Abstract
Introduction: MicroRNAs (miRNAs) has emerged as important factors in osteogenesis and chondrogenesis. This study aimed to determine whether miR-221 is involved in the regulation of osteoporosis and its underlying mechanism. Methods: Total RNA was extracted from fresh femoral neck trabecular bone from women undergoing hip replacement due to either osteoporotic fracture (OP group, n = 12) or osteoarthritis in the absence of osteoporosis (Control group, n = 12). Gene expression was quantified using TaqMan quantitative RT-PCR assays and protein production was determined by western blot analysis. The role of miR-221 in osteoblast differentiation was identified by gain or loss function experiment. MiRNA targets were identified using bioinformatics and luciferase reporter assay. Results: MiR-221 was down-regulated in the osteoporotic samples compared with non-osteoporotic controls, and decreased in a C2C12 cell model of osteogenic differentiation. Overexpression of miR-221 resulted in a decrease in the osteogenic potential, as indicated by the reduced expression levels of key osteoblast markers, including osteocalcin (OC), alkaline phosphatase (ALP) and collagen, type I, α 1 (COL1A1), whereas inhibition of miR-221 promoted the activity of OC, ALP and COL1A1. Then bioinformatic analysis identified potential target sites of the miR-221 located in the 3’ untranslated regions of RUNX2. Western blot analysis demonstrated that miR-221 inhibited RUNX2 gene expression. Furthermore, dual-luciferase reporter assays confirmed that RUNX2 was a direct target of miR-221. Rescue experiments showed that overexpression of RUNX2 significantly attenuated the effect of miR-221 on osteoblast markers providing strong evidence that miR-221 mediated the osteoblast differentiation by targeting RUNX2. Conclusions: Taken together, these data implied that miR-221 played an important part in osteoporosis through regulating RUNX2 expression and osteoblast differentiation.
Keywords: Osteoporosis, osteoblast, osteogenic differentiation, microRNA-221, RUNX2
Introduction
Osteoporosis is a common osteopenic disease in postmenopausal women characterized by low bone mineral density (BMD) and low trauma fractures. It is well known that excessive bone resorption by osteoclasts over bone formation by osteoblasts would lead to the decrease of bone mineral density and disruption of bone microarchitecture [1,2], followed by imbalance of bone remodeling, and finally result in osteoporosis. Therefore, understanding the underlying mechanism involved in the osteoblast differentiation and activation processes is in urgent need for the treatment of osteopenic diseases.
MicroRNAs (miRNAs) are short non-coding RNA molecules of 17-25 nucleotides that control gene expression at the post-transcriptional level and have been shown to play critical roles in a wide variety of biological processes. Moreover, increasing evidences have indicated that bone development and homeostasis is regulated by miRNAs [3-6]. One of the most studied miRNAs in osteoblasts and osteoporosis is miR-2861, which is relatively overexpressed in ST2 stromal cells during bone morphogenetic protein 2-induced (BMP2-induced) osteogenesis [7]. MiR-2861 promotes osteoblast differentiation and bone formation by targeting HDAC5, an enhancer of runt-related transcription factor 2 (RUNX2) degradation [7]. MiR-221 and its homologue miR-222 are expressed in different stem cell lines including bone mesenchymal stem cells (BMSCs), and participate in multiple biological behaviors of stem cells [8-11]. Given that osteoclasts are bone-resorptive multinucleated giant cells derived from hematopoietic stem cells, we speculated that miR-221 may be involved in the osteoblast differentiation.
The aim of the present study is to characterize the expression of miR-221 family in osteoporosis and investigate its effects on osteoblast differentiation, as well as the potential molecular mechanisms.
Materials and methods
Bone samples and RNA extraction
Twelve postmenopausal women undergoing hip replacement due to osteoporotic fracture (OP) were enrolled as the experiment group. Another 12 postmenopausal women who were diagnosed as osteoarthritis in the absence of osteoporosis, according to bone mineral density (BMD) and T-score measurements [mean ± SD]: (0.79 ± 0.14 and -0.28 ± 0.83, respectively), were included as the control group (Table 1). None of the participants had a history of other disease including metabolic or endocrine disease, chronic renal failure, chronic liver disease, malignancies, Paget’s disease of bone, malabsorption syndrome, hormone replacement therapy, anti-resorptive or anabolic agents, oral corticosteroids, anti-epileptic drugs, or treatment with lithium, heparin, or warfarin. This study was approved by The First Affiliated Hospital of Soochow University. Written informed consent was obtained from all participants.
Table 1.
Patient characteristics
| n | Age (years) | T-score | BMD (g/cm2) | |
|---|---|---|---|---|
| Osteoporotic | 12 | 63.16 ± 3.14 | -3.33 ± 0.35 | 0.47 ± 0.07 |
| Non-osteoporotic | 12 | 62.53 ± 7.32 | 0.52 ± 0.47 | 0.84 ± 0.04 |
BMD, Bone mineral density; Data are expressed as the mean ± SD.
Bony fragments were extracted from the transcervical region of the femoral neck. Total fresh bone samples were cut into small fragments, triple washed in phosphate buffered solution (PBS), and stored at -80°C. For RNA extraction, bone samples were put in Trizol® (Life Technologies) and homogenized with a tissue homogenizer (Omni International, Kennesaw, GA). RNA was extracted and purified with RNeasy kit (Qiagen, Venlo, Netherlands). The concentration of the purified RNA was analyzed on a spectrophotometer (Nanodrop, Thermo Fisher Scientific Inc).
Reagents, antibodies and plasmids
Bioactive recombinant human BMP-2 was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Anti-RUNX2 (sc-390351), anti-GAPDH (sc-365062), mouse monoclonal antibodies, and the horseradish peroxide-conjugated mouse IgG secondary antibody (sc-2025) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). MiR-221 mimic/inhibitor, mimic/inhibitor control, and RUNX2-siRNA (sc-37145) were obtained from Santa Cruz Biotechnology, Inc.
Cell culture, differentiation and transfection
The C2C12 cell line is a typical pluripotent mesenchymal precursor cell line that possesses the potential to differentiate into myoblasts, chondroblasts and osteoblasts [12,13], and we applied it for the cellular models of osteogenic differentiation. C2C12 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbeccos modified Eagles medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100 µg/ml streptomycin and 100 U/ml penicillin (Gibco Life Technologies, Carlsbad, CA, USA), maintain at 37°C in a humidified atmosphere with 5% CO2. To induce osteogenic differentiation, cells were treated with 2 nM BMP2 (Invitrogen Life Technologies) for 24 h. Transfection was performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Quantitative polymerase chain reaction (qPCR)
Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies), and for quantification of mRNA expression, cDNA was synthesized from 1 µg total RNA using a Reverse Transcription kit (Invitrogen Life Technologies), according to the manufacturers’ instructions. qPCR was performed using an ABI 7900 Real-Time PCR System (Applied Biosystems Life Technologies, Foster City, CA, USA), using the following protocol: 95°C for 3 min, 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 30 sec. The primers used were as follows: Runx2 forward, 5’-AGATGACATCCCCATCCATC-3’ and 5’-GTGAGGGATGAAATGCTTGG-3’; OC forward, 5’-CTGACAAAGCCTTCATGTCCAA-3’ and reverse, 5’-GGTAGCGCCGGAGTCTGTT-3; COL1A1 forward, 5’-GGGTCTAGACATGTTCAGCTTTGTG-3’ and reverse, 5’-ACCCTTAGGCCATTGTGTATGC-3’; ALP forward, 5’-GACAAGAAGCCCTTCACTGC-3’ and reverse, 5’-AGACTGCGCCTGGTAGTTGT-3’; β-actin forward, 5’-AACAGTCCGCCTAGAAGCAC-3’ and reverse, 5’-CGTTGACATCCGTAAAGACC-3’. For the quantification of miRNA expression, the reverse transcription (RT) reaction was carried out using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). RT reaction was processed at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. Gene expression levels were quantified at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. RNU6B served as the internal control.
ALP staining and measurement
The transfected C2C12 cells were fixed with 95% ethanol (v/v) and then incubated with a substrate solution from an ALP staining kit (Beyotime® Institute of Biotechnology, Shanghai, China) in the dark, according to the manufacturer’s protocol. For ALP activity assays, after incubation, the treated cells were washed twice with PBS, and stained with an ALP staining solution (Sigma-Aldrich) at 37°C for 20 min. ALP activity was assayed by a spectrophotometric method using a p-Nitrophenyl Phosphate Liquid Substrate System (Sigma-Aldrich). The absorbance at 405 nm of each well was measured with a microplate reader (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s instruction.
Western blot analysis
The cells lysates were added with sample buffer and boiled at 95°C for 5 min. Protein samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The membranes were blocked by 5% BSA for 30 min and incubated with the primary antibodies at 4°C overnight, followed by incubation with secondary antibodies. Proteins were visualized using an enhanced chemiluminescence method kit (GE Healthcare Life Sciences, Piscataway, NJ, USA), and the protein band intensity was quantified via densitometric analysis using Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Luciferase reporter assay
TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org), and PicTar database (http://pictar.mdc-berlin.de/) software were used in the study to identify miR-221 targets. Then, cells were seeded in 24-well plates at a density of 5×104 cells per well, one day prior to transfection. MiRNA-221 mimic or inhibitor (50 pmol), 500 ng luciferase reporter and 50 ng pRL-TK were added to each well. Firefly and Renilla luciferase activities were determined 48 h after transfection using the dual-luciferase reporter assay system (Promega Corporation, Madison, WI, USA). The Renilla values were normalized to firefly luciferase.
Statistical analysis
Statistical analyses were performed using SPSS software, version 22.0 (SPSS, Inc., Chicago, IL, USA). Each experiment was repeated three times, with all the data presented as the mean ± standard deviation. Student’s t-test and one-way ANOVA were used in either two or multiple groups for statistical significance. Spearman rank order was used to analyze the correlations between variables. P < 0.05 was considered to indicate a statistically significant difference.
Results
MiR-221 is down-regulated in OP cases
To determine whether miR-221 is related to osteogenesis and osteoporosis, the expression levels of miR-221 in OP and non-osteoporotic patients (diagnosed as osteoarthritis in the absence of osteoporosis) were analyzed using RT-qPCR. Results showed that the expression of miR-221 in patients with osteoporotic fracture was significantly lower than that of the control group (Figure 1A), indicating that miR-221 was involved in the progression of osteoporosis.
Figure 1.
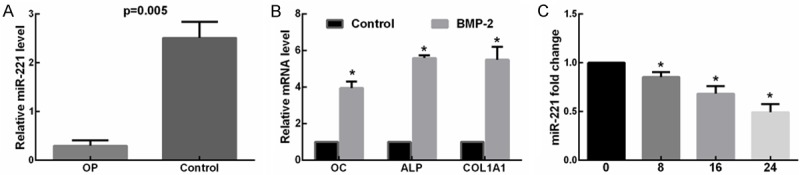
MiR-221 was downregulated in OP and during osteogenic differentiation. A. RT-qPCR was performed to determine the expression levels of miR-221 in patients with osteoporotic fracture and controls showed that miR-221 levels was elevated in bone of OP patients compared with levels observed in non-osteoporotic patients. (P = 0.005). B. RT-qPCR was performed to determine the expression levels of OC, ALP, and COL1A1 in BMP2-induced C2C12 and control cells. *P < 0.05. C. Cells were treated with BMP-2 for 0, 8, 16, and 24 h, and the relative levels of miR-221 to RNU6B were determined by TaqMan MicroRNA expression assay. The control at the 0 h time point was designated as 1. *P < 0.05.
MiR-221 is down-regulated during osteogenic differentiation
Next, we detected the expression levels of miR-221 during osteogenic differentiation. A number of key osteoblast markers, including OC, ALP and COL1A1, were used as phenotypic markers of osteogenic differentiation. The results of RT-qPCR revealed that all the markers above (OC, ALP and COL1A1) were obviously increased in the BMP2 treated cells, indicating that osteogenic differentiation had been successfully induced (Figure 1B). And miR-221 was markedly down-regulated in the BMP2-treated C2C12 cells along with the time course at 0, 8, 16, 24 hours (Figure 1C). Collectively, these results demonstrated that miR-221 was involved in the progression of osteogenic differentiation.
Effects of miR-221 on osteoblast differentiation in C2C12 cells
In order to study the physiological role of miR-221 on osteogenic differentiation, C2C12 cells were transfected with miR-221 mimic or inhibitor. RT-qPCR was performed to determine the efficiency of transfection (Figure 2A). Results showed that miR-221 overexpression markedly decreased the mRNA expression levels of OC, ALP and COL1A1 in the C2C12 cells (Figure 2B). In contrast, C2C12 cells transfected with the miR-221 inhibitor exhibited overexpression of OC, ALP and COL1A1 (Figure 2C). Then, the activities of ALP in the transfected cells were investigated. In miR-221 overexpressed C2C12 cells, the ALP activity was significantly suppressed compared with the miR-NC transfected cells, while a notable increase of the ALP activity was exhibited after the transfection of miR-221 inhibitors (Figure 2D). Collectively, the results indicated that miR-221 was able to inhibit osteogenic differentiation.
Figure 2.
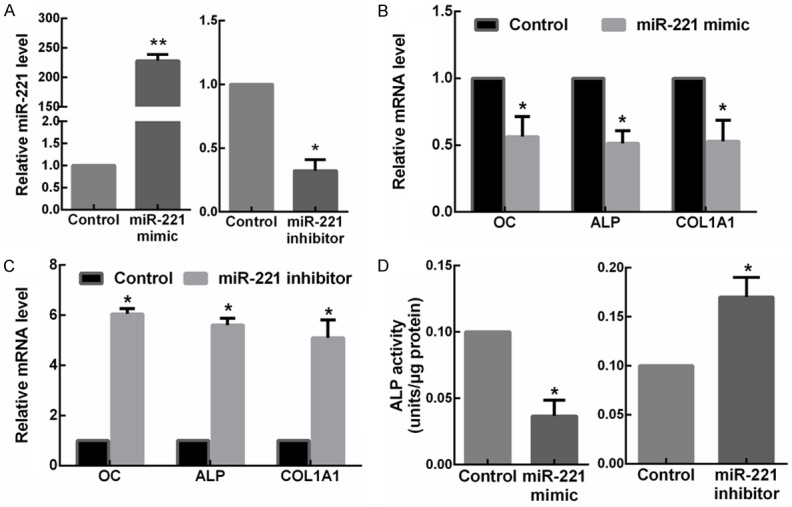
MiR-221 inhibits the osteogenic differentiation of C2C12 cells. A. The expressions of miR-221 in C2C12 cells transfected with miR-221 mimic or inhibitor detected by RT-qPCR. *P < 0.05. B. The mRNA levels of OC, ALP and COL1A1 in C2C12 cells transfected with miR-221 mimic detected by RT-qPCR. *P < 0.05. C. The mRNA levels of OC, ALP and COL1A1 in C2C12 cells transfected with miR-221 inhibitor detected by RT-qPCR. *P < 0.05. D. The ALP activity in C2C12 cells transfected with miR-221 mimic or inhibitor. *P < 0.05.
RUNX2 is a target of miR-221
To identify the target genes of miR-221 in osteogenesis, we searched for candidate genes using the miRNA target prediction database miRanda, TargetScan and PicTar. As a result, one evolutionarily conserved miRNA recognition element (MRE) partially complementary to miR-221 (Figure 3A) in the 3’-UTR of the RUNX2 gene was found. The function of miR-221 on RUNX2 during osteogenic differentiation has not been reported yet. To test whether RUNX2 could be regulated by miR-221, we transfected C2C12 cells with miR-221 mimic and inhibitor, respectively. Results showed that the protein levels of RUNX2 reduced when miR-221 was overexpressed. In contrast, the expression of RUNX2 increased with the knockdown of miR-221 (Figure 3B). These results provided evidences that miR-221 negatively regulated RUNX2 expression. Furthermore, quantitative RT-PCR assays revealed no change of RUNX2 mRNA in miR-221-overexpressing or -knockdown C2C12 cells (Figure 3C), indicating that miR-221 family members regulate RUNX2 gene expression on the basis of translational repression rather than mRNA degradation. To examine whether miR-221 regulate RUNX2 expression directly, C2C12 cells were transfected with a luciferase reporter construct containing the wild-type RUNX2 3’-UTR, together with the miR-221 mimic, mimic control, miR-221 inhibitor, and inhibitors control, respectively. Clearly Renilla luciferase activity decreased in the miR-221-overexpressed cells, while it increased in the miR-221-knockdown cells compared with the negative control (Figure 4A). Then, the predicted target sites in the RUNX2 3’ UTR were mutated (Figure 3A). As expected, miR-221 significantly inhibited the activity of the wild-type reporter gene, whereas mutation of the seed site partially abolished miR-221-mediated repression of reporter gene activity (Figure 4B). These data provided strong evidences that miR-221 inhibit RUNX2 gene expression by directly binding to the distinct seed site within its 3’ UTR.
Figure 3.
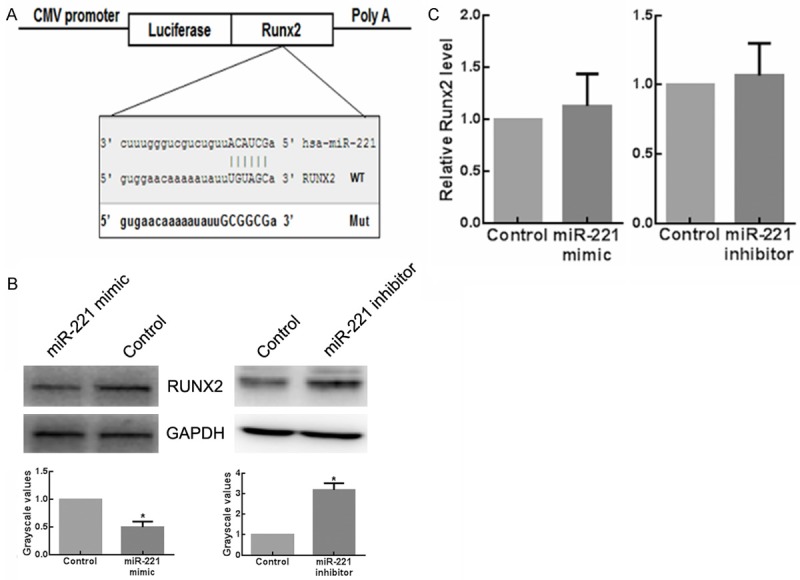
MiR-221 inhibits Runx2 expression in C2C12 cells. A. Putative miR-221-binding sequence of Runx2 RNA. Mutation (Mut) was generated on the Runx2 RNA sequence in the complementary site for the seed region of miR-221. B. The expressions of RUNX2 in C2C12 cells transfected with miR-221 mimic or inhibitor detected by western blot analysis. *P < 0.05. C. The expressions of RUNX2 in C2C12 cells transfected with miR-221 mimic or inhibitor detected by RT-qPCR. *P < 0.05.
Figure 4.
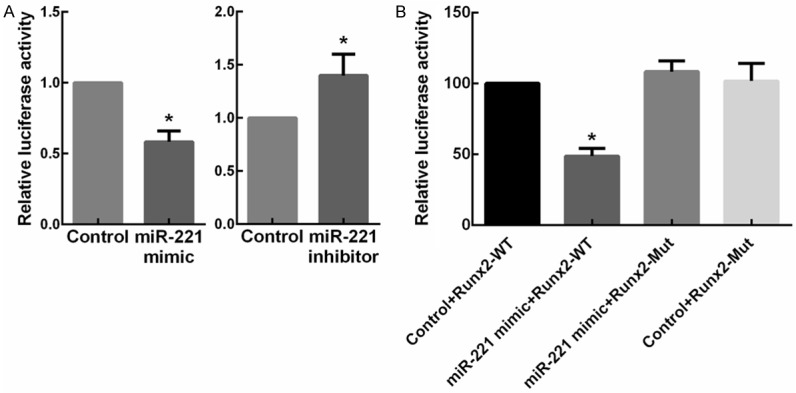
RUNX2 is a direct target of the miR-221 in C2C12 cells. A. Overexpression or knockdown of miR-221 expression inhibited or enhanced the Renilla luciferase activities. C2C12 cells were cotransfected with miR-221 mimic/inhibitor or mimic/inhibitor control, and reporter plasmid containing the wild-type (WT) 3’-UTR of RUNX2. A luciferase activity assay was subsequently performed. *P < 0.05. B. C2C12 cells were cotransfected with the miR-221 mimic and reporter plasmid containing the WT or Mut 3’-UTR of RUNX2. A luciferase activity assay was subsequently performed. *P < 0.05.
MiR-221 inhibits osteogenic differentiation through RUNX2
To better understand the relationship between miR-221 and RUNX2 during osteogenic differentiation, C2C12 cells stimulated with BMP-2 for 24 h were evaluated for changes of endogenous RUNX2 protein expression. The increase of RUNX2 protein level was observed for 8 h after stimulation. The change of RUNX2 protein expression was negatively correlated with that of miR-221 during BMP-2 stimulation (Figure 5A). Furthermore, co-transfection of miR-221 inhibitor with a RUNX2 siRNA significantly decreased the mRNA expression levels of OC, ALP and COL1A1 (Figure 5B). Thus, knockdown of RUNX2 appeared to attenuate the miR-221-knockdown-mediated promotion of osteogenic differentiation. These results indicated that miR-221 suppressed osteogenic differentiation, in part via the downregulation of RUNX2 expression.
Figure 5.
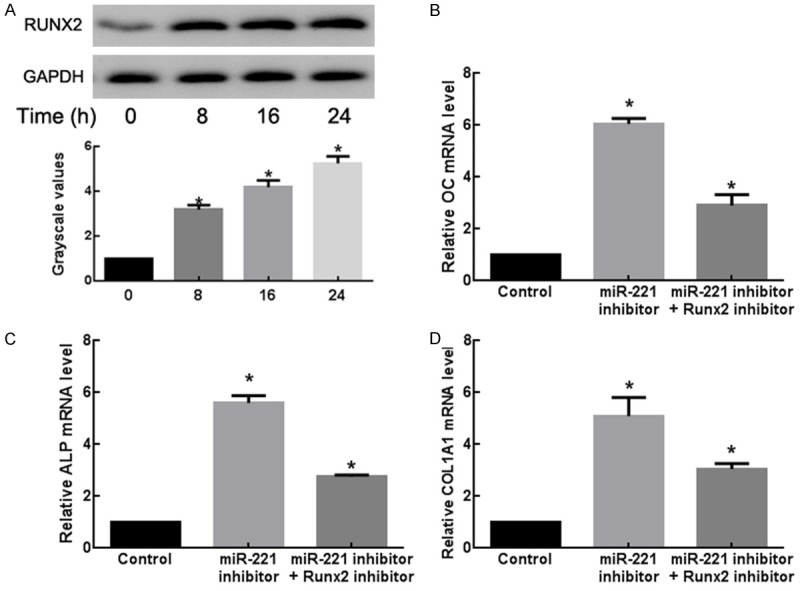
MiR-221 inhibits osteogenic differentiation of C2C12 cells by targeting RUNX2. A. Dynamic changes of RUNX2 protein levels in BMP-2-induced C2C12 cells. The protein samples from Figure 1C were determined by Western blot assays. *P < 0.05. B. The expressions of OC in C2C12 cells transfected with miR-221 control, inhibitor or miR-221 inhibitor + RUNX2 inhibitor detected by RT-qPCR. *P < 0.05. C. The expressions of ALP in C2C12 cells transfected with miR-221 control, inhibitor or miR-221 inhibitor + RUNX2 inhibitor detected by RT-qPCR. *P < 0.05. D. The expressions of COL1A1 in C2C12 cells transfected with miR-221 control, inhibitor or miR-221 inhibitor + RUNX2 inhibitor detected by RT-qPCR. *P < 0.05.
Discussions
Osteoporosis is a common and progressive skeletal disorder-related disease worldwide, especially in countries with large aging populations, such as China [14]. Collaborating with osteoclasts, osteoblasts have been widely accepted as the main cells that regulate bone homeostasis [15]. By secreting alkaline phosphatase (ALP) and bone matrix proteins that induce bone matrix mineralization, osteoblasts act as the major cells that contribute to bone formation [16]. Recently, numerous experiments have focused on the role of miRNAs in the process of osteoporosis and osteoblast differentiation [7,17,18]. This study presents an important finding that the expression of miR-221 is downregulated in the bone from women with postmenopausal osteoporosis (OP), and downregulated in the C2C12 cells during osteoclast differentiation. Importantly, miR-221 functions as an inhibitor of osteogenic differentiation via downregulating osteoblast-specific transcription factor RUNX2, implying a critical function of miR-221 in the development of osteoporosis.
MiRNAs are essential regulatory molecules and can orchestrate gene expressions implicated in the regulation of bone homeostasis through translational inhibition and mRNA stability. They are proved to be regulators of bone resorption and formation, and also participate extensively in the osteoblast differentiation, cell fate, and apoptosis [19]. Among them, miR-221 has been demonstrated to be downregulated during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells [20], while its expression and role in osteoclast differentiation has not been studied. We investigated the effects of miR-221 on BMP-2-induced osteogenic differentiation and found that knockdown of miR-221 could induce osteogenic differentiation, indicated by the increase of the mRNA expression levels of the typical osteoblast differentiation markers, OC, ALP and COL1A1, whereas overexpression of miR-221 led to the decrease of the expression levels of these markers. These data suggested miR-221 as a negative regulator in osteoblast differentiation induced by BMP-2 of preosteoblast cell lines.
RUNX2, a transcription factor that belongs to the runt-domain genes, has been taken as the prodominant transcription factor for osteoblast differentiation and bone formation [21]. RUNX2 is restrictively expressed in fetal development, and disruption of RUNX2 results in a complete lack of bone formation owing to maturational arrest of osteoblasts [22]. It has been reported that emodin treatment can suppress osteoclast differentiation and stimulate osteoblast formation by increasing the expression of RUNX2, indicating that RUNX2 may be a potential target for the treatment of osteoporosis [23]. So far, several miRNAs were reported to target RUNX2 and regulate its expression in different physiologic or pathologic conditions. For example, miR-30a regulates the proliferation, migration, and invasion of human osteosarcoma by targeting RUNX2 [24]. MiR-320 was found to promote adipocytic differentiation and bone marrow adipogenesis via directly targeting on the 3’-UTR of RUNX2 [25]. Moreover, some other miRNAs have been demonstrated to modulate osteoblastic differentiation by regulating RUNX2 directly or indirectly [26-30]. Our studies found new miRNA directly targeting RUNX2 in osteoblast differentiation, indicating that RUNX2 could be regulated by different miRNAs through different mechanisms in different microenviroment.
Besides osteoblast differentiation, it would be interesting to investigate the role of miR-221 in osteoblast and osteoclast function, or particularly in chondrocyte differentiation, as RUNX2 signaling is also involved in chondrogenesis [31]. MiR-221 has been reported to modulate proliferation and matrix synthesis in chondrocyte [32], and exhibit pro-chondrogenic effect in human MSCs [33]. Future experiments are needed to address miR-221 functions in depth.
In summary, the present study demonstrated that miR-221 was downregulated in bone of osteoporotic patients compared with the control osteoarthritic samples, and participated in osteogenic differentiation. Furthermore, miR-221 acts as a negative regulator of osteoblast differentiation through directly targeting RUNX2, which would monitor the pathological process of osteoporosis. Hence, this study illustrated a potential crucial function of miR-221 in the development of osteoporosis, and may provide a promising therapeutic target against disorders associated with bone formation.
Disclosure of conflict of interest
None.
References
- 1.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 2.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Qiu M, Dou C, Cao Z, Dong S. MicroRNAs in Bone Balance and Osteoporosis. Drug Dev Res. 2015;76:235–245. doi: 10.1002/ddr.21260. [DOI] [PubMed] [Google Scholar]
- 4.Fang S, Deng Y, Gu P, Fan X. MicroRNAs regulate bone development and regeneration. Int J Mol Sci. 2015;16:8227–8253. doi: 10.3390/ijms16048227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Y, Zhang L, Gao Y, Ge W, Tang P. The Multiple Roles of Microrna-223 in Regulating Bone Metabolism. Molecules. 2015;20:19433–19448. doi: 10.3390/molecules201019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan MQ, Tye CE, Stein GS, Lian JB. Non-coding RNAs: Epigenetic regulators of bone development and homeostasis. Bone. 2015;81:746–756. doi: 10.1016/j.bone.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, Xu K, Sheng ZF, Zhou HD, Wu XP, Luo XH. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009;119:3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ke J, Zhao Z, Hong SH, Bai S, He Z, Malik F, Xu J, Zhou L, Chen W, Martin-Trevino R, Wu X, Lan P, Yi Y, Ginestier C, Ibarra I, Shang L, McDermott S, Luther T, Clouthier SG, Wicha MS, Liu S. Role of microRNA221 in regulating normal mammary epithelial hierarchy and breast cancer stem-like cells. Oncotarget. 2015;6:3709–3721. doi: 10.18632/oncotarget.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu A, Kang N, He L, Li X, Xu X, Zhang H. MiR-221 and miR-26b regulate chemotactic migration of MSCs toward HGF through activation of Akt and FAK. J Cell Biochem. 2016;117:1370–83. doi: 10.1002/jcb.25428. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Zhao L, Ischenko I, Bao Q, Schwarz B, Niess H, Wang Y, Renner A, Mysliwietz J, Jauch KW, Nelson PJ, Ellwart JW, Bruns CJ, Camaj P. Antisense inhibition of microRNA-21 and microRNA-221 in tumor-initiating stem-like cells modulates tumorigenesis, metastasis, and chemotherapy resistance in pancreatic cancer. Target Oncol. 2015;10:535–48. doi: 10.1007/s11523-015-0360-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, Wang S, Wu X, Yang T, Huang F, Cai J, Zhu Q, Zhu W, Qian H, Xu W. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110:1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin CS, Lecanda F, Sheikh S, Weitzmann L, Cheng SL, Civitelli R. Relative abundance of different cadherins defines differentiation of mesenchymal precursors into osteogenic, myogenic, or adipogenic pathways. J Cell Biochem. 2000;78:566–577. [PubMed] [Google Scholar]
- 14.Feng Z, Liu C, Guan X, Mor V. China’s rapidly aging population creates policy challenges in shaping a viable long-term care system. Health Aff (Millwood) 2012;31:2764–2773. doi: 10.1377/hlthaff.2012.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Li T, Fan J, Li T, Fan L, Wang S, Weng X, Han Q, Zhao RC. miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ. 2015;22:1935–1945. doi: 10.1038/cdd.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De-Ugarte L, Yoskovitz G, Balcells S, Guerri-Fernandez R, Martinez-Diaz S, Mellibovsky L, Urreizti R, Nogues X, Grinberg D, Garcia-Giralt N, Diez-Perez A. MiRNA profiling of whole trabecular bone: identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genomics. 2015;8:75. doi: 10.1186/s12920-015-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Qiu M, Dou C, Cao Z, Dong S. MicroRNAs in Bone Balance and Osteoporosis. Drug Dev Res. 2015;76:235–45. doi: 10.1002/ddr.21260. [DOI] [PubMed] [Google Scholar]
- 20.Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9:e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 22.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Cheon YH, Kwak SC, Baek JM, Yoon KH, Lee MS, Oh J. Emodin regulates bone remodeling by inhibiting osteoclastogenesis and stimulating osteoblast formation. J Bone Miner Res. 2014;29:1541–1553. doi: 10.1002/jbmr.2183. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, Yan S, Wang J, Deng F, Guo Y, Li Y, Fan M, Song Q, Liu H, Weng Y, Shi Q. MiR-30a regulates the proliferation, migration, and invasion of human osteosarcoma by targeting Runx2. Tumour Biol. 2015 doi: 10.1007/s13277-015-4086-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Hamam D, Ali D, Vishnubalaji R, Hamam R, Al-Nbaheen M, Chen L, Kassem M, Aldahmash A, Alajez NM. microRNA-320/RUNX2 axis regulates adipocytic differentiation of human mesenchymal (skeletal) stem cells. Cell Death Dis. 2014;5:e1499. doi: 10.1038/cddis.2014.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du F, Wu H, Zhou Z, Liu YU. microRNA-375 inhibits osteogenic differentiation by targeting runt-related transcription factor 2. Exp Ther Med. 2015;10:207–212. doi: 10.3892/etm.2015.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu N, Feng C, Jiang Y, Miao Q, Liu H. Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and ERK/MAPK Pathway. Int J Mol Sci. 2015;16:10491–10506. doi: 10.3390/ijms160510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, He X, Wei W, Zhou X. MicroRNA-194 promotes osteoblast differentiation via downregulating STAT1. Biochem Biophys Res Commun. 2015;460:482–488. doi: 10.1016/j.bbrc.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Sun Q, Wan C, Li L, Zhang L, Chen Z. MicroRNA-338-3p regulates osteogenic differentiation of mouse bone marrow stromal stem cells by targeting Runx2 and Fgfr2. J Cell Physiol. 2014;229:1494–1502. doi: 10.1002/jcp.24591. [DOI] [PubMed] [Google Scholar]
- 30.Qiao W, Chen L, Zhang M. MicroRNA-205 regulates the calcification and osteoblastic differentiation of vascular smooth muscle cells. Cell Physiol Biochem. 2014;33:1945–1953. doi: 10.1159/000362971. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Yang TL, Li X, Guo Y. Functional analyses reveal the essential role of SOX6 and RUNX2 in the communication of chondrocyte and osteoblast. Osteoporos Int. 2015;26:553–561. doi: 10.1007/s00198-014-2882-3. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Zhang L, Gibson GJ. Chondrocyte miRNAs 221 and 483-5p respond to loss of matrix interaction by modulating proliferation and matrix synthesis. Connect Tissue Res. 2015;56:236–243. doi: 10.3109/03008207.2015.1018384. [DOI] [PubMed] [Google Scholar]
- 33.Lolli A, Lambertini E, Penolazzi L, Angelozzi M, Morganti C, Franceschetti T, Pelucchi S, Gambari R, Piva R. Pro-chondrogenic effect of miR-221 and slug depletion in human MSCs. Stem Cell Rev. 2014;10:841–855. doi: 10.1007/s12015-014-9532-1. [DOI] [PubMed] [Google Scholar]


