Abstract
The protein kinase (PK) encoded by the Epstein-Barr Virus (EBV) BGLF4 gene is the only EBV protein kinase. The expression pattern of EBV PK during the reactivation of the viral lytic cycle and the subcellular localization of the protein were analyzed with a polyclonal antiserum raised against a peptide corresponding to the N terminus of EBV PK. Based on previously published data (E. Gershburg and J. S. Pagano, J. Virol. 76:998-1003, 2002) and the expression pattern described here, we conclude that EBV PK is an early protein that requires viral-DNA replication for maximum expression. By biochemical fractionation, the protein could be detected mainly in the nuclear fraction 4 h after viral reactivation in Akata cells. Nuclear localization could be visualized by indirect immunofluorescence in HeLa cells transiently expressing EBV BGLF4 in the absence of other viral products. Transient expression of 3′-terminal deletion mutants of EBV BGLF4 resulted in cytoplasmic localization, confirming the presence of a nuclear localization site in the C-terminal region of the protein. In contrast to the wild-type EBV PK, all of the mutants were unable to hyperphosphorylate EA-D during coexpression or to phosphorylate ganciclovir, as measured by an in-cell activity assay. Thus, the results demonstrate that the nuclear localization, as well as the kinase activity, of BGFL4 is dependent on an intact C-terminal region.
Many large DNA viruses encode their own protein kinases that manage different aspects of virus replication and virus-cell interactions (21). Phosphorylation of cellular and viral proteins, which has been observed during lytic infection of cells by herpesviruses, seems to be a common phenomenon that involves a number of different protein kinase activities (17). Two groups of viral protein kinases (PKs) have been identified in herpesviruses. Alphaherpesviruses encode members of both groups. The US3 gene of herpes simplex virus type 1 (HSV-1) (28) exemplifies the first group, which was first predicted to encode protein kinases on the basis of strong similarity to the family of eukaryotic serine/threonine protein kinases. Later, a number of targets for this kinase, such as HSV UL34 (35), US9 (14), UL12 (15), and the cellular proapoptotic protein BAD (15), were identified. UL13, the representative of a second group, has been shown to phosphorylate the viral proteins ICP22 (34), gE and gI (30), and ICP0 (31), as well as cellular translation elongation factor 1 delta (20) and p60 (6). Despite the facts that US3 is involved in the inhibition of apoptosis (2, 22) and both US3 and UL13 regulate a number of key factors, both seem to be dispensable for the replication of HSV-1 in vitro (12, 16, 24).
Beta- and gammaherpesviruses encode only protein kinases belonging to the second group, with homology to HSV UL13. This group, identified by sequence homology, includes UL97 of human cytomegalovirus (HCMV), BGLF4 of Epstein-Barr virus (EBV) (36), U69 of human herpesvirus 6 (1), ORF36 of Kaposi's sarcoma-associated herpesvirus (33), and a few others. This subgroup of UL protein kinases is evolutionarily more distant from the cellular protein kinases than are the alphaherpesvirus US protein kinases. Some members of this group, such as UL97, U69, BGLF4, and ORF36, have been shown to phosphorylate ganciclovir (GCV) (1, 7, 23, 26, 37). The functions of herpesvirus PKs are not fully understood; however, the relatively high degree of conservation of these kinases can be attributed to their importance for the replication of these viruses in their natural hosts and their possible role in viral pathogenesis.
EBV BGLF4 was first identified as a Ser/Thr protein kinase-related gene by the use of amino acid sequence alignment of regions conserved within the catalytic domains of protein kinases (36), and the BGLF4 protein is the only potential protein kinase identified in the EBV genome. Recently, several groups have reported protein kinase activity associated with BGLF4-encoded protein (9, 18, 19); however, the characterization of the protein remains incomplete. In this report, we fill several gaps in knowledge about EBV PK: (i) it is detected in immunoblotting as a single band at ∼49 kDa (the predicted molecular mass is 51 kDa); (ii) it is expressed as early as 4 h after viral reactivation in Akata cells, and protein expression peaks between 12 and 24 h; (iii) the protein is localized in the nuclei of induced Akata or transfected HeLa cells; (iv) while computer analysis did not detect any known nuclear localization sites (NLS, 3′-terminal deletion mutants of BGLF4 exhibited cytoplasmic localization, suggesting the existence of a noncanonical NLS in the C terminus of the protein; (v) all mutants failed to hyperphosphorylate EA-D and to phosphorylate GCV.
MATERIALS AND METHODS
Cell culture.
Akata is a Burkitt's lymphoma (BL) cell line latently infected with EBV (38), DG75 is an EBV-negative BL cell line (4), HeLa is a cervical adenocarcinoma cell line (ATCC CCL2), and HEK293 is a permanent line of primary human embryonal kidney cells transformed by sheared human adenovirus type 5 DNA (ATCC CRL1573). The cells were grown as described previously (40).
Treatment of cells with antiviral compounds and viral reactivation.
Maribavir (previously designated 1263W94) was supplied by GlaxoSmithKline. Exponentially growing cells were centrifuged at 800 × g and suspended in fresh medium containing different concentrations of the compound for an appropriate time. At the end of the treatment, cells were harvested and analyzed depending on the aim of the experiment.
To reactivate viral lytic infection, Akata cells were treated with goat anti-human immunoglobulin G (0.1 mg/ml; Sigma). Treatment with the antiviral compound and viral reactivation were begun at the same time.
Plasmids and transfections.
Cloning of the EBV BGLF4 open reading frame (ORF) has been described previously (18). 3′-truncated mutants were constructed by PCR amplification using the cloned EBV BGLF4 ORF as a template, the PK-5′ oligonucleotide (5′-CCGGATCCCAGCGGGTGGAGG-3′) as a 5′ primer for all mutants, and PK-3′ (5′-GAATTCCTATCCACGTCGGCCATC-3′), PK-(1-374)-3′ (5′-GAATTCCTACGTGACATCCACGCAC-3′), PK-(1-169)-3′ (5′-GAATTCCTACTCAATACTACCATC-3′), or PK-(1-74)-3′ (5′-GAATTCCTAATCACAGCGCGTCATG-3′) as a 3′ primer for full-length PK and mutants expressing the first 374, 169, or 74 amino acids (aa), respectively. The amplified fragments were cloned into pFLAG-CMV2 (Sigma); the resulting plasmids were designated pFPK-FL, pFPK-(1-374), pFPK-(1-169), and pFPK-(1-74). The full-length BGLF4 ORF was also cloned into the vector pEGFP-C1 (Clontech); the resulting plasmid was designated pEGFP/PK. The plasmids pcDNA-UL97, pcDNA-UL97(K355 M), and pcDNA-BGLF4-Flag were described previously (26). HeLa or 293 HEK cells were transfected with the use of Effectine (QIAgen) following the manufacturer's protocol. Transfected cells were cultured in Dulbecco's modified Eagle's medium supplemented by 10% fetal bovine serum and antibiotics for 24 to 48 h at 37°C and then harvested for analysis.
Antibodies, immunoblotting, and indirect immunofluorescence (IF).
Monoclonal antibodies against γ-tubulin and FLAG (M2) were purchased from Sigma and used at 1:5,000 and 1:3,000 dilutions, respectively. Anti-GRP-78 was purchased from Santa Cruz Biotechnology and used at 1:500 dilution. Purified anti-PK antibody was used at a dilution of 1:250. Secondary donkey anti-mouse and anti-rabbit horseradish peroxidase-conjugated antibodies were purchased from Amersham (1:5,000).
The preparation of whole-cell lysates and immunoblotting were described previously (18). For IF, cells were fixed with 3.7% formaldehyde for 10 min, followed by additional fixation and permeabilization in cold acetone for 5 min. EBV EA-D was detected by incubation with R3 monoclonal antibody (a gift from J. Luka) at 1:500 dilution, followed by donkey anti-mouse Alexa-594-conjugated antibody (Molecular Probes) (1:100). EBV PK staining was performed by incubation with a 1:100 dilution of the purified anti-PK antibody, followed by donkey anti-rabbit Alexa-488-conjugated antibody at a 1:100 dilution. Nuclei were stained with Hoechst stain (1:100). Samples were analyzed with a Zeiss Axiophot microscope using a 63 or 100× objective, and digital images were captured with Metamorph imaging software (Universal Imaging Corp.) and a MicroMAX 5-MHz cooled charge-coupled device camera (Princeton Instruments).
In-cell activity assay.
The in-cell activity assay was described in detail previously (27). Briefly, 293 HEK cells were transfected with appropriate plasmid DNA according to the Lipofectamine procedure (Lipofectamine Plus reagents; Gibco BRL). Twenty-four hours posttransfection the media were replaced by diluted GCV (concentrations, 0 to 160 μM). On day 7, color changes were quantified by measuring an optical density at 560 nm.
RESULTS
Temporal expression of EBV PK after viral lytic reactivation in Akata cells.
To study the expression pattern of the BGLF4 gene product, we first produced antibody against EBV PK. New Zealand White rabbits were immunized with a peptide corresponding to the N terminus of the EBV PK protein following standard protocols. To achieve the desired specificity, the resulting antiserum was subsequently purified by affinity chromatography on EBV PK-bound resin. Figure 1A illustrates the specificity of the purified antiserum toward EBV PK. The EBV PK antibody detected a specific band at ∼49 kDa in induced Akata cells (Fig. 1A, lane 3) and in HeLa cells transiently expressing EBV PK (Fig. 1A, lanes 1 and 2) but not in EBV-negative DG-75 cells (Fig. 1A, lane 4). The molecular mass of the protein detected by immunoblotting is in agreement with the predicted molecular mass of 51 kDa, which has been calculated based on the amino acid sequence. A nonspecific band at ∼70 kDa was detected in all tested samples, perhaps due to cross-reactivity with a cellular protein. No specific signal was detected with preimmune serum in both induced Akata and BGLF4-expressing HeLa cells (data not shown).
FIG. 1.
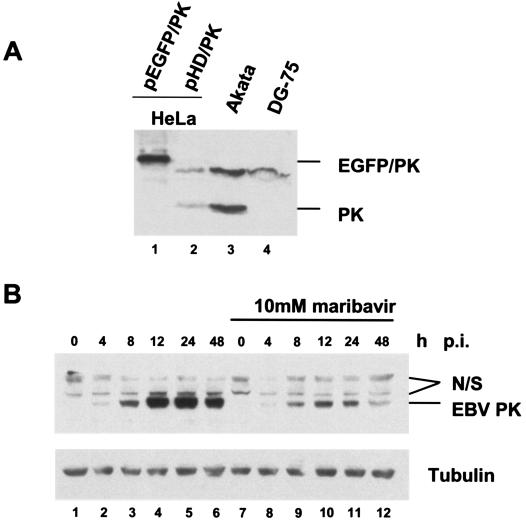
EBV PK expression during viral reactivation in Akata cells. (A) Equal amounts of whole-cell lysates from the HeLa cells transfected with pEGFP/PK or pHD/PK, Akata cells (24 h after viral reactivation), and DG-75 cells were subjected to SDS-PAGE, followed by immunoblotting with the affinity-purified anti-PK antibody. (B) Akata cells were collected at different time points after viral lytic reactivation in the presence or absence of maribavir, and equal amounts of the whole-cell lysates were analyzed by immunoblotting them with anti-PK antibody. Lanes 1 to 6, no maribavir; lanes 7 to 12, samples treated with 10 μM maribavir. Lanes 1 and 7 are latent Akata cells. Nonspecific bands detected by the antibody are marked N/S.
Previously, it was shown that the antiviral compound maribavir affected the kinetics of appearance of pp58, the hyperphosphorylated form of EA-D, in Akata cells during viral reactivation (18). In this study, we used the same treatment in order to assess the effect of maribavir on EBV PK kinetics. The EBV cytolytic cycle was induced by immunoglobulin G treatment (see Materials and Methods) in the presence or absence of 10 μM maribavir, and the expression pattern of the EBV PK protein was analyzed by immunoblotting (Fig. 1B). The analysis revealed that EBV PK appears as early as 4 h postinduction (p.i.) (Fig. 1B, lane 2), peaks between 12 and 24 h p.i. (Fig. 1B, lanes 4 and 5), and is slightly reduced at 48 h p.i. (Fig. 1B, lane 6). The protein levels were significantly reduced after treatment with maribavir (Fig. 1B, compare lanes 2 to 6 to lanes 8 to 12). Previous data (18) indicated that the inhibition of EBV DNA synthesis by maribavir temporally coincided with inhibition by acyclovir, a known inhibitor of EBV replication. Therefore, these results confirm at the protein level that the BGLF4 gene is an early or early-late gene that expresses relatively low levels of protein under early conditions (maribavir treatment), with maximum expression coinciding with viral-DNA replication.
EBV PK intracellular localization during viral reactivation in Akata cells and during transient expression in HeLa cells.
HCMV UL97, an EBV PK homolog, has been detected in the nuclei of infected cells (29). Also, it was recently shown that EBV PK is associated with phosphorylation of the EBV BMRF1 product, EA-D, in vivo (18), and Chen et al. (9) have phosphorylated EA-D with EBV PK in vitro. EA-D is a known nuclear protein (11). However, the intracellular localization of EBV PK protein has not been clearly demonstrated. To address this question, we employed IF analysis and protein extract fractionation, followed by immunoblotting. Akata cells were treated as described above and subjected to biochemical partition by the use of a commercial kit (Pierce) following the manufacturer's protocol. The extracts were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting. The results show that most of the PK is detected in the nuclear fraction (Fig. 2A, lanes 11 to 17), while some of the protein could be detected in the cytoplasm (Fig. 2A, lanes 1 to 4). Treatment with maribavir strongly reduced the EBV PK levels in both the nucleus and the cytoplasm (Fig. 2A, lanes 6 to 9 and 15 to 18).
FIG. 2.
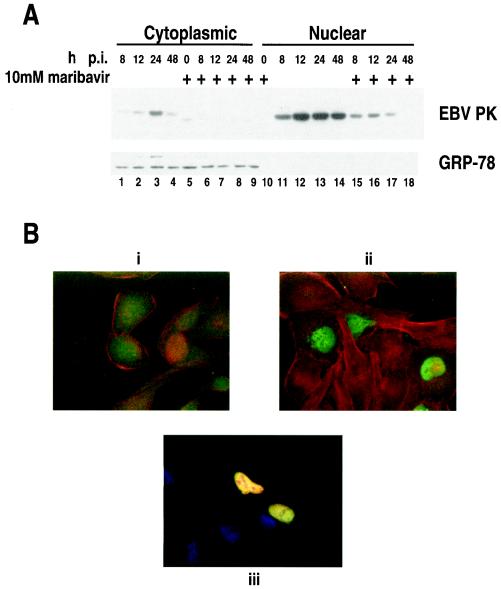
Subcellular localization of EBV PK. (A) Akata cells were collected at the indicated time points after viral reactivation, and nuclear and cytoplasmic fractions were subjected to SDS-PAGE and analyzed by immunoblotting. Lanes 5 to 10 and 15 to 18, samples treated with 10 μM maribavir. GRP-78 served as a cytoplasmic marker. (B) Indirect immunofluorescence of the HeLa cells transfected with pHD/PK alone or cotransfected with pHD/PK and pHD/EA-D. i, preimmune serum; ii, anti-PK serum; iii, anti-PK and anti-EA-D serum. In i and ii, red is actin and green is PK; in iii, red is EA-D, green is PK, and blue is nuclei.
To determine whether the nuclear localization of EBV PK is dependent on other EBV proteins, we performed indirect immunofluorescence analysis of HeLa cells transiently expressing the BGLF4 gene. EBV PK was detected only in the nuclei of transfected cells 24 h posttransfection (Fig. 2B, ii). Moreover, the EBV PK colocalized with EBV EA-D when both were coexpressed in HeLa cells (Fig. 2B, iii). These findings indicate that no other EBV proteins are required for nuclear localization of the EBV PK protein.
Putative NLS of EBV PK protein.
To analyze further the nuclear translocation of the EBV PK protein, a number of C-terminally truncated mutants were created (Fig. 3A). The mutants were transiently expressed in 293 HEK or HeLa cells, and their expression was analyzed by protein extract fractionation. The analysis showed that all of the mutants were localized in the cytoplasm with only trace amounts detected in the nuclei (Fig. 3B). These results suggest the existence of an NLS in the C-terminal region of the protein between aa 374 and 429. Sequence analysis of this region revealed a cluster of basic amino acids that could serve as a putative NLS (RSLKKRFK); however, no significant homology with any of the known NLS was found.
FIG. 3.
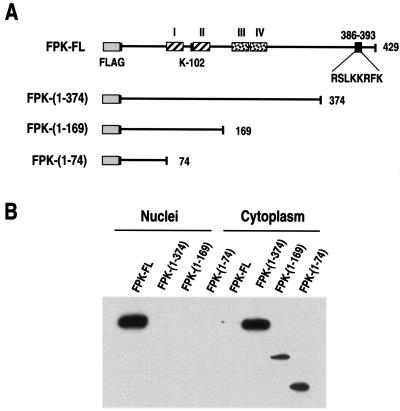
Expression of EBV PK mutants. (A) Summary of BGLF4 mutants. I to IV are the putative functional domains, based on alignment of different kinases. The basic amino acid cluster, catalytic lysine, and FLAG tag are shown as well. The different mutants were generated by PCR, as described in Materials and Methods. (B) The mutants were transfected into HEK 293 cells; 48 h after transfection, the cells were fractionated, and the nuclear and the cytoplasmic fractions were subjected to SDS-PAGE and analyzed by immunoblotting them with anti-FLAG antibody.
Kinase activity of EBV PK mutants.
In order to further characterize the activities of the EBV PK mutants, we used two previously established assays. First, we tested the abilities of the mutants to hyperphosphorylate EBV EA-D during coexpression in 293 HEK cells, and second, we tested their abilities to phosphorylate GCV. The results presented in Fig. 4A demonstrate that all of the mutants failed to hyperphosphorylate EA-D (the detected band corresponds to the pp50 hypophosphorylated form and migrates similarly to EA-D expressed alone), while wild-type PK cloned in the same manner functioned properly (the band detected corresponds to the pp58 hyperphosphorylated form). The in-cell activity assay also showed a lack of GCV phosphorylation by all the EBV PK mutants in comparison to the wild-type EBV PK (Fig. 4B). Thus, the results showed the importance of the intact C terminus of the EBV PK protein for its proper functioning.
FIG. 4.
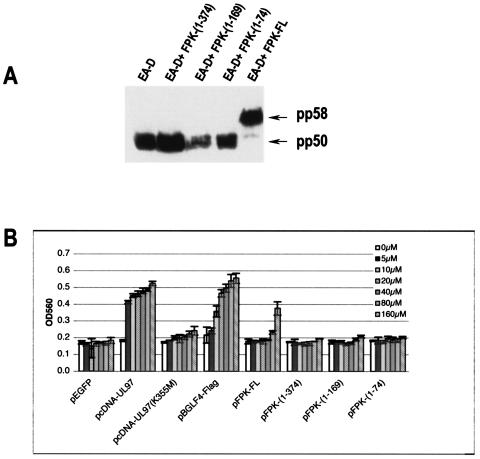
Kinase activities of EBV PK mutants. (A) Hyperphosphorylation of EBV EA-D. The mutants were transfected, along with pHD/BMRF1, into HEK 293 cells; 48 h after transfection, the cells were collected, and the whole-cell lysates were subjected to SDS-PAGE and analyzed by immunoblotting them with EA-D antibody. (B) In-cell activity assay. The cells were treated as described in reference 27 and Materials and Methods. All measurements were based on triplicate transfections in three independent experiments. Mean and standard deviation values are shown. OD560, optical density at 560 nm.
DISCUSSION
Herpesvirus-encoded protein kinases have attracted increasing attention lately due to the growing evidence of their involvement in different aspects of viral life cycles, which makes them appealing targets for antiviral compounds. The HSV-1 UL13 and HCMV UL97 PKs have been studied extensively, whereas the EBV PK has remained largely uncharacterized. In this study, we have addressed a number of questions, including the expression pattern of the protein during viral lytic reactivation and its intracellular localization in different cell lines. To answer these questions, we have used a polyclonal antiserum raised against a peptide corresponding to the N terminus of the protein. The antiserum was affinity purified on a PK-bound column (Fig. 1A). This antiserum made possible direct detection of the protein, in contrast to previous studies in which epitope-tagged proteins and tag-directed antibodies were used (9, 10).
Previously, we reported that EBV BGLF4 is an early gene based on its mRNA levels throughout viral reactivation in Akata cells in the presence of maribavir or acyclovir. The results demonstrated early kinetics comparable to the pattern of expression of the EBV BMRF1 protein, EA-D (18). The results of the present study of the EBV PK protein expression pattern are in agreement with the RNA data (Fig. 1B); interestingly, The PK protein could be detected earlier then EA-D, which correlates with its involvement in EA-D posttranslational modification (18). In contrast to the RNA level, which waned 48 h after viral reactivation, the protein level was substantial at this time (18) (Fig. 1B), which may imply that the protein is relatively stable. Another interesting observation made in the course of this study is that although maribavir was reported to be an enzymatic inhibitor (3, 5), we consistently observed overall reduction in the levels of different viral proteins (Fig. 1B) (18; E. Gershburg and J. S. Pagano, unpublished data), as well as changes in the mRNA profile of at least one EBV gene (Gershburg and Pagano, unpublished). Thus, we cannot exclude the possible effect of the compound on transcription and translation, and we are investigating these possibilities.
Our results reveal that the protein encoded by the EBV BGLF4 gene has the intrinsic capacity for nucleus translocation even in the absence of other EBV proteins. This was clearly shown in HeLa cells, in which the protein was transiently expressed (Fig. 2); furthermore, coexpression of EBV PK with EA-D resulted in their colocalization, as detected by indirect immunofluorescence (Fig. 2). These data are in agreement with previously published results that demonstrated nuclear localization of HSV UL13 (13), HCMV UL97 (29), and Kaposi's sarcoma-associated herpesvirus ORF36 (33). However, they contradict the data published by Chen et al., who reported cytoplasmic localization of EBV BGLF4 (9). The abnormal localization of the BGLF4 product in transient expression could be caused by the EBNA-1 tag system used in that study (10). In contrast, our results are consistent in different cell types and with the use of different methods (immunofluorescence or cell partitioning followed by immunoblotting); in addition, the FLAG-tagged version of the protein was expressed similarly to the wild-type protein (Fig. 1 and 3B).
Nuclear uptake of proteins is a highly selective signal-mediated process. The proteins that are actively translocated into the nucleus have been shown to possess a specific NLS (reviewed in reference 25). A single cluster of basic amino acids (RSLKKRFK) is located in the C terminus of EBV PK, and deletion of the last 55 aa resulted in cytoplasmic retention of the protein (Fig. 3). In contrast to the observation with HCMV UL97, where deletion of the putative NLS resulted in mixed distribution of the protein that was attributed to a second basic region (29), EBV BGLF4 possesses only one basic region, deletion of which resulted in the cytoplasmic localization of all tested mutants. These data suggest that the protein possesses only one putative NLS, which is located in the C terminus; however, we were unable to find significant homology between the basic cluster found in EBV PK and any known NLS. Known viral NLS are the classical monopartite [B4, P(B3X), PXX(B3X), and B3(H/P)], bipartite [BBX10(B3X2)], or arginine-rich (RXXRRX1,2RBR) regions, in which B is a basic residue (K or R) and X is any residue (25, 32, 39), whereas, although it remotely resembles a viral NLS motif, the EBV PK cluster (RXXKKRXK) is not a perfect match. Therefore, we are attempting to elucidate the importance of each one of the residues in this motif for protein nuclear translocation.
In assessing the functional properties of the mutants, we examined their abilities to hyperphosphorylate EBV EA-D and to phosphorylate GCV. None of the mutants were active in these two assays, including pFPK-(1-374), which lacks only 55 aa. Since this mutation did not affect any regions important for the kinase activity (8, 9), it is possible that the lack of activity resulted from the subcellular localization of the kinase mutants. This hypothesis is being tested in our laboratory.
The new data presented in this report improve our understanding of the biology and functions of EBV-PK. Studies to better understand EBV PK nuclear translocation, as well as to identify new viral and cellular targets of the kinase, are under way.
Acknowledgments
We thank J. Luka for the mouse monoclonal antibody (R3) against EBV EA-D.
This study was supported in part by grants AI042371 and HL64851 from the National Institutes of Health.
REFERENCES
- 1.Ansari, A., and V. C. Emery. 1999. The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses. J. Virol. 73:3284-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., J. O'Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baek, M. C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase. Importance of the P+5 position. J. Biol. Chem. 277:29593-29599. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwich, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a ′Burkitt like' malignant lymphoma (line DG-75). Int. J. Cancer 19:27-33. [DOI] [PubMed] [Google Scholar]
- 5.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, I. A. Smith, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruni, R., B. Fineschi, W. O. Ogle, and B. Roizman. 1999. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0, is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J. Virol. 73:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon, J. S., F. Hamzeh, S. Moore, J. Nicholas, and R. F. Ambinder. 1999. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J. Virol. 73:4786-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 9.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, M. R., H. Huang, C. Y. Fen, and J. Y. Chen. 2000. A novel EBNA-1 tag system for high level expression and efficient detection of fusion proteins in vitro and in vivo. J. Virol. Methods 85:35-41. [DOI] [PubMed] [Google Scholar]
- 11.Cho, M. S., G. Milman, and S. D. Hayward. 1985. A second Epstein-Barr virus early antigen gene in BamHI fragment M encodes a 48- to 50-kilodalton nuclear protein. J. Virol. 56:860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulter, L. J., H. W. Moss, J. Lang, and D. J. McGeoch. 1993. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 74:387-395. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 14.Daikoku, T., R. Kurachi, T. Tsurumi, and Y. Nishiyama. 1994. Identification of a target protein of US3 protein kinase of herpes simplex virus type 2. J. Gen. Virol. 75:2065-2068. [DOI] [PubMed] [Google Scholar]
- 15.Daikoku, T., Y. Yamashita, T. Tsurumi, and Y. Nishiyama. 1995. The US3 protein kinase of herpes simplex virus type 2 is associated with phosphorylation of the UL12 alkaline nuclease in vitro. Arch. Virol. 140:1637-1644. [DOI] [PubMed] [Google Scholar]
- 16.de Wind, N., J. Domen, and A. Berns. 1992. Herpesviruses encode an unusual protein-serine/threonine kinase which is nonessential for growth in cultured cells. J. Virol. 66:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feighny, R. J., M. P. Farrell, and J. S. Pagano. 1980. Polypeptide synthesis and phosphorylation in Epstein-Barr virus-infected cells. J. Virol. 34:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershburg, E., and J. S. Pagano. 2002. Phosphorylation of the Epstein-Barr virus (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the l-riboside benzimidazole 1263W94. J. Virol. 76:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1δ (EF-1δ): EF-1δ is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the U(L)13 gene of herpes simplex virus 1. J. Virol. 72:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leader, D. P. 1993. Viral protein kinases and protein phosphatases. Pharmacol Ther. 59:343-389. [DOI] [PubMed] [Google Scholar]
- 22.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 24.Longnecker, R., and B. Roizman. 1987. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science 236:573-576. [DOI] [PubMed] [Google Scholar]
- 25.Macara, I. G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65:570-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2002. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J. Gen. Virol. 83:1013-1023. [DOI] [PubMed] [Google Scholar]
- 27.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2001. Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97. J. Gen. Virol. 82:1439-1450. [DOI] [PubMed] [Google Scholar]
- 28.McGeoch, D. J., and A. J. Davison. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 14:1765-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel, D., I. Pavic, A. Zimmermann, E. Haupt, K. Wunderlich, M. Heuschmid, and T. Mertens. 1996. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J. Virol. 70:6340-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37-48. [DOI] [PubMed] [Google Scholar]
- 31.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406-413. [DOI] [PubMed] [Google Scholar]
- 32.Palmeri, D., and M. H. Malim. 1999. Importin beta can mediate the nuclear import of an arginine-rich nuclear localization signal in the absence of importin alpha. Mol. Cell. Biol. 19:1218-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, J., D. Lee, T. Seo, J. Chung, and J. Choe. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) open reading frame 36 protein is a serine protein kinase. J. Gen. Virol. 81:1067-1071. [DOI] [PubMed] [Google Scholar]
- 34.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc. Natl. Acad. Sci. USA 89:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purves, F. C., D. Spector, and B. Roizman. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 65:5757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. (Errata, 359:85, 1992, and 366:756, 1993.) [DOI] [PubMed] [Google Scholar]
- 38.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt's lymphoma lines. Int. J. Cancer. 33:27-32. [DOI] [PubMed] [Google Scholar]
- 39.Truant, R., and B. R. Cullen. 1999. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol. Cell. Biol. 19:1210-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zacny, V. L., E. Gershburg, M. G. Davis, K. K. Biron, and J. S. Pagano. 1999. Inhibition of Epstein-Barr virus replication by a benzimidazole l-riboside: novel antiviral mechanism of 5,6-dichloro-2-(isopropylamino)-1-beta-l-ribofuranosyl-1H-benzimidazole. J. Virol. 73:7271-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]


