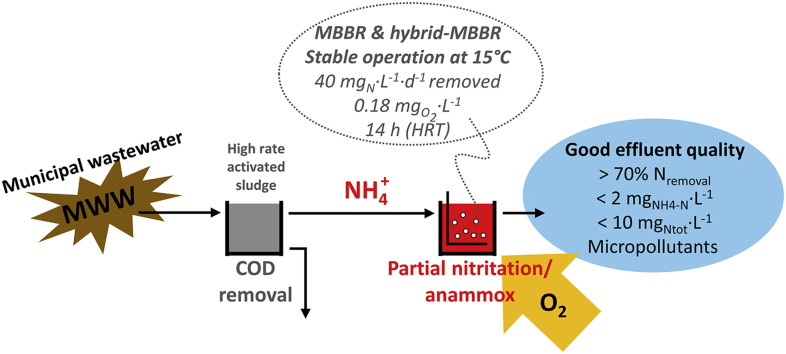
Abstract
The implementation of autotrophic anaerobic ammonium oxidation processes for the removal of nitrogen from municipal wastewater (known as “mainstream anammox”) bears the potential to bring wastewater treatment plants close to energy autarky. The aim of the present work was to assess the long-term stability of partial nitritation/anammox (PN/A) processes operating at low temperatures and their reliability in meeting nitrogen concentrations in the range of typical discharge limits below 2 and 10 mgNtot·L−1. Two main 12-L sequencing batch reactors were operated in parallel for PN/A on aerobically pre-treated municipal wastewater (21 ± 5 and residual 69 ± 19 mgCODtot·L−1) for more than one year, including over 5 months at 15 °C. The two systems consisted of a moving bed biofilm reactor (MBBR) and a hybrid MBBR (H-MBBR) with flocculent biomass. Operation at limiting oxygen concentrations (0.15–0.18 ) allowed stable suppression of the activity of nitrite-oxidizing bacteria at 15 °C with a production of nitrate over ammonium consumed as low as 16% in the MBBR. Promising nitrogen removal rates of 20–40 mgN·L−1·d−1 were maintained at hydraulic retention times of 14 h. Stable ammonium and total nitrogen removal efficiencies over 90% and 70% respectively were achieved. Both reactors reached average concentrations of total nitrogen below 10 mgN·L−1 in their effluents, even down to 6 mgN·L−1 for the MBBR, with an ammonium concentration of 2 mgN·L−1 (set as operational threshold to stop aeration). Furthermore, the two PN/A systems performed almost identically with respect to the biological removal of organic micropollutants and, importantly, to a similar extent as conventional treatments. A sudden temperature drop to 11 °C resulted in significant suppression of anammox activity, although this was rapidly recovered after the temperature was increased back to 15 °C. Analyses of 16S rRNA gene-targeted amplicon sequencing revealed that the anammox guild of the bacterial communities of the two systems was composed of the genus “Candidatus Brocadia”. The potential of PN/A systems to compete with conventional treatments for biological nutrients removal both in terms of removal rates and overall effluent quality was proven.
Keywords: Mainstream anammox, Partial nitritation/anammox, Municipal wastewater, Low temperature, Effluent quality, Nitrogen removal, Micropollutants
Graphical abstract
Highlights
-
•
Stable partial nitritation/anammox of aerobically pre-treated municipal wastewater at 15 °C.
-
•
Effluent ammonia and total nitrogen concentrations meet current discharge limits.
-
•
Successful NOB activity suppression at low dissolved oxygen concentrations (0.15–0.18 mgO2/L).
-
•
Micropollutants removal comparable to conventional biological treatments.
-
•
Reversible but dramatic anammox activity suppression during prolonged operation at 11 °C.
1. Introduction
The implementation of anaerobic ammonium oxidation processes for the autotrophic removal of nitrogen from municipal wastewater (MWW), known as “mainstream anammox”, would allow to segregate the removal of nitrogen and organic matter, and would bring wastewater treatment plants close to energy autarky (Siegrist et al., 2008, van Loosdrecht and Brdjanovic, 2014). In fact, in contrast to conventional activated sludge processes designed for full biological nitrogen removal by nitrification and denitrification, the organic matter contained in raw influent wastewater could be concentrated through physicochemical or biological pre-treatments and further valorized into methane-rich biogas via anaerobic digestion. The nitrogen present in the liquid fraction, together with the concentrated digester supernatant, could then be treated autotrophically via combined partial nitritation/anammox (PN/A) with significant savings in terms of aeration costs, sludge production and external organic carbon source (no amendment needed). To date, anammox-based processes are widely applied and represent a robust method for the treatment of wastewaters with high nitrogen concentrations under mesophilic conditions (Lackner et al., 2014). However, their potential for the direct treatment of MWW has not yet been fully confirmed despite increasing experimental evidence. The main challenges of mainstream applications relate to i) low nitrogen concentrations, ii) variable nitrogen loads, iii) low temperatures, iv) stringent effluent quality requirements, and v) long-term process stability.
Anammox bacteria have been shown to grow on MWW at low temperatures typical of moderate climates (10–15 °C) and with activities relevant for MWW applications when nitrite is dosed (Laureni et al., 2015, Lotti et al., 2014b, Ma et al., 2013). The possibility of PN/A in single-stage reactors has been proven on COD-free synthetic media under mainstream conditions with influent ammonium concentrations below 70 and psychrophilic temperatures (Gilbert et al., 2014, Gilbert et al., 2015, Hu et al., 2013, Lotti et al., 2014a). To date, only few studies have evaluated low-temperature PN/A directly treating actual MWW (De Clippeleir et al., 2013, Lotti et al., 2015). Moreover, in these works, process performance has been limited by the accumulation of nitrite (NO2−) and the production of nitrate (NO3−), resulting in low nitrogen removals (<45%) and effluent qualities not sufficient for direct discharge (ammonium concentrations >7 mgN·L−1). In turn, mainstream applications would require compliance with low effluent ammonium concentrations (<2 mgN·L−1) and high removal efficiencies (90%) (e.g. Switzerland, WPO (1998)) or low total nitrogen concentrations (<10 mgN·L−1) and removal efficiencies (70–80%) (e.g. European Union, Council Directive 91/271/EEC (1991)). In addition, the discharge of organic micropollutants with treated municipal wastewater is well documented (Petrie et al., 2015) and is becoming an issue of major concern (Eggen et al., 2014). In conventional biological treatment systems about half of the organic micropollutants load is eliminated primarily by biotransformation, sorption to the biomass and/or volatilization (Falås et al., 2016, Petrie et al., 2015). Limited information is however available on micropollutants removal in anammox-based systems and is restricted to highly concentrated streams (Alvarino et al., 2014, de Graaff et al., 2011). Overall, the possibility of stably operating mainstream PN/A processes at effluent nitrogen concentrations within the range of discharge limits and determining their potential to remove micropollutants, in comparison to conventional systems, remains unexplored.
Successful PN/A requires a balanced activity between the three main autotrophic guilds involved. The reactor configuration and type of biomass should be chosen in order to favor the retention of aerobic (AOB) and anaerobic ammonium oxidizing (AMX) bacteria and to suppress and/or wash-out nitrite oxidizing bacteria (NOB). Suspended sludge is used for sidestream treatment (Joss et al., 2011) whereas severe biomass losses have been reported for mainstream applications (Laureni et al., 2015). In turn, biofilm-based biomasses involving granules or biofilm carriers have been shown to be more resistant to temperature changes and have been applied for PN/A at conditions relevant for MWW applications (Gilbert et al., 2015). In general, the slow-growing AMX preferentially grow in big aggregates (e.g. thick biofilms) and the aerobic AOB and NOB guilds tend to preferentially populate smaller aggregates (e.g. flocs) with less diffusion limitations (Corbala-Robles et al., 2015, Vlaeminck et al., 2010, Volcke et al., 2010, Winkler et al., 2012). The coexistence of biofilm and flocs has been shown to improve the PN/A performance and the NOB suppression at high temperatures (>25 °C), both on digester supernatant (Veuillet et al., 2014) and pre-treated MWW (Malovanyy et al., 2015). In their modeling efforts, in contrast, Hubaux et al. (2015) have reported marked process deteriorations due to the unfavorable presence of a small fraction of flocs (5% total biomass weight) in a granular biofilm reactor treating concentrated side-streams. Reliable design and operation strategies (e.g. better NOB control) for mainstream PN/A could benefit from the understanding of the causes of biomass segregation, the corresponding implications in terms of substrate competition, and their impact on process performance at low temperatures.
The aim of the present work was to assess the long-term stability of PN/A processes operating at low temperatures (15 °C) on pre-treated MWW and their reliability in meeting effluent nitrogen concentrations within the range of typical discharge limits. Two parallel PN/A reactors, a moving bed bioreactor (MBBR) and a hybrid MBBR (H-MBBR) with flocculent biomass, were operated for more than one year at low dissolved oxygen (DO) concentrations (0.15–0.18 mgO2·L−1). The driving hypothesis was that both the suppression of NOB and the achievement of volumetric process rates relevant for mainstream applications (i.e. 50 mgN·L−1·d−1 at hydraulic retention times – HRT – below 24 h) can successfully be achieved by operation under limiting oxygen conditions. The reactors were compared in terms of overall performance, nitrogen removal rates and efficiencies, and effluent quality (nitrogen species and COD). The results are further discussed on the basis of the relative abundance, actual activity and distribution of the three main autotrophic guilds (AMX, AOB, and NOB). The effects of a sharp and prolonged temperature drop to 11 °C on the maximum anammox activity were investigated in a third reactor. Finally, the micropollutants removal of mainstream PN/A systems was quantified and compared to conventional systems for nutrient removal.
2. Materials and methods
2.1. Long-term reactor operation
Two main sequencing batch reactors (SBR; 12-L working volume) were operated in parallel for PN/A on aerobically pre-treated MWW (see below). The reactors (MBBR and H-MBBR) were inoculated with already established biofilm carriers K5 (protected surface 800 m2·m−3; AnoxKaldnes™, Sweden) originating from two lab-scale side-stream MBBRs treating digester supernatant (Weissbrodt et al., 2015), at a biofilm media filling ratio of 33%. The reactors were run for 400 (MBBR) and 360 (H-MBBR) days respectively. The last 240 days of operation of each reactor are discussed in the main manuscript and the full experimental period is presented in the Supporting Information (Figs. S1, S2). The MBBR and H-MBBR were operated at decreasing temperatures, from mesophilic (29 ± 2 °C) to psychrophilic (15 ± 1 °C), under micro-aerobic conditions at 0.18 ± 0.02 (with airflow 350 mL·min−1, 15 °C) and 0.15 ± 0.05 (100 mL·min−1, 15 °C) respectively (Figs. S1, S2). Each SBR cycle consisted of five steps: settling (10 min for MBBR; 60 min for H-MBBR), simultaneous feeding and effluent discharge (6 L of pre-treated MWW), mixing (10 min), aeration (variable duration based on the fixed DO set-point and terminated when a residual ammonium concentration of 2 was reached), and mixing (40 min). The cycle duration varied between 4 and 6 h, depending on the actual microbial activity, and the aerobic time accounted for 72 ± 9% and 61 ± 10% of the total cycle time at 15 °C in MBBR and H-MBBR respectively (Figs. S1, S2). In the H-MBBR, the longer settling phase allowed the development of a hybrid system with part of the biomass in suspension (<10% of the total suspended solids, TSS). The SRT of the suspended fraction was not controlled and depended on the uncontrolled sludge loss with the effluent e.g. lower influent concentrations during rain events resulted in shorter HRT, higher daily volume exchanges ratios and consequently increased washout. The SRT of the suspended fraction under dry weather conditions (last month of operation) was estimated to be 7 ± 2 days.
A third 12-L sequencing batch reactor (MBBR-2) was inoculated, at an available volumetric surface area of 630 m2·mreactor−3, with a different type of biofilm carrier (FLUOPUR® synthetic porous fleece material, WABAG Water Technology Ltd., Switzerland) originating from a 400-L pilot-scale PN/A reactor treating digester supernatant (unpublished work). MBBR-2 was operated for 270 days as the MBBR on the same pre-treated MWW and under micro-aerobic conditions, 0.17 ± 0.04 (200 mL·min−1) (Fig. S3). The main focus of this additional experiment was to study the behavior of the anammox activity during a sudden and prolonged (26 days) temperature drop from 17 to 11 °C and its capacity to recover after increasing the temperature back to 15 °C. In all reactors, the pH was not controlled and remained stable at 7.4 ± 0.2 throughout the experimental period.
2.2. Municipal wastewater (MWW)
Wastewater from the municipality of Dübendorf (Switzerland) was pre-treated in a primary settler followed by an aerated 12-L SBR operated for COD removal at a sludge retention time (SRT) of 1 day. The characteristics of the primary effluent and pre-treated MWW are presented in Table 1. The pre-treated MWW was first stored in an external buffer tank of 50 L, with no temperature control, to equalize hydraulic loads prior to feeding into the PN/A reactors.
Table 1.
Average compositions of the wastewater after each treatment step: primary effluent, pre-treated (after A-stage) and treated (after PN/A) effluents over the 5 months of operation at 15 °C. The complete time series are available in Fig. S4 in the Supporting Information.
| Units | Primary effluent | Pre-treated MWW | Effluent MBBR | Effluent H-MBBR | |
|---|---|---|---|---|---|
| NH3+ | [mgN∙L−1] | 25.1 ± 6.8 | 21.2 ± 5.2 | 1.8 ± 0.4 | 2.1 ± 0.9 |
| NO2−a | [mgN∙L−1] | <0.2 | <0.2 | <0.2 | <0.2 |
| NO3− | [mgN∙L−1] | 0.3 ± 0.3 | 0.4 ± 0.5 | 3.6 ± 1.4 | 5.7 ± 2.7 |
| Ntot | [mgN∙L−1] | 25.5 ± 6.8 | 21.8 ± 5.2 | 5.7 ± 1.3 | 8.0 ± 2.6 |
| CODsol | [mgCOD∙L−1] | 182 ± 61 | 46 ± 7 | 18 ± 3 | 20 ± 4 |
| CODtotb | [mgCOD∙L−1] | 533 ± 222 | 69 ± 18 | 40 ± 13 | 33 ± 11 |
Less than 10% of the values exceeded 0.2 mgN∙L−1; in the case of the MBBR about 40% of the values exceeded 0.2 mgN∙L−1, with average and standard deviation of 0.29 ± 0.07 mgN∙L−1. The limit of quantification was 0.2 mgN∙L−1.
The total COD concentration was only measured until days 280 (MBBR) and 210 (H-MBBR) and thus refers to operation at temperatures above 15 °C.
2.3. Overall volumetric nitrogen removal rates in the PN/A reactors
The overall volumetric nitrogen removal rate is defined as the amount of total nitrogen (sum of NH4+, NO2− and NO3−) removed per reactor volume and day (mgN·L−1·d−1) and is calculated by dividing the difference between the sum of the dissolved nitrogen species in the influent and effluent by the overall hydraulic retention time (HRT) in the reactor (i.e. including settling and mixing/idle times). The influent and effluent were sampled once to twice a week (Fig. S4), whereas the HRT was calculated on the basis of online data acquisition (Figs. S1, S2, S3).
The relative removals of ammonium and total nitrogen are defined as the difference between the corresponding influent and effluent concentrations divided by the influent concentration and are expressed as percentage.
2.4. Maximum anammox activity
The maximum anammox activity (rAMX,max) is defined as the volumetric nitrogen removal rate (sum of NH4+ and NO2−) in the absence of O2 and under non-limiting concentrations of NH4+ and NO2−. It was measured once or twice a week in situ in batch tests conducted at the end of an SBR cycle in order to avoid excessive residual COD. NH4+ and NO2− were supplied as NH4Cl and NaNO2 (≥15 mgN·L−1 each) and their volumetric consumption rates were calculated by linear regression of off-line measurements of three to four grab samples of bulk liquid phase. The sampling interval of 15 to 60 min depended on the actual rate.
2.5. Actual activity of AMX, AOB, and NOB during PN/A operation
The actual volumetric activities of the three main autotrophic guilds during operation (rAMX,cycle, rAOB,cycle and rNOB,cycle) were estimated on the basis of a nitrogen mass balance over the aerobic phase of an SBR cycle. The consumption of NH4+, accumulation of NO2− and production of NO3− were calculated by linear regression of off-line measurements of three to four grab samples of bulk liquid phase during aeration. Since simultaneous heterotrophic denitrification could be neglected (see below), the actual activities were derived from the full rank stoichiometric matrix presented in the Supporting Information (Table S1) and the following equation:
| (1) |
where A is the matrix of the stoichiometric coefficients, rR is the vector of the unknown process rates (i.e. ρAOB, ρNOB and ρAMX in mgCOD·L−1·d−1), and rC is the vector of the measured net conversion and/or production rates (i.e. rNH4+, rNO2− and rNO3− expressed as ·d−1, ·d−1, and ·d−1 respectively). The actual volumetric activities of the three guilds (i.e. rAMX,cycle, rAOB,cycle and rNOB,cycle expressed as ·d−1, ·d−1, and ·d−1 respectively) were obtained by multiplying the process rate with the appropriate stoichiometric coefficient (for details see Table S1). The actual activities were estimated 8 and 11 times for the MBBR and H-MBBR respectively, during the last two months of operation at 15 °C. In the text, rAMX,cycle is expressed as ·d−1 to allow for a direct comparison with the maximum anammox activity (rAMX,max).
2.6. Heterotrophic denitrification test
To assess the contribution of heterotrophic denitrification to the overall nitrogen removal, the consumption of NO3− was measured in the presence of acetate as a representative of an easily biodegradable carbon source at different DO concentrations (0, 0.2 and 1.5 , anoxic, micro-aerobic and aerobic respectively). The test was performed at the end of the experimental period in situ, at 15 °C, once in the MBBR and twice in the H-MBBR. At the end of a normal SBR cycle, NH4+ was first completely consumed via anammox after addition of NO2−. Non-limiting concentrations of NO3− (30 mgN·L−1) and acetate (40–50 mgCOD·L−1) were then added and their consumption was followed over a period of 1–2 h at the targeted DO set-point.
2.7. 16S rRNA gene-targeted amplicon sequencing
The composition of the bacterial communities of the MBBR and H-MBBR biomasses during the five months of operation at 15 °C was analyzed by high-throughput sequencing of PCR amplicons of the v4 hypervariable region of the 16S rRNA gene pool (primers 515F: 5′-GTGCCAGCMGCCGCGGTAA-3′ and 806R: 5′-GGACTACHVGGGTWTCTAAT-3′), using a MiSeq desktop sequencer (Illumina, USA). A set of four biofilm samples was selected from the MBBR (days 249, 290, 326 and 390) and both the biofilm and suspended floc fractions were sampled from the H-MBBR on days 219, 255, 295 and 350. The v4 hypervariable region was targeted to simultaneously detect anammox populations and cover the diversity of bacterial communities (Gilbert et al., 2014, Weissbrodt et al., 2015). The method was applied here as previously reported (Laureni et al., 2015). Each sample was prepared and sequenced in triplicates. A sequencing depth of 36,500 ± 4,100 reads (min = 25,476, max = 41,844) was achieved on average per sample. The sequencing datasets were mapped and processed using the MiDAS field guide to the microbes of activated sludge (McIlroy et al., 2015). Closest bacterial relatives were assigned to sequencing reads forming operational taxonomic units (OTUs). Amplicon sequencing was used to identify the key bacterial populations composing the AOB, AMX, and NOB guilds, whereas qFISH was then used for quantitative measurements of their relative abundances (see below). Community structures of the MBBR (biofilms solely) and of the H-MBBR (biofilm and floc fractions) were compared via non-metric multidimensional scaling (NMDS) after rarefaction of the sequencing datasets to 25,000 reads.
2.8. Quantitative fluorescence in situ hybridization (qFISH)
Fixation and hybridization of biomass samples were conducted as previously described (Nielsen et al., 2009). Prior to hybridization, the biomass samples were mechanically homogenized with a Potter-Elvehjem tissue grinder (Wheaton, USA) for 1–2 min. The oligonucleotide probes specific to “Candidatus Brocadia anammoxidans” and “Ca. Kuenenia stuttgartiensis” (Amx820) and “Ca. Brocadia fulgida” (Bfu613) were chosen on the basis of the results of the 16S rRNA gene-targeted amplicon sequencing and applied in equimolar mixtures to target the anammox guild. The probes used to detect the ammonium- (AOB) and nitrite- (NOB) oxidizing bacteria as well as the details of their specificity have been previously reported (Laureni et al., 2015). All probes were purchased from Thermo-Fisher Scientific (Ulm, Germany). The hybridized biomass samples were examined and imaged with a confocal laser scanning microscope (Leica, SP5, Germany) and the quantification performed according to Laureni et al. (2015). The relative abundances of AMX, AOB and NOB were estimated by calculating the ratio of their respective specific bacterial biovolumes to the total bacterial biovolume using the Daime software (Daims et al., 2006). A set of four biofilm samples was selected from the MBBR (days 248, 298, 365 and 392) and both the biofilm and suspended floc fractions were sampled from the H-MBBR on days 294, 318 and 349.
2.9. Sampling and analysis of organic micropollutants
In order to investigate the removal of organic micropollutants in the studied PN/A systems, a panel of 27 representative xenobiotic substances was monitored in the primary effluent, pre-treated MWW and effluents. Most of the selected compounds are non-volatile and with low affinity for sorption (Falås et al., 2016). Each sampling campaign lasted between six to nine days and was performed three times for the MBBR (at 20 °C on days 239–244; at 15 °C on days 273–280 and 326–333) and once for the H-MBBR (at 15 °C on days 255–262). The liquid phase samples were collected twice a day and stored at 4 °C for a maximum of three days, after filtration (MN GF-5, 0.4 μm, Macherey-Nagel). The samples from two to three consecutive days were then mixed flow proportionally and stored at −20 °C pending analysis.
An additional fully aerobic (1–4 mgO2·L−1) activated sludge reactor, for COD oxidation and complete nitrification, was established to compare the performance of micropollutant removal in the two PN/A systems with a conventional COD removal and nitrifying system. The reactor received the same primary effluent as the two A-stage + PN/A systems and was operated with an SRT of 15 d (2.1 gTSS·L−1) and an HRT of 12 h. After three months of stable operation, the activated sludge system was sampled in parallel to the two PN/A systems, at a temperature of 15 °C.
The LC-MS/MS method used and the analytical procedure followed for micropollutant analysis are described in (Falås et al., 2016, Rühmland et al., 2015). In brief, sample aliquots of 80 μL were injected into an Agilent 1260 Series liquid chromatography system (Agilent Technologies, Waldbronn, Germany) coupled to a SCIEX QTrap 5500 mass spectrometer (Sciex, Darmstadt, Germany). Chromatographic separation was achieved using a Zorbax Eclipse Plus C-18 (2.1 × 150 mm, 3.5 mm, Agilent Technologies, Waldbronn, Germany). All target compounds were measured within one chromatographic run by scheduled multiple reaction monitoring (sMRM) using electrospray ionization (ESI) in both negative and positive mode. Further details on the LC-MS/MS method and quality assurance are described in the Supporting Information. Compounds displaying removals in the range 0 ± 25% are here considered as persistent. Variations in observed removals are expected to be due to matrix effects as well as sampling and analytical inaccuracies (Joss et al., 2005). For some specific compounds, the occurrence of human metabolites in the influent wastewater that could be retransformed to the parent compound during the biological treatment (e.g. by deconjugation) might result in negative removals (Falås et al., 2016).
2.10. Additional analytical methods for the measurement of global parameters
The concentration of NH4+ was analyzed using a flow injection analyzer (Foss FIA star 5000, Rellingen, Germany). The concentrations of NO2− and NO3− were analyzed by ion chromatography (Compact IC 761, Metrohm, Herisau, Switzerland). The concentration of COD was measured photometrically with test kits (Hach Lange, Düsseldorf, Germany). The samples were filtered using 0.45 μm filters (Macherey-Nagel) prior to analysis. The concentration of total suspended solids (TSS) in the mixed liquors was determined according to standard methods (American Public Health Association, 2005). The biomass on biofilm carriers was estimated from the difference between the weight of a colonized carrier dried at 105 °C and the weight of the same clean carrier dried at 105 °C after immersion during 48 h in a mixture of 2%w/w NaOH and 1% sodium dodecyl sulfate (SDS) solutions. The total biofilm biomass was then obtained by multiplication by the total number of carriers (1032 in MBBR and 1068 in H-MBBR) present in the reactors. In total, six carriers out of the MBBR as well as four carriers and four suspended samples out of the H-MBBR were quantified during the last month of operation at 15 °C.
3. Results
3.1. Aerobic pre-treatment of municipal wastewater to remove COD in the A-stage
The primary effluent was pre-treated in a fully aerated bioreactor to remove organic matter (COD). Removals up to 84 ± 8% and 73 ± 10% were stably obtained for total and dissolved COD respectively (Table 1). Ammonium loss in the pre-treatment varied in a range of 14 ± 20%. The HRT averaged 6 ± 1 h.
3.2. Autotrophic nitrogen removal in the mainstream PN/A reactors from 29 to 15 °C
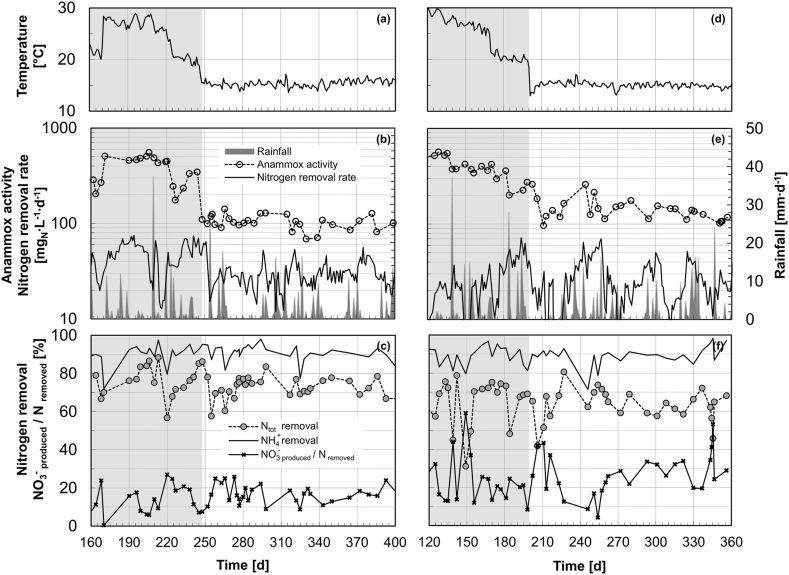
The two PN/A reactors displayed stable operation on pre-treated wastewater during more than one year, including over 5 months at 15 °C (the last 240 days of operation are presented in Fig. 1; for the whole period, see Figs. S1, S2). The reactors were operated at comparable volumetric nitrogen loads: 61 ± 1 and 40 ± 12 mgN·L−1·d−1 (MBBR) and 42 ± 15 and 38 ± 15 mgN·L−1·d−1 (H-MBBR) at medium (15–29 °C) and low (15 °C) temperatures respectively.
Fig. 1.
Conditions and performance of the MBBR (a–c) and H-MBBR (d–f) reactors during the last 240 days of operation. The two reactors were inoculated independently and run in total for 400 and 360 days respectively (the full operational period is presented in Figs. S1, S2). Time series of temperature (a, d); maximum anammox activity (·d−1), overall total nitrogen removal rate (mgN·L−1·d−1), and rainfall (b, e); total and ammonium nitrogen removals, and yield of NO3− production over total nitrogen removed (c, f). Grey areas indicate operation at temperatures above 15 °C. Rainfall data source: Swiss National Air Pollution Monitoring Network (FOEN/NABEL).
The temperature decrease from 25 to 15 °C resulted in a marked drop of the overall nitrogen removal rate in the MBBR from 47 ± 16 to 30 ± 10 mgN·L−1·d−1 (Fig. 1b). However, no major impacts were observed in terms of total and ammonium nitrogen removals, 73 ± 6% and 91 ± 4% respectively, based on the outlet ammonium concentration of 2 mgN·L−1 (set as operational threshold to stop aeration) that meets with discharge quality criteria (Fig. 1c). The yield of NO3− production over nitrogen consumed remained stable at 16 ± 5%. In contrast, the nitrogen removal rates in the H-MBBR were less stable and the step-wise decrease in temperature did not therefore result in any noticeable change (Fig. 1e). The removal rates varied between 26 ± 14 mgN·L−·d−1 at 15 °C with significantly lower total nitrogen removals (63 ± 8%) associated with increased yields of NO3− production (27 ± 11%). However, the consumption of ammonium remained stable in this hybrid reactor at 89 ± 6% (Fig. 1f).
The temperature decrease to 15 °C resulted in an increase in the HRT from 9 ± 1 to 14 ± 3 h in MBBR and from 12 ± 1 to 14 ± 3 h in H-MBBR as a result of a reduced overall PN/A activity and the fixed effluent ammonium threshold value (Figs. S1, S2). In H-MBBR, the SRT of the suspended biomass was estimated as 7 ± 2 d. The measurements were performed during the last month of the experiment under dry weather conditions. In turn, prolonged rain events were observed to result in the progressive washout of the suspended biomass (i.e. shorter HRT due to lower nitrogen concentrations and thus increased volume exchanges and biomass washout). This can partially explain the observed instabilities in the nitrogen removal rate (Fig. 1e).
Interestingly, occasional perturbations such as feeding shutdown and prolonged aeration at DO above 3 (e.g. week-end technical failures), or higher concentrations of COD in the pre-treated wastewater (150–230 mgCODsol·L−1) due to occasional lower performance of the A-stage, did not affect the process performances over the long term.
3.3. Maximum anammox activity in the mainstream PN/A reactors from 29 to 15 °C
At temperatures above 25 °C, both reactors displayed similarly high maximum anammox activities (rAMX,max) of 300–600 ·d−1 (Fig. 1b, e). These dropped significantly when the temperature was decreased to 15 °C. At this temperature, the maximum anammox activity stabilized over five months at 103 ± 18 ·d−1 in the MBBR and at significantly higher values of 138 ± 38 ·d−1 in the H-MBBR. Importantly, the MBBR was run for five additional months (15–20 °C) under stable automated operation without any regular supervision by the operator and maximum anammox activities of 81 and 116 ·d−1 were measured on days 519 and 568 respectively. The overall yield of nitrogen to CODsol consumption at 15 °C amounted to 1.8 ± 0.7 (MBBR) and 2.2 ± 0.8 gN·gCODsol−1 (H-MBBR).
3.4. Effluent quality during mainstream PN/A at 15 °C: N and COD concentrations
According to Table 1, the majority of nitrogen was removed in the PN/A process while most of the COD was removed in the A-stage. Both PN/A reactors stably achieved the effluent value of NH4+ around 2 mgN·L−1, set as the operational threshold to stop aeration, while maintaining an average residual NO2− concentration below 0.2 mgN·L−1. For NO3−, higher concentrations were detected in the H-MBBR effluent (Table 1). Nevertheless, the total nitrogen concentration in the effluent was below 10 mgN·L−1 in both reactors, namely 5.7 ± 1.3 (MBBR) and 8.0 ± 2.6 mgN·L−1 (H-MBBR). Most of the nitrogen removal occurred during the aerobic phase and only minor amounts of NO3− were degraded in the anoxic phases (see Section 3.6). The residual soluble COD was comparable in the effluents of the two systems, whereas the total COD was slightly higher in the MBBR. This was most likely due to the detachment of biomass from the biofilm carriers that was not retained inside the reactor after the only short settling phase of 10 min.
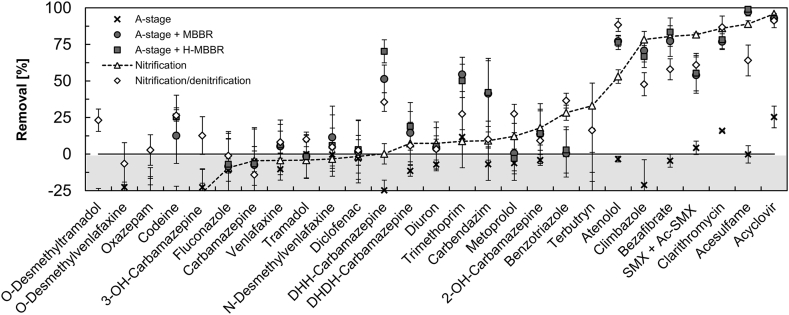
3.5. Effluent quality during mainstream PN/A at 15 °C: organic micropollutants removal
The two studied PN/A systems displayed an almost identical performance in the removal of all investigated micropollutants. Over the global process boundaries, including the A-stage and PN/A, most of the degradation was associated with the PN/A stages (Fig. 2). In the MBBR, the removal did not change over time despite the temperature decrease between the sampling campaigns, from 20 °C (day 239–244) to 15 °C (days 273–280 and 326–333) (for data comparison see Fig. S4).
Fig. 2.
Removal of the studied organic micropollutants at 15 °C in the treatment schemes comprising the A-stage followed by PN/A systems in comparison to a conventional activated sludge reactor. A-stage: pre-treatment for COD removal only; A-stage + MBBR and A-stage + H-MBBR: full treatment schemes; Nitrification: reference reactor for oxidation of organic matter and nitrification (12 h HRT, 15 d SRT); Nitrification/denitrification: literature values from a nitrifying and denitrifying reactor with an HRT of 12 h and an SRT of 10 d (Falås et al., 2016). All removals were calculated for the same time period (sampling campaign days 326–333 MBBR, and 255–262 H-MBBR). The concentrations detected in the MWW served as the initial concentrations C0 for calculating the removal (C/C0) in the different systems. Error bars display standard deviations of 48-h composite samples (n = 3). Compounds displaying removals in the range 0 ± 25% are here considered as persistent. The compounds have been ordered according to their removals in the reference nitrification reactor. Reference removals are connected with a dashed line to facilitate visual comparison. Compounds with removals below −25% (e.g. due to deconjugation during biological treatment) are not visualized on the graph. Micropollutants acronyms: DHH- Carbamazepine: 10,11-dihydro-10-hydroxy-carbamazepine; DHDH-Carbamazepine: 10,11-dihydro-10,11-dihydroxy-carbamazepine; SMX + Ac-SMX: sum of sulfamethoxazole and N4-acetylsulfamethoxazole.
Furthermore, the removal efficiencies of the two PN/A systems were comparable for most compounds to those obtained in the nitrifying activated sludge reactor operated in parallel under similar conditions (15 °C, 12-h HRT, 15-d SRT) and reported in literature for a nitrification/denitrification reactor (Falås et al., 2016). In line with these conventional systems, approximately half of the studied micropollutants displayed negligible or low removals (0 ± 25%), in the range of persistent compounds, and only in few cases removals exceeded 75% (Fig. 2). The negligible transformation of the investigated compounds in the A-stage, characterized by high sludge production and a continuous aeration regime, confirms the minor role played by stripping and sorption processes.
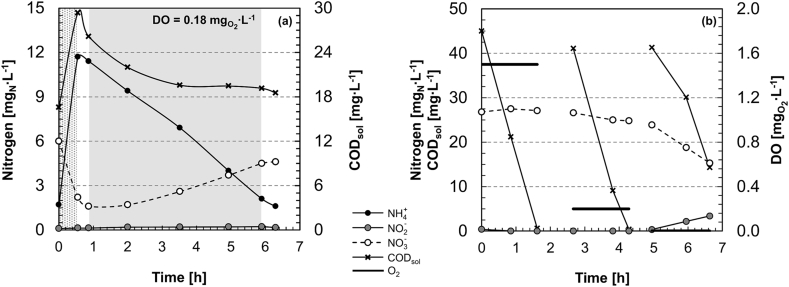
3.6. Actual activities contributing to nitrogen turnover during a PN/A cycle
A representative PN/A SBR cycle at 15 °C of the MBBR reactor is presented in Fig. 3a (the H-MBBR displayed similar profiles). During feeding, the NH4+ concentration increased while the NO3− was progressively diluted and partially denitrified. Denitrification also occurred in the pre-anoxic step with simultaneous consumption of CODsol and NO3−, partially contributing to the overall low concentration of nitrogen in the effluent. Most of the nitrogen was transformed during aeration with a minor effect of the post-anoxic phase. During aeration, the residual CODsol was further consumed. Specific tests were performed by spiking acetate as a representative readily biodegradable carbon source to confirm that, even at a low DO concentration of 0.18 , oxygen was the dominant terminal electron acceptor for the oxidation of the residual organic matter (Fig. 3b). In the presence of O2 and absence of NO2− and NH4+, the acetate was fully consumed without simultaneous reduction of NO3−. Conversely, NO3− was used as the electron acceptor for acetate oxidation under anoxic conditions (Fig. 3b). It is therefore reasonable to neglect the contribution of heterotrophic denitrification to the nitrogen turnover during the aeration phase. Thus, the volumetric activities of the three autotrophic guilds during SBR operation (rAMX,cycle, rAOB,cycle and rNOB,cycle) can be estimated on the basis of the concentration changes of the main nitrogen species during aeration, according to the stoichiometric matrix presented in Table S1. The activities during operation at 15 °C are reported in Table 2 together with the maximum anammox activity (averaged over the five months at 15 °C; rAMX,max). The activity of the AOB guild was comparable during the cycles of the two systems, whereas the activity of the NOB guild was significantly higher during the H-MBBR cycle. In turn, the activity of the AMX guild was significantly higher during the MBBR cycle.
Fig. 3.
Evolution of the concentrations of nitrogen species (NH4+, NO2−, NO3−) and dissolved organic matter (CODsol) during representative SBR cycles at 15 °C (dotted area: initial settling + feeding phase; white areas: pre- and post-anoxic phases; grey area: aeration phase at 0.18 ) (a). Nitrogen species, CODsol and DO set-point during in situ batch tests conducted by spiking acetate as a representative readily biodegradable organic compound under different DO conditions (set at 1.5, 0.2 and 0 ), at 15 °C and in absence of ammonium (b).
Table 2.
Average volumetric activities of the three autotrophic guilds of interest (rAMX,cycle, rAOB,cycle and rNOB,cycle) during normal operation for PN/A at 15 °C (MBBR: n = 8, H-MBBR: n = 11). The maximum anammox activity (rAMX,max) was averaged over the five months of operation at 15 °C (MBBR: n = 29, H-MBBR: n = 28). The relative abundances of the three guilds were measured by qFISH on the biofilm (biofilm) of both reactors and additionally on the suspended floc fraction (flocs) of the H-MBBR.
| Actual activity |
Relative abundances (qFISH) and Total Suspended Solids |
|||||||
|---|---|---|---|---|---|---|---|---|
| Units | MBBR | H-MBBR | Units | MBBRbiofilm | H-MBBRbiofilm | H-MBBRflocs | ||
| rAMX,max | [mg(NH4+NO2)-N∙L−1∙d−1] | 103 ± 18 | 138 ± 38 | AMX | [%] | 16.1 ± 3.1 | 15.5 ± 3.2 | 1.8 ± 1.9 |
| rAMX,cycle | [mg(NH4+NO2)-N∙L−1∙d−1] | 40 ± 11 | 23 ± 9 | |||||
| rNOB,cycle | [mgNO3-N∙L−1∙d−1] | 16 ± 9 | 25 ± 12 | NOB | [%] | 1.6 ± 0.6 | 0.7 ± 0.2 | 1.9 ± 0.7 |
| rAOB,cycle | [mgNO2-N∙L−1∙d−1] | 44 ± 10 | 41 ± 9 | AOB |
[%] |
1.7 ± 0.6 |
0.4 ± 0.1 |
1.9 ± 0.5 |
| TSS | [g/L] | 1.87 ± 0.14 | 2.47 ± 0.55 | 0.28 ± 0.07 | ||||
3.7. Bacterial community composition and guilds segregation in the PN/A reactors at 15 °C
Both reactors displayed comparable concentrations of total solids, i.e. 1.9 and 2.7 gTSS·L−1 in the MBBR and H-MBBR respectively, and the flocculent sludge represented about 10% of the global TSS of the H-MBBR (Table 2). Analyses of 16S rRNA gene-targeted amplicon sequencing (v4 hypervariable region) qualitatively revealed that, over the experimental phase at 15 °C, the AMX, AOB, and NOB guilds were dominated by the known genera “Ca. Brocadia” (100% of the guild-specific sequencing reads), Nitrosomonas (97 ± 4% of guild-specific reads) and Nitrospira (59 ± 23% of guild-specific reads, while all reads affiliated with the order Nitrospirales), respectively. “Ca. Brocadia” AMX relatives and Nitrospira-affiliated NOB were primarily detected on the biofilm carriers of the two reactors (Fig. 4a–b). Nitrosomonas-related AOB were substantially detected on the carriers of the MBBR, whereas the H-MBBR exhibited a shared presence of this population on both biofilm and floc fractions (Fig. 4b–c).
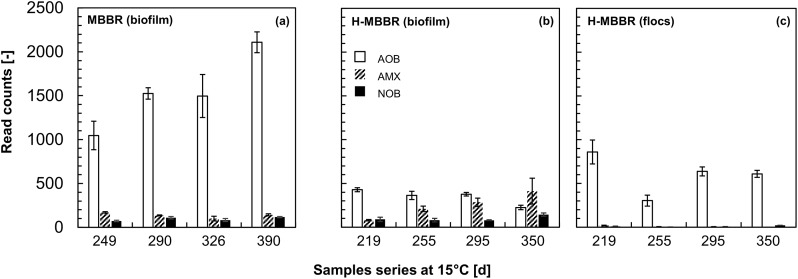
Fig. 4.
Preferential localization of “Ca. Brocadia”-related AMX, Nitrosomonas-related AOB, and Nitrospira-related NOB guilds over the four biomass samples collected along the operation at 15 °C in the MBBR (biofilm carriers only – a) and in the H-MBBR (biofilm carriers and flocs – b and c respectively) examined by 16S rRNA gene-based amplicon sequencing analyses (error bars display the standard deviation of biological technical triplicates). The sequencing results are only qualitatively displayed as read counts (out of 25,000 reads per sample), whereas the relative abundances of these guilds were estimated by qFISH-CLSM (Table 2).
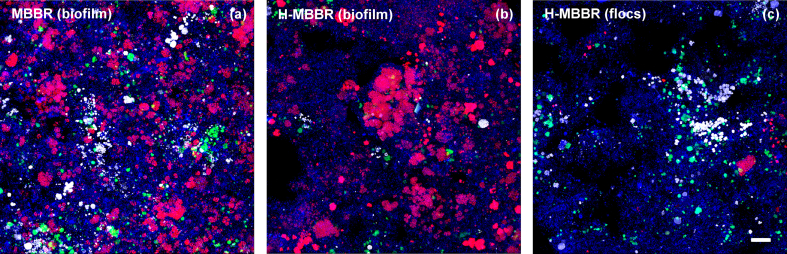
Quantitatively, the relative abundances of the AMX, AOB, and NOB guilds were estimated by qFISH-CLSM (Table 2). AMX represented about 15–16% of the biofilm bacterial community of both reactors while only a minor fraction (<2%) was found in the floc suspension of the H-MBBR. AOB and NOB were present in comparable relative abundances on the biofilm carriers of the MBBR, 1.6% and 1.7%, respectively. In the H-MBBR, unlike in the amplicon sequencing results, both aerobic guilds were detected in the suspended and attached fractions with about 25% of NOB and 35% of AOB found in suspension (as estimated by multiplying the qFISH relative abundances and the TSS of the biofilm and flocs, Table 2). Over the whole period at 15 °C, the relative abundances of all three autotrophic guilds remained stable in all biomass fractions. Fig. 5 provides representative FISH-CLSM images of the two types of attached and suspended biomasses collected at the end of the experimental period from the MBBR (day 400) and H-MBBR (day 359).
Fig. 5.
Representative FISH-CLSM digital images illustrating the distribution of AMX, AOB and NOB in the different biomass fractions, namely MBBR biofilm (a), H-MBBR biofilm (b) and flocs (c) at the end of the experimental period at 15 °C. Anammox populations (AMX; Amx820 + Bfu613 oligonucleotides labeled with the fluorescent probe Cy5) are displayed with purple color allocation, aerobic ammonium-oxidizing bacteria (AOB; AOB-mix, Cy3) in white, aerobic nitrite-oxidizing bacteria (NOB; NOB-mix, FLUOS) in green, and DAPI stain in blue. Each image is the maximum intensity projection of a single z-stack. Biomasses were homogenized prior to imaging (scale bars: 20 μm). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Amplicon sequencing further indicated that the bacterial community of mainstream PN/A systems is far from being composed solely of AMX, AOB, and NOB (Fig. S6a in the Supporting Information). In addition, non-metric multidimensional scaling (NMDS) computation revealed that, in contrast to a more variable floc fraction of the H-MBBR, the bacterial community compositions of the biofilm carriers did not display significant dissimilarities over time at 15 °C in both the MBBR and the H-MBBR (Fig. S6b).
3.8. Impacts of a drop in temperature and prolonged operation at 11 °C on anammox activity
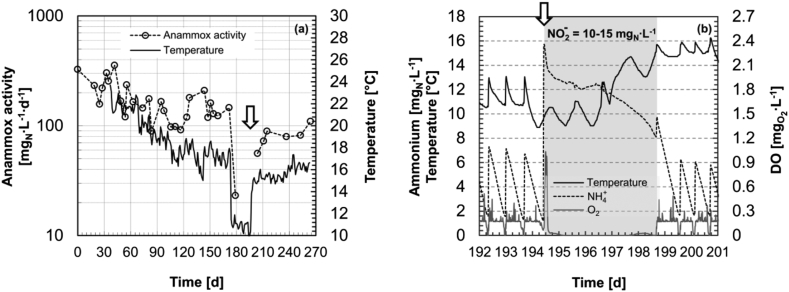
The third additional reactor MBBR-2, operated in parallel under similar conditions to the two main PN/A reactors, was used to test the effect of a sharp temperature decrease to 11 °C on anammox activity (Fig. 6a). The anammox activity was stable in the range of 100–200 ·d−1 at temperatures above 15 °C. The sudden temperature drop to 11 °C resulted in a dramatic decrease in activity below 20 ·d−1. The anammox activity was further barely detectable during a month at 11 °C, as confirmed by the activity test performed on day 194 (Fig. 6b). Initially, the added NH4+ and NO2− (white arrow in Fig. 6) were almost completely oxidized to nitrate due to residual bulk O2 (grab sample, data not shown). Once the oxygen was consumed, the concentrations of ammonia (12–14 mgN·L−1, online signal; Fig. 6b) and nitrite (10–15 mgN·L−1, test stripes; data not shown) remained stable during two days. Nevertheless, when the temperature was slowly increased to about 15 °C on day 197, ammonia started to be consumed again together with nitrite (Fig. 6b). From then on, anammox activity increased rapidly and was almost completely recovered after one week. The effluent characteristics of the MBBR-2 are plotted in Fig. S4. The aerobic activity was also negatively affected by the imposed temperature decrease as clearly suggested by the reduction of the time the air valve was open during a batch (i.e. slower oxygen consumption; Fig. S3(d)) and the increase in the average HRT (i.e. longer cycles due to slower ammonia oxidation; Fig. S3(c)).
Fig. 6.
Temporal evolution of the maximum volumetric anammox activity (expressed as the sum of NH4+, NO2− consumption) in response to the temperature step variation during the operation for PN/A in MBBR-2 (a). Online NH4+, O2 and temperature signals of MBBR-2 between day 192 and 201 (b). For completeness, three normal SBR cycles are included before and after the in situ anammox activity performed on days 194–199 (grey area; non-limiting nitrite concentration 10–15 mgN·L−1; test stripes). The white arrow highlights the beginning of the anammox activity on day 194, namely when SBR operation was stopped, the reactor was set to mixing mode and non-limiting concentrations of NH4+ and NO2− were added. Note: the minor temperature fluctuations around the set value in (b) are due to the feeding events (the influent tank was not temperature controlled) or to the diurnal ambient temperature variations when the reactor was not fed (i.e. during the batch anammox test).
4. Discussion
4.1. Mainstream PN/A performs comparably to conventional treatments at 15 °C in terms of nitrogen removal and effluent quality
Stable PN/A was successfully maintained for more than one year on aerobically pre-treated MWW in two parallel SBRs (Fig. 1). The net volumetric nitrogen removal rates averaged 47 mgN·L−1·d−1 at higher temperatures (20–30 °C) and 30 mgN·L−1·d−1 at 15 °C (MBBR). These values are comparable to the typical values achieved in municipal wastewater treatment (Lotti et al., 2015, Metcalf & Eddy et al., 2013) and thus would result in comparable reactors size. Settling and idle/mixing times accounted for about 30–40% of the total operating time at 15 °C (Figs. S1, S2). Therefore, an optimized process design and operation would result directly in improved rates. Higher removal rates have been previously reported in similar studies investigating low temperature PN/A on MWW. In a rotating biological contactor (RBC), operated continuously at 15 °C and 3–4 , activities between 300 and 500 mgN·L−1·d−1 have been obtained on diluted raw wastewater containing 50–60 and 0.5–2 gCOD·−1 (De Clippeleir et al., 2013). Similarly, volumetric rates in the range of 150–200 mgN·L−1·d−1 at 19 °C and 0–2 have been achieved in a plug-flow granular pilot-scale reactor treating the settled effluent of a pilot-scale A-stage (30 , 0.7 gCOD·−1) (Lotti et al., 2015). However, the overall nitrogen removal was limited (<45%) in both studies, mainly due to high NO3− production and, to a lesser extent, to NO2− accumulation. In contrast, the NOB activity was consistently low in our study. At 15 °C, the average yield of nitrate produced per ammonium consumed was as low as 16%, i.e. close to the stoichiometric value of 11% for PN/A (Strous et al., 1999). Accordingly, removal efficiencies were significantly higher, >90% for NH4+ and >70% for total nitrogen, and thus comparable with the ones reported e.g. for the Demon plant in Strass, Austria, treating digester supernatant (Wett, 2007) and complying with a prerequisite for mainstream applications (Council Directive 91/271/EEC, 1991, WPO, 1998).
In terms of effluent quality at 15 °C, both reactors achieved residual average concentrations of total nitrogen below 10 mgN·L−1 (below 6 mgN·L−1 in MBBR), which is comparable to the performance of conventional treatments for nitrogen removal. These values are by far the lowest reported in the literature for mainstream PN/A under cold conditions (De Clippeleir et al., 2013, Gilbert et al., 2015, Hu et al., 2013, Lotti et al., 2015). The minimum effluent concentrations of NH4+ reported in the literature at 10–15 °C are in the range of 5–8 with significant accumulation of nitrite and nitrate (De Clippeleir et al., 2013, Gilbert et al., 2015). In turn, NO2− was not observed to accumulate in the PN/A systems operated here and was in most cases below 0.2 , with the exception of rare cases of O2-sensor failures. It is to be noted that, in line with typical effluent quality criteria, a fixed threshold value of 2 for ammonium was used as a control parameter to end the aeration phase. Further optimization of effluent quality and removal rate are beyond the scope of this work but are deemed to be feasible.
4.2. Organic micropollutants are removed comparably to conventional biological treatments
In addition to more stringent nutrients discharge limits, the removal of organic micropollutants is gaining increased relevance in the design and upgrade of wastewater treatment plants (Eggen et al., 2014). The discharge of organic micropollutants with MWW may in fact trigger unwanted ecological effects in the receiving waters (Brodin et al., 2013, Jobling et al., 1998). In this perspective, the micropollutants removal potential of mainstream PN/A systems has been quantified in the present work for the first time. Interestingly, the two PN/A reactors performed almost identically and removed trace contaminants to a similar extent as conventional treatments (Fig. 2). The discussion about the specific removal of individual compounds was beyond the scope of the present work. Nevertheless, the high removals observed for trimethoprim and DHH-carbamazepine (50–70%), in line with e.g. the ones reported for staged conventional systems (Batt et al., 2006), are highlighted and might be worth further investigation. Moreover, another intriguing open question is whether anammox bacteria (which represented 15% of the bacterial community) were directly responsible for the observed removals or some other microorganisms developing under these conditions.
The high effluent quality obtained in the studied PN/A systems with respect to organic carbon (COD) and NO2− (Table 1) would allow effective tertiary treatment of micropollutants with ozone, where both organic matter and NO2− act as O3 scavengers, and with activated carbon, where the organic carbon can result in premature saturation of available sorption sites (Margot et al., 2013). Thus, this study highlights that PN/A systems perform comparably to conventional nutrient elimination with respect to the biological removal of micropollutants, and are expected to be comparably suited wherever tertiary treatment of organic micropollutants is required.
4.3. A robust anammox population can be stably maintained in the biofilm under mainstream conditions
The anammox guild was stably present and active in the biofilm of both the MBBR and H-MBBR throughout the experimental period over the temperature range 15–29 °C. Moreover, anammox remained the main process governing the observed nitrogen removal, with heterotrophic denitrification playing only a minor role (i.e. limited and mainly aerobic COD oxidation in PN/A reactors Fig. 3). Prolonged operation at 15 °C resulted in lower but stable maximum activities with values in the range of similar studies (e.g. (De Clippeleir et al., 2013, Lotti et al., 2014a)). A dramatic decrease and almost complete suppression of anammox activity was observed only when the temperature was suddenly reduced to 11 °C (Fig. 6), in good agreement with the literature (Gilbert et al., 2015, Hu et al., 2013, Lotti et al., 2014a). Nonetheless, after one month at 11 °C, the anammox activity rapidly recovered as soon as the temperature was increased back to 15 °C. This proved that the anammox populations were successfully retained in the ecosystem of the biofilm carriers and their activity was resilient after only temporary inhibition. However, the causes of the observed anammox activity suppression e.g. direct temperature effect, indirect oxygen inhibition or a combination thereof, remain unclear and deserve further investigation.
Several months of operation at 15 °C did not result in any apparent change in the predominant anammox genus nor in the relative abundance of this population in the biofilms. qFISH analysis showed that the AMX guild stably accounted for about 15% of the biofilm fraction of both reactors (Table 2). Similarly, Gilbert et al. (2015) observed a relatively stable bacterial community composition at phylum level and comparable relative abundances of anammox (25–35%; qPCR) on carrier media treating COD-free synthetic wastewater at low temperatures. In the present study, the preferential localization of the AMX guild in the biofilm fractions was further qualitatively confirmed by amplicon sequencing. Overall, qFISH data and the relative read abundance of the amplicon sequences were consistent between the various time points, although with significantly lower values for the latter. The observed discrepancies can most likely be ascribed to differences in gene copy numbers and extraction biases combined with possible overestimations by FISH due to differences in ribosomal content and fluorochrome intensities.
Interestingly, “Ca. Brocadia” dominated the AMX guild of the sidestream biofilm carriers used as inocula (Weissbrodt et al., 2015) and remained the main anammox genus throughout the experiment. The predominance of “Ca. Brocadia” has been reported in many independent mainstream studies (e.g. (Gilbert et al., 2014, Laureni et al., 2015, Lotti et al., 2015)), so the putative important role of this candidate genus under mainstream conditions is further supported by this work.
4.4. A low DO concentration allows successful suppression of NOB activity in the MBBR biofilm
The MBBR and H-MBBR were operated at comparably low O2 concentrations of 0.18 and 0.15 mgO2·L−1 respectively, and at an identical effluent ammonium concentration of 2 mgN·L−1 (set as operational threshold to stop aeration). Under these conditions, stable suppression of NOB activity was achieved in the biofilm carriers of the MBBR (Fig. 1). Substrate gradients and competition for oxygen have been shown numerically to act as the main mechanisms for the control of NOB in biofilm-based reactors (Brockmann and Morgenroth, 2010, Isanta et al., 2015, Pérez et al., 2014). In addition, maintaining a minimum residual bulk ammonium concentration (according to the operational DO set-point) has been recently reported as a prerequisite to promote the growth of AOB over NOB (Pérez et al., 2014). It was shown here that effluent ammonium concentrations complying with discharge limits can be stably achieved at the imposed low oxygen concentrations. At the same time, the low DO set-point most likely limited AOB activity and thus the production of nitrite. As a result, the anammox activity during the SBR cycle operation (rAMX,cycle) was limited to only 39% of their maximum potential (rAMX,max; Table 2).
In contrast, similar operating conditions with even slightly more stringent O2 availability did not allow proper suppression of NOB activity in the H-MBBR, where about 10% of the total biomass was present as flocs. In fact, aerobic guilds tend to prefer smaller aggregates (e.g. flocs) with less diffusion limitations than biofilms (Corbala-Robles et al., 2015, Vlaeminck et al., 2010, Volcke et al., 2010, Winkler et al., 2012). In the H-MBBR, about 35% of AOB and 25% of NOB grew in suspension (as estimated by multiplying the qFISH relative abundances and the TSS of the biofilm and flocs, Table 2). Most likely, NOB benefited from a more direct access to O2 and NO2− because of lower diffusion limitations in flocs and were thus favored over AMX. The AOB activity was comparable in the two systems, whereas the NOB activity was substantially higher in H-MBBR in spite of being about two times less abundant in the hybrid reactor. This led to higher NO3− effluent concentrations and poorer process performance in the H-MBBR. Nevertheless, anammox were maintained in the system even if their activity during operation was only 16% of their maximum potential. A combination of increased competition with NOB for NO2− and deeper O2 penetration in the biofilm (e.g. reduced aerobic layer on biofilm surface) is hypothesized here as the reason for the observed behavior.
4.5. Practical implications
The presented results strongly support the feasibility of MWW treatment schemes with anammox-based autotrophic nitrogen removal. Stable PN/A was demonstrated on pre-treated MWW, even with a relatively high residual content of organic matter (2.2 gCODsol·gN−1 of which 1.3 gCODrb·gN−1 readily biodegradable). Effluent total nitrogen concentrations below 10 mgN·L−1 and micropollutants removals comparable to conventional nitrification treatments were achieved.
Operation at variable hydraulic and nitrogen loads while permanently complying with the discharge limits constitutes the next main challenge toward process scale-up. In the present study, the PN/A systems were operated at varying nitrogen loads (depending on MWW concentrations) but the HRT varied according to the PN/A activity and not to the actual MWW flow. An additional challenge is the management of winter times with prolonged periods at temperatures close to or below 10 °C. Here, the anammox populations were shown to survive with strongly reduced activity, but they recovered rapidly after one month at 11 °C (typical duration of minimum temperatures in moderate climates). Elucidating the mechanisms that limited anammox activity (e.g. direct temperature effect or indirect oxygen inhibition due to reduced aerobic activity) would allow appropriate low-temperature operational strategies to be derived.
Finally, the identification and design of engineered solutions for NOB control, suppression, and wash-out is a prerequisite towards the implementation of anammox microbial processes under mainstream conditions. In the present study, stable NOB suppression was obtained at low DO concentration in the MBBR, whereas the development of an uncontrolled suspended biomass fraction in the H-MBBR seemed to favor nitrite oxidation, in agreement with the numerical results of Hubaux et al. (2015). It should however be noted that the hybrid system was in this case intentionally operated with only a minor fraction of biomass in suspension, periodically exposed to washout, in order to study its impacts on process performance. Therefore, the presented results do not permit a comparison between pure MBBR and true hybrid systems such as the integrated fixed-film activated sludge (IFAS) systems (Veuillet et al., 2014) for mainstream applications. Moreover, it is here speculated that hybrid systems with proper control of suspended biomass and segregation of microbial activities (i.e. anoxic in biofilm and aerobic in flocs) bear the potential for a more versatile control of NOB, for higher volumetric rates with lower oxygen requirements (e.g. reduced diffusion limitations in flocs), and possibly for a more flexible operation towards varying loads as opposed to pure MBBRs.
5. Conclusions
The long-term stability and effluent quality of mainstream PN/A processes treating municipal wastewater at low temperature were studied in three main parallel lab-scale reactors operated as pure and hybrid MBBRs respectively. The obtained results led to the following main conclusions:
-
•
PN/A processes can be stably operated on pre-treated MWW at 15 °C over several months with nitrogen removal rates of 30 mgN·L−1·d−1 in the range of conventional nutrient removal systems;
-
•
good total nitrogen removal efficiencies (>70%) and effluent concentrations (2 mgNH4-N·L−1 and 6 mgNtot·L−1), complying with the current discharge limits, are achievable under mainstream conditions;
-
•
the removal of organic micropollutants in mainstream PN/A systems is comparable to the removal achieved in conventional processes for biological nutrients removal;
-
•
“Ca. Brocadia” remained the dominant anammox genus in the biofilm throughout the study, further confirming its apparent metabolic advantage on real substrates under mainstream conditions;
-
•
prolonged operation at 11 °C may result in a reversible but dramatic suppression of anammox activity and thus operation under winter conditions remains an open challenge towards full-scale implementation;
-
•
NOB activity can be stably suppressed at low oxygen concentrations (0.18 mgO2·L−1) in MBBR systems, whereas the development of suspended biomass fraction in hybrid MBBRs requires proper control to prevent nitrite oxidation.
Acknowledgements
This study was funded by the European Research Council ERC via the ATHENE project (grant agreement 267897). We are extremely grateful to Ilona Szivák for valuable discussions and experience exchange on the FISH method. We sincerely thank Kris Villez for fruitful discussions on different mathematical approaches, Marco Kipf for his support in the laboratory, and Claudia Baenninger-Werffeli and Karin Rottermann at Eawag for their assistance with the physicochemical analyses of all the samples. We further acknowledge the excellent assistance of Nadieh de Jonge with amplicon sequencing analyses at Aalborg University.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.watres.2016.05.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alvarino T., Suarez S., Katsou E., Vazquez-Padin J., Lema J.M., Omil F. Removal of PPCPs from the sludge supernatant in a one stage nitritation/anammox process. Water Res. 2014;68C:701–709. doi: 10.1016/j.watres.2014.10.055. [DOI] [PubMed] [Google Scholar]
- American Public Health Association E.A.D.A.W.W.A.W.E.F. APHA-AWWA-WEF; Washington, D.C: 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Batt A.L., Kim S., Aga D.S. Enhanced biodegradation of iopromide and trimethoprim in nitrifying activated sludge. Environ. Sci. Technol. 2006;40(23):7367–7373. doi: 10.1021/es060835v. [DOI] [PubMed] [Google Scholar]
- Brockmann D., Morgenroth E. Evaluating operating conditions for outcompeting nitrite oxidizers and maintaining partial nitrification in biofilm systems using biofilm modeling and monte carlo filtering. Water Res. 2010;44(6):1995–2009. doi: 10.1016/j.watres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Brodin T., Fick J., Jonsson M., Klaminder J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science. 2013;339(6121):814–815. doi: 10.1126/science.1226850. [DOI] [PubMed] [Google Scholar]
- Corbala-Robles L., Picioreanu C., van Loosdrecht M.C., Perez J. Analysing the effects of the aeration pattern and residual ammonium concentration in a partial nitritation-anammox process. Environ. Technol. 2015:1–22. doi: 10.1080/09593330.2015.1077895. [DOI] [PubMed] [Google Scholar]
- Council Directive 91/271/EEC of 21 May 1991 concerning urban waste-water treatment. Off. J. Eur. Commun. L. 30/5/1991;135:40–52. [Google Scholar]
- Daims H., Lücker S., Wagner M. daime, a novel image analysis program for microbial ecology and biofilm research. Environ. Microbiol. 2006;8(2):200–213. doi: 10.1111/j.1462-2920.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- De Clippeleir H., Vlaeminck S.E., De Wilde F., Daeninck K., Mosquera M., Boeckx P., Verstraete W., Boon N. One-stage partial nitritation/anammox at 15°C on pretreated sewage: feasibility demonstration at lab-scale. Appl. Microbiol. Biotechnol. 2013;97(23):10199–10210. doi: 10.1007/s00253-013-4744-x. [DOI] [PubMed] [Google Scholar]
- de Graaff M.S., Vieno N.M., Kujawa-Roeleveld K., Zeeman G., Temmink H., Buisman C.J.N. Fate of hormones and pharmaceuticals during combined anaerobic treatment and nitrogen removal by partial nitritation-anammox in vacuum collected black water. Water Res. 2011;45(1):375–383. doi: 10.1016/j.watres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Eggen R.I., Hollender J., Joss A., Scharer M., Stamm C. Reducing the discharge of micropollutants in the aquatic environment: the benefits of upgrading wastewater treatment plants. Environ. Sci. Technol. 2014;48(14):7683–7689. doi: 10.1021/es500907n. [DOI] [PubMed] [Google Scholar]
- Falås P., Wick A., Castronovo S., Habermacher J., Ternes T.A., Joss A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016;95:240–249. doi: 10.1016/j.watres.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert E.M., Agrawal S., Karst S.M., Horn H., Nielsen P.H., Lackner S. Low temperature partial nitritation/anammox in a moving bed biofilm reactor treating low strength wastewater. Environ. Sci. Technol. 2014;48(15):8784–8792. doi: 10.1021/es501649m. [DOI] [PubMed] [Google Scholar]
- Gilbert E.M., Agrawal S., Schwartz T., Horn H., Lackner S. Comparing different reactor configurations for partial nitritation/anammox at low temperatures. Water Res. 2015 doi: 10.1016/j.watres.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Hu Z., Lotti T., de Kreuk M., Kleerebezem R., van Loosdrecht M.C.M., Kruit J., Jetten M.S.M., Kartal B. Nitrogen removal by a nitritation-anammox bioreactor at low temperature. Appl. Environ. Microbiol. 2013;79(8):2807–2812. doi: 10.1128/AEM.03987-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubaux N., Wells G., Morgenroth E. Impact of coexistence of flocs and biofilm on performance of combined nitritation-anammox granular sludge reactors. Water Res. 2015;68:127–139. doi: 10.1016/j.watres.2014.09.036. [DOI] [PubMed] [Google Scholar]
- Isanta E., Reino C., Carrera J., Perez J. Stable partial nitritation for low-strength wastewater at low temperature in an aerobic granular reactor. Water Res. 2015;80:149–158. doi: 10.1016/j.watres.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Jobling S., Nolan M., Tyler C.R., Brighty G., Sumpter J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998;32(17):2498–2506. [Google Scholar]
- Joss A., Derlon N., Cyprien C., Burger S., Szivák I., Traber J., Siegrist H., Morgenroth E. Combined nitritation–anammox: advances in understanding process stability. Environ. Sci. Technol. 2011;45(22):9735–9742. doi: 10.1021/es202181v. [DOI] [PubMed] [Google Scholar]
- Joss A., Keller E., Alder A.C., Gobel A., McArdell C.S., Ternes T., Siegrist H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005;39(14):3139–3152. doi: 10.1016/j.watres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Lackner S., Gilbert E.M., Vlaeminck S.E., Joss A., Horn H., van Loosdrecht M.C.M. Full-scale partial nitritation/anammox experiences – an application survey. Water Res. 2014;55:292–303. doi: 10.1016/j.watres.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Laureni M., Weissbrodt D.G., Szivak I., Robin O., Nielsen J.L., Morgenroth E., Joss A. Activity and growth of anammox biomass on aerobically pre-treated municipal wastewater. Water Res. 2015;80:325–336. doi: 10.1016/j.watres.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti T., Kleerebezem R., Hu Z., Kartal B., de Kreuk M., van Erp Taalman Kip C., Kruit J., Hendrickx T.L.G., van Loosdrecht M.C.M. Pilot-scale evaluation of anammox-based mainstream nitrogen removal from municipal wastewater. Environ. Technol. 2015;36(9):1167–1177. doi: 10.1080/09593330.2014.982722. [DOI] [PubMed] [Google Scholar]
- Lotti T., Kleerebezem R., Hu Z., Kartal B., Jetten M.S.M., van Loosdrecht M.C.M. Simultaneous partial nitritation and anammox at low temperature with granular sludge. Water Res. 2014;66:111–121. doi: 10.1016/j.watres.2014.07.047. [DOI] [PubMed] [Google Scholar]
- Lotti T., Kleerebezem R., van Erp Taalman Kip C., Hendrickx T.L.G., Kruit J., Hoekstra M., van Loosdrecht M.C.M. Anammox growth on pretreated municipal wastewater. Environ. Sci. Technol. 2014;48(14):7874–7880. doi: 10.1021/es500632k. [DOI] [PubMed] [Google Scholar]
- Ma B., Peng Y., Zhang S., Wang J., Gan Y., Chang J., Wang S., Wang S., Zhu G. Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour. Technol. 2013;129:606–611. doi: 10.1016/j.biortech.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Malovanyy A., Trela J., Plaza E. Mainstream wastewater treatment in integrated fixed film activated sludge (IFAS) reactor by partial nitritation/anammox process. Bioresour. Technol. 2015;198:478–487. doi: 10.1016/j.biortech.2015.08.123. [DOI] [PubMed] [Google Scholar]
- Margot J., Kienle C., Magnet A., Weil M., Rossi L., de Alencastro L.F., Abegglen C., Thonney D., Chèvre N., Schärer M., Barry D.A. Treatment of micropollutants in municipal wastewater: ozone or powdered activated carbon? Sci. Total Environ. 2013;461–462:480–498. doi: 10.1016/j.scitotenv.2013.05.034. [DOI] [PubMed] [Google Scholar]
- McIlroy S.J., Saunders A.M., Albertsen M., Nierychlo M., McIlroy B., Hansen A.A., Karst S.M., Nielsen J.L., Nielsen P.H. MiDAS: the field guide to the microbes of activated sludge. Database. 2015;2015:bav062. doi: 10.1093/database/bav062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf & Eddy, Inc., Tchobanoglous G., Stensel H.D., Tsuchihashi R., Burton F. McGraw-Hill Education; 2013. Wastewater Engineering: Treatment and Resource Recovery. [Google Scholar]
- Nielsen P.H., Daims H., Lemmer H. IWA Publishing; London: 2009. FISH Handbook for Biological Wastewater Treatment : Identification and Quantification of Microorganisms in Activated Sludge and Biofilms by FISH. [Google Scholar]
- Pérez J., Lotti T., Kleerebezem R., Picioreanu C., van Loosdrecht M.C.M. Outcompeting nitrite-oxidizing bacteria in single-stage nitrogen removal in sewage treatment plants: a model-based study. Water Res. 2014 doi: 10.1016/j.watres.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Petrie B., Barden R., Kasprzyk-Hordern B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015;72:3–27. doi: 10.1016/j.watres.2014.08.053. [DOI] [PubMed] [Google Scholar]
- Rühmland S., Wick A., Ternes T.A., Barjenbruch M. Fate of pharmaceuticals in a subsurface flow constructed wetland and two ponds. Ecol. Eng. 2015;80:125–139. [Google Scholar]
- Siegrist H., Salzgeber D., Eugster J., Joss A. Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci. Technol. 2008;57(3):383–388. doi: 10.2166/wst.2008.048. [DOI] [PubMed] [Google Scholar]
- Strous M., Fuerst J.A., Kramer E.H.M., Logemann S., Muyzer G., van de Pas-Schoonen K.T., Webb R., Kuenen J.G., Jetten M.S.M. Missing lithotroph identified as new planctomycete. Nature. 1999;400(6743):446–449. doi: 10.1038/22749. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M.C.M., Brdjanovic D. Water treatment. Anticipating the next century of wastewater treatment. Science. 2014;344(6191):1452–1453. doi: 10.1126/science.1255183. [DOI] [PubMed] [Google Scholar]
- Veuillet F., Lacroix S., Bausseron A., Gonidec E., Ochoa J., Christensson M., Lemaire R. Integrated fixed-film activated sludge ANITAMox process – a new perspective for advanced nitrogen removal. Water Sci. Technol. 2014;69(5):915–922. doi: 10.2166/wst.2013.786. [DOI] [PubMed] [Google Scholar]
- Vlaeminck S.E., Terada A., Smets B.F., De Clippeleir H., Schaubroeck T., Bolca S., Demeestere L., Mast J., Boon N., Carballa M., Verstraete W. Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and anammox. Appl. Environ. Microbiol. 2010;76(3):900–909. doi: 10.1128/AEM.02337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volcke E.I., Picioreanu C., De Baets B., van Loosdrecht M.C.M. Effect of granule size on autotrophic nitrogen removal in a granular sludge reactor. Environ. Technol. 2010;31(11):1271–1280. doi: 10.1080/09593331003702746. [DOI] [PubMed] [Google Scholar]
- Weissbrodt D.G., Wells G.F., Goel R.K., Laureni M., Bürgmann H., Johnson D.R., Men Y., Fischer S., Minder A., Aluri S., Harhangi H.R., Kipf M., Joss A., Christensson M., Nielsen J.L., Morgenroth E. In: A Process Engineering Vista in the Ecogenomics of Aerobic-anaerobic Ammonium Oxidation. Oleszkiewicz, editor. IWA; Gdańsk, Poland: 2015. May 18–21, 2015. [Google Scholar]
- Wett B. Development and implementation of a robust deammonification process. Water Sci. Technol. 2007;56(7):81–88. doi: 10.2166/wst.2007.611. [DOI] [PubMed] [Google Scholar]
- Winkler M.K., Yang J., Kleerebezem R., Plaza E., Trela J., Hultman B., van Loosdrecht M.C. Nitrate reduction by organotrophic Anammox bacteria in a nitritation/anammox granular sludge and a moving bed biofilm reactor. Bioresour. Technol. 2012;114:217–223. doi: 10.1016/j.biortech.2012.03.070. [DOI] [PubMed] [Google Scholar]
- WPO . UNECE; Geneva: 1998. Water Protection Ordinance of 28 October 1998 (814.201) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.