Abstract
Seminal fluid enhanced human T-cell leukemia virus type 1 (HTLV-1) infection by transactivating the HTLV-1 long terminal repeat promoter, which is chromosomally integrated in a cell-type-dependent manner. Our data may indicate a potential role for seminal fluid in the sexual transmission of HTLV-1 and imply complex features of regulation of HTLV-1 expression.
Human T-cell leukemia virus type 1 (HTLV-1), the causative agent of adult T-cell leukemia and HTLV-associated myelopathy, is transmitted vertically via breastfeeding and horizontally via sexual intercourse. Male-to-female transmission occurs exceedingly more frequently than female-to-male transmission (15). Given such a disproportion between genders in susceptibility to sexual transmission of HTLV-1, it is possible that a semen-derived factor(s) facilitates male-to-female transmission (10-12). We show here that seminal fluid enhances in vitro HTLV-1 infection. We also report that the seminal fluid-mediated effect on HTLV-1 expression requires its chromosomal integration and is cell type specific.
(This report was previously presented in part at the 11th International Conference on Human Retrovirology: HTLV and Related Viruses, 2003 [abstract P96].)
Seminal fluid enhances HTLV-1 replication and transmission.
Seminal fluid was prepared from healthy male volunteers as described previously (1). Peripheral blood mononuclear cells (PBMC) obtained from asymptomatic HTLV-1 carriers were cultured in the presence or absence of seminal fluid.
HTLV-1 p19 antigen levels were measured as described previously (10). Cellular DNA was extracted by a QIAamp blood DNA kit (QIAGEN K.K., Tokyo, Japan) and subjected to PCR with a QuantiTect SYBR Green PCR kit (QIAGEN). The upstream and downstream primer sequences in the HTLV-1 tax gene that were selected for PCR analysis were 5′-CCCACTTCCCAGGGTTTGGACAGAG-3′ and 5′-CTGTAGAGCTGAGCCGATAACGCG-3′, respectively. Quantitative determination of the amplified products was done with the iCycler iQ Real-Time Detection System (Bio-Rad Laboratories, Inc., Hercules, Calif.). Heat activation (15 min at 95°C) of hot-start Taq polymerase was followed by 50 cycles of denaturation (30 s at 95°C), annealing (30 s at 50°C), and extension (30 s at 72°C). The cell numbers used for PCR analysis were confirmed by simultaneous PCR of the tubulin gene. Cellular RNA was extracted with a QIAamp RNA kit (QIAGEN) and subjected to PCR with a QuantiTect SYBR Green reverse transcription (RT)-PCR kit (QIAGEN). One-step RT-PCR was performed at 50°C for 30 min, followed by the same reactions as above. Tax mRNA levels were standardized by using those of tubulin mRNA.
HTLV-1 p19 antigen production and Tax mRNA levels were markedly enhanced by seminal fluid while proviral loads were modestly enhanced (Table 1). Although semen may be diluted in and poured out of the female genital tract after sexual intercourse, the concentration used in these experiments (1%) could be easily achieved in vivo. The viability of PBMC was found by trypan blue staining to be 80 to 90% and 85 to 95% in the presence and absence of 1% seminal fluid, respectively, throughout the experiments. These results suggest that seminal fluid can enhance HTLV-1 replication
TABLE 1.
Seminal fluid enhances replication and expression of HTLV-1a
| Donor | Seminal fluid | Proviral load (copies/100 PBMC) | (Tax mRNA/tubulin mRNA) × 100 | p19 antigen (pg/ml) |
|---|---|---|---|---|
| 1 | − | 0.35 | 1.08 | <25 |
| + | 1.25 | 4.18 | 68 | |
| 2 | − | 2.86 | 3.63 | 254 |
| + | 10.6 | 42.5 | 2,880 | |
| 3 | − | 0.12 | 0.76 | <25 |
| + | 0.19 | 9.24 | 186 | |
| 4 | − | 1.02 | 2.20 | 45 |
| + | 1.44 | 6.86 | 122 | |
| 5 | − | 0.08 | 0.022 | <25 |
| + | 0.30 | 1.32 | 32 | |
| 6 | − | 2.42 | 2.11 | 102 |
| + | 4.41 | 10.1 | 654 |
PBMC from six asymptomatic HTLV-1 carriers were propagated, and 3 million PBMC each were cultured in the presence or absence of seminal fluid (1%). Cell-free supernatants and cell pellets were collected on day 5. DNA and RNA were purified from the cell pellets, and real-time PCR and real-time RT-PCR were performed to estimate proviral loads and Tax mRNA levels, respectively. HTLV-1 p19 antigen levels in cell-free supernatants were determined by enzyme-linked immunosorbent assay.
To demonstrate whether seminal fluid can enhance viral transmission, PBMC from HTLV-1-uninfected individuals were cocultured with carriers' PBMC that had been treated with mitomycin C (MMC). Since MMC rendered the carriers' PBMC incapable of proliferating and supporting de novo HTLV-1 infection, HTLV-1 replication in the coculture largely depends on viral transmission to PBMC from HTLV-1-uninfected individuals (4, 10). Seminal fluid increased HTLV-1-infected cell numbers in this coculture system but had little effect on infected cell numbers in MMC-treated PBMC alone (Fig. 1A), suggesting that seminal fluid facilitated de novo HTLV-1 infection in PBMC derived from uninfected donors.
FIG. 1.
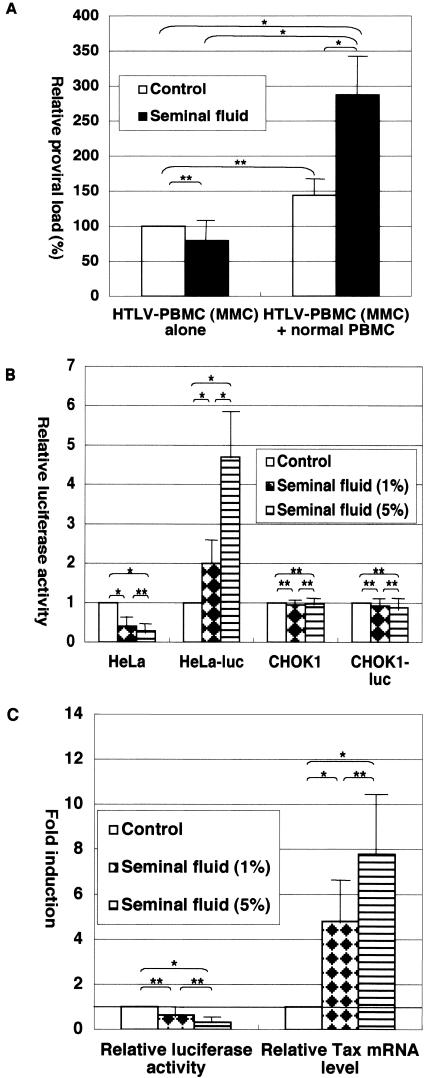
Seminal fluid-mediated effects on HTLV-1 infection. (A) Seminal fluid facilitates de novo HTLV-1 infection. PBMC were isolated from three asymptomatic HTLV-1 carriers and treated with MMC as described previously (2). MMC-treated, HTLV-1-infected PBMC were cultured either alone or with PBMC derived from healthy uninfected donors at a ratio of 1:1. Where indicated, seminal fluid (1%) was added to the cultures. On day 7, whole cultures were harvested for DNA purification and proviral loads were determined by real-time PCR. Proviral loads in MMC-treated, HTLV-1-infected PBMC in the absence of seminal fluid were between 0.82 and 1.3 copies per 100 PBMC, and the results shown are means ± standard errors shown as proviral loads relative to them. Student t tests were performed for statistical significance (*, P < 0.05; **, P ≥ 0.05). (B) Differential effects of seminal fluid on HTLV-1 transcription. HeLa and CHOK1 cells were transfected with pHTLV-luc and pMT-Tax, while HeLa-luc and CHOK1-luc cells were transfected with pMT-Tax alone. The transfected cells were left untreated or treated with the indicated concentrations of seminal fluid and harvested for luciferase assays 2 days after transfection. Data are means ± stand errors from six independent experiments, and results are shown as fold induction relative to the luciferase activity in untreated (control) cells. Student t tests were performed for statistical significance (*, P < 0.05; **, P ≥ 0.05). (C) Seminal fluid increases expression from integrated provirus but decreases expression from transfected plasmid in PBMC. PBMC derived from asymptomatic HTLV-1 carriers were transfected with pHTLV-luc and either left untreated or treated with the indicated concentrations of seminal fluid. The transfected cells were harvested 2 days later, cell lysates were subjected to luciferase assays, and RNA purified from the cells was subjected to real-time RT-PCR for Tax and tubulin mRNAs. The data shown are means ± standard errors from six independent experiments, and results are shown as fold induction relative to those in untreated (control) cells. Student t tests were performed for statistical significance (*, P < 0.05; **, P ≥ 0.05).
Seminal fluid upregulates expression from the HTLV-1 LTR.
Since we have previously demonstrated that certain seminal fluid-derived factors can transactivate the HTLV-1 long terminal repeat (LTR) (10-12), we investigated the effects of seminal fluid on the HTLV-1 LTR. Plasmid pHTLV-luc, provided by K.-T. Jeang (National Institute of Allergy and Infectious Diseases, Bethesda, Md.) (7), was transfected into PBMC with a Human T-Cell Nucleofector kit (Amaxa Biosystems) as described previously (13). Transfections of HeLa (cervical carcinoma) cells, CHOK1 cells, HeLa-luc cells, or CHOK1-luc cells (containing a chromosomally integrated HTLV-1 LTR-driven luciferase gene), also gifts of K.-T. Jeang (14), were performed by a modified calcium phosphate method (9). Transfection efficiency in these adherent cells was tested by cotransfection with pMACS14.1 (Miltenyi Biotec, Bergisch Gladbach, Germany), followed by flow cytometry for CD14 expression, and was found to be comparable (data not shown).
Unexpectedly, seminal fluid downregulated HTLV-1 LTR activity in HeLa cells (Fig. 1B). Interestingly, however, it enhanced HTLV-1 LTR activity in HeLa-luc cells (Fig. 1B), indicating that seminal fluid has opposing effects on the HTLV-1 LTR, depending on whether it exists episomally or is chromosomally integrated. The effects mediated by seminal fluid appear to be cell type dependent, because it had no effect on HTLV-1 LTR activity in CHO or CHO-luc cells (Fig. 1B). Trypan blue staining demonstrated that the seminal fluid concentrations used in these experiments were not toxic to those cell lines (data not shown). To clarify how seminal fluid influences HTLV-1 LTR activity in PBMC, we transfected carriers' PBMC with pHTLV-luc and treated the transfected cells with seminal fluid. LTR activity from the episomal plasmid was determined by luciferase assay, and LTR activity from the integrated proviral DNA was inferred on the basis of Tax mRNA levels. As shown in Fig. 1C, seminal fluid downregulated the activity of the episomal HTLV-1 LTR while upregulating expression from the integrated HTLV-1 LTR. These results suggest that seminal fluid can enhance the transcriptional activation of proviral DNA in carriers' PBMC, probably contributing to seminal fluid-induced HTLV-1 replication and transmission.
Sexual, particularly male-to-female, transmission has been critical for the coexistence of HTLV-1 with the host because infected females subsequently transmit the virus to the next generation. Male-to-female transmission is exceedingly more efficient than female-to-male transmission, at least in part because this virus is highly cell associated (15), although involvement of cell-free virus in sexual transmission was not ruled out. Furthermore, male-to-female transmission may also be potentiated by the fact that the target tissue in the female genital tract is greater in size than that in the male genital tract.
This study suggests that seminal fluid-derived factors may play a role in sexual transmission. We have previously demonstrated that prostaglandin E2 (10), lactoferrin (11), and transforming growth factor β (12), all of which are major constituents of seminal fluid, could enhance in vitro HTLV-1 replication. However, while they upregulated HTLV-1 LTR activity in transient-expression assays (10-12), seminal fluid-mediated activity upregulated chromosomally integrated HTLV-1 LTR but not transiently transfected pHTLV-luc. Therefore, the effect of crude seminal fluid may not be simple addition of those factors but has more complex features. Our preliminary studies, including size fractionation and treatment with RNase A or proteinase K, indicate that not a single factor but a combination of several factors is involved in the effects of seminal fluid on the HTLV-1 LTR (data not shown).
Differential requirements for activation of the integrated and transiently transfected HTLV-1 LTR (14) and the human immunodeficiency virus type 1 LTR (3, 8) have been reported. The HeLa-luc cells used in this study were a pool of three independent HeLa clones with integration of two to four copies of pHTLV-luc, to minimize biases stemming from particular cellular integration sites (14). We also used PBMC from several different HTLV-1-infected donors to perform the experiments whose results are shown in Fig. 1B. Therefore, it is unlikely that the discrepancy in seminal fluid-mediated effects between the integrated and transiently transfected HTLV-1 LTR depends on integration sites.
Since seminal fluid-mediated transactivation of the HTLV-1 LTR was observed in PBMC and HeLa cells but not in CHOK cells, cell-type-specific mechanisms must be considered. Interestingly, induction of the expression of certain genes in cervical epithelial cells by seminal fluid has also been reported (5, 6). We confirmed this observation and extended the targets of this seminal fluid activity to PBMC. It is of note that seminal fluid induces expression of heat shock protein 70 (Hsp70) in cervical epithelial cells (5) and that HTLV-1 expression is enhanced following the cellular stress response that results in production of Hsp70 family proteins (2). Therefore, Hsp70 may play a role in the cell-type-dependent effect of seminal fluid on HTLV-1 infection.
Our preliminary studies suggested that the observed activity of seminal fluid on HTLV-1 LTR transactivation appears to result not from a single factor but from a combination of several factors. This complex feature of the effect of seminal fluid was not unexpected, considering the fact that seminal fluid contains a number of factors, including nucleases, proteases, and many other bioactive factors. Further studies are necessary to determine by what mechanisms and which factor(s) in seminal fluid mediates chromosomal integration-dependent transactivation of the HTLV-1 LTR.
Acknowledgments
We thank K.-T. Jeang for precious materials; S. Chiyoda, H. Okuda, and K. Deguchi (Nagasaki Red Cross Blood Center) for providing blood samples; and M. Yokoyama for excellent technical assistance.
This work was supported in part by grants provided by a Research for the Future Program (JSPS-RFTF97L00705) of the Japan Society for the Promotion of Science, The Japan Leukemia Research Foundation, The Mother and Child Health Foundation, and The ATL Prevention Program Nagasaki. This study was approved by the Institutional Review Board of the Nagasaki University School of Medicine.
REFERENCES
- 1.Anderson, D. J., J. A. Politch, L. D. Tucker, R. Fichorova, F. Haimovici, R. E. Tuomala, and K. H. Mayer. 1998. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S43-S49. [PubMed] [Google Scholar]
- 2.Andrews, J. M., M. J. Oglesbee, A. V. Trevino, D. J. Guyot, G. C. Newbound, and M. D. Lairmore. 1995. Enhanced human T-cell leukemia virus type I expression following induction of the cellular stress response. Virology 208:816-820. [DOI] [PubMed] [Google Scholar]
- 3.Benkirane, M., R. F. Chun, H. Xiao, V. V. Ogryzko, B. H. Howard, Y. Nakatani, and K.-T. Jeang. 1998. Activation of integrated provirus requires histone acetyltransferase. P300 and P/CAF are coactivators for HIV-1 Tat. J. Biol. Chem. 273:24898-24905. [DOI] [PubMed] [Google Scholar]
- 4.Busch, M. P., T. H. Lee, and J. Heitman. 1992. Allogeneic leukocytes but not therapeutic blood elements induce reactivation and dissemination of latent human immunodeficiency virus type 1 infection: implications for transfusion support of infected individuals. Blood 80:2128-2135. [PubMed] [Google Scholar]
- 5.Jeremias, J. C., A. M. Bongiovanni, and S. S. Witkin. 1999. Induction of heat shock protein expression in cervical epithelial cells by human semen. Infect. Dis. Obstet. Gynecol. 7:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Jeremias, J., and S. S. Witkin. 1999. Effect of human seminal fluid on production of messenger ribonucleic acid for metalloproteinase 2 and metalloproteinase 9 in cervical epithelial carcinoma cells. Am. J. Obstet. Gynecol. 181:591-595. [DOI] [PubMed] [Google Scholar]
- 7.Kibler, K. V., and K.-T. Jeang. 2001. CREB/ATF-dependent repression of cyclin A by human T-cell leukemia virus type 1 Tax protein. J. Virol. 75:2161-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller, S. C., A. Taylor, K. Watanabe, K. Mok, and F. M. Torti. 1997. Regulation of NF-κB and HIV-1 LTR activity in mouse L cells by ultraviolet radiation: LTR trans-activation in a nonirradiated genome in heterokaryons. Exp. Cell Res. 230:9-21. [DOI] [PubMed] [Google Scholar]
- 9.Moriuchi, M., H. Moriuchi, S. E. Straus, and J. I. Cohen. 1994. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology 200:297-300. [DOI] [PubMed] [Google Scholar]
- 10.Moriuchi, M., H. Inoue, and H. Moriuchi. 2001. Reciprocal interactions between human T-lymphotropic virus type 1 and prostaglandins: implications for viral transmission. J. Virol. 75:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moriuchi, M., and H. Moriuchi. 2001. A milk protein lactoferrin enhances human T-cell leukemia virus type I and suppresses HIV-1 infection. J. Immunol. 166:4231-4236. [DOI] [PubMed] [Google Scholar]
- 12.Moriuchi, M., and H. Moriuchi. 2002. Transforming growth factor-β enhances human T-cell leukemia virus type I infection. J. Med. Virol. 71:343-346. [DOI] [PubMed] [Google Scholar]
- 13.Moriuchi, M., and H. Moriuchi. 2003. YY1 transcription factor downregulates the expression of CCR5, a co-receptor for HIV-1 entry. J. Biol. Chem. 278:13003-13007. [DOI] [PubMed] [Google Scholar]
- 14.Okada, M., and K.-T. Jeang. 2002. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 76:12564-12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuver, S. O., N. Tachibana, A. Okayama, S. Shioiri, Y. Tsunetoshi, K. Tsuda, and N. E. Mueller. 1993. Heterosexual transmission of human T cell leukemia/lymphoma virus type 1 among married couples in southwestern Japan: an initial report from the Miyazaki cohort study. J. Infect. Dis. 167:57-65. [DOI] [PubMed] [Google Scholar]


