140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mejia SP, Cano LE, Lopez JA, Hernandez O, Gonzalez A. Human neutrophils produce extracellular traps against Paracoccidioides brasiliensis. Microbiology. 2015;161:1008–1017. doi: 10.1099/mic.0.000059. [DOI] [PubMed] [Google Scholar]
- 31.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchner T, Moller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm. 2012;2012:849136. doi: 10.1155/2012/849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wysong DR, Christin L, Sugar AM, Robbins PW, Diamond RD. Cloning and sequencing of a Candida albicans catalase gene and effects of disruption of this gene. Infect Immun. 1998;66:1953–1961. doi: 10.1128/iai.66.5.1953-1961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim MB, Kuiper JW, Katchky A, Goldberg H, Glogauer M. Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol. 2011;90:771–776. doi: 10.1189/jlb.1010549. [DOI] [PubMed] [Google Scholar]
- 35.Kim C, Dinauer MC. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol. 2001;166:1223–1232. doi: 10.4049/jimmunol.166.2.1223. [DOI] [PubMed] [Google Scholar]
- 36.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 37.Munafo DB, Johnson JL, Brzezinska AA, Ellis BA, Wood MR, Catz SD. DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J Innate Immun. 2009;1:527–542. doi: 10.1159/000235860. [DOI] [PMC free article] [PubMed] [Google Scholar]


