Abstract
Background: Acute graft rejection mediated by alloreactive memory CD4+ T cells is a major obstacle to transplantation tolerance. It has been reported that CD8+ T regulatory cells (Tregs) have the ability to induce graft tolerance by restraining the function of activated CD4+ T cells, but not including memory T cells. The aim of this study is to elucidate the effect of CD8+ Tregs on alloreactive memory CD4+ T cells. Methods: We detected Qa-1 expression and performed proliferative assay on memory CD4+ T cells. All memory CD4+ T cells were purified from mice receiving skin allografts. We performed inhibitory and cytotoxic assays on CD8+ Tregs, which were isolated from a T cell vaccination mouse model, and IL-2, IL-4, IL-10 and IFN-γ levels were measured in co-culture supernatants by ELISA. To confirm CD8+ Tregs inhibition of memory CD4+ T cells in-vivo, we utilized a murine model of cardiac allograft transplantation. Results: Memory CD4+ T cells mediated acute allograft rejection, and CD8+ Tregs suppressed the proliferation of memory CD4+ T cells. In vitro, memory CD4+ T cells were inhibited and lysed by CD8+ Tregs. There was a positive correlation between IFN-γ levels, and cell lysis rate induced by CD8+ Tregs. In-vivo studies demonstrated CD8+ Tregs prolonged graft survival times, by inhibiting CD4+ memory T cells, through a Qa-1-peptide-TCR pathway. Conclusions: CD8+ Tregs inhibit CD4+ memory T cell-mediated acute murine cardiac allograft rejection, and further prolong graft survival times. These results provide new insights into immune regulation of organ rejection.
Keywords: Qa-1, T regulatory cells (Tregs), memory T cells, transplantation
Introduction
Organ transplantation is an effective treatment for end-stage organ failure [1-3]. However, almost all allograft patients require long-term continuous immunosuppressive to prevent graft rejection, making recipients susceptible to infection and more vulnerable to cancer [4-6]. Although major advances have been made in patient management, graft rejection remains a significant problem. T cells, including pre-existing memory T cells in the recipients, play a central role in allograft rejection [7-11]. Memory T cells consist of alloantigen specific clones that can be generated as a result of organ transplantation, blood transfusion or pregnancy [12-14], as well as cross-recognition [15]. Alloantigen specific memory T cells are promptly activated and proliferate into effector T cells more efficiently than naive T cells [16,17]. Thus, a better understanding of memory T cell-mediated rejection processes will be important for effective control of allograft rejection [14,16-18].
Clinical and experimental data indicate that current immunosuppressive treatments fail to inhibit transplant rejection mediated by memory CD4+ T cells [14,18-20]. Indeed, much research has focused on T regulatory cells (CD4+CD25+FOXP3+ Tregs) [21,22], which primarily target at naive T cells and the initial stages of an immune reaction. These CD4+ Tregs have a limited effect on the regulation of memory CD4+ T cells [23]. Here, we investigate the effect of CD8+ T regulatory cells on memory CD4+ T cells. CD8+ T regulatory cells have been extensively investigated in autoimmune diseases, such as EAE (experimental autoimmune encephalomyelitis) [24], murine autoimmune diabetes [25], and inflammatory bowel disease [26]. The TCR of these CD8+ T regulatory cells can recognize the Qa-1 peptide complex expressed on the surface of activated T cells, and prevent delayed type hypersensitivity (DTH) by deleting auto-reactive CD4+ T cells [27-30].
However, although CD8+ T regulatory cells are known to be involved in transplant tolerance [31-34], little is known about the interaction between CD8+ T regulatory cells and memory CD4+ T cells in alloimmunity. Here, we investigate the regulation of memory CD4+ T cells by CD8+ T regulatory cells in vitro and in vivo.
Materials and methods
Mice
6-8 weeks old C57BL/6J mice were purchased from Wuhan University center for animal experiment/A3-lab (Wuhan, China). C3H mice were purchased from Vital River Laboratories (VRL) (Beijing, China), B6.129S7-Rag1tm1Mom/J mice were purchased from The Jackson Laboratory (USA). All animals were housed in the specific pathogen-free environment at Tongji Medical college center for animal experiment, Huazhong University of Science and Technology. All mouse experiments were approved by the ethics committee of Tongji Medical College and conducted according to the guidelines of the National Institutes of Health.
Murine skin allograft transplantation
We used a murine skin allograft transplantation model to produce CD4+CD44+CD62L-CCR7- effector memory CD4+ T cells, which specifically reacted against C3H mouse antigens [35]. Skin grafts without hair and connective tissue from C3H mice were cut into patches with the size of 1cm in diameter, and then immersed into the saline. Two patches of 1 cm diameter skin were removed from the back of C57BL/6J mice. The wounds were covered with C3H mice skin patches, vaseline gauze and medical patches in sequence, and fixed by medical adhesive tape. Medical adhesive tape, medical drip patches and vaseline gauze were removed after 7 days. Each recipient was maintained separately in a single cage, and continued to be housed for two months.
Purification of memory CD4+ T cells
Memory CD4+ T cells were isolated from spleens of C57BL/6J skin allograft recipients using magnetic negative selection (MAGM206, R&D, USA), which were confirmed by markers CD4 (FITC, 50 µl, 100405, BioLegend) CD44 (PE, 100 tests, 103024, BioLegend), CCR7 (PE, 200 µg, 120106, BioLegend) and CD62L (FITC, 500 µg, 104406, BioLegend) using Flow Cytometry as we have previously described [35].
Production of CD8+ T regulatory cells
Naive CD4+ T cells purified from the spleens of wildtype C57BL/6J mice were cultured in 10% FBS RPMI 1640 with 1% penicillin, 1% streptomycin, concanavalinA (conA 4 µg/ml, SIGMA-ALDRICH) and rmIL-15 (100 ng/ml, R&D) for 96 hours at 37°C and 5% CO2 humidified environment. Activated CD4+ T cells were collected and washed three times by RPMI 1640 medium, resuspended in RPMI medium at 107 cells/ml with mitomycin C (25 µg/ml) used to attenuate the function of activated CD4+ T cells [36-38], and incubated for 30 minutes in 37°C and 5% CO2 humidified environment before the reaction was terminated by 40% FBS. Attenuated CD4+ T cells were transferred into C57BL/6J mice by tail vein injection at a concentration of 2×106 cells/200 µl per mouse after washing three times with RPMI medium. The CD8+ T cells (more than 95%) were purified from the spleens of these C57BL/6J mice two weeks later using Mag Cellect Mouse CD8+ T Cell Isolation Kit (MAGM203, R&D, USA) [27,39].
Identification of memory CD4+ T cells and their proliferation in vivo
Memory CD4+ T cells were labeled with CFSE (Carboxyfluorescein succinimidyl ester, 10 mM, 21888, SIGMA-ALDRICH) (around 95% positive). 5×106 memory CD4+ T cells were transferred into recipient mice by tail vein injection one day before heart transplantation. Proliferation of CFSE-labeled memory CD4+ T cells was measured daily, from 1 day before transplantation to 5 days post-operation. At day 6, CD44, CCR7 and CD62L on these CFSE labeled cells were detected by Flow Cytometry.
Hematoxylin-eosin (HE) and immunofluorescence staining
For histological examination, cardiac graft blocks were placed in 10% buffered formaldehyde solution 48 hours before being embedded in paraffin. Four μm heart sections were stained with HE staining, evaluated by a pathologist blinded to the experimental groups, in accordance with the standardization of nomenclature in the diagnosis of heart rejection [40] (The International Society of the Heart and Lung Transplantation, ISHLT). Donor cardiac histologic tissues were scored by a semiquantitative histology scoring system. Sections were graded 0-3 based on damage of donor cardiac allografts including epicardial, myocardial, endocardial damage, and inflammatory cell infiltration, 0 (no damage), 1 (mild damage), 2 (moderate damage), and 3 (severe damage). Five sections of each group were chosen and three fields of each section were captured to score. Data was shown as mean ± SEM. Immunofluorescence staining was performed following the specification: rat anti-mouse CD4 antibody (500 µg, 100510, BioLegend).
Purification of anti-mouse Qa-1 and anti-mouse CD8 antibodies
Hybridoma cell lines producing anti-mouse Qa-1b antibodies (CRL-2743™ built by Professor MJ Soloski in Johns Hopkins University) and hybridoma producing anti-mouse CD8 antibodies (against mouse Lyt 2.2, CRL-1971™) were purchased from American type culture collection (ATCC, USA). The hybridoma were cultured in Dulbecco’s Modified Eagle’s Medium (Gbico, USA) with 10% FBS. Purification of anti-mouse Qa-1 and anti-mouse CD8 antibodies from the hybridoma was performed by Proteintech Group, Inc (Wuhan, China) and the antibodies were dissolved in glycerol (1 µg/µl).
Qa-1 expression on memory CD4+ T Cells
The expression of Qa-1 on memory CD4+ T cells (experiment group with 4 µg/ml conA) was detected by Flow Cytometry using PE-anti-Qa-1 antibodies (1 µg/106 cells, SC-23889, Santa Cruz Biotechnology)at 0 h, 24 h, 48 h, 72 h, 96 h, and 120 h time point.
Proliferation assay of memory CD4+ T cells with different stimuli
Memory CD4+ T cells labeled with CFSE at a concentration from 2×104 to 1×105 cells/well were assigned to control group, positive group (4 µg/ml conA as stimulus), and experiment group(co-cultured with C3H spleen cells pretreated with mitomycin C as stimulus at 1×105 cells/well). Proliferation was measured by Flow Cytometry at 0 h, 24 h, 48 h, 72 h, and 96 h time points [41,42].
Suppression assay of CD8+ T cells on memory CD4+ T cells
Memory CD4+ T cells labeled with CFSE (105 cells/well) were cocultured with CD8+ T cells at the presence of C3H spleen cells pretreated with mitomycin C at different E/T ratios of 1:1, 1:2, 1:4 and at different time points. The most effective ratio and time point was chosen for the following experiments. The inhibitory effect of CD8+ T regulatory cells on memory CD4+ T cells was detected by Flow Cytometry. In blocking experiments, memory CD4+ T cells were incubated with an anti-Qa-1 antibody (10 µg/ml) and CD8+ T regulatory cells were incubated with an anti-CD8 antibody (25 µg/ml) for 1 h at 4°C. Then memory CD4+ T cells and CD8+ T regulatory cells were co-cultured for 96 h, the proliferation effect of memory CD4+ T cells was measured by Flow Cytometry [42].
In vitro cytotoxic assay
Memory CD4+ T cells labeled with CFSE were incubated with CD8+ T regulatory cells at different ratio (1:5, 1:10, 1:20) for 2 hours at 37°C and a 5% CO2 humidified environment, then all the cells were stained with PI (Propidium Iodide, Sigma). CFSE and PI double positive was considered as percentage of lysis, and cytotoxic ability was calculated by the formula: sample lysis-control lysis [43,44].
ELISA assay
Detection of IL-2, IL-4, IL-10 and IFN-γ levels from supernatant of cocultured cells was performed following the protocol of ELISA kit (Dakewei Biotech Company, Shenzhen, China).
Murine cardiac allograft transplantation
All the donor cardiac grafts were from C3H mice. Mouse recipients were divided into seven groups (5-8 mice per group): control group (C57BL/6J), control group (Rag1-/-), mCD4 group (Rag1-/- mouse received 5×106 mCD4+ T cells), CD8 group (Rag1-/- mouse received 5×106 CD8+ Tregs), mCD4+CD8 group (Rag1-/- mouse received 5×106 CD8+ Tregs and 5×106 mCD4+ T cells at the same time), mCD4+ CD8+ anti-Qa-1 mAb group (Rag1-/- mouse received anti-Qa-1 mAb, mCD4 and CD8), mCD4+ CD8+ anti-CD8 mAb group (Rag1-/- mouse received anti-CD8 mAb, mCD4 and CD8). All cells were transferred into recipients 24 hours before heart transplantation by tail vein injection. Anti-Qa-1 mAb and anti-CD8 mAb were administered one day before operation by peritoneal cavity injection (500 µg/mouse). The procedure of acquiring donor cardiac allografts and placing them in the abdominal cavity of recipients was performed as previously described [45]. Heart graft function was assessed daily by manual palpation. Graft rejection was defined as cessation of beating as determined by manual palpation, and was confirmed by visual inspection. All grafts were harvested at time of rejection or 100 days after transplantation.
Statistical analysis
All experiments were performed at least three independent times. The data were presented as Mean ± SEM and analyzed by a student’s t-test or one-way analysis of variance (ANOVA). Kaplan-Meier analysis with a log-rank test was used to compare heart grafts survival between groups. Statistical analysis was performed using Prism 5.0 (GraphPad Software, La Jolla, 263 CA, USA) or SPSS 13.0 (Chicago, IL, USA). P value<0.05 was considered to be statistically significant.
Results
Identification of memory CD4+ T cells and its mediating murine cardiac allograft rejection
Memory CD4+ T cells isolated from C57BL/6J skin allograft recipient consisted of more than 95% of CD4+CD44+CD62L-CCR7- T cells [35]. Memory CD4+ T cells labeled with CFSE were transferred into Rag1-/- mice by tail vein injection one day before C3H cardiac allografts transplantation. We found that fluorescence intensity of CFSE on memory CD4+ T cells from recipients decreased with time after surgery, and the ratio of CFSE positive cells increased (data not shown). This data indicated that CFSE labeled memory CD4+ T cells began to proliferate 24 hours after surgery when they were confronted with alloantigens. The surface markers on CD4+ T cells were measured 6 days after surgery: CD44+ accounted for 52.09%, CCR7- for 96.01%, and CD62L- for 97.02%.
To elucidate the role of memory CD4+ T cells in murine cardiac allograft transplantation, we performed two groups of cardiac transplants: mCD4 group (C3H to B6/RAG+mCD4) and negative control (C3H to B6/RAG). Memory CD4+ T cells mediated allograft rejection, and shortened survival times as compared to the negative control group (15 d vs. more than 100 d, P=0.001, Figure 1A). CD4+ T cell infiltration into allografts was confirmed using immunofluorescence staining (Figure 1B).
Figure 1.
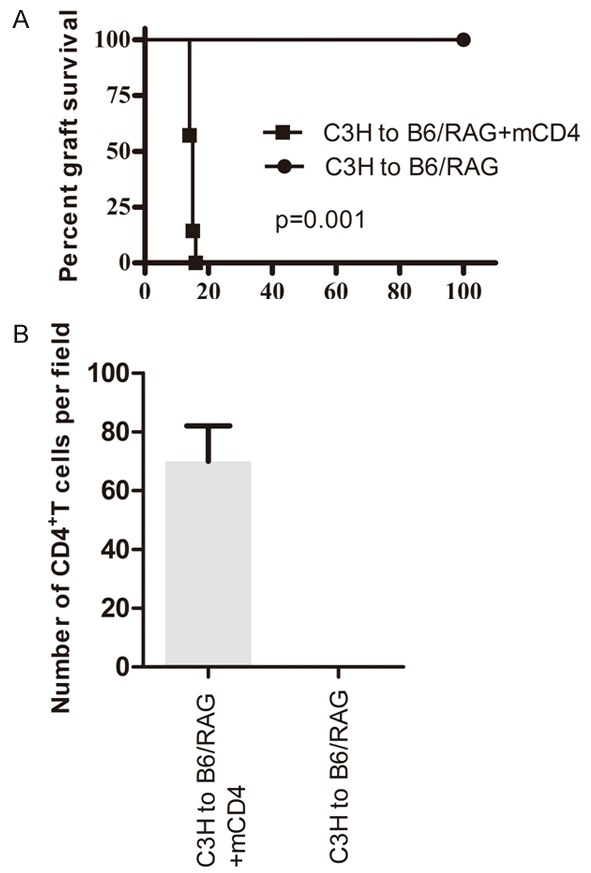
Memory CD4+ T cells had the ability to induce acute rejection in mice model. A. Two group of mice received C3H cardiac allograft and surviving times were observed: mCD4 group (C3H to B6/RAG+mCD4) (n=7, MST=15 days); Negative control (C3H to B6/RAG) (n=5, MST>100 days). B. Comparison of memory CD4+ T cells between mCD4 group and negative control.
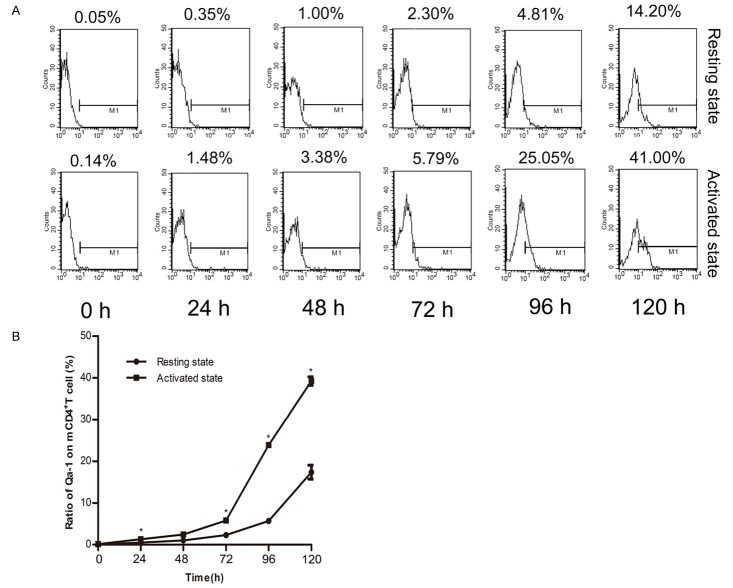
Qa-1 expression on memory CD4+ T cells increased as time went by in both activated and resting groups
To assess Qa-1 expression on C3H mouse-antigen specific memory CD4+ T cells, memory CD4+ T cells were divided into a resting group and an activated group (activated by 4 µg/ml conA). Cells collected were for Flow Cytometry detection at different time points. The Qa-1 expression on these cells in both groups increased as time went by (Figure 2A), showing a higher proportion after stimulation with conA after 48 hours (Figure 2B).
Figure 2.
Expression of Qa-1 on memory CD4+ T cells. A. Memory CD4+ T cells were cultured in 96-well plate with or without Con A, 30000 cells/well. Qa-1 was detected every 24 hours for 120 hours in a row. B. Expression of Qa-1 on memory CD4+ T cells was shown in resting state and activated state (*, P<0.05, comparison between resting state and activated state at each time point).
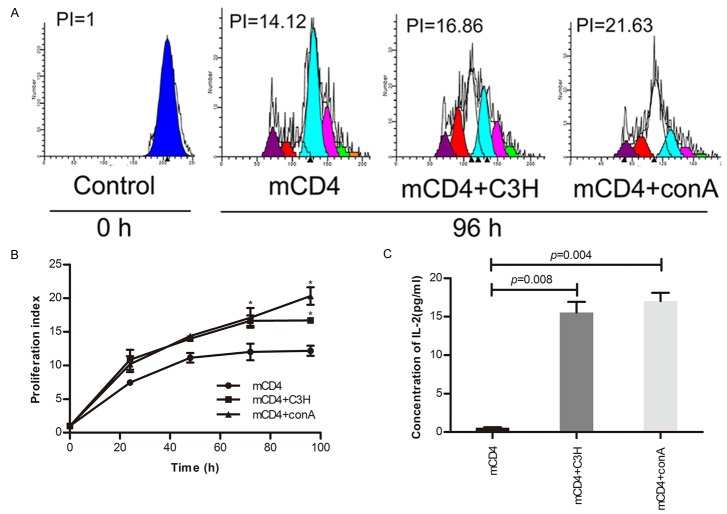
Memory CD4+ T cells proliferated with C3H spleen cells stimulation as time went by
The number of mCD4+ T cells increased as time went by and this proliferative effect was enhanced after stimulation by conA or C3H spleen cells pretreated with mitomycin C for 72 hours (Figure 3A, 3B). Figure 3 indicated that conA and C3H spleen cells had the similar ability to stimulate mCD4+ T cells to proliferate significantly (P<0.05). This phenomenon was also confirmed by the ELISA assay of IL-2 indirectly at 96 h (Figure 3C).
Figure 3.
Proliferation effect of C3H mouse-antigen specific mCD4. A. CFSE labeled memory CD4+ T cells were cultured in 96-well plates 105/well with control, con A. (4 µg/ml), C3H spleen cells (105/well). B. Cells were harvested 0 h, 24 h, 48 h, 72 h, and 96 h for proliferation index measurement. Proliferation index at 0 hour was considered as 1. C. Supernatants were collected for the detection of IL-2 by ELISA at 96 h.
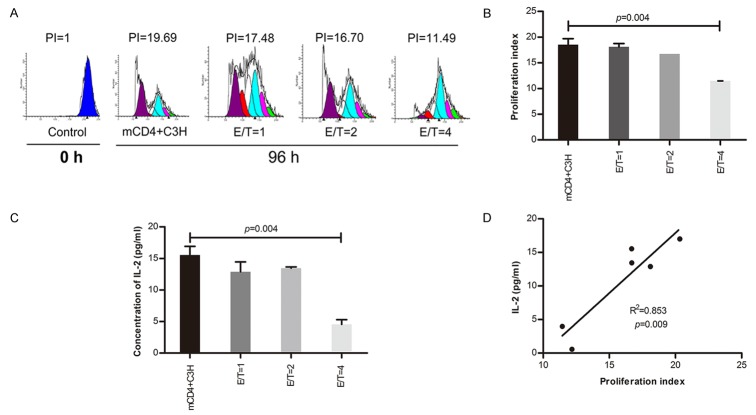
CD8+ regulatory T cells suppressed the proliferation of C3H mouse-antigen specific memory CD4+ T cells
Figure 3 showed C3H mouse-antigen specific memory CD4+ T cells proliferated significantly compared with the control at 96 h. So, we further investigated the suppressive effect of Qa-1-restricted CD8+ T cells on C3H mouse-antigen specific memory CD4+ T cells according to different E/T ratios. Our results showed that the proliferation index and IL-2 levels of supernatants decreased while the E/T ratio increased, especially at E/T ratio=4 (Figure 4A-C). There was a significantly positive relationship between IL-2 levels and the proliferation index at the 96 h time point (R2=0.853, P=0.009, Figure 4D).
Figure 4.
Effect of CD8+ regulatory T cells on antigen specific memory CD4+ T cells (E/T). A. Qa-1-restricted CD8+ T cells were cultured with memory CD4+ T cells at various E/T ratio=1, 2, 4. B. Proliferative effect was detected by Flow Cytometery at 96 hour. C. IL-2 in supernatant was measured by ELISA at 96h. D. The relationship between supernatants IL-2 level and proliferation index of Memory CD4+ T cells.
Anti-Qa-1 and anti-CD8 antibodies reduced the suppressive activity of CD8+ regulatory T cells on memory CD4+ T cells
Figure 4 Showed that CD8+ Tregs had the ability to suppress the proliferation of antigen specific memory CD4+ T cells at E/T ratio=4 at the 96 hour time point. This phenomenon leads us to ask how CD8+ T cells inhibited the activity of C3H mouse-antigen specific memory CD4+ T cells. Studies in autoimmunity have described a role for CD8+ Tregs [24-26]. In these studies CD8+ Tregs have been shown to suppress autoreactive CD4+ cells via recognition of Qa-1-peptide complex [24,26]. Given these reports we analyzed the role of Qa-1 and CD8+ Tregs. Our data shows that anti-Qa-1 or anti-CD8 antibodies inhibited the suppressive activity of CD8+ Tregs, and combined administration of anti-Qa-1 and anti-CD8 also had a blockade effect (Figure 5A, 5B). These data indicated that CD8+ regulatory T cells also inhibited the proliferative activity of alloreactive memory CD4+ T cells through Qa-1-peptide-CD8-TCR pathway.
Figure 5.
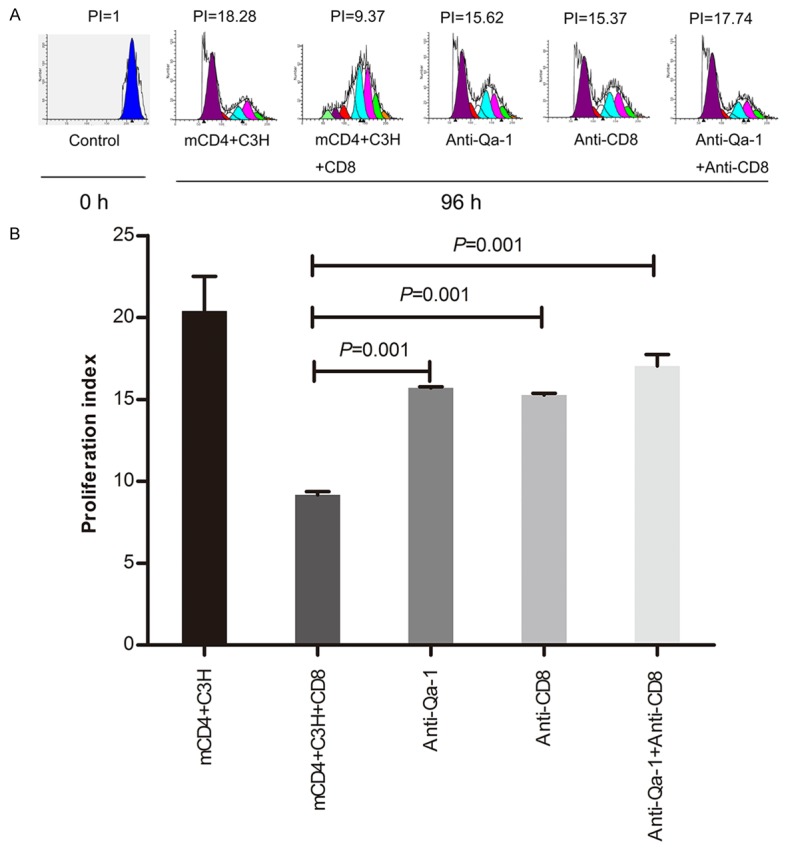
Interruption of inhibitive effect of CD8+ regulatory T cells on memory CD4+ T cells by anti-Qa-1 and anti-CD8 antibodies. A. Qa-1 restricted CD8+ T cells were incubated with anti-CD8 mAb (25 µg/ml) and CFSE-labeled memory CD4+ T cells with Qa-1 mAb (10 µg/ml) (ratio of Qa-1 restricted CD8+ T cells to memory CD4+ T cells=4) for 96 hours. B. The proliferation effect was tested as previously described.
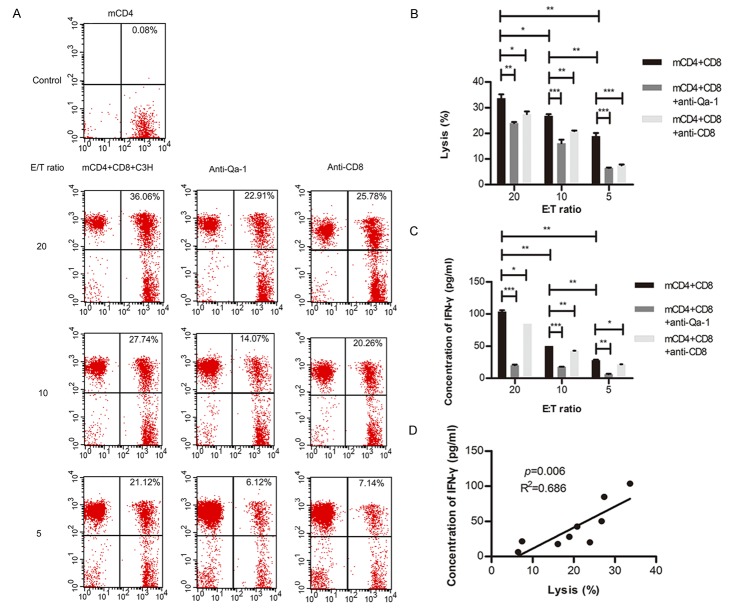
Killing effect of CD8+ regulatory T cells on C3H mouse-antigen specific memory CD4+ T cells
The lysis rate of memory CD4+ T cells was used to evaluate the killing effect of Qa-1-restricted CD8+ T cells in this experiment. The data showed that the lysis rate of memory CD4+ T cells (CFSE labeled) at different E/T ratios, and this killing effect could be blocked by anti-Qa-1 or anti-CD8 mAb (Figure 6A, 6B). The higher E/T ratio was, the more obvious killing effect and the lower concentration of IFN-γ could be detected (Figure 6B and 6C). There was an obvious positive relationship between lysis rate and IFN-γ level (R2=0.686, P=0.006, Figure 6D). However, some inhibitory cytokines, such as IL-4 and IL-10 were not measurable in these supernatant (data not shown).
Figure 6.
Lysis function of CD8+ Tregs on memory CD4+ T cells. A. CFSE labeled memory CD4+ T cells and CD8+ T cells were incubated at various E/T ratio, accompanied by inactivated C3H spleen cells. B. Lysis of memory CD4+ T cells by CD8+ T cells. C. IFN-γ from cultured supernatants was detected by ELISA. D. The relationship between supernatants IFN-γ level and lysis of memory CD4+ T cells. (*, P<0.05; **, P<0.01; ***P<0.001).
Protective effect of CD8+ regulatory T cells on murine cardiac allografts
In order to test the inhibitory effect of CD8+ Tregs on memory CD4+ T cells in vivo, mice were divided into seven groups before they were given specific treatments (Figure 7A). All heart allografts from C3H to B6/Rag1-/- group (n=6, 0R, Figure 7B) survived more than 100 days, except one, which failed at 57 days, obviously longer than those from C3H to B6/WT group (n=8, MST=8 days, P<0.01) (Figure 7B). After injection with mCD4, cardiac allograft survival time (n=7, MST=15 days, P<0.01, Figure 7B) was shorter compared with the C3H to B6/Rag1-/- group. However, the survival time was prolonged significantly by treatment with CD8+ Tregs (C3H to B6/Rag+mCD4+CD8 group, n=5, MST=37 days, P<0.05, Figure 7B), even longer in the CD8 group (n=5, MST=58 days, P<0.05, Figure 7B). These data indicated that CD8+ Tregs exerted their protection against rejection by inhibiting C3H mouse-antigen specific memory CD4+ T cells. Protective function of CD8+ Tregs was diminished by anti-Qa-1 mAb (n=5, MST=13 days, P<0.05, Figure 7B) and anti-CD8 mAb treatments (n=5, MST=16, P<0.05, Figure 7B), which confirms that CD8+ Tregs inhibited C3H mouse-antigen specific memory CD4+ T cells by Qa-1-peptite-CD8-TCR complexes.
Figure 7.
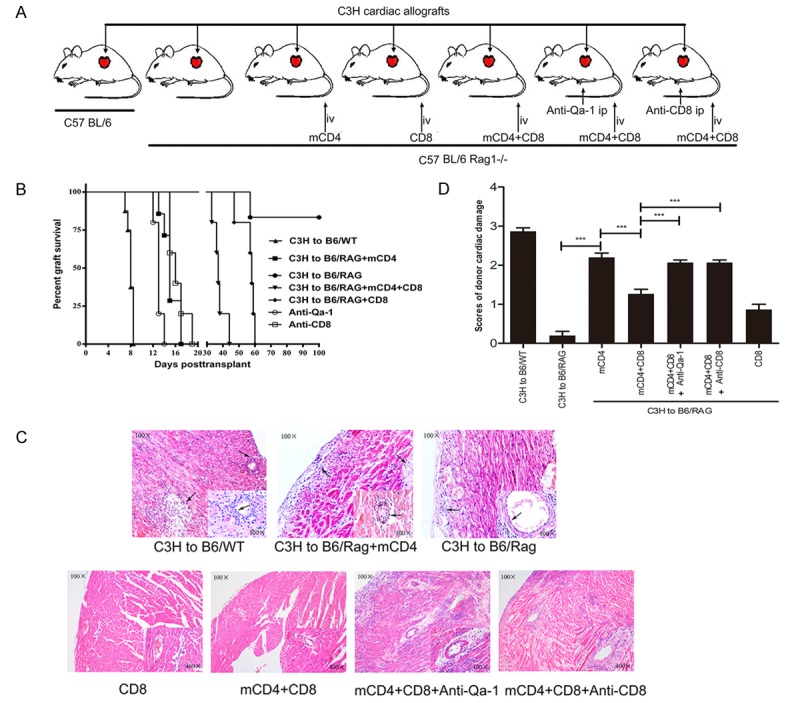
Protective effect of CD8+ Tregs on murine cardiac allografts. A. Mice were divided into seven groups. B. Survival time was calculated by Kaplan-Meier analysis with a log-rank test for the seven groups. C. Histological examination of donor cardiac allografts at terminal or determined time points. D. Scores for damage of donor cardiac allografts. (***P<0.001).
Histological evaluations of donor cardiac allografts are presented in Figure 7C. Cardiac allograft damage in the mCD4 group was more severe than in the negative control while less serious compared with the mCD4+ CD8 group. Both anti-Qa-1 and anti-CD8 decreased histological scores as compared with mCD4+ CD8 group (Figure 7D).
Discussion
Alloreactive memory CD4+ T cells belong to a sublineage of CD4+ T cells, but they have their own characteristics, which include, lower activation threshold, long term (usually couple of years) survival independent of the antigen, and MHC complexes, which are normally circulating in secondary lymphoid tissue and non-lymphoid tissue, and directly hypersensitive reaction to intruding specific antigens [46]. Transplant rejection is the main cause of the loss of heart transplant function. Strict human leukocyte antigen (HLA) matching before transplantation, and the application of non-specific immunosuppressive drugs after surgery, are used to prevent transplant rejection. However, despite these approaches, acute transplant rejection still occurs and is still one of the common complications, which leads to organ failure. Alloreactive memory CD4+ T cells that preexist in transplant recipients play an important role in this process, and are largely unresponsive to conventional immunosuppressive regimes. Therefore, there is a need to understand the mechanisms that lead to memory cell expansion, and how to control alloreactive memory CD4+ T cell-mediated acute transplant rejection.
Memory T cells are distinct from naive T cells due to their expression of CD45RAlow, CD45ROhi, CD44hi, and CD11ahi. Memory T cells are divided into central memory lymphocytes (TCM) (CCR7hi and CD62Lhi) and effector memory T lymphocytes (TEM) (CCR7low and CD62Llow) [47,48]. The memory CD4+ T cells isolated from spleens in this study were more than 95% CD4+CD44+CD62L-CCR7- T cells, belonging to TEM. Alloreactive memory CD4+ T cells have the ability to induce acute transplant rejection rapidly because they can migrate into the donor graft and mediate graft rejection, independent of secondary lymphoid tissues such as spleen, lymph nodes and mucosa lymphoid tissues [49]. Our data showed that alloreactive CD4+ TEM became activated and proliferated as early as 24 hours after surgery, and that the ratio of CD4+ T cells increased with time. These results indicated that CD4+ TEM were activated and could proliferate at an early stage when stimulated with specific antigen [46]. The expression of CCR7- (96.01%) and CD62L- (97.02%) on CD4+ T cells 6 days after transplantation remained high, but CD44+ expression decreased (from more than 95% to 52.09%). CD44, which is a single glycoprotein located on the surface of cell membrane, belongs to the family of cell adhesive molecules [23]. CD44 takes part in activation of T cells by increasing Ca2+ influx through combining with its ligand, facilitates T cell adhesion to capillary endothelial cell, and mediates T cell migration to target tissues [23]. Moreover, acute rejection mediated by TEM in mCD4 group was confirmed by pathological character of allografts. The survival time of allografts also definitely confirmed that alloreactive CD4+ TEM took part in transplant rejection and had a negative impact on graft survival time (Figure 1A). Additionally, our immunofluorescence staining data showed abundant CD4+ T lymphocyte infiltration into donor heart in mCD4 group, with CD4 cells predominantly located in the central layer of myocardium (Figure 1B). Taken together, these data support a role for memory CD4+ T cells mediating transplant rejection.
These studies, and that of others, support a key role for CD4+ TEM in graft rejection, and therefore strategies to diminish or control these cells may be of great therapeutic significance. In spite of improved immunosuppressive regimes, donor-specific memory CD4+ T cells can still be detected [50]. Researchers also tried other potential therapeutic approaches to prolong allograft survival, such as clearing donor-specific memory CD4+ T cells by antibodies, immunotoxins, and radiation, preventing migration of memory CD4+ T cells via administration of FTY720 [51], blocking co-stimulatory pathway [52-54]. However, in this study, we performed a new approach to modulate CD4+ TEM. Here we utilized CD8+ Tregs, and demonstrate the suppressive capacity of these cells on alloreactive memory CD4+ T cells in vitro and in vivo.
Our data showed that alloreactive memory CD4+ T cells proliferate promptly post transplantation when confronted with specific antigens. Qa-1 expression on memory CD4+ T cells began to increase significantly after 48 hours of conA stimulation (Figure 2), and it increased with time, which was consistent with endogenous CD4+ T cells [55].
CD8+ T regulatory cells, firstly described in autoimmune diseases, were shown to be capable of preventing autoimmune diseases, and can be generated by a T cell vaccination process [27,39,55,56]. Inhibitory function of CD8+ T regulatory cells mainly depends on its recognition of Qa-1 (HLA-E in human) complexes that have the ability to present a mass of self and foreign peptide [27,57]. Our data indicated that CD8+ T regulatory cells required a minimum quantity (E/T ratio=4) and time (96 h) in vitro to exert their suppressive function. In the antibody-blocking assay, both anti-Qa-1 and anti-CD8 mAb could efficiently reverse the inhibitory function of CD8+ T regulatory cells, which reflected CD8+ Tregs recognized memory CD4+ T cells via TCR-Qa-1-peptide pathway (Figures 4 and 5). In killing assays, we found there was an obvious positive relationship between lysis rate of memory CD4+ T cells and IFN-γ levels (Figure 6). IFN-γ, associated with its ability to elevate Qa-1 expression on activated cells [58], plays an inhibitory role in the interaction of CD8+ T regulatory cells on CD4+ T cells [39,59,60], which was confirmed by our data (Figure 6). IFN-γ plays paradoxical roles in immune reactions [59], and its role here still requires further investigation. However, whatever kind of role it plays in the inhibitory effects of CD8+ Tregs, IFN-γ could be used as a direct index for the lysis function of CD8+ Tregs. Taken together, these data provide intriguing evidence for a novel approach to protect the heart allograft from memory CD4 T cell mediated graft injury.
Our data confirms that CD8+ Tregs can inhibit the function of memory CD4+ T cells, and importantly prolong survival time of allografts (Figure 7B). The important role of Qa-1 and CD8 Tregs was confirmed by anti-Qa-1 and anti-CD8 mAb blockade, which inhibited protective effects on CD8+ Tregs and significantly shorten allograft survival times (Figure 7B). These results indicated that the TCR-Qa-1-peptide pathway is involved in the inhibitory function of CD8+ Tregs. We also found that CD8 cells could shorten survival time compared to the negative group (C3H toB6/RAG) (Figure 7B), which might be attributed to the purity of CD8+ Tregs or plasticity of their regulatory phenotype. These isolated CD8+ T cells might also contain a small fraction of cytotoxic CD8+ T cells. However, this method of CD8+ regulatory T cell purification has been used in similar studies [27,39,55].
In conclusion, our data demonstrates that CD8+ T regulatory cells inhibit the proliferation of memory CD4+ T cells by recognizing Qa-1-peptide through an IFN mediated killing mechanism, and protect allografts from rejection mediated by memory CD4+ T cells. Both Qa-1 and CD8 mAb can interrupt this effect. Further studies on mechanisms of CD8+ Tregs regulating memory CD4+ T cells may yield new approaches to fight allograft rejection.
Acknowledgements
We are sincerely grateful to Dr. Gang Chen and Lu Wang (Organ Transplantation Institute, Tongji Hospital) for valuable suggestions for the design of the experiment; Dr. Xiaolan Li (Department of Surgery and Molecule Medical Center, Tongji Hospital) for Flow Cytometry analysis. This work was supported by National Natural Science Foundation of China (NO. 81001305).
Disclosure of conflict of interest
None.
References
- 1.Sayegh MH, Carpenter CB. Transplantation 50 years later-progress, challenges, and promises. N Engl J Med. 2004;351:2761–2766. doi: 10.1056/NEJMon043418. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Q, He SQ, Gao D, Lei B, Long X, Liang HF, Zhu P, Jin JF, Tang B, Tomlinson S, Wu ZY, Chen XP. Early application of auxiliary partial orthotopic liver transplantation in murine model of Wilson disease. Transplantation. 2015;99:2317–2324. doi: 10.1097/TP.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 3.Dabrowska-Bender M, Michalowicz B, Paczek L. Assessment of the quality of life in patients after liver transplantation as an important part of treatment results. Transplant Proc. 2016;48:1697–1702. doi: 10.1016/j.transproceed.2015.12.139. [DOI] [PubMed] [Google Scholar]
- 4.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941–948. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 5.Tsai HI, Yu HP. A review of nationwide population study of organ transplantation in Taiwan. Acta Anaesthesiol Taiwan. 2016;54:70–74. doi: 10.1016/j.aat.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Kittleson MM, Kobashigawa JA. Long-term care of the heart transplant recipient. Curr Opin Organ Transplant. 2014;19:515–524. doi: 10.1097/MOT.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen FT, Velardi A. Progress in understanding and exploiting the immune response in solid organ and hemopoietic stem cell transplantation. Curr Opin Immunol. 2009;21:522–524. doi: 10.1016/j.coi.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 9.Gerlach UA, Vogt K, Schlickeiser S, Meisel C, Streitz M, Kunkel D, Appelt C, Ahrlich S, Lachmann N, Neuhaus P, Pascher A, Sawitzki B. Elevation of CD4+ differentiated memory T cells is associated with acute cellular and antibody-mediated rejection after liver transplantation. Transplantation. 2013;95:1512–1520. doi: 10.1097/TP.0b013e318290de18. [DOI] [PubMed] [Google Scholar]
- 10.Gorbacheva V, Fan R, Fairchild RL, Baldwin WM 3rd, Valujskikh A. Memory CD4 T cells induce antibody-mediated rejection of renal allografts. J Am Soc Nephrol. 2016;27:3299–3307. doi: 10.1681/ASN.2015080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalache S, Lakhani P, Heeger PS. Effects of preexisting autoimmunity on heart graft prolongation after donor-specific transfusion and anti-CD154. Transplantation. 2014;97:12–19. doi: 10.1097/TP.0b013e3182a77eba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 14.Tang AL, Bingaman AW, Kadavil EA, Leeser DB, Farber DL. Generation and functional capacity of polyclonal alloantigen-specific memory CD4 T cells. Am J Transplant. 2006;6:1275–1284. doi: 10.1111/j.1600-6143.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- 15.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 16.Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, Wood KJ, Turka LA, Jones ND. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid-Adam J, Yang N, Song Y, Cravedi P, Li XM, Heeger P. Immunosuppressive effects of the traditional Chinese herb Qu Mai on human alloreactive T cells. Am J Transplant. 2013;13:1159–1167. doi: 10.1111/ajt.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Hippen KL, Lemire AL, Gu J, Wang W, Ni X, Ranganathan P, Levine BL, Riley JL, June CH, Turka LA, Munn DH, Garzon R, Lu L, Blazar BR. miR-146b antagomir treated human Tregs increase TRAF6-NFkB expression, suppressor function and GVHD inhibitory potency. Blood. 2016;128:1424–35. doi: 10.1182/blood-2016-05-714535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lifshitz GV, Zhdanov DD, Lokhonina AV, Eliseeva DD, Lyssuck EY, Zavalishin IA, Bykovskaia SN. Ex vivo expanded regulatory T cells CD4+CD25+FoxP3+CD127 Low develop strong immunosuppressive activity in patients with remitting-relapsing multiple sclerosis. Autoimmunity. 2016;49:388–396. doi: 10.1080/08916934.2016.1199020. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Ahn C, Jung HK, Kim EK, Kim JY, Kim YS, Han JS, Kim S, Lee JS. The expression patterns of CD44 and CD45RB on peripheral blood T lymphocytes in the rejection of allogeneic murine skin transplantation. Transpl Immunol. 2003;11:197–206. doi: 10.1016/S0966-3274(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 24.Leavenworth JW, Schellack C, Kim HJ, Lu L, Spee P, Cantor H. Analysis of the cellular mechanism underlying inhibition of EAE after treatment with anti-NKG2A F(ab’)2. Proc Natl Acad Sci U S A. 2010;107:2562–2567. doi: 10.1073/pnas.0914732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, Chess L. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010;120:3641–3650. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Y, Han W, Liang J, Ji J, Wang J, Cantor H, Lu L. Glatiramer acetate ameliorates inflammatory bowel disease in mice through the induction of Qa-1-restricted CD8+ regulatory cells. Eur J Immunol. 2013;43:125–136. doi: 10.1002/eji.201242758. [DOI] [PubMed] [Google Scholar]
- 27.Panoutsakopoulou V, Huster KM, McCarty N, Feinberg E, Wang R, Wucherpfennig KW, Cantor H. Suppression of autoimmune disease after vaccination with autoreactive T cells that express Qa-1 peptide complexes. J Clin Invest. 2004;113:1218–1224. doi: 10.1172/JCI20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagarajan NA, Gonzalez F, Shastri N. Nonclassical MHC class Ib-restricted cytotoxic T cells monitor antigen processing in the endoplasmic reticulum. Nat Immunol. 2012;13:579–586. doi: 10.1038/ni.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YM, Zhang GY, Hu M, Polhill T, Sawyer A, Zhou JJ, Saito M, Watson D, Wu H, Wang Y, Wang XM, Harris DC, Alexander SI. CD8+ regulatory T cells induced by T cell vaccination protect against autoimmune nephritis. J Am Soc Nephrol. 2012;23:1058–1067. doi: 10.1681/ASN.2011090914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Zhang C, Zhou Z, Zhang J, Tian Z. Small intestinal intraepithelial lymphocytes expressing CD8 and T cell receptor γδ are involved in bacterial clearance during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2012;80:565–574. doi: 10.1128/IAI.05078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krupnick AS, Lin X, Li W, Higashikubo R, Zinselmeyer BH, Hartzler H, Toth K, Ritter JH, Berezin MY, Wang ST, Miller MJ, Gelman AE, Kreisel D. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124:1130–1143. doi: 10.1172/JCI71359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ophir E, Or-Geva N, Gurevich I, Tal O, Eidelstein Y, Shezen E, Margalit R, Lask A, Shakhar G, Hagin D, Bachar-Lustig E, Reich-Zeliger S, Beilhack A, Negrin R, Reisner Y. Murine anti-third-party central-memory CD8(+) T cells promote hematopoietic chimerism under mild conditioning: lymph-node sequestration and deletion of anti-donor T cells. Blood. 2013;121:1220–1228. doi: 10.1182/blood-2012-07-441493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avivi I, Stroopinsky D, Rowe JM, Katz T. A subset of CD8+ T cells acquiring selective suppressive properties may play a role in GvHD management. Transpl Immunol. 2013;28:57–61. doi: 10.1016/j.trim.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Louvet C, Heslan JM, Merieau E, Soulillou JP, Cuturi MC, Chiffoleau E. Induction of Fractalkine and CX3CR1 mediated by host CD8+ T cells in allograft tolerance induced by donor specific blood transfusion. Transplantation. 2004;78:1259–1266. doi: 10.1097/01.tp.0000140482.20336.77. [DOI] [PubMed] [Google Scholar]
- 35.Peng Zhu, Yifa Chen, Dan Li, Chen X. Production, purification, identification and function of mouse CD4+ memory T lymphocytes. Chin J Microbiol Immunol. 2007;27:1046–1049. [Google Scholar]
- 36.Mor F, Cohen IR. Experimental aspects of T cell vaccination. Clin Exp Rheumatol. 1993;11(Suppl 8):S55–57. [PubMed] [Google Scholar]
- 37.Knickelbein JE, Divito S, Hendricks RL. Modulation of CD8+ CTL effector function by fibroblasts derived from the immunoprivileged cornea. Invest Ophthalmol Vis Sci. 2007;48:2194–2202. doi: 10.1167/iovs.06-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohwasser S, Kubota A, Salcedo M, Lian RH, Takei F. The non-classical MHC class I molecule Qa-1(b) inhibits classical MHC class I-restricted cytotoxicity of cytotoxic T lymphocytes. Int Immunol. 2001;13:321–327. doi: 10.1093/intimm/13.3.321. [DOI] [PubMed] [Google Scholar]
- 39.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci U S A. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Bocharov G, Luzyanina T, Cupovic J, Ludewig B. Asymmetry of Cell Division in CFSE-Based Lymphocyte Proliferation Analysis. Front Immunol. 2013;4:264. doi: 10.3389/fimmu.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran DQ. In vitro suppression assay for functional assessment of human regulatory T cells. Methods Mol Biol. 2013;979:199–212. doi: 10.1007/978-1-62703-290-2_16. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JH, Liu LQ, He YL, Kong WJ, Huang SA. Cytotoxic effect of trans-cinnamaldehyde on human leukemia K562 cells. Acta Pharmacol Sin. 2010;31:861–866. doi: 10.1038/aps.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jedema I, van der Werff NM, Barge RM, Willemze R, Falkenburg JH. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 2004;103:2677–2682. doi: 10.1182/blood-2003-06-2070. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Q, Li D, Liang H, Yang H, Lei D, Gao D, Long X, Chen Y, Zhu P, Chen X. Effects of long-term administration of low-dose FTY720 on survival of murine cardiac allograft. J Huazhong Univ Sci Technolog Med Sci. 2012;32:199–204. doi: 10.1007/s11596-012-0035-5. [DOI] [PubMed] [Google Scholar]
- 46.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 47.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 48.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Chalasani G, Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A. 2002;99:6175–6180. doi: 10.1073/pnas.092596999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 51.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 52.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 53.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant. 2008;8:497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 54.Yuan X, Salama AD, Dong V, Schmitt I, Najafian N, Chandraker A, Akiba H, Yagita H, Sayegh MH. The role of the CD134-CD134 ligand costimulatory pathway in alloimmune responses in vivo. J Immunol. 2003;170:2949–2955. doi: 10.4049/jimmunol.170.6.2949. [DOI] [PubMed] [Google Scholar]
- 55.Varthaman A, Clement M, Khallou-Laschet J, Fornasa G, Gaston AT, Dussiot M, Caligiuri G, Cantor H, Kaveri S, Nicoletti A. Physiological induction of regulatory Qa-1-restricted CD8+ T cells triggered by endogenous CD4+ T cell responses. PLoS One. 2011;6:e21628. doi: 10.1371/journal.pone.0021628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holderried TA, Lang PA, Kim HJ, Cantor H. Genetic disruption of CD8+ Treg activity enhances the immune response to viral infection. Proc Natl Acad Sci U S A. 2013;110:21089–21094. doi: 10.1073/pnas.1320999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 58.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest. 2007;117:871–873. doi: 10.1172/JCI31860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95:2711–2719. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]