Abstract
Aims
Exposure to particulate air pollution is associated with increased blood pressure (BP), a well-established risk factor for cardiovascular disease. To elucidate the mechanisms underlying this relationship, we investigated whether the effects of particulate matter of less than 10 μm in aerodynamic diameter (PM10) on BP are mediated by microRNAs.
Methods and results
We recruited 90 obese individuals and we assessed their PM10 exposure 24 and 48 h before the recruitment day. We performed multivariate linear regression models to investigate the effects of PM10 on BP. Using the TaqMan® Low-Density Array, we experimentally evaluated and technically validated the expression levels of 377 human miRNAs in peripheral blood. We developed a mediated moderation analysis to estimate the proportion of PM10 effects on BP that was mediated by miRNA expression.
PM10 exposure 24 and 48 h before the recruitment day was associated with increased systolic BP (β=1.22 mmHg, P=0.019; β=1.24 mmHg, P=0.019, respectively) and diastolic BP (β=0.67 mmHg, P=0.044; β=0.91 mmHg, P=0.007, respectively). We identified nine miRNAs associated with PM10 levels 48 h after exposure. A conditional indirect effect (CIE=−0.1431) of PM10 on diastolic BP, which was mediated by microRNA-101, was found in individuals with lower values of mean body mass index.
Conclusions
Our data provide evidence that miRNAs are a molecular mechanism underlying the BP-related effects of air pollution exposure, and indicate miR-101 as epigenetic mechanism to be further investigated.
Abbreviations: CVD, Cardiovascular disease; BP, Blood pressure; PM, Particulate matter; PM10, PM of less than 10 μm in aerodynamic diameter; miRNAs, MicroRNAs; MI, Myocardial infarction; BMI, Body mass index; CIE, Conditional indirect effect; FARM, Flexible Air Quality Regional Model; TLDA, TaqMan Low-Density Array; FDR, False Discovery Rate; IPA, Ingenuity Pathway Analysis
keywords: Air pollution, Microrna, Blood pressure, Cardiovascular disease, Obesity
Highlights
-
•
We investigated whether the effects of PM10 on BP are mediated by microRNAs.
-
•
PM10 exposure was associated with increased systolic and diastolic BP.
-
•
Nine blood miRNAs were associated with PM10 levels 48 h after exposure.
-
•
miR-101 mediated the effects of particle exposure on diastolic BP.
1. Introduction
According to the World Health Organization (WHO), the effects of exposure to ambient air pollution result in approximately 7 million premature deaths worldwide each year, representing one out of every eight deaths from any cause. In 2010, there were an estimated 600,000 premature deaths in Europe, including 32,447 deaths in Italy (WHO Regional Office for Europe, 2015).
Previous studies of the public health relevance of exposure to air pollution have shown that this factor is one of the main triggers of cardiovascular disease (CVD), with incidence and risk rates in CVD similar to those of alcohol consumption and cocaine abuse (Nawrot et al., 2011). Elevated blood pressure (BP) is a well-established risk factor for CVD. An increase in systolic BP of 1 mmHg has been estimated to raise the risk of CVD death by 2–4% (Stamler et al., 1993, Van den Hoogen et al., 2000). Data from epidemiological and controlled human studies have shown that exposure to particulate matter (PM), a major air pollutant made of small particles emitted from motor vehicles and industries (Brook and Rajagopalan, 2009a, Brook et al., 2009b, Dvonch et al., 2009, Urch et al., 2005), increases BP after exposure times ranging from 2 h to 8 days. Although several studies have demonstrated the harmful effects of air pollution exposure on cardiovascular outcomes (Pulliero et al., 2014, Izzotti et al., 2015), including BP (Jardim et al., 2009), the intermediate mechanisms by which these effects occur remain largely unexplored.
Blood microRNAs (miRNAs) have been suggested to be an active component of the cellular response to PM exposure. MiRNAs are single-stranded short noncoding RNA sequences of ~22 nucleotides that regulate gene expression at the posttranscriptional level. Altered miRNA expression is an established mechanism by which chemical agents induce alterations in target cells. Protective mechanisms such as the p53-related activation of DNA-repair processes, cell-cycle arrest, and apoptosis have been suggested to be activate by miRNAs belonging to the let-7 and miR-34 families (Izzotti and Pulliero, 2014). It has been shown experimentally that PM triggers local pulmonary inflammation and oxidative stress, leading to the release of pro-inflammatory signals, such as cytokines (Nemmar et al., 2001), and alterations of miRNA levels in the blood. Moreover, numerous miRNAs are known to be expressed in the cardiovascular system, supporting the possibility that most physiological and pathological events in the cardiovascular system are controlled, at least in part, by miRNAs (Hata, 2013). Due to their easy withdrawal accessibility, circulating blood miRNAs have been investigated as potential biomarkers of CVDs, such as hypertension (Dickinson et al., 2013), heart failure (Tijsen et al., 2010), acute myocardial infarction (MI) (Wang et al., 2014), coronary artery disease (Ren et al., 2013), and stroke (Li et al., 2014).
Obesity, defined as a body mass index (BMI) of 30 kg/m2 or greater, is a strong risk factor for CVD. Obesity has been demonstrated to modify the effects of PM exposure on heart rate variability and markers of inflammation, oxidative stress, and acute phase response (Schwartz et al., 2005, Dubowsky et al., 2006, Schneider et al., 2008). These effects could be due, at least in part, to an higher PM dose rate in obese individuals, as has been demonstrated in children by Bennett and Zeman (2004). These findings suggest that obese individuals might represent a suitable population in which to investigate PM-mediated cardiovascular effects and related pathogenic mechanisms.
To the best of our knowledge, few studies have documented changes in BP levels after PM10 exposure, and no study has investigated the possible role of miRNAs as mediators of this association. In the present study, we investigated the effects of exposure to PM of less than 10 μm in aerodynamic diameter (PM10) on cardiovascular outcomes, such as systolic and diastolic BP, in overweight and obese participants living in Lombardia, Italy. We experimentally evaluated the expression levels of 377 human miRNAs in the peripheral blood of participants and analyzed the effects of PM10 exposure on miRNA patterns. Furthermore, we performed a mediated moderation analysis to estimate the proportion of PM10 effects on BP that are mediated by miRNA expression. Findings from this study have the potential to enhance current understanding of the molecular mechanisms that drive the effects of air pollution exposure on BP.
2. Material and methods
2.1. Study population
Ninety overweight and obese subjects were recruited at the Center for Obesity and Work (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico in Milan, Lombardia, Italy) from September 2010 to January 2011 as part of the cross-sectional SPHERE study (Bollati et al., 2014). Each participant provided written informed consent before inclusion in the study, which was approved by the Ethics Committee of Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico (approval number 1425). The investigation is conforming to the principles outlined in the Declaration of Helsinki.
As part of the routine protocol, each subject presenting at the Center underwent physical and anthropometric examinations on the day of recruitment. These examinations included measurements of weight, height, and BP (measured with the participant supine, after 5 min of rest). Each subject provided a 7-ml blood sample for analyses of miRNA expression (see Supplemental file: Blood collection and RNA isolation).
2.2. Assessment of PM exposure
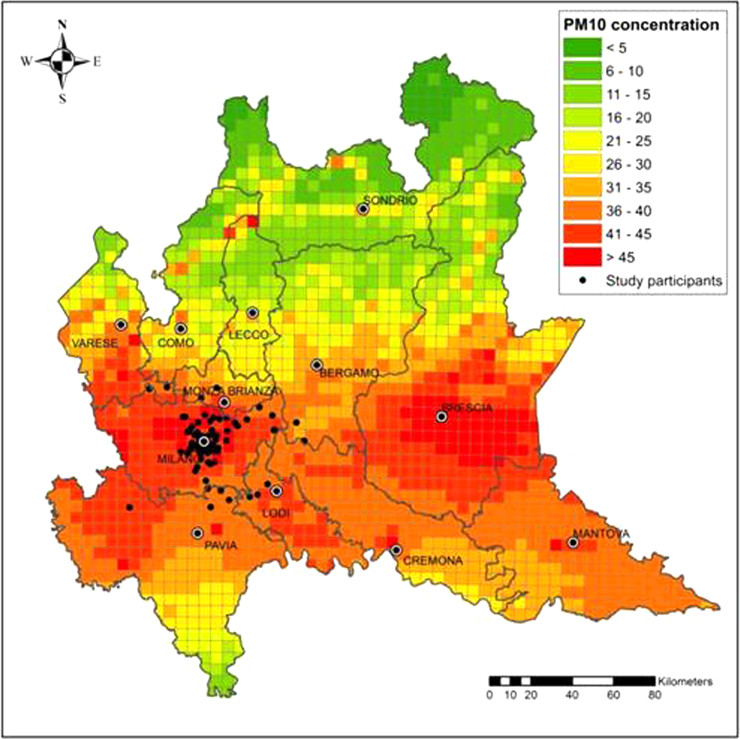
Daily PM10 concentrations were measured at each participant's residential address from September 1, 2010 to January 28, 2011. PM10 levels were estimated with the Flexible Air Quality Regional Model (FARM), a three-dimensional Eulerian 4×4 km2 grid model that considers the dispersion, transformation, and deposition of particulates. According to this model, the Lombardia Region was divided into 1678 cells, each associated with an estimated PM10 level. To assign a PM10 exposure level to each subject, the residential addresses were georeferenced, and the resulting map was superimposed on the FARM grid (Fig. 1).
Fig. 1.
Graphical representation of mean PM10 concentration levels and the distribution of participants’ residences. Mean PM10 concentrations in the Lombardia region (Italy) were predicted by the FARM model in each 4×4 km2 grid during the study period, from September 1, 2010 to January 28, 2011.
2.3. Profiling and validation of miRNA expression
MiRNA expression was profiled with the TaqMan® Low-Density Array (TLDA) (TaqMan® Array Human MicroRNA A Cards Set v2.1; Life Technologies, Carlsbad, CA). Each TLDA card detects 384 features, including 377 human miRNAs, three endogenous small RNA controls (RNU6, RNU44, and RNU48, the first in quadruplicate), and a negative control (Ath-miR159a). All reactions were performed as specified in the manufacturer's protocols. Briefly, after reverse transcription (RT) (see Supplemental file: RNA reverse transcription for miRNA expression profiling), a total reaction mixture containing RT products and the TaqMan Universal PCR Master Mix (Life Technologies) was added to each line of TLDA after gentle vortexing. Each card was centrifuged and mechanically sealed with a Life Technologies sealer device. TLDAs were run in a 7900HT Fast Real-Time PCR System (Life Technologies) under the following thermal cycler conditions: 50 °C for 2 min, 94.5 °C for 10 min, 40 cycles of 97 °C for 30 s, and 59.7 °C for 1 min. Twenty-eight candidate miRNAs were validated with a custom TLDA, on which the TaqMan® miRNA assays were preplated in triplicate (see Supplemental file: RNA RT for miRNA expression validation). The coefficient of variation for the triplicates was 0.62%. After RT, all reactions were performed as described above.
2.4. Analysis of miRNA expression
Real-time PCR quantifies miRNA expression in terms of cycle threshold (Ct). We applied restrictive miRNA selection criteria. Values that were missing, had a Ct greater than 33, or had an ampscore less than 1 were set equal to the detection limit (35 Ct). To reduce background noise, we excluded miRNAs with at least 90% of samples not expressed (Ct=35), producing a final dataset of 122 miRNAs. For each miRNA, the expression level was normalized to the average Ct of three endogenous controls (RNU6, RNU44, and RNU48). The fold change relative to the calibrator (pool of all 90 samples) was calculated for expression data analysis.
2.5. Statistical analysis
Descriptive statistical data were obtained for the demographic, physical, and anthropometric variables. The mean and standard deviation (±SD) were calculated for normally distributed data.
Associations between the daily PM10 concentrations 24 and 48 h before the recruitment day and cardiovascular parameters, such as systolic and diastolic BP, were assessed. We performed multivariate linear regression analyses adjusting for age, sex, BMI, and smoking habits. The interaction term between BMI and PM10 exposure (BMI×PM10) was tested and included in the models because it was statistically significant (P<0.05). We used linear regression models to verify the association between PM10 exposure and miRNA expression in the screening and validation phases. MiRNA expression values were log2-transformed to achieve a normal distribution. Multivariate regression analyses adjusted for age, sex, BMI, smoking habits (current smoker, non-smoker, or ex-smoker), % of granulocytes, recruitment date, and ambient apparent temperature were applied to evaluate changes in miRNA expression in association with daily PM10 concentrations.
Due to the high number of comparisons, we applied a multiple comparison correction based on the False Discovery Rate (FDR) control. A threshold of 0.10 was applied to the FDR P-value significance level to identify the set of top miRNAs. The same analyses were repeated in the validation phase on the set of 28 candidate miRNAs selected for technical validation. All statistical analyses were performed with SAS software, version 9.3.
2.6. Mediated moderation analysis
We performed mediated moderation analysis to estimate the effect of the interaction between PM10 exposure and BMI on BP, mediated by miRNA. We considered PM10 as the independent variable, miRNAs as possible mediators, BMI as a moderator, and systolic and diastolic BP as dependent variables. All variables except BP were centered before being entered into the regression models (Fig. 2 – Conceptual diagram).
Fig. 2.
Mediated moderation analysis. In the conceptual diagram, PM10 is the independent variable, miR-101 is the possible mediator, BMI is the moderator, and BP is the dependent variable. The statistical diagram shows the estimated linear regression coefficients.
Linear regression coefficients were estimated by three equations (Fig. 2 – Statistical diagram) (Muller et al., 2005):
-
•
, which estimates the total exposure effect when a moderation effect occurs between PM10 and BP;
-
•
, which estimates part of the indirect exposure effect (i.e., whether moderation occurs between PM10 and miRNAs);
-
•
, which estimates part of the indirect exposure effect (i.e., whether moderation occurs between miRNAs and BP, when the PM10 effect is controlled) and the direct exposure effect (i.e., whether moderation occurs between PM10 and BP, when the miRNAs are controlled).
MiRNA expression was log2-transformed to achieve a normal distribution. All linear regression models were adjusted for age, sex, and smoking habits.
To apply mediated moderation models, there must not be a correlation between the independent variable (PM10) and the moderator (BMI). In addition, three criteria must be satisfied. First, there must be a statistically significant moderation effect between the exposure and outcome. In other words, the magnitude of the overall PM10 exposure effect on BP must depend on the value of BMI. Second, either 1) there must be a moderation effect between PM10 exposure and miRNAs, and the miRNAs must have an effect on BP when PM10 exposure is controlled; and/or 2) there must be a moderation effect between miRNAs and BP, and PM10 exposure must have an effect on miRNAs. Third, moderation of the direct effect must be reduced in magnitude (or even nonsignificant) compared to moderation of the total effect. Only if all three criteria are satisfied we can evaluate the conditional direct and indirect effects. The conditional indirect effect (CIE) estimates the effect size of PM10 exposure on BP that is mediated by miRNAs at different BMI levels.
Mediated moderation analyses were executed on the SAS version 9.3 macro, while utilizing the PROCESS program (model 59) provided by Hayes (2013). Bootstrap confidence intervals (CIs) were provided with the number of bootstrap samples equal to 10,000.
3. Results
3.1. Characteristics and exposure of study participants
This study included 90 overweight and obese participants (16.7% men, 83.3% women), who had a mean age of 51.6 years. BMI was calculated as the weight divided by the height squared (kg/m2). Participants were classified by BMI as overweight, class I obese, class II obese and class III obese, or severe obese. Mean BMI for 90 subjects was 32.9±5.7 Kg/cm2 and mean waist circumference was 103.11±12.5 cm. The mean systolic and diastolic BP values were 124.8 and 79.2 mmHg, respectively (Table 1).
Table 1.
Characteristics of study participants (n=90).
| Characteristics | |
|---|---|
| Age,years | 51.6±11.9 |
| Sex | |
| Male | 15 (16.7%) |
| Female | 75 (83.3%) |
| BMI*,kg/m2 | 32.9±5.7 |
| Overweight (25≤BMI<30) | 34 (37.8%) |
| Class I obesity (30≤BMI<35) | 28 (31.1%) |
| Class II obesity (35≤BMI<40) | 19 (21.1%) |
| Class III obesity (BMI≥40) | 9 (10.0%) |
| Waist Circumference,cm | 103.11±12.5 |
| Smoking habits | |
| Never smoker | 48 (53.3%) |
| Ex-smoker | 26 (28.9%) |
| Current smoker | 16 (17.8%) |
| Systolic blood pressure,mmHg | 124.8±16.5 |
| Diastolic blood pressure,mmHg | 79.2±1.2 |
| Apparent temperature,°C | 8.7±7.4 |
| Granulocyte,% | 61.3±7.6 |
BMI: Body Mass Index. Continuous variable are expressed as mean±standard deviation (SD). Discrete variables are expressed as counts (%).
Under the hypothesis that the variation in miRNA expression level is a short-term mechanism (Ruegger and Grosshans, 2012), we chose to investigate a 1-week lag exposure time window before the recruitment day. We estimated the daily mean PM10 exposure levels at each participant's residential address for the 7 days before recruitment (see Supplemental file: Supplemental Table 1). Most participants (60.9%) were enrolled during the winter season, and most lived in Milan (84.9%).
3.2. Influence of PM10 exposure on BP
We studied the association of ambient PM10 lag exposure each of the seven days before the recruitment day with cardiovascular risk factors, such as systolic and diastolic BP. Only mean levels of ambient PM10 at 24 and 48 h before recruitment resulted to be significantly associated with elevated systolic and diastolic BP levels. An increase in ambient PM10 of 1 μg/m3 at 24 h before the recruitment was associated with an mean increase in systolic BP of 1.22 mmHg (SE=0.51; P=0.019) and an mean increase in diastolic BP of 0.67 mmHg (SE=0.33; P=0.044) (Table 2). An increase in ambient PM10 of 1 μg/m3 at 48 h before the recruitment was associated with an average increase in systolic BP of 1.24 mmHg (SE=0.52; P=0.019) and an average increase in diastolic BP of 0.91 mmHg (SE=0.33; P=0.007) (Table 2). Significant interaction terms between BMI and PM10 exposure 48 h before recruitment (48 h PM10*BMI ) were obtained for systolic BP (P=0.015) and diastolic BP (P=0.006). The regression model results supported the hypothesis that BMI moderates the effect of PM10 exposure on systolic and diastolic BP.
Table 2.
Association of PM10 lag exposure with systolic and diastolic BP levels.
| β | SE | 95%CI | P-value | ||
|---|---|---|---|---|---|
| Systolic BP | 24 h PM10 | 1.22 | 0.51 | 0.20;2.24 | 0.019 |
| BMI | 1.40 | 0.55 | 0.31;2.49 | 0.013 | |
| 24 h PM10*BMI | −0.04 | 0.02 | −0.07;-0.01 | 0.012 | |
| Diastolic BP | 24 h PM10 | 0.67 | 0.33 | 0.02;1.32 | 0.044 |
| BMI | 0.74 | 0.35 | 0.04;1.44 | 0.037 | |
| 24 h PM10*BMI | −0.02 | 0.01 | −0.04;-0.003 | 0.021 | |
| Systolic BP | 48 h PM10 | 1.24 | 0.52 | 0.21;2.27 | 0.019 |
| BMI | 1.67 | 0.67 | 0.35;3.00 | 0.014 | |
| 48 h PM10*BMI | −0.04 | 0.02 | −0.07;−0.008 | 0.015 | |
| Diastolic BP | 48 h PM10 | 0.91 | 0.33 | 0.25;1.56 | 0.007 |
| BMI | 1.10 | 0.42 | 0.26;1.94 | 0.011 | |
| 48 h PM10*BMI | −0.03 | 0.01 | −0.05;−0.01 | 0.006 |
3.3. Influence of PM10 exposure on miRNA expression levels
To determine whether there is a specific miRNA signature associated with exposure to ambient PM10, we used TLDA to screen for miRNAs whose expression levels are correlated with the daily mean PM10 values. We chose to investigate PM10 lag exposure levels 24 and 48 h before the recruitment day since they were significantly associated with elevated systolic and diastolic BP levels. After data cleaning (see Section 2.4), we obtained 122 miRNAs. We investigated their association with PM10 exposure values using multivariate analysis. Using a Benjamini–Hochberg FDR adjustment for multiple comparisons (FDR P<0.1), we found 19 miRNAs that were significantly associated with the PM10 exposure level 48 h before recruitment. A negative association was observed for all miRNAs (see Supplemental file: Supplemental Table 2), suggesting a suppressive effect of PM10 exposure on the miRNA expression levels.
3.4. Validation of candidate miRNAs with PM10 exposure levels
We used a custom RT-qPCR assay to confirm the expression levels of candidate miRNAs that we identified in the screening analysis. To avoid the exclusion of some relevant miRNAs involved in PM10 exposure and BP regulation, 28 candidate miRNAs were chosen for validation, on the basis of their significant FDR P-values and their roles in BP regulation, CVD, and air pollution exposure (Pan et al., 2012, Deng et al., 2014, Neppl and Wang, 2014, Cakmak et al., 2015, Cengiz et al., 2015) (see Supplemental file: Supplemental Table 3).
Nineteen of the 28 candidate miRNAs resulted not to be significantly associated with PM10 exposure. Nine miRNAs were negatively associated with PM10 lag exposure levels 48 h before the recruitment day (FDR P<0.1; Table 3). Every 1-µg/m3 increase in PM10 exposure levels was associated with a 1.48% decrease in miR-26a expression (FDR P=0.069) and a 1.14% decrease in miR-101 expression (FDR P=0.088). The remaining seven miRNAs (miR-145, miR-197, miR-30b, miR-345, miR-425–5p, miR-331, and miR-140–3p) had decreases in expression ranging from 0.61% to 0.95% (Table 3).
Table 3.
List of 28 miRNAs selected for validation in association with PM10 lag exposure of 48 h.
| MicroRNA | PM10 lag exposure (h) | Δ% | Δ% 95%CI | P | FDR*P |
|---|---|---|---|---|---|
| miR-145 | 48 | −0.95 | −1.50;−0.40 | 0.001 | 0.025 |
| miR-197 | 48 | -0.66 | −1.15;−0.18 | 0.008 | 0.062 |
| miR-30b | 48 | −0.83 | −1.43;−0.22 | 0.009 | 0.062 |
| miR-345 | 48 | −0.95 | −1.65;−0.25 | 0.009 | 0.062 |
| miR-26a | 48 | −1.48 | −2.61;−0.33 | 0.012 | 0.069 |
| miR-425-5p | 48 | −0.61 | −1.11;−0.11 | 0.017 | 0.080 |
| miR-331 | 48 | −0.79 | −1.46;−0.12 | 0.022 | 0.080 |
| miR-140-3p | 48 | −0.82 | −1.52;−0.12 | 0.023 | 0.080 |
| miR-101 | 48 | −1.14 | −2.15;−0.13 | 0.028 | 0.088 |
| miR-339-5p | 48 | −0.79 | −1.54;−0.04 | 0.040 | 0.110 |
| miR-574-3p | 48 | −0.57 | −1.12;−0.02 | 0.043 | 0.110 |
| miR-451 | 48 | −0.81 | −1.60;−0.01 | 0.048 | 0.112 |
| miR-320 | 48 | −0.47 | −0.97;0.03 | 0.063 | 0.136 |
| miR-532-3p | 48 | −0.54 | −1.14;0.07 | 0.080 | 0.151 |
| miR-301 | 48 | −1.81 | −3.83;0.26 | 0.086 | 0.151 |
| miR-16 | 48 | −1.03 | −2.20;0.16 | 0.089 | 0.151 |
| miR-24 | 48 | −0.60 | −1.30;0.10 | 0.092 | 0.151 |
| miR-125a-5p | 48 | −0.54 | −1.19;0.11 | 0.102 | 0.159 |
| miR-222 | 48 | −0.67 | −1.51;0.17 | 0.117 | 0.168 |
| miR-183 | 48 | −1.45 | −3.26;0.39 | 0.120 | 0.168 |
| miR-182 | 48 | −0.91 | −2.13;0.31 | 0.141 | 0.188 |
| miR-99b | 48 | −1.12 | −2.72;0.52 | 0.176 | 0.218 |
| miR-21 | 48 | −0.80 | −1.98;0.40 | 0.188 | 0.218 |
| miR-362-3p | 48 | −1.20 | −2.98;0.62 | 0.193 | 0.218 |
| miR-15b | 48 | −0.79 | −1.97;0.41 | 0.194 | 0.218 |
| miR-486-3p | 48 | −0.72 | −2.64;1.24 | 0.467 | 0.502 |
| miR-150 | 48 | −0.13 | −0.53;0.27 | 0.527 | 0.547 |
| miR-296 | 48 | −0.21 | −1.30;0.90 | 0.711 | 0.711 |
Δ% estimates the percentage change in miRNA expression for a 1-μg/m3 increase in PM10 exposure level. Associations with FDR P<0.10 are shown in bold.
FDR: False Discovery Rate
3.5. Mediated moderation analysis
We performed mediated moderation analysis to estimate the extent to which the top nine validated miRNAs mediated the effects of PM10 exposure on BP, considering the moderator effect of BMI. The relationship between PM10 and BMI was not significant, Pearson's correlation coefficient was 0.011 (P=0.920). Of the top nine miRNAs, only miR-101 satisfied the underlying assumptions for mediated moderation analysis. In the analysis, miR-101 was negatively associated with PM10 (β21=−0.015, SE=0.004, P=0.001) and with the PM10×BMI interaction term (β23=−0.003, SE=0.001, P=0.002) (see Supplemental file: Supplemental Table 4). Subsequently, diastolic BP was predicted by the miR-101×BMI interaction (β35=0.624, SE=0.274, P=0.025) (see Supplemental file: Supplemental Table 4, Eq. (3)).
On the basis of these findings, we hypothesized that PM10 exposure, in a manner moderated by BMI, leads to reduced miR-101 expression and increased diastolic BP. We estimated the CIE. Given the overall significant interaction term, significance tests were conducted to test the hypothesis that CIE=0 at specified values (mean±1 SD) of the moderator. A bootstrapped 95% CI confirmed that the indirect effects of PM10 on diastolic BP by miR-101 were significant only for low values of mean BMI (Mean−1 SD) (CIE=−0.1431, 95% CI=−0.3091 to −0.0288) (Table 4 and Supplemental Fig. 1). The association between systolic BP and the interaction term (miR-101×BMI) was not statistically significant. Moreover, the CIE was not significant at different values of BMI (see Supplemental file: Supplemental Table 4).
Table 4.
Conditional indirect effect (CIE) of PM10 exposure on blood pressure (BP) via miR-101 at different body mass index (BMI) levels.
| Systolic BP | CIE | SEa | 95% CIb | |
|---|---|---|---|---|
| BMI [+ 1 SD] | 0.002 | 0.034 | −0.051 | 0.101 |
| Average BMI | −0.005 | 0.036 | −0.069 | 0.071 |
| BMI [−1 SD] | −0.150 | 0.112 | −0.409 | 0.053 |
| Diastolic BP | CIE | SEa | 95% CIb | |
| BMI [+ 1 SD] | 0.001 | 0.017 | −0.030 | 0.046 |
| Average BMI | −0.018 | 0.022 | −0.069 | 0.024 |
| BMI [−1 SD] | −0.143 | 0.069 | −0.309 | −0.029 |
SE: Standard error.
95% CI: Confidence interval.
3.6. miRNA functional analysis
The top nine miRNAs (miR-101, miR-140–3p, miR-145, miR-197, miR-26a, miR-30b, miR-331, miR-345, and miR-425–5p) that were significantly associated with PM10 exposure levels were further investigated, together with their target genes, by functional analysis using Ingenuity Pathway Analysis (IPA) software (see Supplemental material: Prediction of miRNA Targets).
Target enrichment analysis of the nine miRNAs identified 3759 unique mRNAs that were experimentally observed or predicted with a high confidence level. Numbers of downstream regulated mRNAs for each miRNA are shown in Supplemental file, Supplemental Table 5.
To investigate whether the mRNAs targeted by the nine miRNAs were potentially involved in biological processes connected to BP regulation, we performed functional analyses for diseases and functions involved in cardiovascular disorders. CVDs and functions were selected according to their P-values and the number of molecules involved. Target genes of the nine CVD-associated miRNAs were largely involved in artery disorders, vascular disease, hypertension, and occlusion of arteries (see Supplemental file: Supplemental Table 6).
As miR-101 was found to mediate the effects of PM10 exposure on BP, we performed a Tox Lists analysis with IPA to uncover pathways and molecular mechanisms that may be altered by toxicant exposure to PM10. The miR-101 targets (n=849) mapped to five toxicological pathways, belonging to RAR activation, cardiac hypertrophy, cardiac necrosis/cell death, increased heart failure, and p53 signaling (see Supplemental file: Supplemental Table 7).
4. Discussion
In this study of 90 overweight and obese adults in Lombardia, Italy, we found an increase in systolic and diastolic BP levels 24 and 48 h after exposure to levels of PM10. Obese individuals were chosen for this study due to their increased susceptibility to the effects of air pollution (due to a higher particle accumulation rate) (Bennett and Zeman, 2004, Bollati et al., 2014). We identified and validated a distinct signature of nine miRNAs (miR-145, miR-197, miR-30b, miR-345, miR-26a, miR-425–5p, miR-331, miR-140–3p, and miR-101) in the peripheral blood, which were associated with PM10 exposure levels 48 h after exposure. Among these nine miRNAs, only miR-101 mediated the effects of particle exposure on diastolic BP, possibly influencing the risk of developing CVD.
Identification of a PM exposure time of 48 h in association with BP is consistent with findings from previous studies focused on cardiovascular outcomes. Dominici et al. (2006) showed a higher risk of CVD hospitalizations across 204 United States counties 24–48 h after exposure to PM with an aerodynamic diameter of 2.5 μm or less (PM2.5), using single-day average measures . The strongest association was found for heart failure, which had a 1.28% increased risk for each 10-μg/m3 increase in PM2.5. Zanobetti et al. (2009) estimated the association between 2-day mean PM2.5 levels and emergency hospital admissions for CVD, MI, and congestive heart failure in 26 US communities, reporting larger effect sizes than Dominici.
We found that an increase in ambient PM10 of 1 μg/m3 was associated with an increase in mean systolic and diastolic BP levels of 0.67–1.24 mmHg 48 h after exposure. Consistent with our results, Kubesch et al. (2015) reported a PM10-associated increase in systolic BP of 0.98 mmHg after 5 h of exposure. Dvonch et al. (2009) found an average increase in systolic BP of 3.2 mmHg 2 days after exposure to urban PM2.5 .
The molecular pathways that determine the systemic changes in blood pressure levels after PM exposures are still largely unknown. We propose that inhaled air particles provoke alveolar inflammation which causes the release of mediators such as miRNAs, able to modulate systemic inflammation and related health effects. Alveolar macrophages and pulmonary epithelial cells constitute the first line of defense against inhaled noxious compounds. They have been suggested to initiate a cascade of pro-inflammatory cytokines (TNF, IL-1, IL-6, and GM-CSF), upon PM exposure, that can rapidly extend to systemic circulation (Van Eeden et al., 2001, Wang et al., 2005). These systemic reactions can be assessed through expression analysis, in circulating leukocytes, of miRNAs related to inflammatory pathways and oxidative stress, which are exquisitely sensitive to PM exposure (Jardim et al., 2009). Recent data from healthy human subjects suggest that circulating miRNAs may be an active component of the cellular response to PM exposure. Vrijens et al. demonstrated that miR-10b and miR-128 are distinct miRNAs that are frequently overexpressed after exposure to air pollution in in-vitro and human studies (Vrijens et al., 2015). Our group previously published results on a population of foundry workers who were highly exposed to metal-rich PM. We identified four differentially expressed inflammatory miRNAs after short-term exposure to PM10 for 4 days (Motta et al., 2013). In a population of elderly males, an association was discovered between PM2.5 and expression levels of eight miRNAs involved in the immune response after exposure to PM2.5 for 4 h to 28 days (Fossati et al., 2014).
The present study reports the results of regression models as the percent change in miRNA expression for each 1-μg/m3 increase in PM10 exposure level. Small percent changes were found for the nine identified miRNAs, indicating tight regulation of the miRNA expression mechanism. Bioinformatics analysis with IPA further showed that these nine miRNAs are largely involved in artery disorders/occlusion, vascular diseases, and hypertension. Among the nine miRNAs identified in this study, the literature provides distinct and well-established roles for miR-145, miR-30b, and miR-26a in CVD. MiR-145 plays a role in vascular smooth muscle cell development and function. Altered expression of this miRNA has been linked to pathological changes in the vasculature, such as vascular remodeling, aortic aneurysm, and atherosclerosis (Hata, 2013). MiR-145–null mice exhibit blood vessels with reductions in the medial layer thickness, vascular tone, and BP, suggesting that miR-145 is required for normal contractility of the arteries and normal BP (Boettger et al., 2009). The miR-30 family, including miR-30b, is encoded by six genes that are abundantly expressed in the heart under physiological conditions. Involvement of the miR-30 family in cardiovascular pathophysiology has only emerged recently. Using a well-established rat model of AMI to investigate the role of aberrantly expressed miRNAs in different areas of infarcted rat hearts, Dong et al. (2009) observed that miR-30b expression was upregulated in the noninfarcted areas, suggesting an important role in the pathophysiology of ischemic heart disease. MiR-26a is involved in vascular remodeling. Studies using experimental mouse models identified increased miR-26a levels in association with reduced phosphatase and tensin homolog (PTEN) expression (a target of miR-26a), as well as activation of the signal transduction Akt-pathway, resulting in altered myogenic tone and growth of the vessel wall (Bhattachariya et al., 2014).
The results of our mediated moderation analysis are consistent with the hypothesis that, among the nine studied miRNAs, only miR-101 mediates the effects of particle exposure on diastolic BP. Based on the a priori assumption that a mediated effect is biologically plausible, this approach decomposes the total observed effect of exposure on BP into direct and indirect effects, with the latter acting via miR-101. We found that miR-101 mediates the indirect effect of PM10 on diastolic BP, and that this effect is significant only in patients with lower values of mean BMI. These results indicate possible beneficial roles of miR-101 in counteracting the acute adverse effects of PM10 on diastolic BP, but only in subjects with higher BMI mean levels.
These findings might open up two different scenarios. The first scenario indicates obesity (BMI) as modifier of the association between ambient air pollution exposure and blood pressure. A clinical controlled study conducted on 348 participants ages ≥25 years in the United States showed that exposure to PM2.5 at lags 2 and 3 was associated with an increased risk for elevated pulse pressure among those who were determined to be obese according to their BMI. Results from this study suggest that obesity may be a modifier of the association between ambient air pollution exposure and blood pressure (Kannan et al., 2010). Therefore, we hypothesize that obesity would modify the association between air pollution exposure and blood pressure, with a stronger association in obese subjects with higher BMI than in individuals of lower BMI.
The second scenario suggests that the clinical conditions related to obesity might somehow interfere with the role of miRNA-101. Adipose tissue produces high serum concentrations of various pro-inflammatory cytokines, chemokines and adipokines, contributing to endothelial dysfunction, renin–angiotensin system activation, sympathetic activation, and oxidative stress (Sood, 2010). The pro-inflammatory state can be also associated to the failure of anti-inflammatory mechanisms such as miR-101 that is involved in the regulation of endothelial function and atherosclerosis. Pan et al. identified miR-101 as a cardiac fibrosis-related miRNA. In postinfarct rats, miR-101 overexpression mitigated interstitial fibrosis and the deterioration of cardiac performance, indicating the therapeutic potential of miR-101 for fibrosis-associated cardiac disease (Pan et al., 2012). The complex inflammatory state associated to obesity may be the reason why the mediating beneficial role of miR-101 on the relationship between air pollution exposure and blood pressure differs in individuals with different classes of obesity. However, we did not find any direct evidence in this study.
Growing evidence suggests that the evaluation of miRNA expression levels in the peripheral blood of healthy individuals may be a useful tool in elucidating the mechanisms leading to changes in BP and in predicting human CVD. Blood RNA is derived from a mixed cell population containing different types of circulating white blood cells. Although exposure to PM10 had no significant effects on the proportions of the major leukocyte cell types, the models were adjusted for the proportion of granulocytes.
Our study aimed at investigating the entire population of a region, rather than selected locations near monitoring sites, as was commonly done in previous studies. By selecting PM10 as the exposure value, we aimed to detect the effects of different sources and components of PM that might trigger cardiovascular outcomes. The average daily PM10 exposure concentration in the study period was 36.4 µg/m3. This value is higher than the WHO Air Quality Guideline (AQG) of 20 μg/m3, but considerably lower than previously reported occupational (Bonzini et al., 2010) and chamber studies, (Gamble and Lewis, 1996) in which levels exceeded hundreds of micrograms per cubic meter. Our findings suggest that even levels close to the WHO AQG limits can lead to effects on BP and can have major effects on miRNA expression levels.
The strength of this study was the use of a nontargeted, exploratory approach to select candidate miRNAs. We chose to use a real-time PCR approach for miRNA expression analysis because of the high precision and sensitivity required to detect closely related miRNAs, which might differ in sequence by only one base (Mackay et al., 2002). Also, to limit false positives in the detection of differentially-expressed miRNAs, we used restrictive statistical significance cut-offs. In addition, the personal levels of exposure to PM10, assessed by the FARM Eulerian grid model, provided us with a unique method to efficiently study PM effects in humans.
However, our study has some limitations. First, from the initial list of 122 miRNAs, we chose to validate a group of 28 miRNAs. Although we used different selection criteria based on statistical significance, biological role and consideration of our preliminary data on the whole SPHERE population, it remains possible that we may have excluded some relevant miRNAs involved in PM10 exposure and BP regulation. In our analysis, we adjusted for potential confounding variables. We did not include in our models clinical variables such as diabetes, cholesterol levels, physical activity, alcohol consumptions and medications. Even though individual factors that may affect PM exposure estimation (such as information on residence area and floor of residence) have been collected in a questionnaire, we chose not to use these variables because of the small sample size. Moreover, the relatively small sample size might have limited our ability to detect significant effects, and larger studies are needed to confirm our findings.
5. Conclusions
The mediated moderation analysis we performed allowed us to conclude that the air pollution effect on BP is likely to be regulated by a change in miRNA expression levels in blood. Future studies could build upon the approach presented here to investigate similar events in other tissues or in diverse exposure conditions. Additionally, we performed a target analysis with the aim of providing a list of possible genes and pathways to be further investigated with in-vitro and animal models. It would be necessary to experimentally test our results in order to provide a mechanistic role for miR-101.
Funding sources
This work was supported by the EU Programme “Ideas” [ERC-2011-StG 282413 to Dr. V. Bollati].
Conflict of interest
None declared.
Ethic approval
Each participant provided written informed consent before inclusion in the study, which was approved by the Ethics Committee of Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico (approval number 1425).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2016.01.010.
Contributor Information
Valeria Motta, Email: valeria.motta@unimi.it.
Chiara Favero, Email: chiara.favero@unimi.it.
Laura Dioni, Email: laura.dioni@unimi.it.
Simona Iodice, Email: simona.iodice@unimi.it.
Cristina Battaglia, Email: laura.angelici@unimi.it.
Laura Angelici, Email: laura.angelici@unimi.it.
Luisella Vigna, Email: luisella.vigna@policlinico.mi.it.
Angela Cecilia Pesatori, Email: angela.pesatori@unimi.it.
Valentina Bollati, Email: valentina.bollati@unimi.it.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- Bennett W.D.,, Zeman K.L. Effect of body size on breathing pattern and fine-particle deposition in children. J. Appl. Physiol. 2004;973:821–826. doi: 10.1152/japplphysiol.01403.2003. [DOI] [PubMed] [Google Scholar]
- Bhattachariya A., Dahan D., Turczynska K.M., Sward K., Hellstrand P., Albinsson S. Expression of microRNAs is essential for arterial myogenic tone and pressure-induced activation of the PI3-kinase/Akt pathway. Cardiovasc. Res. 2014;1012:288–296. doi: 10.1093/cvr/cvt253. [DOI] [PubMed] [Google Scholar]
- Boettger T., Beetz N., Kostin S., Schneider J., Kruger M., Hein L. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J. Clin. Invest. 2009;1199:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati V., Iodice S., Favero C., Angelici L., Albetti B., Cacace R. Susceptibility to particle health effects, miRNA and exosomes: rationale and study protocol of the SPHERE study. BMC Public Health. 2014;14:1137. doi: 10.1186/1471-2458-14-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzini M., Tripodi A., Artoni A., Tarantini L., Marinelli B., Bertazzi P.A. Effects of inhalable particulate matter on blood coagulation. J. Thromb. Haemost. 2010;84:662–668. doi: 10.1111/j.1538-7836.2009.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R.D., Rajagopalan S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009;35:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Urch B., Dvonch J.T., Bard R.L., Speck M., Keeler G. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;543:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak H.A., Coskunpinar E., Ikitimur B., Barman H.A., Karadag B., Tiryakioglu N.O. The prognostic value of circulating microRNAs in heart failure: preliminary results from a genome-wide expression study. J. Cardiovasc. Med. 2015;166:431–437. doi: 10.2459/JCM.0000000000000233. [DOI] [PubMed] [Google Scholar]
- Cengiz M., Yavuzer S., Kilickiran Avci B., Yuruyen M., Yavuzer H., Dikici S.A. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin. Exp. Hypertens. 2015:1–7. doi: 10.3109/10641963.2015.1036064. [DOI] [PubMed] [Google Scholar]
- Deng Q., Huang S., Zhang X., Zhang W., Feng J., Wang T. Plasma microRNA expression and micronuclei frequency in workers exposed to polycyclic aromatic hydrocarbons. Environ. Health Perspect. 2014;1227:719–725. doi: 10.1289/ehp.1307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson B.A., Semus H.M., Montgomery R.L., Stack C., Latimer P.A., Lewton S.M. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur. J. Heart Fail. 2013;156:650–659. doi: 10.1093/eurjhf/hft018. [DOI] [PubMed] [Google Scholar]
- Dominici F., Peng R.D., Bell M.L., Pham L., McDermott A., Zeger S.L. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;29510:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Cheng Y., Yang J., Li J., Liu X., Wang X. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J. Biol. Chem. 2009;28443:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowsky S.D., Suh H., Schwartz J., Coull B.A., Gold D.R. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ. Health Perspect. 2006;1147:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvonch J.T., Kannan S., Schulz A.J., Keeler G.J., Mentz G., House J. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;535:853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati S., Baccarelli A., Zanobetti A., Hoxha M., Vokonas P.S., Wright R.O. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology. 2014;251:68–78. doi: 10.1097/EDE.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J.F., Lewis R.J. Vol. 1048. 1996. Health and respirable particulate (PM10) air pollution: a causal or statistical association? pp. 838–850. (Environ. Health Perspect.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A. Functions of microRNAs in cardiovascular biology and disease. Annu. Rev. Physiol. 2013;75:69–93. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. Guilford Press, New York, NY; 2013. An introduction to mediation, moderation, and conditional process analysis: a regression-based approach. [Google Scholar]
- Izzotti A., La Maestra S., Micale R.T., Longobardi M.G., Sacca S.C. Genomic and post-genomic effects of anti-glaucoma drugs preservatives in trabecular meshwork. Mutat. Res. 2015;772:1–9. doi: 10.1016/j.mrfmmm.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Izzotti A., Pulliero A. The effects of environmental chemical carcinogens on the microRNA machinery. Int. J. Hyg. Environ. Health. 2014;2176:601–627. doi: 10.1016/j.ijheh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Jardim M.J., Fry R.C., Jaspers I., Dailey L., Diaz-Sanchez D. Disruption of microRNA expression in human airway cells by diesel exhaust particles is linked to tumorigenesis-associated pathways. Environ. Health Perspect. 2009;11711:1745–1751. doi: 10.1289/ehp.0900756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S., Dvonch J.T., Schulz A.J., Israel B.A., Mentz G., House J. Exposure to fine particulate matter and acute effects on blood pressure: effect modification by measures of obesity and location. J. Epidemiol. Community Health. 2010;641:68–74. doi: 10.1136/jech.2008.081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubesch N., De Nazelle A., Guerra S., Westerdahl D., Martinez D., Bouso L. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur. J. Prev. Cardiol. 2015;225:548–557. doi: 10.1177/2047487314555602. [DOI] [PubMed] [Google Scholar]
- Li W.Y., Jin J., Chen J., Guo Y., Tang J., Tan S. Circulating microRNAs as potential non-invasive biomarkers for the early detection of hypertension-related stroke. J. Hum. Hypertens. 2014;285:288–291. doi: 10.1038/jhh.2013.94. [DOI] [PubMed] [Google Scholar]
- Mackay I.M., Arden K.E., Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002;306:1292–1305. doi: 10.1093/nar/30.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta V., Angelici L., Nordio F., Bollati V., Fossati S., Frascati F. Integrative analysis of miRNA and inflammatory gene expression after acute particulate matter exposure. Toxicol. Sci. 2013;1322:307–316. doi: 10.1093/toxsci/kft013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Judd C.M., Yzerbyt V.Y. When moderation is mediated and mediation is moderated. J. Personal. Soc. Psychol. 2005;896:852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Nawrot T.S., Perez L., Kunzli N., Munters E., Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;3779767:732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- Nemmar A., Vanbilloen H., Hoylaerts M.F., Hoet P.H., Verbruggen A., Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001;1649:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- Neppl R.L., Wang D.Z. The myriad essential roles of microRNAs in cardiovascular homeostasis and disease. Genes Dis. 2014;11:18–39. doi: 10.1016/j.gendis.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Sun X., Shan H., Wang N., Wang J., Ren J. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-beta1 pathway. Circulation. 2012;1267:840–850. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- Pulliero A., Marengo B., Fenoglio D., Parodi A., Cereda C., Domenicotti C. Prevention of lymphocyte neurotoxic effects by microRNA delivery. MicroRNA. 2014;23:187–193. doi: 10.2174/22115366113029990010. [DOI] [PubMed] [Google Scholar]
- Ren J., Zhang J., Xu N., Han G., Geng Q., Song J. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One. 2013;812:e80738. doi: 10.1371/journal.pone.0080738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger S., Grosshans H. MicroRNA turnover: when, how, and why. Trends Biochem. Sci. 2012;3710:436–446. doi: 10.1016/j.tibs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Schneider A., Neas L., Herbst M.C., Case M., Williams R.W., Cascio W. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environ. Health Perspect. 2008;11612:1666–1674. doi: 10.1289/ehp.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Park S.K., O'Neill M.S., Vokonas P.S., Sparrow D., Weiss S. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am. J. Respir. Crit. Care Med. 2005;17212:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. Obesity, adipokines, and lung disease. J. Appl. Physiol. 2010;1083:744–753. doi: 10.1152/japplphysiol.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J., Stamler R., Neaton J.D. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch. Intern. Med. 1993;1535:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- Tijsen A.J., Creemers E.E., Moerland P.D., de Windt L.J., van der Wal A.C., Kok W.E. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010;1066:1035–1039. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- Urch B., Silverman F., Corey P., Brook J.R., Lukic K.Z., Rajagopalan S. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ. Health Perspect. 2005;1138:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen P.C., Feskens E.J., Nagelkerke N.J., Menotti A., Nissinen A., Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven countries study research group. N. Engl. J. Med. 2000;3421:1–8. doi: 10.1056/NEJM200001063420101. [DOI] [PubMed] [Google Scholar]
- Van Eeden S.F., Tan W.C., Suwa T., Mukae H., Terashima T., Fujii T. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am. J. Respir. Crit. Care Med. 2001;1645:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Vrijens K., Bollati V., Nawrot T.S. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ. Health Perspect. 2015;1235:399–411. doi: 10.1289/ehp.1408459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Long G., Zhao C., Li H., Chaugai S., Wang Y. Atherosclerosis-related circulating miRNAs as novel and sensitive predictors for acute myocardial infarction. PLoS One. 2014;99:e105734. doi: 10.1371/journal.pone.0105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Neuburg D., Li C., Su L., Kim J.Y., Chen J.C. Global gene expression profiling in whole-blood samples from individuals exposed to metal fumes. Environ. Health Perspect. 2005;1132:233–241. doi: 10.1289/txg.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Regional Office for Europe, 2015. Economic cost of the health impact of air pollution in Europe: clean air health wealth.
- Zanobetti A., Franklin M., Koutrakis P., Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ. Health. 2009;8:58. doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material