Abstract
We previously showed that bone mesenchymal stem cells (BMSCs) inhibit interleukin-1 beta (IL-1β) induced degenerative effects in NP cells by their paracrine activity, but the anti-inflammatory and anti-apoptotic effect of BMSC paracrine activity and the relative signaling pathway were not further investigated in annulus fibrosus (AF) cells. In this study, AF cells were exposed to IL-1β, which was applied to mimic intervertebral disc degeneration (IDD) in vitro. Indirect co-culture with BMSCs in a transwell co-culture system reduced the activity of nuclear factor-κB-p65 (NF-κB-p65) through the restoration of its inhibitor IκBa. Real time polymerase chain reaction (PT-PCR) and Western blotting revealed that the up-regulation of MMP-3 and MMP-13 induced by IL-1β were impeded by BMSC co-culture, and the decrease in aggrecan, collagen I and TIMP-1 were reversed. An ELISA showed that the increased inflammatory factors, such as nitrite, prostaglandin E-2 (PGE-2), IL-6 and cyclooxygenase-2 (COX-2), were decreased by the BMSC co-culture. Furthermore, the apoptosis rate of AF cells were detected by flow cytometry, and the apoptosis-related proteins, such as Bax, Bcl-2 and caspase-3, were analyzed by Western blotting and ELISA. The changes in mitochondrial membrane potentials were also detected by confocal microscopy. The results showed that IL-1β induced apoptosis of AF cells was attenuated by co-culturing, which suppressed the functions of the mitochondria function. We suggest that BMSC paracrine activity has an anti-inflammation effect and anti-apoptotic effect on IDD, and it is mediated, at least in part, via the relative NF-κF and mitochondrial apoptotic pathways in AF cells.
Keywords: Interleukin-1 beta (IL-1β), annulus fibrosus, BMSCs, disc degeneration, apoptosis, paracrine
Introduction
Lower back pain is one of the major reasons for job-related disability in the United States. It is estimated that nearly 80% of adults will experience some form of back pain over their lifetime. In addition, almost 5% of sufferers become chronically disabled [1,2]. Intervertebral disc degeneration (IDD) is one of the dominating contributing factors to lower back pain, which is characterized by increased activity of catabolic enzymes, decreased synthesis of proteoglycan, and excessive apoptosis of the disc cells [3-5].
Inflammatory mediators, such as interleukin-1 beta (IL-1β), tumor necrosis factor-alpha, IL-8 and IL-6, play a key role in IDD [6]. Many investigations have pointed out that IL-1β is a significant catabolic cytokine involved in IDD, and it not only increased matrix-degrading enzyme activity but also enhanced excessive apoptosis in the NP and AF tissues [7,8]. It has also been reported that IL-1β sensitizes annulus fibrosus (AF) cells to fluid-induced shear stress [9]. Thus, to disrupt the harmful effects of IL-1β, it is important to identify signaling pathways in charge of the effects of IL-1β and inhibit these pathways.
In the pathological process of IDD, the NF-κB signal pathway plays a crucial role in the activity of catabolic genes and inflammatory mediators [10]. NF-κB inhibition reversed the changes in protein expression of matrix metalloproteinase-3/13 (MMP-3/13), which are two major aggrecanases, a disintegrin and metalloproteinase with thrombospondin motifs 4/5 (ADAMTS-4/5), aggrecan and collagen II induced by IL-1β in NP tissues [10,11]. With disc degeneration and age, the expression of NF-κB has also been shown to be promoted in human intervertebrals (both NP and AF tissues), which correlates with accumulated oxidative stress [12]. In addition, a recent study confirmed that, with exposure to NF-κB inhibition, NF-κB nuclear translocation induced by IL-1β was decreased in AF cells and the expression of MMP-3, inducible nitric oxide synthase (INOS), prostaglandin E2 (PGE2) production and cyclooxygenase-2 (COX-2) responded to combined inflammatory and mechanical stimulation [13]. Thus, it is necessary to block the catabolic effects of IL-1β and the NF-κB signal pathway in treatment of IDD.
There are two main caspase-dependent pathways in the signaling pathways of apoptosis; i.e., intrinsic and extrinsic, which are mediated by mitochondria and death-receptors, respectively [14]. The mitochondrial intrinsic pathway is mainly activated by various apoptotic signals including abnormal mechanical stress, serum deprivation, oxidative stress, cytokines, and nitric oxide [15]. The pro-apoptotic proteins (Bax and Bak) and the anti-apoptotic proteins (Bcl-2 and Bcl-xl) can result in the release of mitochondrial cytochromec, which combines with procaspase-9, apoptotic protease activating factor-1 (Aparf-1) and ATP to form the apoptosome [16]. Many assays have revealed that the intrinsic pathway, also known as the mitochondria pathway, plays an important part in apoptosis in IDD [15]. Previous studies have emphasized the significance of apoptosis in IDD by demonstrating an association between ECM degradation and intervertebral disc cellular loss [14]. Therefore, it is necessary to inhibit apoptosis and relative signaling pathways in the treatment of IDD.
Numerous studies have demonstrated the therapeutic effect of mesenchymal stem cells (MSCs) on IDD in experimental models. These studies have drawn the conclusion that MSCs could promote the synthesis of the remaining ECM of NP and AF tissues as well as impedance of inflammatory mediator expression and matrix-degrading enzyme activity, resulting in the structure of the IVD being maintained. The use of MSCs as a choice of treatment for disc degeneration is under extensive investigation [17]. Animal studies have also shown beneficial effects of MSCs on NP cell morphology and histology in various disc degeneration models, although their mechanism of action remains unclear [18]. MSCs have recently been reported to secrete anti-inflammatory cytokines and growth factors [19]. Some investigations have suggested that paracrine plays a vital role in the therapeutic capacity of BMSCs and emphasized that the effect of MSCs may be due in part to paracrine factors [20,21]. The paracrine effect of BMSCs on IDD treatment has not been fully investigated, so we focused on the paracrine mechanisms of BMSC treatment for disc degeneration.
To better understand the therapeutic capacity of BMSCs on the pathogenesis of IDD, the aim of our study was to explore the protective effects of BMSCs on AF cells in vitro. By means of a transwell co-culture system, both BMSCs and AF cells were challenged with the inflammatory cytokine IL-1β. We hypothesized that paracrine factors secreted by BMSCs may have an anti-inflammatory and anti-apoptotic effect on IL-1β induced AF cells and its relative signal pathways were studied.
Materials and methods
Ethics statement
The male Sprague-Dawley rats (weight, 100-120 g) used in the present investigation were provided by the Second Military Medical University Laboratory Animal Center (Shanghai, China). All experiments were permitted by the Animal Ethical Committee of the Second Military Medical University (No. 13071002114).
Isolation and culture of BMSCs and AF cells
Using the method reported previously [22], primary BMSCs were isolated and cultured. The harvested cells were centrifuged at 500× g for 10 min at 4°C and then resuspended in complete Dulbecco’s modified Eagle’s medium (DMEM)/F-12 with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco Life Technologies, Carlsbad, CA, USA). Cells at passage 3 were subjected to flow cytometric analysis (Cytomics FC500; Beckman Coulter, USA) to estimate the activity of the surface markers, including cluster of differentiation 29 (CD29), cluster of differentiation 90 (CD90), cluster of differentiation 29 (CD31) and cluster of differentiation 45 (CD45).
According to the method described previously reported, cells were isolated from AF tissues in the lumbar disc [23]. The cells were treated with 0.2% pronase and 0.025% collagenase P (Sigma, Milwaukee, WI) overnight for digestion. AF cells were isolated and cultured with Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (Gibco Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA, USA) and 1% (vol/vol) penicillin and streptomycin in a 37°C, 5% CO2 atmosphere. The medium was changed every 24 hours. When they reached 80% to 90% confluence about 2 weeks later, the cells were trypsinized and subcultured in 6-well plates (1×105 cells/well).
Experimental groups and cell treatment
BMSCs (1×105 cells/compartment) were seeded into the upper compartment of a six-well transwell system (pore size of 0.4 mm; Costar, Cambridge, MA). AF cells were placed into the lower compartment of the six-well transwell system (2×105 cells/well, the ratio was 2:1 to BMSCs). Before the co-culture study started, both cells were cultured separately in complete medium (Dulbecco’s modified Eagle’s medium (DMEM)/F-12 containing 10% fetal bovine serum, 2 ml/well) for 2 days so that cells were 70%-80% confluent at the time of co-culture. Although IL-1β could not induce annular cell damage and apoptosis in the presence of 10% FBS, the complete culture medium had to be replaced by low serum medium (Dulbecco’s modified Eagle’s medium/F-12 supplemented with 1% FBS) before the experiment began [7]. Our experiment was divided into three groups. The cells of the control group were treated with low serum DMEM throughout the experiment. The cells in IL-1β group were treated with 10 ng/mL recombinant human IL-1β (R&D Systems, Minneapolis, MN). In the co-culture group, AF cells were co-cultured with BMSCs in the presence of IL-1β (10 ng/ml). The harvesting time for AF cells of each group was 24 h. Cells were then harvested for messen ribonucleic acid (mRNA), protein analysis and flow cytometry. Meanwhile, inflammatory cytokines were assayed with an ELISA.
Gene expression analysis
Cells were collected from the three groups at different time points and lysed with Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). Total RNA was extracted and its concentration was measured photometrically (NanoDrop 2000/2000c; Thermo Fisher Sci entific, Inc, Wilmington, DE, USA). Synthesis of the cDNA was performed using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. PCR analysis was performed using genespecific primers (Table 1) for GAPDH, MMP-3, MMP-13, ADAMTS-4, TIMP-1, Aggrecan, and collagen I (Sangon Biotech Co., Ltd., Shanghai, China). Real-time PCR reactions were performed in triplicate in 96-well plates in a final volume of 20 µL using a SYBR Premix Ex Taq Kit (TaKaRa, Shanghai, China). Real-time PCR amplifications were carried out according to the following protocol: 95°C for 15 minutes, followed by 40 cycles of amplifications, consisting of a denaturation step at 94°C for 15 seconds, an annealing step at 55°C for 30 seconds, and an extension step at 72°C for 30 seconds. The cycle threshold values were obtained, and data were normalized to GAPDH expression using the 2-ΔΔCt method. All samples were run in triplicate.
Table 1.
Primer sequences used for RT-PCR
| Gene | Primer name | Sequence |
|---|---|---|
| GAPDH | GAPDH-F | CCATCAACGACCCCTTCATT |
| GAPDH-R | ATTCTCAGCCTTGACTGTGC | |
| MMP-3 | MMP-3-F | AAAGAACCCGCTGAGAGCAG |
| MMP-3-R | AACCTCCATGCCAGCATCTT | |
| MMP-13 | MMP-13-F | CCCTGGAGCCCTGATGTTT |
| MMP-13-R | CTCTGGTGTTTTGGGGTGCT | |
| ADAMTS-4 | ADAMTS-4-F | CGTTCCGCTCCTGTAACACT |
| ADAMTS-4-R | TTGAAGAGGTCGGTTCGGTG | |
| Aggrecan | Aggrecan-F | GCCTGCAAGGGAAATGTGTG |
| Aggrecan-R | GGCGGAAAGATTTGCCGTTAG | |
| Collagen II | Collagen-II-F | GACCTCCGGCTCCTGCTCCTCTTAG |
| Collagen-II-R | GACAGCACTCGCCCTCCCGTTTTTG | |
| TIMP | TIMP-F | ATAGTGCTGGCTGTGGGGTGTG |
| TIMP-R | TGATCGCTCTGGTAGCCCTTCTC |
All sequences are given in 5’-3’ direction.
Western blot analysis
AF cells were washed twice with PBS and lysed using ProteoJET Mammalian Cell Lysis Reagent (Thermo Fisher Scientific, Inc.). The protein concentration was determined using the Pierce BCA protein assay, and equivalent amounts of protein (20 μg) were separated and analyzed with a SDS-PAGE assay, probing with primary antibodies. Antibodies against NF-κB p65 (ab131485, abcam, USA; 1:400 dilution), IκBa (ab32518, abcam, USA; 1:400 dilution), and antibodies for MMP-3 (ab52915, abcam, USA; 1:400 dilution), MMP-13 (ab39012, abcam, USA; 1:400 dilution), TIMP-1 (ab61224, abcam, USA; 1:400 dilution), aggrecan (ab36861, bcam, USA; 1:400 dilution), collagen I (ab34710, abcam, USA; 1:400 dilution), and GAPDH (ab8245, abcam, USA; 1:400 dilution). The membranes were then incubated with the respective horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibodies (beyotime, Shanghai, China) at room temperature for 1 h. Immunoreactive bands were detected using an enhanced chemiluminescence system (EMD Millipore, Billerica, MA, USA) and quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Cytokine assessment by ELISA and NO assays
AF cells harvested from each group and levels of COX-2, IL-6 and PGE-2 were determined with a corresponding ELISA kit (R&D Systems, inneapolis, MN) according to the manufacturer’s instructions. In addition, based on the methods reported previously, the production of NO in AF cells was evaluated as CM nitrite using the Griess Reaction [23]. Briefly, 100 μl of the supernatant was incubated with 100 μl of Griess reagent [equal volumes of 1% (w/v) sulfanilamide (Wako, Osaka, Japan) and 0.1% (w/v) N-1-naphtyl ethylenediamine dihydrochloride (Wako) in 2.5% (w/v) H3PO4] for 10 min at room temperature, and the NO2 concentration was measured by detecting the optical density at 550 nm (A550) in reference to the A550 of the standard NaNO2 solution.
Detection of apoptotic incidence by flow cytometry
The apoptotic rate of the AF cells was detected using the Annexin V-Fluorescein Isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit I (BD Pharmingen, San Diego, CA, USA) with double staining according to the manufacturer’s instructions and as previously described [7]. Briefly, the cells of the three groups were collected by trypsinization and centrifugation, and then washed with ice-cold PBS twice and resuspended in 500 µl binding buffer. Then the cells were incubated with 5 µl of fluorescein-conjugated Annexin V and 5 µl of PI for 15 min at room temperature in the dark. The apoptotic rate was analyzed by a Fluorescence activated cell sorter (Cytomics FC500; Beckman Coulter) within 1 h. Apoptotic cells, including those staining positive for Annexin V and negative for PI, were identified as early apoptotic cells and those positive for double staining were identified as late apoptotic cells. The cells were counted and represented as a percentage of the total cell count.
Caspase-3 activity assay
Caspase-3 activity was detected using a caspase-3 activity kit (Beyotime, Shanghai, China), which is based on the ability of caspase-3 to change acetyl-Asp-Glu-Val-Asp p-nitroanilide into the yellow formazan product, p-nitroaniline. According to the product description, treated cells were lysed with lysis buffer (100 μl per 2×106 cells) for 15 min on ice and then washed with cold HBSS. After incubating the mixture composed of 10 μl of cell lysate, 80 μl of reaction buffer and 10 μl of 2 mM caspase-3 substrate in 96-well microtiter plates at 37°C for 4 h, the activity of caspase 3 was quantified on a microplate spectrophotometer (Biotek Instruments, Inc., Winooski, VT, USA) at 405 nm. Caspase-3 activity was expressed as the fold-change in enzyme activity compared with that of synchronized cells.
Assay of mitochondrial membrane potential (Δψm)
The mitochondrial membrane potential was determined by JC-1 staining (Beyotime) according to the manufacturer’s protocol. The changes in the mitochondrial membrane potential were detected by confocal microscopy. In physiological conditions, JC-1 accumulates in the intact mitochondrial membrane, where it forms pronounced red aggregates. When the mitochondrial membrane potential is disturbed, the red fluorescence decreases and there is an increase in green fluorescence of the free JC-1 form.
Statistical analysis
Data were analyzed with the use of SPSS 10 statistical software. Data are presented as the mean ± standard deviation and a Student’s t test or one-way ANOVA were used for comparisons between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Identification of isolated BMSCs
Flow cytometric analysis was used to detect the expression of CD90, CD45, CD29, and CD31 (Figure 1). BMSCs (>90%) were positive for CD29 or CD90, whereas <5% were positive for CD45 or CD31.
Figure 1.

Identification of isolated BMSCs. Flow cytometric analysis of CD90, CD45, CD29 and CD31. The results showed >90% of the BMSCs were positive for CD90 or CD29, whereas <5% of the BMSCs were positive for CD45 or CD31.
Effects of BMSC co-culture on IκBa and NF-κB p65 activity induced by IL-1β
Western blot analysis showed that the nuclear protein level of p65, which is a subunit of NF-κB [8], was increased by IL-1β stimulation and the increase was largely decreased by co-culture with BMSCs. IL-1β stimulation decreased the protein level of IκBa in AF cells and co-culture with BMSCs rescued the decreased IκBa protein mediated by IL-1β stimulation in AF cells (Figure 2).
Figure 2.

Effects of co-culture with BMSCs on the expression of NF-κB p65 and IκBa of AF cells induced by IL-1β. A. The protein levels of NF-κB p65 and IκBa were detected by Western blotting. B. The protein concentration was quantified. Values are mean ± SD. IL-1β control = 1. A student’s t test was performed to evaluate the difference. *P<0.05; **P<0.01.
Effects of BMSC co-culture on AF cell gene expression induced by IL-1β
IL-1β significantly up-regulated the expression of catabolic genes. The fold changes of gene expression induced by IL-1β relative to controls were: MMP-3, 30 and MMP-13, 68. BMSCs significantly reduced the expression of MMP-3 and MMP-13. The expression of ADAMTS-4 was not significantly different from the controls, and this increase was not affected by BMSC co-culture (Figure 3A). IL-1β stimulation moderately down-regulated the levels of matrix component genes and anticatabolic genes. The fold changes in gene expression induced by IL-1β relative to controls were: TIMP-1, 0.56; aggrecan, 0.62; and collagen I, 0.44; and when followed by BMSC treatment this decrease was reversed, respectively (Figure 3B).
Figure 3.

Effects of co-culture with BMSCs on gene expression of AF cells induced by IL-1β. Control value = 1. The data for MMP-3 and MMP-13 are plotted as 1/10 of the actual fold change. A. The change in catabolic gene expression; B. The data for matrix component genes and anticatabolic genes. Values are mean ± SE (n = 9 samples). A student’s t test was performed to evaluate the difference. NS: no sense, *P<0.05; **P<0.01.
Effects of BMSC co-culture on the protein expression of MMP-3, MMP-13, TIMP-1, aggrecan and collagen I in AF cells induced by IL-1β
To evaluate the effects of the BMSC co-culture on protein expression of some key components involved in IDD, we examined the protein expression of MMP-3, MMP-13, TIMP-1, aggrecan and collagen I (Figure 4A). IL-1β significantly up-regulated the protein expression of MMP-3 and MMP-13, and down-regulated the protein expression of TIMP-1, aggrecan and collagen I. These effects were impeded by BMSC co-culture (Figure 4B).
Figure 4.

Effects of co-culture with BMSCs on the expression of MMP-3, MMP-13, TIMP-1, collagen I, and aggrecan in AF cells. A. The protein levels of MMP-3, MMP-13, TIMP-1, collagen I and aggrecan were assayed by Western blotting. B. The protein concentration was quantified. Values are mean ± SD. IL-1β control = 1, A student’s t test was performed to evaluate the difference. *P<0.05; **P<0.01.
Effects of BMSC co-culture on the expression of conditioned media nitrite, PGE-2, IL-6 and COX-2
As the index of NO production, CM concentrations of nitrite were measured after co-culture with BMSCs. The increase in nitrite accumulation induced by IL-1β was significantly suppressed by the BMSC co-culture (Figure 5A). Likewise, the increasing production of PGE-2, IL-6 and COX-2 stimulated by IL-1β were reversed by the BMSC co-culture (Figure 5B-D).
Figure 5.
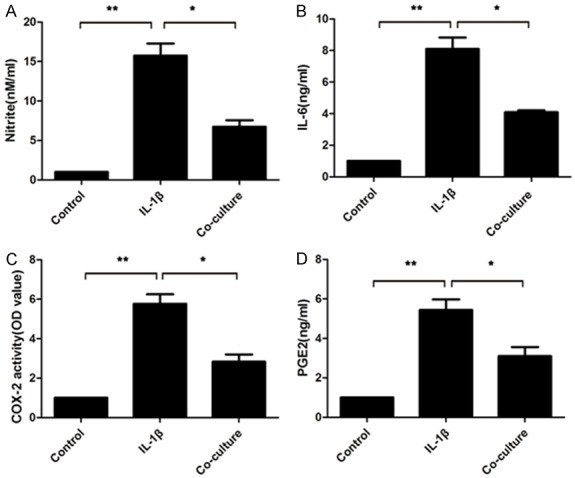
Effects of the BMSC co-culture on the production of NO, PGE2, IL-6 and COX-2. A. The nitrite levels in the culture medium were assessed by Griess reaction. B-D. The levels of IL-6, COX-2 and PGE2 were investigated using a commercially available ELISA kit. Values are mean ± SD. A student’s t test was performed to evaluate the difference. *P<0.05; **P<0.01.
Co-culture with BMSCs decreased apoptosis of AF cells induced by IL-1β
By using flow cytometric analysis of annexin V-FITC/PI staining, the effects of the BMSC co-culture on AF cells were analyzed (Figure 6). Quantitative analysis indicated that, compared with the cells in the control group, the apoptotic rate was significantly elevated in the AF cells induced by IL-1β; and the up-regulation of the apoptotic rate was suppressed by the BMSC co-culture (control, 4.20±1.01%; IL-1β, 20.23±2.04%; co-culture, 12.87±2.21%).
Figure 6.

Effects of the co-culture with BMSCs on apoptosis in the control, IL-1β, co-culture groups. Changes in apoptotic incidence in the control, IL-1β and co-culture groups. Data are presented as the mean ± SD. A student’s t test was performed to evaluate the difference. *P<0.05; **P<0.01.
During the apoptotic process, pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2 emerged as key regulators. As shown in Figure 7A, Western blots from the present study revealed a significant up-regulation in the expression of Bax and down-regulation in the expression of Bcl2 stimulated by IL-1β. This imbalance of Bax/Bcl-2 was partially reversed by coculture of the AF cells with BMSCs.
Figure 7.

Effects of the co-culture with BM-MSCs on apoptosis and the mitochondrial membrane potential in the control, IL-1β and coculture groups. A. The changes in expression of Bax and Bcl-2 in AF cells from both groups were detected by Western blotting. B. Changes in the mitochondrial membrane potential in AF cells in each group. Bar = 100 μm. C. Changes in caspase-3 activity in AF cells from the control, IL-1β, co-culture groups were assayed with an ELISA Data are presented as the mean ± SD. A student’s t test was performed to evaluate the difference. *P<0.05; **P<0.01.
The activation of caspase 3 has been identified as an early indicator of apoptosis, and it acts as an executioner in the final steps of the apoptotic program. Our results revealed that the expression of caspase-3 was significantly elevated following IL-1β, and co-culture with BMSCs inhibited the upregulation of caspase-3 (Figure 7C).
Co-culture with BMSCs increased Δψm in IL-1β induced AF cells
The normal AF cells stained with JC-1 exhibited red mitochondrial fluorescence with a little green fluorescence, suggesting that the cells were in a natural condition. In contrast, the cells stimulated with IL-1β showed a weak red fluorescence with a stronger green fluorescence, reflecting the loss of Δψm. Co-culture with BMSCs increased the proportion of cells with a healthy Δψm (Figure 7B). The changes in fluorescence color from red to green demonstrated that IL-1β can induce a decrease in the mitochondrial membrane potential, and following co-culture with BMSCs, it could reverse the effect.
Discussion
In this study, we first looked at the protective capacity of BMSC-secreted factors on IL-1β damaged AF cells. With the inhibiting effect of NF-κB signaling, the anti-inflammatory effects of BMSCs were investigated. Likewise, the anti-apoptotic effect was also assessed, and mitochondria signals were inhibited by BMSC paracrine activity.
To clarify the role of the paracrine mechanism on treatment for IDD, both BMSCs and AF cells were stimulated by IL-1β in a transwell co-culture system that we established in vitro. It was found that the up-regulation of NF-κB-p65 induced by IL-1β was significantly reduced in the co-cultured group. Conversely, the expression of IκBa was induced in the co-cultured group. We also confirmed that combined treatment with BMSCs and IL-1β led to lower gene and protein expression than IL-1β alone, and that all changes to MMP-3, MMP-13, TIMP-1, and inflammatory mediators involving NO, PGE-2, COX-2, IL-6 were reversed. In addition, following BMSC co-culture, there was a reduction in the apoptosis rate of AF cells and pro-apoptotic caspase-3 and BAX, and there was up-regulated expression of anti-apoptotic Bcl-2. The mitochondrial membrane potential was suppressed after the co-culture. Our results suggested that the paracrine mechanism of BMSCs plays a potentially important role in the disc degeneration delaying effect.
First, we established a precise co-cultured transwell system in vitro. By interfering with IL-1β, we imitated the inflammatory micro-environment in disc degeneration in vitro. With this, we had a better approach for clarifying the paracrine function of BMSCs on AF cells. Many researchers have emphasized the paracrine function of MSCs, concluding that this function plays a significant role in the beneficial effects observed in both in vitro and in vivo experiments [20]. This indirect co-culture system could guarantee these two types of cells only communicated with each other by the factors they secreted. According to the literature, the effect of BMSCs and their signaling pathway toward AF cells have not been studied further. A recent study co-cultured AF cells and BMSCs in a 3D co-culture system, and there was up-regulated expression of extracellular matrix synthesis and proliferation after a 3-week co-culture [24]. By establishing a transwell co-culture system, our experiments focused on the therapeutic mechanism of BMSCs on AF cells exposed to IL-1β in vitro. The choice of IL-1β (10 ng/ml) as an inflammatory stimulus represents the simplification of a complex inflammatory milieu in IDD [23]. By eliciting the expression of matrix-degrading enzymes, inflammatory mediators and apoptosis, IL-1β leads to ECM degeneration in AF tissues. Our results confirmed that these paracrine factors, including anti-inflammatory and anti-apoptotic factors, were accessed by the co-culture system. This indicated that paracrine mechanisms play an essential role in diminishing the pathology of IDD.
Next, we performed a further in-depth study to demonstrate the NF-κF signal pathway as well as inflammatory effects were impeded with the treatment of BMSCs. Our previous study showed that BMSCs could attenuate the expression of MMP-3, ADAMTS-4/5 and the apoptotic rate of inflammatory factor stimulated NP cells via paracrine mechanisms [25]. However, the signaling pathway has not been shown to be involved. Many assays have investigated this, as a significant signaling pathway, and NF-κF was implicated as a key mediator of disc degeneration [10]. A study reported that, by co-culturing BMSCs and NP cells in a contact co-culture system, the expression of aggrecan, CII, and SOX-9 were increased and the NF-κB content was significantly lowered [26]. Association of IκB with the NF-κB p65/p50 dimer plays a significant role in regulating nuclear translocation and target gene transcription by NF-κB. It is well established that IκB degradation induces the nuclear translocation of p65 [27]. Thus, we assessed the level of IκBa and NF-κB-p65 in IL-1β-stimulated AF cells following BMSC treatment. Our data showed that IL-1β increased nuclear p65 and decreased the level of IκBa. Meanwhile, with NF-κF inhibition by the BMSC co-culture, the expression of MMP-3 and MMP-13 were decreased with increasing collagen I and aggrecan synthesis in AF cells. Correspondingly, inflammatory cytokines, such as iNOS, L-6, PGE2 and COX-2, had a similar trend. These factors were stimulated by IL-1β, which also takes part in disc degradation of the proteoglycan matrix. In addition, we also studied the expression of ADAMTS-4, which plays crucial roles in aggrecan degradation of nucleus pulposus cells. However, this factor did not show a similar trend with other factors.
Another important finding from this study was that BMSC treatment decreased the level of apoptosis by inhibiting the mitochondrial pathway. By transplanting mesenchymal stem cells into a degenerative disc model, a study suggested that both disc height index decreases and the quantity of nucleus pulposus were inhibited in the MSC transplanted group. This effect may be the result of the inhibition of apoptosis by MSCs [28]. However, the signaling pathway that is involved in this anti-apoptosis effect of disc degeneration has not been reported in the literature. In this study, we revealed that the up-regulation of both the apoptosis rate and caspase-3 activated by IL-1β were reversed after treatment with the BMSC co-culture. In addition, we reported Bcl-2 family proteins, such as pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2, play a crucial role in apoptosis by regulating the permeabilization of the mitochondrial membrane. ΔΨm is another important factor regulating mitochondrial function and it acts as an indicator of the status of AF cell apoptosis [29]. We observed that the expression of pro-apoptotic Bax was markedly declined, and that of anti-apoptotic Bcl-2 was concomitantly raised in AF cells after exposure to the BMSC co-culture. Also, Δψm decreased significantly during the BMSC co-culture with AF cells compared with IL-1β. Taken together, we concluded that the mitochondrial pathway is impeded in the apoptotic process of AF cells with treatment of BMSCs.
A limitation to this study was that there was no precise in vivo model to examine the therapeutic effects of disc degeneration after treatment with BMSC paracine factors. With small spaces in the disc, it is hard to transplant BMSC-conditioned media to a degenerative disc. A recent study has reported that BMSC derived extracellular vesicles (BMSC-EVs) transplanted in a colitis model by intravenous injection, attenuated the severity of colitis as evidenced by a decrease in the disease activity index (DAI) and histological colonic damage. Their data revealed that the therapeutic effect of BMSC-EVs resulted from the reduction of pro-inflammatory mediator levels, modulation of the anti-oxidant/oxidant balance, and a decreased occurrence of apoptosis [19]. According to the special structure of the discs, a novel method should be developed and applied in the treatment of IDD.
In conclusion, our study revealed that, with the inhibiting effect of NF-κB signaling and mitochondria signaling, an anti-inflammatory effect and anti-apoptotic effect of BMSC paracrine activity was observed. Thus, our information provides new insight into the therapeutic effects of the BMSC paracrine mechanism and its relative signals in disc degeneration. Further in vivo studies are warranted.
Acknowledgements
The study was supported by the International Cooperation Program of Shanghai Science and Technology Committee (13430721000) and the Chinese National Natural Science Foundation (Grant No. 81401836). The authors thank Accdon for its linguistic assistance during the preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Modic MT, Ross JS. Lumbar degenerative disk disease. Radiology. 2007;245:43–61. doi: 10.1148/radiol.2451051706. [DOI] [PubMed] [Google Scholar]
- 2.Meisel HJ. The lumbar vertebral disc. Eur Spine J. 2006;15(Suppl 3):S301–302. doi: 10.1007/s00586-006-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- 4.Gruber HE, Hanley EN Jr. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine (Phila Pa 1976) 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ariga K, Miyamoto S, Nakase T, Okuda S, Meng W, Yonenobu K, Yoshikawa H. The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine (Phila Pa 1976) 2001;26:2414–2420. doi: 10.1097/00007632-200111150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao CQ, Liu D, Li H, Jiang LS, Dai LY. Interleukin-1 beta enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis. 2007;12:2155–2161. doi: 10.1007/s10495-007-0137-x. [DOI] [PubMed] [Google Scholar]
- 8.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 9.Elfervig MK, Minchew JT, Francke E, Tsuzaki M, Banes AJ. IL-1 beta sensitizes intervertebral disc annulus cells to fluid-induced shear stress. J Cell Biochem. 2001;82:290–298. doi: 10.1002/jcb.1153. [DOI] [PubMed] [Google Scholar]
- 10.Tisherman R, Coelho P, Phillibert D, Wang D, Dong Q, Vo N, Kang J, Sowa G. NF-kappaB signaling pathway in controlling intervertebral disk cell response to inflammatory and mechanical stressors. Phys Ther. 2016;96:704–711. doi: 10.2522/ptj.20150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, Risbud MV. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am J Pathol. 2013;182:2310–2321. doi: 10.1016/j.ajpath.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nerlich AG, Bachmeier BE, Schleicher E, Rohrbach H, Paesold G, Boos N. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann N Y Acad Sci. 2007;1096:239–248. doi: 10.1196/annals.1397.090. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Choi H, Suh MJ, Shin JH, Hwang MH, Lee HM. Effect of biphasic electrical current stimulation on IL-1 beta-stimulated annulus fibrosus cells using in vitro microcurrent generating chamber system. Spine (Phila Pa 1976) 2013;38:E1368–1376. doi: 10.1097/BRS.0b013e3182a211e3. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YH, Zhao CQ, Jiang LS, Dai LY. Cyclic stretch-induced apoptosis in rat annulus fibrosus cells is mediated in part by endoplasmic reticulum stress through nitric oxide production. Eur Spine J. 2011;20:1233–1243. doi: 10.1007/s00586-011-1718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang SD, Yang DL, Sun YP, Wang BL, Ma L, Feng SQ, Ding WY. 17beta-estradiol protects against apoptosis induced by interleukin-1 beta in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis. 2015;20:348–357. doi: 10.1007/s10495-015-1086-4. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Rong Z, Zeng M, Cao Y, Gong X, Lin L, Chen Y, Cao W, Zhu L, Dong W. Pyrroloquinoline quinone protects nucleus pulposus cells from hydrogen peroxide-induced apoptosis by inhibiting the mitochondria-mediated pathway. Eur Spine J. 2015;24:1702–1710. doi: 10.1007/s00586-014-3630-2. [DOI] [PubMed] [Google Scholar]
- 17.Acosta FL Jr, Lotz J, Ames CP. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: a critical overview. Neurosurg Focus. 2005;19:E4. doi: 10.3171/foc.2005.19.3.5. [DOI] [PubMed] [Google Scholar]
- 18.Freeman BJ, Kuliwaba JS, Jones CF, Shu CC, Colloca CJ, Zarrinkalam MR, Mulaibrahimovic A, Gronthos S, Zannettino AC, Howell S. Allogeneic mesenchymal stem cells promote healing in postero-lateral annular lesions and improve indices of lumbar intervertebral disc degeneration in an ovine model. Spine (Phila Pa 1976) 2016;41:1331–9. doi: 10.1097/BRS.0000000000001528. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, Liu YJ. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10:e0140551. doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16:579–585. doi: 10.1016/j.jcyt.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, Yu T, Wang CM, An G, Sha WH, Chen QK. Pre-activation of mesenchymal stem cells with TNF-alpha, IL-1 beta and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep. 2015;5:8718. doi: 10.1038/srep08718. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Bu F, Li T, Ding Y, Sun L, Tu T, Zhou F, Qi W, Jiang X, Fang J, Hu J, Zhu W, Sun X. Cytotoxic effects of 4-methylimidazole on bone marrow mesenchymal stem cells in vitro. Am J Transl Res. 2015;7:1736–1746. [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Zhi-Hong W, Gui-Xing Q, Bin Y, Jun C, Yi-Peng W. Extracellular signal-regulated kinase inhibition modulates rat annulus fibrosus cell response to interleukin-1. Spine (Phila Pa 1976) 2013;38:E1075–1081. doi: 10.1097/BRS.0b013e31829a6930. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Zhang XJ, Fang L, Zhao TB. Co-culture of annulus fibrosus cells and bone marrow mesenchymal stem cells. Genet Mol Res. 2015;14:3932–3938. doi: 10.4238/2015.April.27.7. [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Deng G, Tian Y, Pu Y, Cao P, Yuan W. An in vitro investigation into the role of bone marrowderived mesenchymal stem cells in the control of disc degeneration. Mol Med Rep. 2015;12:5701–5708. doi: 10.3892/mmr.2015.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao C, Zou J, Liu X, Shapiro A, Moral M, Luo Z, Shi Q, Liu J, Yang H, Ebraheim N. Bone marrow mesenchymal stem cells slow intervertebral disc degeneration through the NF-kappaB pathway. Spine J. 2015;15:530–538. doi: 10.1016/j.spinee.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002;64:883–888. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, Tang T, Ebraheim NA. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010;10:802–810. doi: 10.1016/j.spinee.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Tang Y, Zhang Z, Zhang X, Yao Y, Fu C, Wang X, Ma G. Inhibiting the mobilization of Ly6C (high) monocytes after acute myocardial infarction enhances the efficiency of mesenchymal stromal cell transplantation and curbs myocardial remodeling. Am J Transl Res. 2015;7:587–59. [PMC free article] [PubMed] [Google Scholar]


